Sugarcane Bagasse and Orange Peels as Low-Cost Biosorbents for the Removal of Lead Ions from Contaminated Water Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Instrumentation and Equipment
2.3. Biosorbents’ Collection and Preparation
2.4. Characterisation Studies
2.4.1. Characterisation by Zeta Potential Analyser
2.4.2. Characterisation by Fourier Transform Infrared Spectroscopy
2.4.3. Characterisation by Thermogravimetric Analysis
2.4.4. Characterisation by Scanning Electron Microscopy-Energy Dispersive Spectroscopy
2.4.5. Characterisation by Transmission Electron Microscopy
2.4.6. Characterisation by Powder X-ray Diffraction
2.4.7. Characterisation by Brunauer–Emmett–Teller
2.5. Preparation of Samples
2.5.1. Preparation of Pb(II) Adsorbate Solutions
2.5.2. Preparation of Calibration Standards for Analysis by FAAS
2.6. Batch Adsorption Studies
2.6.1. Removal of Pb(II) Using SCB and OPS Individually
Effect of pH
Effect of Contact Time
Effect of Adsorbent Dosage
Effect of Initial Metal Ion Concentration
2.6.2. Removal of Pb(II) Using a Combination of Homogenised SCB and OPS Biosorbents
Effect of Contact Time
Effect of Adsorbent Dosage Ratio Variation
Initial Metal Ion Concentration
2.6.3. Effect of Real Water Sample Matrix
2.6.4. Regeneration of SCB and OPS
2.7. Evaluation of Analytical Figures of Merit
2.7.1. Determination of Limit of Detection, Limit of Quantification, and Linearity
2.7.2. Determination of Method Accuracy and Precision
3. Results and Discussion
3.1. Characterisation of Biosorbents
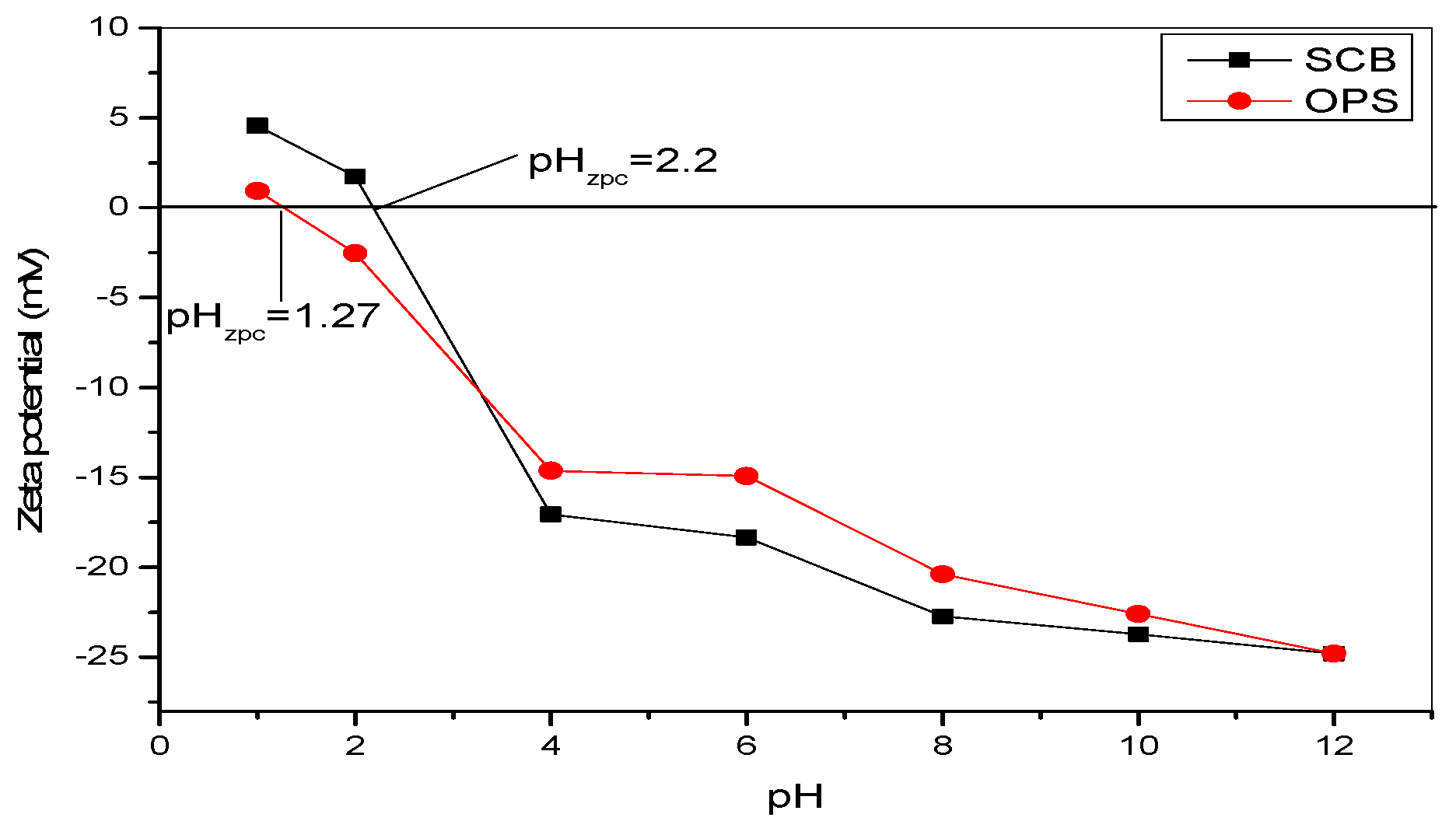
3.1.1. Zeta Potential Analysis
3.1.2. FTIR Spectroscopy Analysis
3.1.3. TGA
3.1.4. SEM and EDS Analyses
3.1.5. TEM Analysis
3.1.6. pXRD Analysis
3.1.7. BET Analysis
3.2. Batch Adsorption Studies
3.2.1. Adsorption Studies Using SCB and OPS Individually, and in a Homogenised Combination of Bisorbents
Evaluation of pH Effect on Adsorption
Evaluation of Contact Time Effect on Adsorption
Evaluation of Adsorbent Dosage Effect on Adsorption
Evaluation of Initial Metal Ion Concentration on Adsorption
3.2.2. Adsorption of Pb(II) in Real Water Samples
3.2.3. Comparison of Pb(II) Removal by Biosorbents
3.2.4. Regeneration of SCB and OPS
3.2.5. Comparison of Adsorption Capacities of SCB, OPS, and Homogenised SCB:OPS with Other Biosorbents
| Biosorbent | Initial Concentration (mg/L) | pH | Contact Time (minutes) | Adsorbent Dosage | Percentage Removal (%) | Adsorption Capacity (mg/g) | Reference |
|---|---|---|---|---|---|---|---|
| Wheat bran | 200 | 7 | 60 | 10 g/L | 98.4 | 49.2 | [69] |
| Rice husks | 0.05 | 9 | 60 | 1 g/30 mL (33.33 g/L) | 96.8 | 0.0622 | [70] |
| Bark | 10 | 5 | 60 | 7.5 g/L | 86.7 | 88.5 | [71] |
| Banana peels | 50 | 5 | 20 | 40 g/L | 85.3 | 2.18 | [72] |
| OPS | 6 | 5 | 15 | 1 g/L | >40 | 27.9 | [39] |
| SCB | 100 | 6 | 120 | 10 g/L | 23.4 | 23.8 | [73] |
| SCB | 10 | 7 | 60 | 0.2 g/100 mL (2 g/L) | 100 | 5 | This study |
| OPS | 20 | 7 | 120 | 0.17 g/100 mL (1.7 g/L) | 100 | 11.8 | This study |
| Homogenised SCB and OPS | 10 | 7 | 120 | 0.2 g/100 mL (2 g/L) | 100 | 5 | This study |
| Initial Pb(II) Concentration (mg/L) | Biosorbent | Dosage | Treated with | Number of Cycles | Adsorption Efficiency (%) | Reference |
|---|---|---|---|---|---|---|
| 50 | Fig sawdust | 0.5 g/50 mL (10 g/L) | 0.1 M HCl | 5 | 93 to 87 | [66] |
| 50 | SCB | 1 g/50 mL (1 g/L) | 0.1 M HNO3 | 3 | 97 to 78 | [74] |
| 57 | SCB | 1 g/L | 1 M HNO3 | 5 | 100 to >85 | [39] |
| 57 | OPS | 1 g/L | 1 M HNO3 | 5 | 100 to >90 | [39] |
| 25 | Groundnut husk | 2 g/L | 0.1 M H2SO4 | 5 | 81.3 to 26.65 | [61] |
| 10 | SCB | 0.2 g/100 mL (2 g/L) | 0.3 HNO3 | 4 | 100 to 49.63 | This study |
| 10 | OPS | 0.17 g/100 mL (1.7 g/L) | 0.3 HNO3 | 3 | 100 to 93.22 | This study |
| 10 | Homogenised SCB and OPS | 0.2 g/100 mL (2 g/L) | 0.3 HNO3 | 3 | 100 to 68.26 | This study |
3.2.6. Analytical Figures of Merit
Determination of Limit of Detection and Limit of Quantification
Linearity
Evaluation of Method Accuracy
Evaluation of Method Precision
4. Conclusions and Recommendations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- du Preez, C.C.; van Huyssteen, C.W. Threats to soil and water resources in South Africa. Environ. Res. 2020, 183, 109015. [Google Scholar] [CrossRef]
- Kumari, P. Application of Sugarcane Bagasse for the Removal of Chromium(VI) and Zinc(II) From Aqueous Solution. Int. Res. J. Eng. Technol. 2017, 4, 1670–1673. [Google Scholar]
- Linos, A.; Petralias, A.; Christophi, C.A.; Christoforidou, E.; Kouroutou, P.; Stoltidis, M.; Veloudaki, A.; Tzala, E.; Makris, K.C.; Karagas, M.R. Oral ingestion of hexavalent chromium through drinking water and cancer mortality in an industrial area of Greece-An ecological study. Environ. Heal. A Glob. Access Sci. Source 2011, 10, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brink, H.G.; Hörstmann, C.; Peens, J. Microbial Pb(II)-precipitation: The influence of oxygen on Pb(II)-removal from aqueous environment and the resulting precipitate identity. Int. J. Environ. Sci. Technol. 2020, 17, 409–420. [Google Scholar] [CrossRef]
- Shah, G.M.; Nasir, M.; Imran, M.; Bakhat, H.F.; Rabbani, F.; Sajjad, M.; Farooq, A.B.U.; Ahmad, S.; Song, L. Biosorption potential of natural, pyrolysed and acid-assisted pyrolysed sugarcane bagasse for the removal of lead from contaminated water. PeerJ 2018, 6, e5672. [Google Scholar] [CrossRef]
- Van Tran, T.; Bui, Q.T.P.; Nguyen, T.D.; Le, N.T.H.; Bach, L.G. A comparative study on the removal efficiency of metal ions (Cu2+, Ni2+, and Pb2+) using sugarcane bagasse-derived ZnCl2-activated carbon by the response surface methodology. Adsorpt. Sci. Technol. 2017, 35, 72–85. [Google Scholar] [CrossRef]
- Molele, L.S.; Magadzu, T.; Ambushe, A.A. Removal of Pb2+ from contaminated water using modified multiwalled carbon nanotubes. Dig. J. Nanomater. Biostruct. 2021, 16, 995–1009. [Google Scholar]
- Niu, Y.; Qu, R.; Sun, C.; Wang, C.; Chen, H.; Ji, C.; Zhang, Y.; Shao, X.; Bu, F. Adsorption of Pb(II) from aqueous solution by silica-gel supported hyperbranched polyamidoamine dendrimers. J. Hazard. Mater. 2013, 244–245, 276–286. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Bautista-Toledo, I.; Ferro-García, M.A.; Moreno-Castilla, C. Bioadsorption of Pb(II), Cd(II), and Cr(VI) on activated carbon from aqueous solutions. Carbon 2003, 41, 323–330. [Google Scholar] [CrossRef]
- Habib, K. Effect of pH and Initial pb(II) Concentration on The Lead Removal Efficiency from Industrial Wastewater Using Ca(OH)2. Int. J. Water Wastewater Treat 2017, 3, 1–4. [Google Scholar]
- SANS 241-1:2015; Drinking Water. South African National Standards: Pretoria, South Africa, 2015.
- World Health Organization. Lead in Drinking-Water: Background Document for Development of Who Guidelines for Drinking-Water Quality, 2nd ed.; World Health Organisation: Geneva, Switzerland, 2003; pp. 1–16. [Google Scholar]
- Chinyelu, E. Use of unmodified orange peel for the adsorption of Cd(II), Pb(II) and Hg(II) ions in aqueous solutions. Am. J. Phys. Chem. 2015, 4, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Bhagat, M.; Yadav, A.; Yadav, V. Removal of Pb(II) ions from Aqueous Solution using Natural Orange Peel and Activated Orange Peel. Int. J. Res. Advent Technol. 2018, 6, 688–693. [Google Scholar]
- Lasheen, M.R.; Ammar, N.S.; Ibrahim, H.S. Adsorption/desorption of Cd(II), Cu(II) and Pb(II) using chemically modified orange peel: Equilibrium and kinetic studies. Solid State Sci. 2012, 14, 202–210. [Google Scholar] [CrossRef]
- Santucci, R.J.; Scully, J.R. The pervasive threat of lead (Pb) in drinking water: Unmasking and pursuing scientific factors that govern lead release. Proc. Natl. Acad. Sci. USA 2020, 117, 23211–23218. [Google Scholar] [CrossRef]
- Matabane, D.; Ambushe, A.A.; Godeto, T.W.; Mampa, R.M. Identification and Determination of Potentially Toxic Elements in Water and Sediments from BLOOD and Mokolo Rivers In Limpopo Province, South Africa. Ph.D. Thesis, University of Limpopo, Polokwane, South Africa, 2018. [Google Scholar]
- Greenfield, R.; van Vuren, J.H.J.; Wepener, V. Heavy metal concentrations in the water of the Nyl River system, South Africa. African J. Aquat. Sci. 2012, 37, 219–224. [Google Scholar] [CrossRef]
- Edokpayi, J.N.; Odiyo, J.O.; Olasoji, S.O. Assessment of Heavy Metal Contamination of Dzindi River, in Limpopo province, South Africa. Int. J. Nat. Sci. Res. 2014, 2, 185–194. [Google Scholar]
- Dikole, M. Seasonal Analysis of Water and Sediment Along the Umgeni River, South Africa. Ph.D. Thesis, University of KwaZulu-Natal, Durban, South Africa, 2014. [Google Scholar]
- Sarker, T.C.; Azam, S.M.G.G.; El-Gawad, A.M.A.; Gaglione, S.A.; Bonanomi, G. Sugarcane bagasse: A potential low-cost biosorbent for the removal of hazardous materials. Clean Technol. Environ. Policy 2017, 19, 2343–2362. [Google Scholar] [CrossRef]
- Renu, A.M.; Singh, K. Heavy metal removal from wastewater using various adsorbents: A review. J. Water Reuse Desalin. 2017, 7, 387–419. [Google Scholar] [CrossRef]
- Bajpai, M.; Bajpai, M.; Katoch, S.S. Treatment of first-flush rainwater by natural low-cost adsorbents using multimedia filter technology. Eng. Sci. Int. Res. J. 2017, 5, 58–62. [Google Scholar]
- Montero, J.I.Z.; Monteiro, A.S.; Gontijo, E.S.; Bueno, C.C.; de Moraes, M.A.; Rosa, A.H. High efficiency removal of As(III) from waters using a new and friendly adsorbent based on sugarcane bagasse and corncob husk Fe-coated biochars. Ecotoxicol. Environ. Saf. 2018, 162, 616–624. [Google Scholar] [CrossRef] [Green Version]
- Ugbe, F.A.; Pam, A.A.; Ikudayisi, A.V. Thermodynamic Properties of Chromium(III) Ion Adsorption by Sweet Orange (Citrus sinensis) Peels. Am. J. Anal. Chem. 2014, 5, 666–673. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A review on reverse osmosis and nanofiltration membranes for water purification. Polymers 2019, 11, 1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwuozor, K.O.; Oyekunle, I.P.; Oladunjoye, I.O.; Ibitogbe, E.M.; Olorunfemi, T.S. A Review on the Mitigation of Heavy Metals from Aqueous Solution using Sugarcane Bagasse. Sugar Tech. 2022, 24, 1167–1185. [Google Scholar] [CrossRef]
- Rafiq, S.; Kaul, R.; Sofi, S.A.; Bashir, N.; Nazir, F.; Nayik, G.A. Citrus peel as a source of functional ingredient: A review. J. Saudi Soc. Agric. Sci. 2018, 17, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Thabethe, L.E.M.; Marlene Labuschagne, M. Estimation of Technical, Economic and Allocative Efficiencies in Sugarcane Production in South Africa: A Case of Mpumalanga Growers. J. Econ. Sustain. Dev. 2014, 5, 86–95. [Google Scholar]
- Molepo, N.S. Impact of international trade on employment in orange industry of South Africa. J. Agribus. Rural Dev. 2021, 60, 193–201. [Google Scholar] [CrossRef]
- Letsoalo, M.R.; Ambushe, A.A.; Mamo, M.A. Novel Chemoresistive Sensor for Sensitive Detection of Pb2+ Ions Using an Interdigital Gold Electrode Fabricated with a Reduced Graphene Oxide-Based Ion-Imprinted Polymer. ACS Omega 2021, 6, 31528–31538. [Google Scholar] [CrossRef]
- Letsoalo, M.R.; Mamo, M.A.; Ambushe, A.A. Simultaneous quantitative speciation of selected toxic elements in water using high performance liquid chromatography coupled to inductively coupled plasma-mass spectrometry (HPLC-ICP-MS). Phys. Chem. Earth 2021, 124, 103011. [Google Scholar] [CrossRef]
- Mokgohloa, C.P.; Thomas, M.S.; Mokgalaka, N.S.; Ambushe, A.A. Speciation of inorganic chromium in river water by graphite furnace-atomic absorption spectrometry after chroma-bond NH2 column based solid phase extraction. Int. J. Environ. Anal. Chem. 2020, 18, 1–16. [Google Scholar]
- Chen, Q.; Yao, Y.; Li, X.; Lu, J.; Zhou, J.; Huang, Z. Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J. Water Process Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Moubarik, A.; Grimi, N. Valorization of olive stone and sugar cane bagasse by-products as biosorbents for the removal of cadmium from aqueous solution. Food Res. Int. 2015, 73, 169–175. [Google Scholar] [CrossRef]
- Salopek, B.; Krasic, D.; Filipovic, S. Measurement and application of zeta-potential. Rud. Zb. 1992, 4, 147–151. [Google Scholar]
- Nie, S.; Zhang, C.; Zhang, Q.; Zhang, K.; Zhang, Y.; Tao, P.; Wang, S. Enzymatic and cold alkaline pretreatments of sugarcane bagasse pulp to produce cellulose nanofibrils using a mechanical method. Ind. Crops Prod. 2018, 124, 435–441. [Google Scholar] [CrossRef]
- Norulaini, N. FTIR study and bioadsorption kinetics of bioadsorbent for the analysis of metal pollutants. RSC Adv. 2014, 4, 58156–58163. [Google Scholar]
- Abdelhafez, A.A.; Li, J. Removal of Pb(II) from aqueous solution by using biochars derived from sugar cane bagasse and orange peel. J. Taiwan Inst. Chem. Eng. 2016, 61, 367–375. [Google Scholar] [CrossRef]
- Indulekha, J.; Siddarth, M.S.G.; Kalaichelvi, P. Characterization of Citrus Peels for Bioethanol Production. Mater. Energy Environ. Eng. 2017, 2017, 3–12. [Google Scholar]
- Kamsonlian, S.; Sundaramurthy, S.; Balomajumder, C.; Chand, S. Characterization of banana and orange peels: Biosorption mechanism. Int. J. Sci. Technol. Manag. 2011, 2, 1–7. [Google Scholar]
- Ahmed, S.A.; El-Roudi, A.M.; Salem, A.A.A. Removal of Mn(II) from ground water by solid wastes of sugar industry. J. Environ. Sci. Technol. 2015, 8, 338–351. [Google Scholar] [CrossRef] [Green Version]
- Licona-Aguilar, Á.I.; Torres-Huerta, A.M.; Domínguez-Crespo, M.A.; Palma-Ramírez, D.; Conde-Barajas, E.; Negrete-Rodríguez, M.X.L.; Rodríguez-Salazar, A.E.; García-Zaleta, D.S. Reutilization of waste biomass from sugarcane bagasse and orange peel to obtain carbon foams: Applications in the metal ions removal. Sci. Total Environ. 2022, 831, 154883. [Google Scholar] [CrossRef]
- Lugo-Lugo, V.; Hernández-López, S.; Barrera-Díaz, C.; Ureña-Núñez, F.; Bilyeu, B. A comparative study of natural, formaldehydetreated and copolymer-grafted orange peel for Pb(II) adsorption under batch and continuous mode. J. Hazard. Mater. 2009, 161, 1255–1264. [Google Scholar] [CrossRef]
- Akbarzadeh, F.; Motaghi, M.; Chauhan, N.P.S.; Sargazi, G. A novel synthesis of new anti-bacterial nanostructures based on Zn-MOF compound: Design, characterization and a high performance application. Heliyon 2020, 6, e03231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phromsuwan, U.; Sirisisathitkul, C.; Sirsisathitkul, Y.; Uyyanonvara, B.; Muneesawang, P. Application of image processing to determine size distribution of magnetic nanoparticles. J. Magn. 2013, 18, 311–316. [Google Scholar] [CrossRef]
- Cui, L.; Wei, X.; Li, J.; Chang, G. Structure and Saccharification of Sugarcane Bagasse Pre-treated with Acid Coupled Alkaline. Proc. Int. Symp. Mech. Eng. Mater. Sci. 2017, 2017, 17–19. [Google Scholar]
- Ash, S.; Fungaro, D.A.; Reis, T.V.S.; Logli, M.A.; Oliveira, N.A. Synthesis and Characterization of Zeolitic Material Derived from Sugarcane Straw Ash. Am. J. Environ. Prot. 2014, 2, 16–21. [Google Scholar]
- Babu, B.V.; Gupta, S. Adsorption of Cr(VI) using activated neem leaves: Kinetic studies. Adsorption 2008, 14, 85–92. [Google Scholar] [CrossRef]
- Mohan, D.; Kumar, H.; Sarswat, A.; Alexandre-Franco, M.; Pittman, C.U. Cadmium and lead remediation using magnetic oak wood and oak bark fast pyrolysis biochars. Chem. Eng. J. 2014, 236, 513–528. [Google Scholar] [CrossRef]
- Samsuri, A.W.; Sadegh-Zadeh, F.; Seh-Bardan, B.J. Adsorption of As(III) and As(V) by Fe coated biochars and biochars produced from empty fruit bunch and rice husk. J. Environ. Chem. Eng. 2013, 1, 981–988. [Google Scholar] [CrossRef]
- Kerrou, M.; Bouslamti, N.; Raada, A.; Elanssari, A.; Mrani, D.; Slimani, M.S. The Use of Sugarcane Bagasse to Remove the Organic Dyes from Wastewater. Int. J. Anal. Chem. 2021, 2021, 5570806. [Google Scholar] [CrossRef]
- Machida, M.; Yamazaki, R.; Aikawa, M.; Tatsumoto, H. Role of minerals in carbonaceous adsorbents for removal of Pb (II) ions from aqueous solution Role of minerals in carbonaceous adsorbents for removal of Pb (II) ions from aqueous solution. Se Purif. Technol. 2005, 46, 88–94. [Google Scholar] [CrossRef]
- Pang, F.M.; Teng, S.P.; Teng, T.T.; Mohd Omar, A.K. Heavy metals removal by hydroxide precipitation and coagulation-flocculation methods from aqueous solutions. Water Qual. Res. J. Can. 2009, 44, 174–182. [Google Scholar] [CrossRef]
- Badmus, M.A.O.; Audu, T.O.K.; Anyata, B.U. Removal of Lead Ion from Industrial Wastewaters by Activated Carbon Prepared from Periwinkle Shells (Typanotonus fuscatus). Turkish J. Eng. Enc. Sci. 2007, 31, 251–263. [Google Scholar]
- Maity, S.; Patil, P.B.; SenSharma, S.; Sarkar, A. Bioremediation of heavy metals from the aqueous environment using Artocarpus heterophyllus (jackfruit) seed as a novel biosorbent. Chemosphere 2022, 307, 136115. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, A.; Vidhyadevi, T.; Kirupha, S.D.; Ravikumar, L.; Sivanesan, S. Removal of chromium (VI) from aqueous solution using chemically modified corncorb-activated carbon: Equilibrium and kinetic studies. Environ. Prog. Sustain. Energy 2013, 32, 673–680. [Google Scholar] [CrossRef]
- Jisha, T.J.; Lubna, C.H.; Habeeba, V. Removal of Cr(VI) using Orange Peel as an Adsorbent. Int. J. Adv. Res. Innov. Ideas Educ. 2017, 3, 276–283. [Google Scholar]
- Bharti, S.K.; Kumar, N. Kinetic study of lead (Pb2+) removal from battery manufacturing wastewater using bagasse biochar as biosorbent. Appl. Water Sci. 2018, 8, 119. [Google Scholar]
- Maluleleke, T.; Ambushe, A. The Investigation of Borehole Water Quality in Nwadzekudzeku Village, Giyani, Limpopo Province; Honours, University of Johannesburg: Johannesburg, South Africa, 2021. [Google Scholar]
- Bayuo, J.; Abukari, M.A.; Pelig-Ba, K.B. Desorption of chromium (VI) and lead (II) ions and regeneration of the exhausted adsorbent. Appl. Water Sci. 2020, 10, 171. [Google Scholar] [CrossRef]
- Wołowicz, A.; Hubicki, Z. Adsorption characteristics of noble metals on the strongly basic anion exchanger Purolite A-400TL. J. Mater. Sci. 2014, 49, 6191–6202. [Google Scholar] [CrossRef]
- Kikuchi, T.; Tanaka, S. Biological removal and recovery of toxic heavy metals in water environment. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1007–1057. [Google Scholar] [CrossRef]
- Hussain, D.; Khan, S.A.; Alharthi, S.S.; Khan, T.A. Insight into the performance of novel kaolinite-cellulose/cobalt oxide nanocomposite as green adsorbent for liquid phase abatement of heavy metal ions: Modelling and mechanism. Arab. J. Chem. 2022, 15, 103925. [Google Scholar] [CrossRef]
- Farah, K.; Rabia, R.; Jamil, A.; Muhammad, S. Removal of lead (II) from water by adsorption on novel composites of polyaniline with maize bran, wheat bran and rice bran. Asian J. Chem. 2013, 25, 2399–2404. [Google Scholar]
- Ghasemi, M.; Naushad, M.; Ghasemi, N.; Khosravifard, Y. A novel agricultural waste based adsorbent for the removal of Pb(II) from aqueous solution: Kinetics, equilibrium and thermodynamic studies. J. Ind. Eng. Chem. 2014, 20, 454–461. [Google Scholar] [CrossRef]
- Kabir, M.M.; Akter, M.M.; Khandaker, S.; Gilroyed, B.H.; Didarul-Alam, M.; Hakim, M.; Awual, M.R. Highly effective agro-waste based functional green adsorbents for toxic chromium(VI) ion removal from wastewater. J. Mol. Liq. 2022, 347, 118327. [Google Scholar] [CrossRef]
- Hussin, F.; Aroua, M.K.; Szlachta, M. Biochar derived from fruit by-products using pyrolysis process for the elimination of Pb(II) ion: An updated review. Chemosphere 2022, 287, 132250. [Google Scholar] [CrossRef] [PubMed]
- Bulut, Y.; Baysal, Z. Removal of Pb(II) from wastewater using wheat bran. J. Environ. Manag. 2006, 78, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Asrari, E.; Tavallali, H.; Hagshenas, M. Removal of Zn(II) and Pb(II) ions using rice husk in food industrial wastewater. J. Appl. Sci. Environ. Manag. 2010, 14, 159–162. [Google Scholar]
- Naiya, T.K.; Bhattacharya, A.K.; Das, S.K. Adsorption of Pb(II) by sawdust and neem bark from aqueous solutions. Environ. Prog. Sustain. Energy 2008, 27, 313–328. [Google Scholar] [CrossRef]
- Anwar, J.; Shafique, U.; Salman, M.; Dar, A.; Anwar, S. Removal of Pb(II) and Cd(II) from water by adsorption on peels of banana. Bioresour. Technol. 2010, 101, 1752–1755. [Google Scholar] [CrossRef]
- Hamza, I.A.A.; Martincigh, B.S.; Ngila, J.C.; Nyamori, V.O. Adsorption studies of aqueous Pb(II) onto a sugarcane bagasse/multiwalled carbon nanotube composite. Phys. Chem. Earth 2013, 66, 157–166. [Google Scholar] [CrossRef]
- Homagai, P.L.; Ghimire, K.N.; Inoue, K. Preparation and characterization of charred xanthated sugarcane bagasse for the separation of heavy metals from aqueous solutions. Se Sci. Technol. 2011, 46, 330–339. [Google Scholar] [CrossRef]
- Leite, V.D.S.A.; de Jesus, B.G.L.; de Oliveira Duarte, V.G.; Constantino, V.R.L.; Izumi, C.M.S.; Tronto, J.; Pinto, F.G. Determination of chromium (VI) by dispersive solid-phase extraction using dissolvable Zn-Al layered double hydroxide intercalated with L-Alanine as adsorbent. Microchem. J. 2019, 146, 650–657. [Google Scholar] [CrossRef]
- Saçmaci, Ş.; Kartal, Ş. Determination of some trace metal ions in various samples by FAAS after separation/preconcentration by copper(II)-BPHA coprecipitation method. Microchim. Acta 2010, 170, 75–82. [Google Scholar] [CrossRef]
- Baig, J.A.; Kazi, T.G.; Elci, L.; Afridi, H.I.; Khan, M.I.; Naseer, H.M. Ultratrace determination of Cr(VI) and Pb(II) by microsample injection system flame atomic spectroscopy in drinking water and treated and untreated industrial effluents. J. Anal. Methods Chem. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Demirtaş, I.; Bakirdere, S.; Ataman, O.Y. Lead determination at ng/mL level by flame atomic absorption spectrometry using a tantalum coated slotted quartz tube atom trap. Talanta 2015, 138, 218–224. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Contract Laboratory Program for National Functional Guidelines for Inorganic Data Review. TAPPI J. 2010, 9, 36–109. [Google Scholar]















| Parameters | |
|---|---|
| pH | SCB: 7 |
| OPS: 7 | |
| SCB:OPS (5:5): 7 | |
| Contact time (min) | SCB: 60 |
| OPS: 120 | |
| SCB:OPS (5:5): 120 |
| Sample | Surface Area (m2/g) | Pore Size (Å) | Pore Volume (cm3/g) |
|---|---|---|---|
| SCB | 4.90 | 2417 | 0.30 |
| OPS | 1.24 | 131 | 0.22 |
| Parameters | SCB | OPS | SCB:OPS |
|---|---|---|---|
| pH | 7 | 7 | 7 |
| Contact time (min) | 60 | 120 | 120 |
| Adsorbent dosage (g) | 0.2 | 0.17 | 0.2 (1:1) |
| Initial metal ion concentration for 100% removal (mg/L) | 10 | 20 | 10 |
| Adsorbent | LOD (mg/L) | LOQ (mg/L) |
|---|---|---|
| SCB | 0.109 | 0.364 |
| OPS | 0.287 | 0.957 |
| Homogenised SCB and OPS | 0.237 | 0.788 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molaudzi, N.R.; Ambushe, A.A. Sugarcane Bagasse and Orange Peels as Low-Cost Biosorbents for the Removal of Lead Ions from Contaminated Water Samples. Water 2022, 14, 3395. https://doi.org/10.3390/w14213395
Molaudzi NR, Ambushe AA. Sugarcane Bagasse and Orange Peels as Low-Cost Biosorbents for the Removal of Lead Ions from Contaminated Water Samples. Water. 2022; 14(21):3395. https://doi.org/10.3390/w14213395
Chicago/Turabian StyleMolaudzi, Ntsieni Romani, and Abayneh Ataro Ambushe. 2022. "Sugarcane Bagasse and Orange Peels as Low-Cost Biosorbents for the Removal of Lead Ions from Contaminated Water Samples" Water 14, no. 21: 3395. https://doi.org/10.3390/w14213395
APA StyleMolaudzi, N. R., & Ambushe, A. A. (2022). Sugarcane Bagasse and Orange Peels as Low-Cost Biosorbents for the Removal of Lead Ions from Contaminated Water Samples. Water, 14(21), 3395. https://doi.org/10.3390/w14213395









