Abstract
Current knowledge about the potential impacts of microplastics (MP) on vadose zone hydrology is scarce. The primary goal of this study was to address some of the limitations of previous research by developing more reliable and conclusive statistical evidence to better understand whether MP pollution can potentially cause hydrological impacts. We examined the effects of MP shape (type), as well as the magnitude of pollution (MP/soil mass ratio, λ) on water holding capacity (WHC) and bare soil water evaporation (ER) of fine sand, under controlled laboratory conditions. Three different shapes (types) of MP—fiber (polyacrylic), strand (polymethyl methacrylate), and pellet (acrylonitrile butadiene styrene), with six environmentally relevant MP concentration levels (MP/soil mass ratio), all ≤1.5%, were studied. Statistical regressions and non-parametric analyses of variance (i.e., Kruskal–Wallis analysis) indicate that MP pollution has a substantial potential to change WHC and late-stage evaporation, even at relatively low MP concentrations, but has minimal impacts on early stage evaporation of the studied fine sand. The magnitude of the impacts depends on individual MP shape (type) and concentration, connoting those MP impact mechanisms are complex. These findings suggest that the global issue of growing soil–MP pollution should be regarded as a concerning environmental and water resources stressor that could potentially cause widespread environmental change by altering soil-water dynamics at the watershed scale.
1. Introduction
Over the last fifty years, plastics have increasingly replaced traditional materials such as metal, wood, and glass because of their desirable qualities, such as malleability, durability, and cost-efficiency. It is estimated that the yearly rate of plastic production has exponentially increased from 1.5 million tons in 1950 to 259 million tons in 2018 [1], with predictions of a further tripling by 2050 [2]. Witnessing the alarming rate of plastic generation and knowing that traditional and current plastic waste management practices have been incapable of preventing their dissemination into the environment, many researchers have raised concerns about the effects of the enormous amount of plastics accumulating in the environment [3,4,5].
In the environmental pollution literature, plastic pollutants are commonly categorized by size as macroplastics (>5 mm), microplastics (1 µm–5 mm), henceforth called MP, and nanoplastics (<1 µm) [4,6]. MPs in the environment are typically found in a range of shapes (pellets, fragments of irregular shape, fibers, films, and foams) and consist of different synthetic polymers such as polyethylene, polypropylene, polystyrene, polyamide (e.g., nylon), polyester, and acrylic [7]. MP can originate directly from products such as detergents, cosmetics, and industrial washing materials, which contain manufactured MP (also known as primary MP) by design, and indirectly by the continuous degradation and fragmentation of plastics in films, vehicle tires, and fabrics (also known as secondary MP) [8,9,10,11]. For example, studies have shown that one load of laundry clothes made from common synthetic fabrics such as polyester, polyester-cotton blends, and acrylic can release, on average, 700,000 MP items (fibers) to the sewer system [12]. Accumulated MPs may stay for a long time on the land or can then spread to other locations beyond their original disposal point by water, wind, and other transport mechanisms, during floods, windstorms, or soil erosion events [8,13,14,15,16,17,18].
Several scientists and the general public have recently raised concerns about MP pollution in the marine environment [19,20,21,22]. It is evident that most of the plastics discharged to aquatic systems originate from terrestrial sources. Hence, MP contamination is more likely to occur in terrestrial systems than in the marine environment [23,24]. Despite its importance, terrestrial MP pollution (hereafter called soil–MP pollution) has received much less attention than aquatic MP pollution. A literature survey about MP pollution reveals that only 5% of 1331 articles published between 2004 and early 2019 are devoted to terrestrial systems [10]. Data and knowledge about MP pollution in soil systems are quite restricted and not in keeping with the growing rate of plastic pollution in the terrestrial environment.
In turn, most works on terrestrial MP pollution focus on MP impacts on terrestrial organisms, i.e., on biological effects, rather than on hydrologically and ecologically important soil-water dynamics. The first quantitative study showing the significant fingerprint of MP on soil systems was by Zubris and Richards (2005), who found that MP in their studied farmlands had originated from wastewater sludge applied 15 years earlier [25]. However, broader concerns about terrestrial MP pollution only arose after the publication of Rillig’s (2012) outstanding viewpoint letter, emphasizing the need to also study MP pollution in soils and terrestrial systems because (1) MP are small enough to be taken up by biota and thus accumulate in the food chain, and (2) MP can sorb pollutants on their surfaces and negatively impact the terrestrial ecosystem [26]. Since then, several researchers have addressed these concerns, providing revealing evidence on how MP impacts the ecosystem from a biological perspective [27,28,29,30,31]. The main goal of this study is to provide evidence for a third argument about the need to better understand terrestrial MP pollution that: MP could potentially impact the environment by pervasively affecting soil-water dynamics.
So far, there is only a handful of studies on MP impacts on the physical characteristics of soils. For example, one study determined that small polyester fibers increase the volume of larger pores (>30 µm) while reducing the volume of smaller (<30 µm) pores [32]. Another study reported that soil bulk density decreased in all of their experiments after mixing soil samples with MP [31]. Studies about the impacts of plastic films and microfibers on clayey soils concluded that MP could significantly enhance water evaporation by creating channels facilitating water movement [32,33]. It has been reported that plastic fibers added to garden soils could potentially change the soils’ water-retention capacity [31], even though no clear trend was identified in this study due to limited data. Finally, one study showed that MP significantly altered the bulk density, porosity, saturated hydraulic conductivity, field capacity, and soil water repellency of sandy soils, while the pH, electrical conductivity, and aggregate stability were not substantially changed [10].
The potential hydrological impacts of soil–MP pollution are relevant for three reasons. First, due to the integration and non-linearity of the processes involved in the water and energy cycles, e.g., upper soil moisture, latent heat flux, and soil surface temperature [34,35,36], slight but widespread changes in soil-water dynamics, e.g., on water holding capacity or soil evaporation, could plausibly affect water budgets at the watershed scale. Second, several studies suggest that MP pollution is a global and large-scale problem, showing how it is possible for agricultural farms and industrial lands to be significantly polluted by MP worldwide [24,37,38,39]. According to USDA (2012), about 52 percent of the U.S. land cover is used for agricultural purposes [40]; a sizeable proportion of this area could become polluted with microplastics through the application of wastewater sludge (biosolids) as fertilizer, plastic mulching practices, or MP-polluted irrigation water [26,41,42,43]. Studies have shown that up to 99% of the MP entering a wastewater treatment plant can be concentrated in the sewage sludge that is later dumped on farms [44,45,46,47,48,49]. Third, witnessing the alarming rate of plastic waste generation and anticipating no dramatic shifts in either plastic production or waste management, it is rational to conclude that the accumulation of plastic pollutants in the environment, and specifically soil–MP pollution in farms and industrial areas, will continue growing for a long time. For these reasons, research is needed on how soil systems respond to this new, growing, and fast-accumulating type of pollution.
This study addresses some of the limitations of previous works by developing more reliable, statistically conclusive evidence to assess the potential of soil–MP pollution to affect soil-water dynamics. Specifically, we focus on the effects of two factors: (1) the shape (and thus type) of MP and (2) the magnitude of MP pollution, which is indexed by the MP concentration expressed as an MP/soil mass ratio, λ (g/g). One specific limitation of many earlier studies (e.g., [31]) is that they typically conducted garden experiments, meaning that an actual soil sample was taken from the field, mixed with MP, and in some cases placed back at its previous location. Using actual soil is required for studies involving organisms or biological processes since these need food and nutrients. However, soil-water dynamics are governed mainly by pore-scale water, vapor, and heat transport processes that strongly depend on soil pore structure [50,51,52]. Although using actual soils might better simulate natural systems, they have heterogeneous pore structures that display high variation within and across replicates (soil samples). In addition, the interconnected organic and microbial changes (e.g., variation in hydrophobicity) would also add uncontrollable uncertainties when investigating the interaction between MP, soil mineral particles, and water. Studying the physics of soil-water dynamics in garden soils would be excessively complex, resulting in high uncertainty due to the number of variables that cannot be controlled. Hence, we only used a single homogenous soil type to control and minimize such uncertainties. This allowed us to use a high number of replicates, N = 10, for each experiment versus the typical 3 to 5 in previous studies, making the total 490 measurements, resulting in more robust statistical results. All experiments were performed under controlled and effectively constant laboratory conditions. A further contribution of the present work is that earlier studies only investigated MP impacts on porosity and soil-water holding capacity (WHC) [31,53], while we add early and late-stage bare soil evaporation (ER) as another critical and fundamental soil-water process.
2. Materials and Methods
2.1. Soil and MP
The entire study was performed in a laboratory environment, using a single homogeneous fine sand as soil, with uniformity and curvature coefficients estimated at 1.45 and 0.95, respectively [54]. The grain size distribution characteristics, D10, D30, D50, and D60, were determined to be 0.32, 0.37, 0.42, and 0.45 (mm). The porosity of loosely packed samples was found to be 0.43. Although the soil was found to be clean under visual inspection, we still treated it for 24 h with 30% hydrogen peroxide to digest any organic residuals [55]. We then repeatedly treated it using saltwater (CaCl2) with a density of ~1.3 (g cm−3) to remove any residual MP particles by floatation [56]. Finally, we rinsed it with distilled water before drying it in the oven.
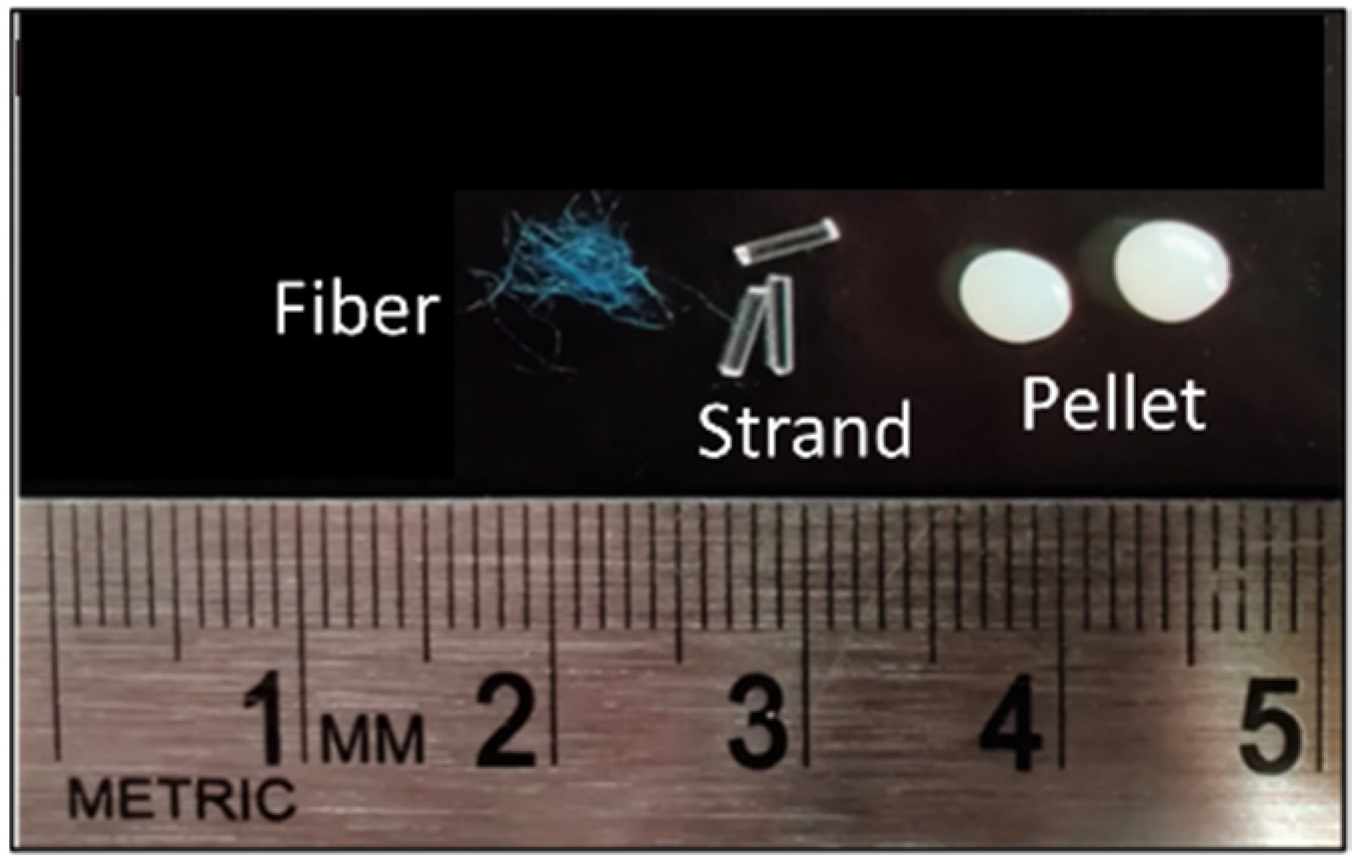
We studied three common shapes (types) of MP with the same size range of 3 mm: fibers (polyacrylic), strands (polymethyl methacrylate), and pellets (acrylonitrile butadiene styrene) (Figure 1). Fibers were fabricated manually by cutting 100% acrylic “Caron H970039767 Simply Soft” yarn to an average length of 3 mm. Strands were obtained by cutting the commercially available “AKEPO 16W Twinkle Fiber Optic Lights” (0.75 mm diameter) to an average length of 3 mm. Manufactured plastic pellets with an average diameter of 3 mm were purchased from the Victory Pellet company.
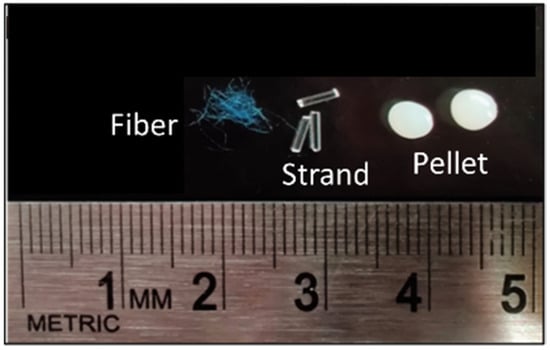
Figure 1.
Types of MP used in this experiment.
2.2. Environmentally Relevant Levels of MP Pollution
We aim to use environmentally relevant λ values (MP to soil mass ratios) so that our outcomes have practical relevance. However, only a few soil–MP pollution studies discuss the ranges of λ measured in natural environments. The main reason is that current techniques for quantifying MP loads in soil samples are quite challenging, costly, and time-consuming. MP quantification includes two main steps: first, MP must be fully separated from the soil minerals, including fine clay and silt particles that may resist separation processes; then, the separated MP must be quantified by counting or weighing or else by estimating their volume. Currently, no effective and consistent protocols or guidelines are available for measuring λ in the environment; therefore, most results reported in the literature are not comparable. Moreover, many authors reported MP pollution using counts in terms of MP items per unit weight or volume of soil, which cannot be converted to mass- or volume-based values [57]. Despite these challenges and the overall lack of data, researchers conclude that the areas with the highest MP pollution (highest λ values) are industrial and agricultural lands. For example, one study conducted in Australia showed that λ values in soils near an industrial area ranged between 0.03% and 6.7% [58]. Biosolids produced in wastewater treatment facilities applied in agricultural and non-agricultural lands are extremely contaminated by MP and are heavily used as fertilizer frequently and, in some cases, every year [8,59]. A study conducted in the UK showed that biosolids had 37.7–286.5 MP particles per one gram [8]. Estimates of MP concentrations in biosolids in the USA are higher, at 324–444 particles per gram [60]. Other studies in Austria and Germany found 3000 particles per gram of biosolid (average of 6 samples) and 12,700 plastic particles per gram (average of 11 samples) [61]. From the literature, it can be inferred that many researchers consider λ ≤ 2% to be a current or soon-to-come environmentally relevant range [4,17,27,31,53,58,62,63,64,65]. Hence, in this study, we used λ ≤ 1.5%. It should also be noted that MP continuously accumulate in the environment; because of the exponentially growing output of plastic products and their high resiliency to degradation in the natural environment, it is expected that λ values will continue to increase in the coming years unless dramatic shifts take place in the plastic industry or waste management sector.
2.3. Experimental Design
As summarized in Table 1, for each combination of three MP types and five environmentally relevant MP/soil mass ratios λ, ten 750 (g) dry fine sand samples were used to study MP effects on WHC and ER. This mass of soil was chosen as it fitted the glass cylinders used in the experiments. Soil samples with no MP, λ = 0%, were studied and labeled as control samples. The same λ ratios were used for samples contaminated with pellets and strands, while relatively smaller λ values were considered for fiber because adding too much fiber to the soil significantly increased its bulk volume, changing soil structure [31].

Table 1.
Experimental design. Three different shapes (types) of MP—polyacrylic (fibers), polymethyl methacrylate (strands), and acrylonitrile butadiene styrene (pellets), with six levels of λ (g/g), were studied for each experiment.
2.4. Soil-Water Measurements
The WHC and ER experiments were designed to be conducted consecutively on the same samples to avoid disturbing the soil structure between one experiment and the next. Below, we explain each experimental setup and measurement procedure in detail.
- -
- Experiment 1: Water Holding Capacity (WHC)
Glass holders (open-end glass cylinders) with an internal diameter of 6.91 (cm) and a height of 11.55 (cm) and their bottom end screened with a nylon mesh were used to prepare the samples (Supplementary Information (SI) S1). The sand was poured into thin layers (~1 cm), dipping the cylinder in a water bath after adding each layer, to effectively remove any air bubbles through it, allowing for full saturation. The cylinder was then raised within the bath, bringing only the sand’s surface out of the water, manually adding a measured amount of MP to each layer, which was carefully mixed with a thin glass rod to effectively distribute the MP within each layer (see SI S2). Our measurements showed that manually adding and then mixing MP, layer by layer, leads to a more uniform MP distribution than mechanical mixing, which could also fragment MP, changing their sizes. The top surface of the finalized sample was flattened using a straight spatula. Saturated samples were then taken out of the water and placed on a grated surface for 24 h to let the excess water drain gravitationally through the soil column. A plastic cap covered each sample to inhibit evaporation from the sand’s surface without sealing it hermetically to avoid possible vacuum effects during drainage (SI S3). The WHC of samples was computed as the difference between the weight of the sand after 24 h of draining and the initial 750 (g) of dry sand, divided by the weight of the dry sand [53,66].
- -
- Experiment 2: Bare Soil Evaporation (ER)
After reaching field capacity in Experiment 1, each sample was placed with its surface at precisely 10 cm below a 50-watt infrared light for 41 h (see SI S4). This provided radiative energy to exacerbate evaporation from the fine sand’s surface, allowing us to create an accelerated-evaporation condition consistent across all samples. Bare soil evaporation was then determined by measuring and tracking the weight of the samples 23 times within 41 h, using a 0.01-g-accurate scale. Averaged evaporation data are shown in SI S4.
2.5. Statistical Tools: Linear Regression and Non-Parametric Analysis of Variance (Kruskal–Wallis ANOVA)
We used two statistical tools, linear regression, and non-parametric ANOVA, also known as the Kruskal–Wallis test, to interpret the collected data. Linear regression models represent the relationship between each dependent variable (i.e., changes in WHC and ER) and the explanatory variable (λ, considered as a continuous variable), helping identify trends (i.e., increasing or decreasing) as well as the association between dependent and independent variables using the slope and correlation coefficient [67].
Kruskal–Wallis model is a well-known non-parametric statistical tool that determines if there are any statistical differences between two or more non-normally distributed datasets by analyzing the mean ranks [68,69]. This test is typically used when the parametric ANOVA’s assumption of the normality of residues is violated. Thus, it is considered the nonparametric equivalent of one-way ANOVA. This tool provided quantitative information about the impacts that MP shape (type) and λ have on changing WHC and ER. In this study, Kruskal–Wallis models considered each λ level to be categorical variables, while dependent WHC and ER variables were continuous. As explained in the next section, the null hypothesis of no effect of MP shape or λ on WHC and ER was tested using the Kruskal–Wallis test. The treatment groups were considered significantly different when the estimated p-value indicated that the null hypothesis’s probability of being true was less than 5%.
3. Results and Discussion
We modeled all measured soil-water dynamics data as a function of two variables: (1) MP shape (type) and (2) MP/soil mass ratio, λ. These two factors were studied independently using linear regressions through the origin and Kruskal–Wallis tests. To help interpret and understand the results, we use the following color and symbol code: orange and circle refer to pellets, green and square to strands and blue and triangle to fibers. Moreover, darker colors refer to larger λ ratios and vice versa. The detailed descriptive statistics for all datasets are given in SI S5.
3.1. Linear Regression Analyses
3.1.1. Experiment 1: Water Holding Capacity (WHC)
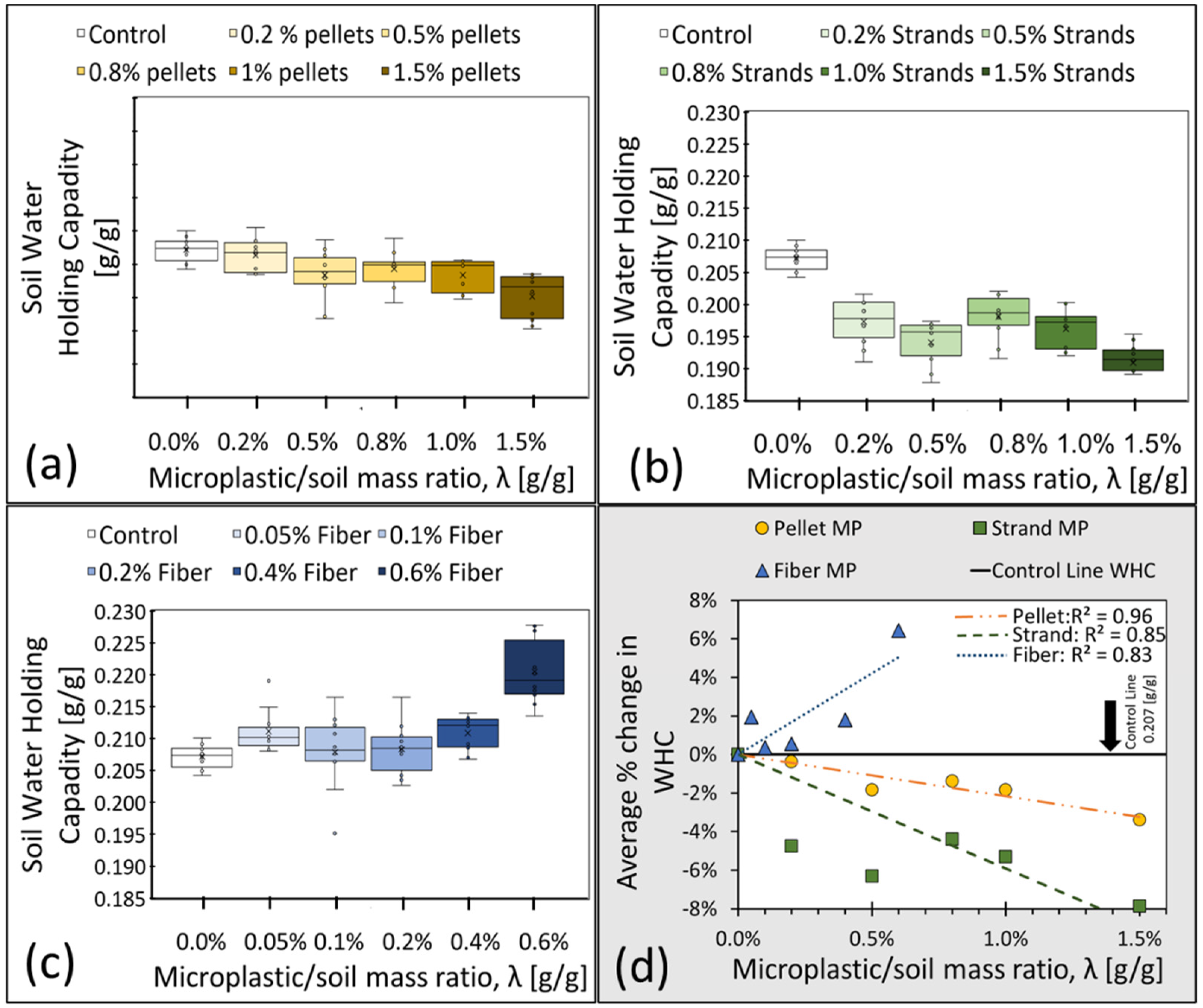
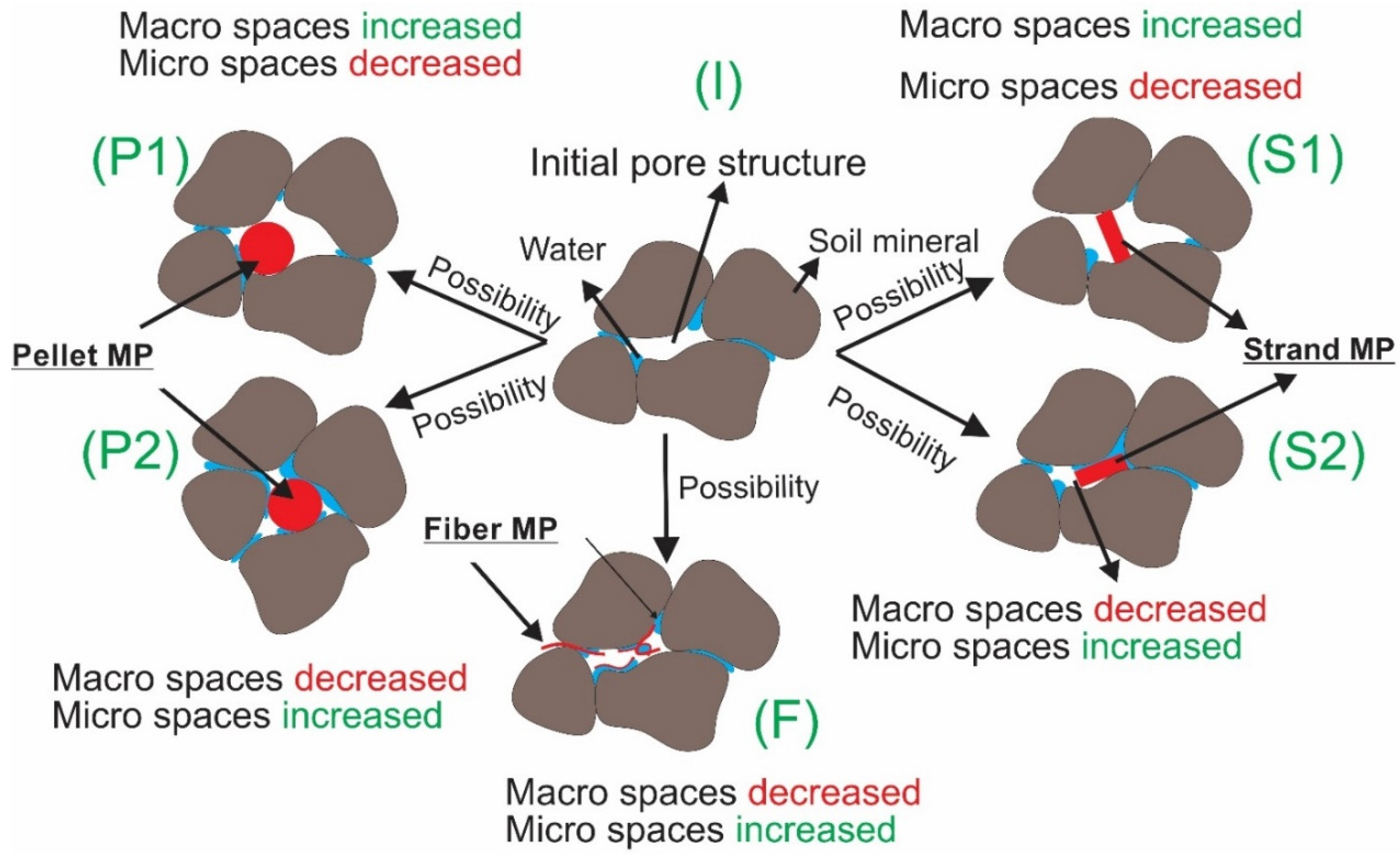
The WHC results (N = 10 for each λ) are summarized by MP type in Figure 2a–c. Figure 2d represents the average percentage change in WHC with respect to the average control value of 0.207, obtained from clean control samples, λ = 0% (solid black line). The linear regressions through the origin depict noticeable trends in the WHC of contaminated samples, with a decreasing (negative) trend for pellets and strands but an increasing (positive) one for fibers. This is possibly related to the fibers’ pliable characteristics that allow them to adapt to the shape of macropores, creating additional microscale spaces that can hold water with possibly minimal impacts on general mineral arrangements. In order to study the changes in pore structure caused by MP pollution, advanced imaging techniques are needed, such as X-rays and CT scans (Computerized Tomography scans). Comparing fiber MP with strands and pellets, our results indicate that fiber MP increases the total number of micro spaces that can hold water from gravity. An overview of only some of the potential impacts of the studied MP on fine sandy soil pore structure can be seen in Figure 3. The pore structure of clean soil is shown in Figure 3 (I). Figure 3 (F) illustrates how fiber MP can become tangled and increase the total number of micro spaces inside the pore structure. Solid pellets and strands, however, can either increase or decrease micro spaces depending on their arrangements in relation to mineral particles (Figure 3 (P1–2) and (S1–2)). Nevertheless, Figure 2d shows a decreasing (negative) trend of WHC for pellets and strands, which can mean that the total volume of micro spaces within all pellet and strand-contaminated samples decreased.
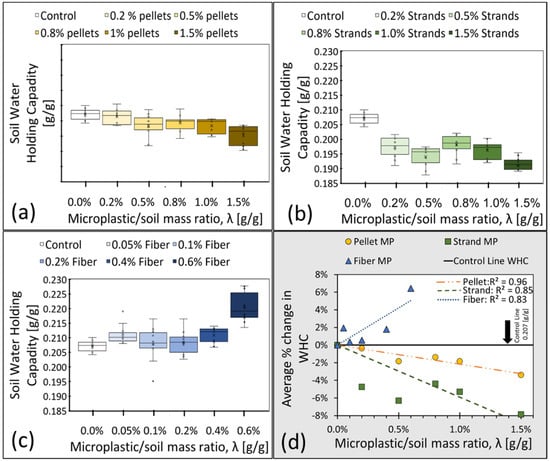
Figure 2.
(a–c) depict the observed WHC as a function of λ (g/g) with standard Box and Whisker plots of WHC for samples contaminated by pellets, strands, and fibers, respectively. Each boxplot displays the median (middle line), mean (cross), first and third quartiles (lower and upper box lid), and collected data with dots. (d) shows through-the-origin linear regressions of the average values (N = 10 samples).
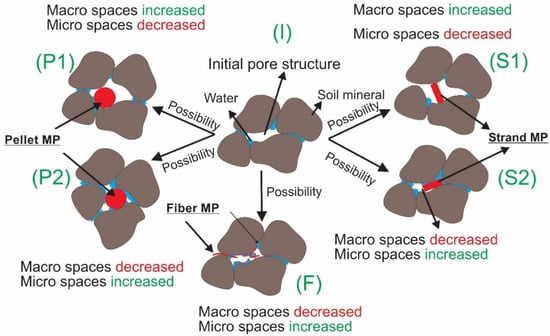
Figure 3.
An illustration of a few scenarios of how pellet, strand, and fiber MP can alter the pore structure of sandy soil. A clean soil’s initial pore structure is shown in (I). As shown in Figures (P1 and 2) and (S1 and 2), soil micropore and macro pore structure can increase or decrease depending upon different soil mineral arrangements, while polluted by pellet and strand MP, respectively. F illustrates how fiber can become tangled and increase the total number of micro spaces inside the pore structure.
3.1.2. Experiment 2: Bare Soil Evaporation (ER)
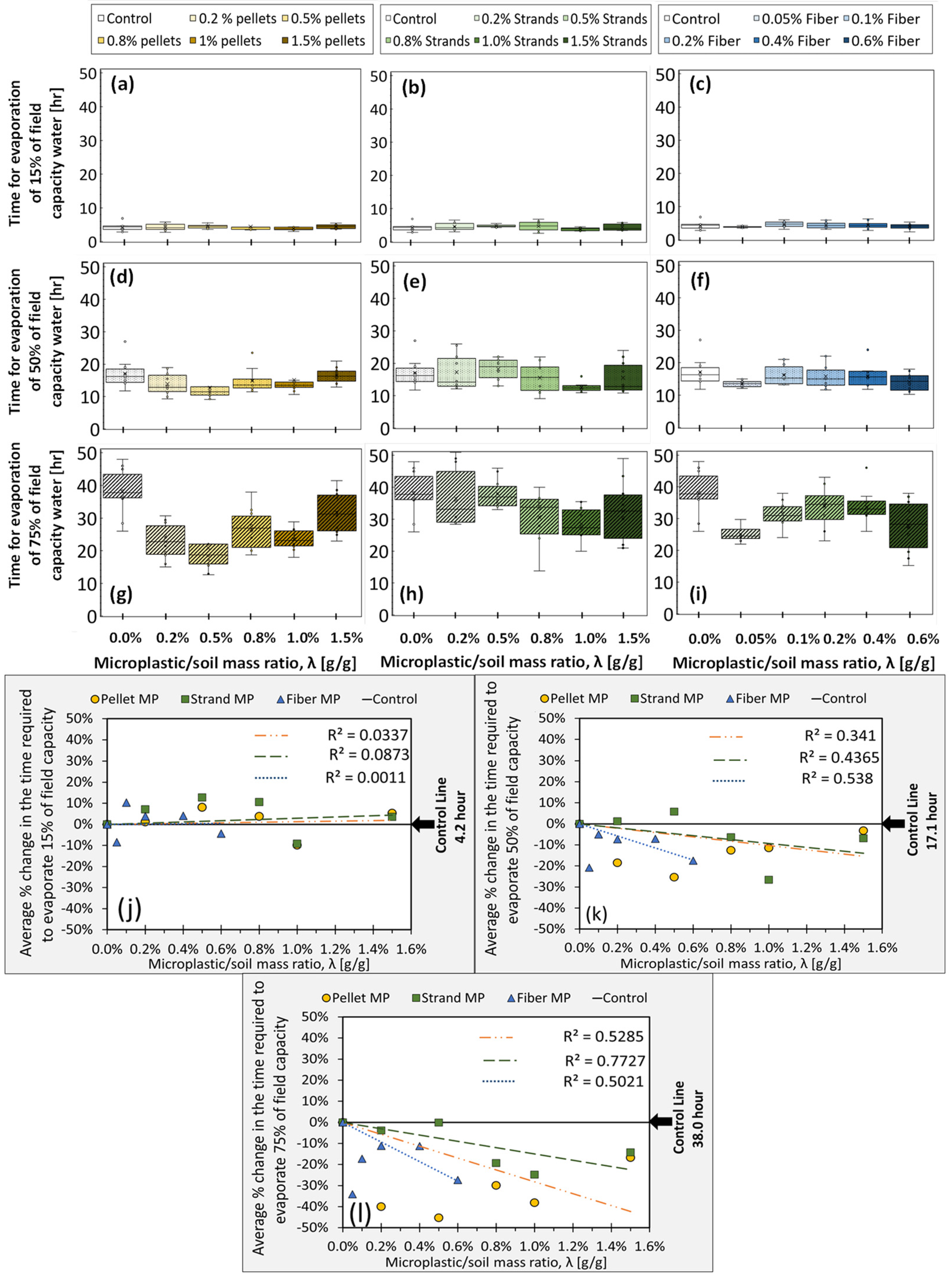
The results for the ER experiments (N = 10 for each λ) are summarized in Figure 4a–i, categorized by λ value and MP shape. Evaporation is quantified as the time required for each sample to evaporate 15%, 50%, and 75% of the water retained at field capacity (Experiment 1), labeled as ER15, ER50, and ER75, respectively. The measured evaporation time is proportional to the total energy received from the infrared light, e.g., if a sample takes longer to evaporate 50% of its moisture at field capacity, it means that it requires more energy from the bulb to do so. These values (15%, 50%, and 75%) are selected to express the three stages of the bare-soil water evaporation process, as discussed in the literature [70,71,72,73]. Stage 1 is the beginning phase of the evaporation process, during which the evaporation rate is high and affected by ambient, atmospherically controlled conditions (e.g., radiation, wind, air moisture); Stage 2 is a transition stage in which the evaporation rate decreases; and finally, Stage 3 corresponds to a soil-controlled, very low and almost constant evaporation rate, in which water within deeper pores starts to diffuse and evaporate. Figure 4j–l represents the average percentage change in evaporation times with respect to the average values obtained from control samples (solid black line), which are 4.2, 17.1, and 38.0 (hr) for 15%, 50%, and 75% evaporation, respectively. Regression analyses show no significant trend for the early stage of the evaporative process (Stage 1, 15% evaporation). It could be mainly because, in Stage 1, water available for evaporation is close to the top of the sample so that atmospheric conditions (mainly radiation in these experiments) dominantly control the process. However, in the latter evaporation stages, capillary forces, surface tension, and integrated thermal and isothermal diffusive characteristics of soil, water, and vapor begin to play a role and impact the evaporation rate [52,73,74,75,76]. Our results show slightly decreasing (negative) trends for ER50 and stronger decreasing (negative) trends for ER75 for all MP types. In other words, MP-polluted soil samples dry faster in the 50% and 75% ranges. These results show that MP could have a noticeable impact on the late-stage evaporation of bare soil, which could affect irrigation planning, sustainable agriculture management, or watershed management.
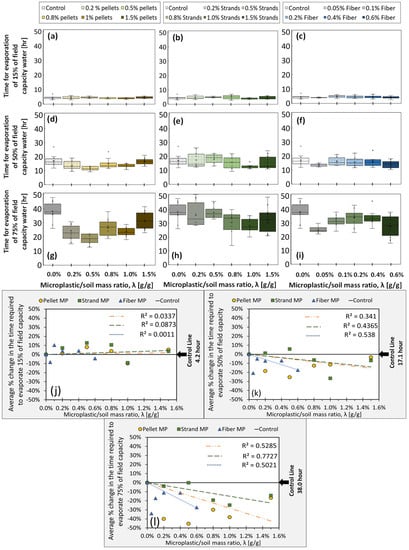
Figure 4.
(a–i) depict the time required to evaporate 15%, 50%, and 75% of field capacity as a function of λ (g/g), for samples contaminated by pellets, strands, and fibers, respectively. Each boxplot displays the median (middle line), mean (cross), first and third quartiles (lower and upper box lid), and collected data with dots. (j–l) show through-the-origin linear regressions of the average values (N = 10 samples).
3.2. Kruskal–Wallis Statistical Analyses
The second statistical tool employed to analyze the impacts of MP shape (type) and λ on soil-water dynamics was the Kruskal–Wallis test. We first examined the normality of each residual of several groups by conducting the Shapiro–Wilks test using a conservative significance level of α = 0.05, as recommended for small sample sizes [77,78]. Results from the Shapiro–Wilks test indicated that a few groups did not comply with the normality requirement of parametric ANOVA; thus, the non-parametric Kruskal–Wallis was used that does not have the normality assumption [79,80,81]. For the reasons explained earlier, fiber tests differed from pellets and strands experiments. Therefore, Kruskal–Wallis was applied twice for pellets and strands combined, and once for fibers only. For the strands-pellets analyses, the dependent variables were sorted individually based on two different factors, λ values with six levels (i.e., 0%, 0.2%, 0.4%, 0.8%, 1.0%, and 1.5%), and shapes with two levels (pellet and strand). The former and latter tests quantified the role of λ values and shapes in changing WHC and ER, respectively. Kruskal–Wallis was conducted once for fiber only when the λ values were the factor with six levels (i.e., 0%, 0.05%, 0.1%, 0.2%, 0.4%, and 0.6%). All tests were conducted using the IBM SPSS Statistics package [82]. Then, we conducted post hoc pairwise comparisons among the different factors. The descriptive tables are placed in SI S5.
The pairwise comparison test results of Kruskal–Wallis models for strands-pellets analyses are summarized in Table 2a,b, considering α = 0.05. The pairwise comparison analysis provided further information about where the significant differences occurred. Table 2a showed a significant difference between different shapes (i.e., pellets and strands) for WHC and ER75, while insignificant difference for ER15 and ER50. This indicated that MP shape (pellets vs. strands) played a role in WHC and ER75 but not in the early stage evaporation processes, ER15 and ER50. Table 2b summarizes the comparison of control samples, , and contaminated samples. It shows that there were significant differences between the control and contaminated samples, indicating that λ is an influential parameter in changing soil-water dynamics.

Table 2.
(a) Pairwise comparison of WHC and ER of strands-pellets experiments once shape (type) of MP was a factor. We found statistically significant differences for each WHC and ER75. (b) Pairwise comparisons of contaminated samples with control samples, λ (g/g) = 0, once λ was considered as a factor. There were statistically significant differences for each WHC and ER75. In both tables, the significance level was considered as α = 0.05.
Table 3 shows the results of the Kruskal–Wallis test performed on the fiber experiments. The comprehensive descriptive tables are available in Supplementary Information. Except for ER15 and ER50, there were statistically significant differences in both studied soil-water properties. The pairwise comparison analyses provided further information about where these differences occurred. Same as in Table 2b no significant difference was observed in ER15 and ER50 for the fiber experiments. However, unlike Table 2b, where there was a significant difference between the control sample and all WHC and ER75 cases, some cases in Table 3 displayed insignificant differences from the control sample (highlighted in red). When comparing Table 2 and Table 3, it should be noted that the fiber ratios were significantly smaller than those for pellets and strands. Excepting WHC and λ = 0.1% and 0.2%, and ER75 and λ = 0.2%, significant differences between the control and contaminated samples occurred for all other λ.

Table 3.
Results of the Kruskal–Wallis (fiber) analyses. Considering α = 0.05, we find, excepting the highlighted ones, there are statistically significant differences for each subject factor.
4. Conclusions: Environmental Implications and Future Directions
Our results show that MP pollution at environmentally relevant levels is capable of causing noticeable changes in soil-water properties. As soil–MP pollution continues growing exponentially worldwide, especially in agricultural and industrial areas, scientists and decision-makers should be more concerned about its potential impacts on the environment and water resources. There are only a few studies and limited available data about the effects of MP pollution on soil-water dynamics; our current knowledge about the possible impacts of MP on the water cycle needs further development. It is important to note that even slight changes in soil-water dynamics within a large-scale hydrological unit could affect its water balance. Further efforts must be made to collect data from different locations to better estimate the range of environmentally relevant λ values as well as the shapes, types, and size distributions of MP discharged into the environment.
It is recommended that future studies use high levels of replication (e.g., our N = 10 or even higher), or else the quality of data, effectiveness of the analyses, and accuracy of the conclusions can remain controversial. This study collected relatively large datasets that allowed us to use statistical models (i.e., regression analysis and Kruskal–Wallis test) for more reliable and objective interpretations. We concluded that for our studied fine sand and ranges of MP concentration, MP pollution has the potential to noticeably change WHC and late-stage evaporation while having minimal effects on early stage evaporation. Moreover, to the best of our knowledge, it had never been quantitatively (statistically) shown before these effects depend both on the MP shape and concentration λ.
However, this study has limitations; its results cannot be generalized to the different soil types and MP sizes, types, and concentrations than those we used. It was limited to a few shapes and sizes of MP (pellets, strands, and fibers ~3 mm in size) and a single type of homogenous soil, and focused on what we considered to be an environmentally relevant range for λ. Moreover, we did not consider the potential impacts of organic matter that would be present in actual soils. Furthermore, we did not look into how the plastic types that we used could have different surface chemistry and hydrophobicity characteristics. Future studies need to address these limitations in order to increase the accuracy and certainty of predictions about the impacts of MP on soil-water dynamics. Generalization will only be possible by combining more laboratory and field studies; this study is a step forward in that direction. We found that the impacts of fiber MP, which are widely discharged to the environment, might have specific characteristics compared with other shapes because of their unique pliable characteristics that allow the fibers to create additional microscale spaces. This might be worth considering in future studies.
Our conclusions concur with previous studies in that MP can potentially change soil-water dynamics. However, since these used different soils as well as MP shapes and sizes, it is difficult to draw clear analogies between the findings. For example, it was previously found that plastic films and fibers in clayey soils significantly enhance water evaporation [32,33], while our study in fine sand shows that pellets and strands enhance evaporation, but fibers decrease it. Another study had showed that adding fiber MP to garden soils could noticeably change their water-retention capacity but could not identify a clear trend [31]; for our fine sand samples, we document a relatively meaningful increasing trend in water-retention capacity.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w14213430/s1. Figure S1: An image of a glass holder and soil sample, Figure S2: Creating saturated soil samples using a water bath and layer-by-layer filling of the sample holder, Figure S3: Saturated samples were then taken out of the water and placed on a grated surface for 24 h to let the excess water drain away by gravity through the soil column. A plastic cap covered each sample to inhibit evaporation from the soil’s surface but without sealing it hermetically, to avoid possible vacuum pressure effects due to water draining, Figure S4: After reaching the field capacity in Experiment 1, every sample was placed with its surface at precisely 10 cm below a 50-Watt infrared light for 41 h, Figure S5: (a–c) shows the averaged values of cumulative evaporation percentage of water vs. time (hr) for Pellets, Strands, and Fibers, respectively. Horizontal dotted lines represent ER15, 50, and 75. The average value of cumulative percentage of water was determined by measuring and recording the weight of the samples for 41 h, Table S1: Detailed descriptive statistics (Pellet and Strands), Table S2: Detailed descriptive statistics (Fibers).
Author Contributions
T.J.C. conducted the laboratory experiments and collected and preprocessed the data. F.J. and T.J.C. post-processed and analyzed the data. F.J. prepared the first draft of the article. F.J., T.J.C. and M.S. provided ideas and revised multiple drafts of this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by the United States Department of Agriculture, National Institute of Food and Agriculture (USDA/NIFA) (67019-31166-2020). The Herff College of Engineering at the University of Memphis supported TJC through its graduate student assistantship program.
Data Availability Statement
The descriptive datasets are provided in the Supplementary Materials section. Further data and information are available on request from the corresponding author [F.J.].
Acknowledgments
In addition, we would like to acknowledge Yves Coquet, for his valuable comments and suggestions in better analyzing the collected data during the revision of this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.amazon.com/gp/product/B00CB39ICA (accessed on 2 May 2022).
- Banimahd, S.A.; Zand-Parsa, S.H. Simulation of evaporation, coupled liquid water, water vapor and heat transport through the soil medium. Agric. Water Manag. 2013, 130, 168–177. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, P.; Huerta-Lwanga, E.; Corradini, F.; Geissen, V. Sewage sludge application as a vehicle for microplastics in eastern Spanish agricultural soils. Environ. Pollut. 2020, 261, 114198. [Google Scholar] [CrossRef] [PubMed]
- Bigelow, D.; Borchers, A. Major Uses of Land in the United States, 2012; United States Department of Agriculture: Washington, DC, USA, 2017. [Google Scholar]
- Bittelli, M.; Ventura, F.; Campbell, G.S.; Snyder, R.L.; Gallegati, F.; Pisa, P.R. Coupling of heat, water vapor, and liquid water fluxes to compute evaporation in bare soils. J. Hydrol. 2008, 362, 191–205. [Google Scholar] [CrossRef]
- Bläsing, M.; Amelung, W. Plastics in soil: Analytical methods and possible sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef]
- Bonan, G. Ecological Climatology: Concepts and Applications; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef] [PubMed]
- Brutsaert, W. Evaporation into the Atmosphere: Theory, History and Applications; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Büks, F.; Kaupenjohann, M. Global concentrations of microplastics in soils—A review. Soil 2020, 6, 649–662. [Google Scholar] [CrossRef]
- Crawford, C.B.; Quinn, B. Microplastic Pollutants; Elsevier Limited: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Crossman, J.; Hurley, R.R.; Futter, M.; Nizzetto, L. Transfer and transport of microplastics from biosolids to agricultural soils and the wider environment. Sci. Total Environ. 2020, 724, 138334. [Google Scholar] [CrossRef]
- Deb, S.K.; Shukla, M.K.; Sharma, P.; Mexal, J.G. Coupled liquid water, water vapor, and heat transport simulations in an unsaturated zone of a sandy loam field. Soil Sci. 2011, 176, 387–398. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, M.; Qiu, W.; Song, Z. Effect of microplastics and arsenic on nutrients and microorganisms in rice rhizosphere soil. Ecotoxicol. Environ. Saf. 2021, 211, 111899. [Google Scholar] [CrossRef] [PubMed]
- Elliott, A.C.; Hynan, L.S. A SAS® macro implementation of a multiple comparison post hoc test for a Kruskal–Wallis analysis. Comput. Methods Programs Biomed. 2011, 102, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, Q.; Sun, Y.; Zhang, S.; Wang, F. Microplastics change soil properties, heavy metal availability and bacterial community in a Pb-Zn-contaminated soil. J. Hazard. Mater. 2022, 424, 127364. [Google Scholar] [CrossRef] [PubMed]
- Forero-López, A.; Rimondino, G.; Truchet, D.; Colombo, C.; Buzzi, N.; Malanca, F.; Spetter, C.; Fernández-Severini, M. Occurrence, distribution, and characterization of suspended microplastics in a highly impacted estuarine wetland in Argentina. Sci. Total Environ. 2021, 785, 147141. [Google Scholar] [CrossRef]
- Fuller, S.; Gautam, A. A procedure for measuring microplastics using pressurized fluid extraction. Environ. Sci. Technol. 2016, 50, 5774–5780. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.R. Plastic scrubbers’ in hand cleansers: A further (and minor) source for marine pollution identified. Mar. Pollut. Bull. 1996, 32, 867–871. [Google Scholar] [CrossRef]
- Han, J.; Zhou, Z. Dynamics of soil water evaporation during soil drying: Laboratory experiment and numerical analysis. Sci. World J. 2013, 2013, 240280. [Google Scholar] [CrossRef]
- Harley-Nyang, D.; Memon, F.; Jones, N.; Galloway, T. Investigation and analysis of microplastics in sewage sludge and biosolids: A case study from one wastewater treatment works in the UK. Sci. Total Environ. 2022, 823, 153735. [Google Scholar] [CrossRef] [PubMed]
- Hauer, F.R.; Lamberti, G.A. Methods in Stream Ecology; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- He, D.; Luo, Y.; Lu, S.; Liu, M.; Song, Y.; Lei, L. Microplastics in soils: Analytical methods, pollution characteristics and ecological risks. TrAC Trends Anal. Chem. 2018, 109, 163–172. [Google Scholar] [CrossRef]
- Hecke, T.V. Power study of anova versus Kruskal-Wallis test. J. Stat. Manag. Syst. 2012, 15, 241–247. [Google Scholar] [CrossRef]
- Helsel, D.R.; Hirsch, R.M. Statistical Methods in Water Resources; Elsevier: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Hurley, R.R.; Nizzetto, L. Fate and occurrence of micro(nano)plastics in soils: Knowledge gaps and possible risks. Curr. Opin. Environ. Sci. Health 2018, 1, 6–11. [Google Scholar] [CrossRef]
- Iden, S.C.; Blöcher, J.R.; Diamantopoulos, E.; Durner, W. Capillary, film, and vapor flow in transient bare soil evaporation (1): Identifiability analysis of hydraulic conductivity in the medium to dry moisture range. Water Resour. Res. 2021, 57, e2020WR028513. [Google Scholar] [CrossRef]
- de Jesus Piñon-Colin, T.; Rodriguez-Jimenez, R.; Rogel-Hernandez, E.; Alvarez-Andrade, A.; Wakida, F.T. Microplastics in stormwater runoff in a semiarid region, Tijuana, Mexico. Sci. Total Environ. 2020, 704, 135411. [Google Scholar] [CrossRef]
- Jiang, X.J.; Liu, W.; Wang, E.; Zhou, T.; Xin, P. Residual plastic mulch fragments effects on soil physical properties and water flow behavior in the Minqin Oasis, northwestern China. Soil Tillage Res. 2017, 166, 100–107. [Google Scholar] [CrossRef]
- Kershaw, P. Sources, Fate and Effects of Microplastics in the Marine Environment: A Global Assessment; International Maritime Organization: London, UK, 2015. [Google Scholar]
- Kim, S.W.; An, Y.J. Soil microplastics inhibit the movement of springtail species. Environ. Int. 2019, 126, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, M.B. Principles of Soil and Plant Water Relations; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Konert, M.; Vandenberghe, J.E.F. Comparison of laser grain size analysis with pipette and sieve analysis: A solution for the underestimation of the clay fraction. Sedimentology 1997, 44, 523–535. [Google Scholar] [CrossRef]
- Koutnik, V.; Alkidim, S.; Leonard, J.; DePrima, F.; Cao, S.; Hoek, E.; Mohanty, S. Unaccounted microplastics in wastewater sludge: Where do they go? ACS EST Water 2021, 1, 1086–1097. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; He, M.; Tsang, D.C.W.; Gupta, J.; Khan, E.; Harrad, S.; Hou, D.; Ok, Y.S.; Bolan, N.S. Microplastics as pollutants in agricultural soils. Environ. Pollut. 2020, 265, 114980. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, P.; Bandyopadhyay, S. Evidence of microplastics in wetlands: Extraction and quantification in Freshwater and coastal ecosystems. J. Water Process Eng. 2021, 40, 101966. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, P.; Manna, C.; Jain, M. Abundance, interaction, ingestion, ecological concerns, and mitigation policies of microplastic pollution in riverine ecosystem: A review. Sci. Total Environ. 2021, 782, 146695. [Google Scholar]
- Lebreton, L.C.M.; Van Der Zwet, J.; Damsteeg, J.-W.; Slat, B.; Andrady, A.; Reisser, J. River plastic emissions to the world’s oceans. Nat. Commun. 2017, 8, 15611. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, A.; Fitschen, K.; Rillig, M.C. Abiotic and biotic factors influencing the effect of microplastic on soil aggregation. Soil Syst. 2019, 3, 21. [Google Scholar] [CrossRef]
- Li, J.; Ouyang, Z.; Liu, P.; Zhao, X.; Wu, R.; Zhang, C.; Lin, C.; Li, Y.; Guo, X. Distribution and characteristics of microplastics in the basin of Chishui River in Renhuai, China. Sci. Total Environ. 2021, 773, 145591. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Huang, S.; Wang, H.; Liu, M.; Xue, S.; Tang, D.; Cheng, W.; Fan, T.; Yang, X. Effects of plastic particles on germination and growth of soybean (Glycine max): A pot experiment under field condition. Environ. Pollut. 2021, 272, 116418. [Google Scholar] [CrossRef]
- Lian, J.; Liu, W.; Meng, L.; Wu, J.; Zeb, A.; Cheng, L.; Lian, Y.; Sun, H. Effects of microplastics derived from polymer-coated fertilizer on maize growth, rhizosphere, and soil properties. J. Clean. Prod. 2021, 318, 128571. [Google Scholar]
- Liang, Y.; Lehmann, A.; Ballhausen, M.-B.; Muller, L.; Rillig, M.C. Increasing temperature and microplastic fibers jointly influence soil aggregation by saprobic fungi. Front. Microbiol. 2019, 10, 2018. [Google Scholar] [CrossRef]
- Lindeque, P.K.; Cole, M.; Coppock, R.L.; Lewis, C.N.; Miller, R.Z.; Watts, A.J.R.; Wilson-McNeal, A.; Wright, S.L.; Galloway, T.S. Are we underestimating microplastic abundance in the marine environment? A comparison of microplastic capture with nets of different mesh-size. Environ. Pollut. 2020, 265, 114721. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Lehnert, T.; Linck, L.T.; Lehmann, A.; Rillig, M.C. Microplastic shape, polymer type, and concentration affect soil properties and plant biomass. Front. Plant Sci. 2021, 12, 169. [Google Scholar]
- Maaß, S.; Daphi, D.; Lehmann, A.; Rillig, M.C. Transport of microplastics by two collembolan species. Environ. Pollut. 2017, 225, 456–459. [Google Scholar]
- MacArthur, E. The New Plastics Economy Rethinking the Future of Plastics. 2016. Available online: https://ellenmacarthurfoundation.org/the-new-plastics-economy-rethinking-the-future-of-plastics (accessed on 1 February 2022).
- Magnusson, K.; Norén, F. Screening of Microplastic Particles in and Down-Stream a Wastewater Treatment Plant, IVL Swedish Environmental Research Institute. 2014. Available online: http://urn.kb.se/resolve?urn=urn:nbn:se:naturvardsverket:diva-2226 (accessed on 10 February 2022).
- Meier, C.I.; Hauer, F.R. Strong effect of coarse surface layer on moisture within gravel bars: Results from an outdoor experiment. Water Resour. Res. 2010, 46, W05507. [Google Scholar] [CrossRef]
- Meixner, K.; Kubiczek, M.; Fritz, I. Microplastic in soil–current status in Europe with special focus on method tests with Austrian samples. AIMS Environ. Sci. 2020, 7, 174–191. [Google Scholar] [CrossRef]
- Montgomery, D.C.; Peck, E.A.; Vining, G.G. Introduction to Linear Regression Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.L.; Huerta Lwanga, E.; Eldridge, S.M.; Johnston, P.; Hu, H.W.; Geissen, V.; Chen, D. An overview of microplastic and nanoplastic pollution in agroecosystems. Sci. Total Environ. 2018, 627, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.; Claveau-Mallet, D.; Hernandez, L.M.; Xu, E.G.; Farner, J.M.; Tufenkji, N. Separation and analysis of microplastics and nanoplastics in complex environmental samples. Acc. Chem. Res. 2019, 52, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Nizzetto, L.; Bussi, G.; Futter, M.N.; Butterfield, D.; Whitehead, P.G. A theoretical assessment of microplastic transport in river catchments and their retention by soils and river sediments. Environ. Sci. Process. Impacts 2016, 18, 1050–1059. [Google Scholar] [CrossRef]
- Or, D.; Lehmann, P.; Shahraeeni, E.; Shokri, N. Advances in soil evaporation physics—A review. Vadose Zone J. 2013, 12, vzj2012-0163. [Google Scholar] [CrossRef]
- Padha, S.; Kumar, R.; Dhar, A.; Sharma, P. Microplastic pollution in mountain terrains and foothills: A review on source, extraction, and distribution of microplastics in remote areas. Environ. Res. 2022, 207, 112232. [Google Scholar] [CrossRef]
- Peters, A.; Iden, S.C.; Durner, W. Revisiting the simplified evaporation method: Identification of hydraulic functions considering vapor, film and corner flow. J. Hydrol. 2015, 527, 531–542. [Google Scholar] [CrossRef]
- Piehl, S.; Leibner, A.; Löder, M.G.J.; Dris, R.; Bogner, C.; Laforsch, C. Identification and quantification of macro-and microplastics on an agricultural farmland. Sci. Rep. 2018, 8, 17950. [Google Scholar] [CrossRef] [PubMed]
- Plastics—The Facts. 2018. Available online: https://plasticseurope.org/wp-content/uploads/2021/10/2018-Plastics-the-facts.pdf (accessed on 15 February 2022).
- Qi, R.; Jones, D.L.; Li, Z.; Liu, Q.; Yan, C. Behavior of microplastics and plastic film residues in the soil environment: A critical review. Sci. Total Environ. 2020, 703, 134722. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.Y.; Ben-Asher, J. Experimental determination of soil evaporation stages with soil surface temperature. Soil Sci. Soc. Am. J. 2010, 74, 13–22. [Google Scholar] [CrossRef]
- Rillig, M.C. Microplastic in terrestrial ecosystems and the soil? Environ. Sci. Technol. 2012, 46, 6453–6454. [Google Scholar] [CrossRef]
- Rochman, C.M.; Cook, A.M.; Koelmans, A.A. Plastic debris and policy: Using current scientific understanding to invoke positive change. Environ. Toxicol. Chem. 2016, 35, 1617–1626. [Google Scholar] [CrossRef]
- Rolsky, C.; Kelkar, V.; Driver, E.; Halden, R.U. Municipal sewage sludge as a source of microplastics in the environment. Curr. Opin. Environ. Sci. Health 2020, 14, 16–22. [Google Scholar] [CrossRef]
- Saito, H.; Šimunek, J.; Mohanty, B.P. Numerical analysis of coupled water, vapor, and heat transport in the vadose zone. Vadose Zone J. 2006, 5, 784–800. [Google Scholar] [CrossRef]
- Selonen, S.; Dolar, A.; Kokalj, A.J.; Skalar, T.; Dolcet, L.P.; Hurley, R.; van Gestel, C.A.M. Exploring the impacts of plastics in soil–The effects of polyester textile fibers on soil invertebrates. Sci. Total Environ. 2020, 700, 134451. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M.C. Impacts of microplastics on the soil biophysical environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Chang. Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- de Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics can change soil properties and affect plant performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.M.; Ni, B.-J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Terzaghi, K.; Peck, R.B.; Mesri, G. Soil Mechanics in Engineering Practice; John Wiley & Sons: Hoboken, NJ, USA, 1996. [Google Scholar]
- Wagner III, W.E. Using IBM® SPSS® Statistics for Research Methods and Social Science Statistics; Sage Publications: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Wan, Y.; Wu, C.; Xue, Q.; Hui, X. Effects of plastic contamination on water evaporation and desiccation cracking in soil. Sci. Total Environ. 2019, 654, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Weithmann, N.; Möller, J.N.; Löder, M.G.J.; Piehl, S.; Laforsch, C.; Freitag, R. Organic fertilizer as a vehicle for the entry of microplastic into the environment. Sci. Adv. 2018, 4, eaap8060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.S.; Zhang, F.X.; Li, X.T. Effects of polyester microfibers on soil physical properties: Perception from a field and a pot experiment. Sci. Total Environ. 2019, 670, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, X.; Gertsen, H.; Peters, P.; Salánki, T.; Geissen, V. A simple method for the extraction and identification of light density microplastics from soil. Sci. Total Environ. 2018, 616–617, 1056–1065. [Google Scholar] [CrossRef]
- Zubris, K.A.V.; Richards, B.K. Synthetic fibers as an indicator of land application of sludge. Environ. Pollut. 2005, 138, 201–211. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).