A Novel CYP4 Gene Identified in the Polychaete Sternaspis scutata and Its Transcriptional Levels along the Coasts of the Liaodong Peninsula
Abstract
:1. Introduction
2. Materials and Methods
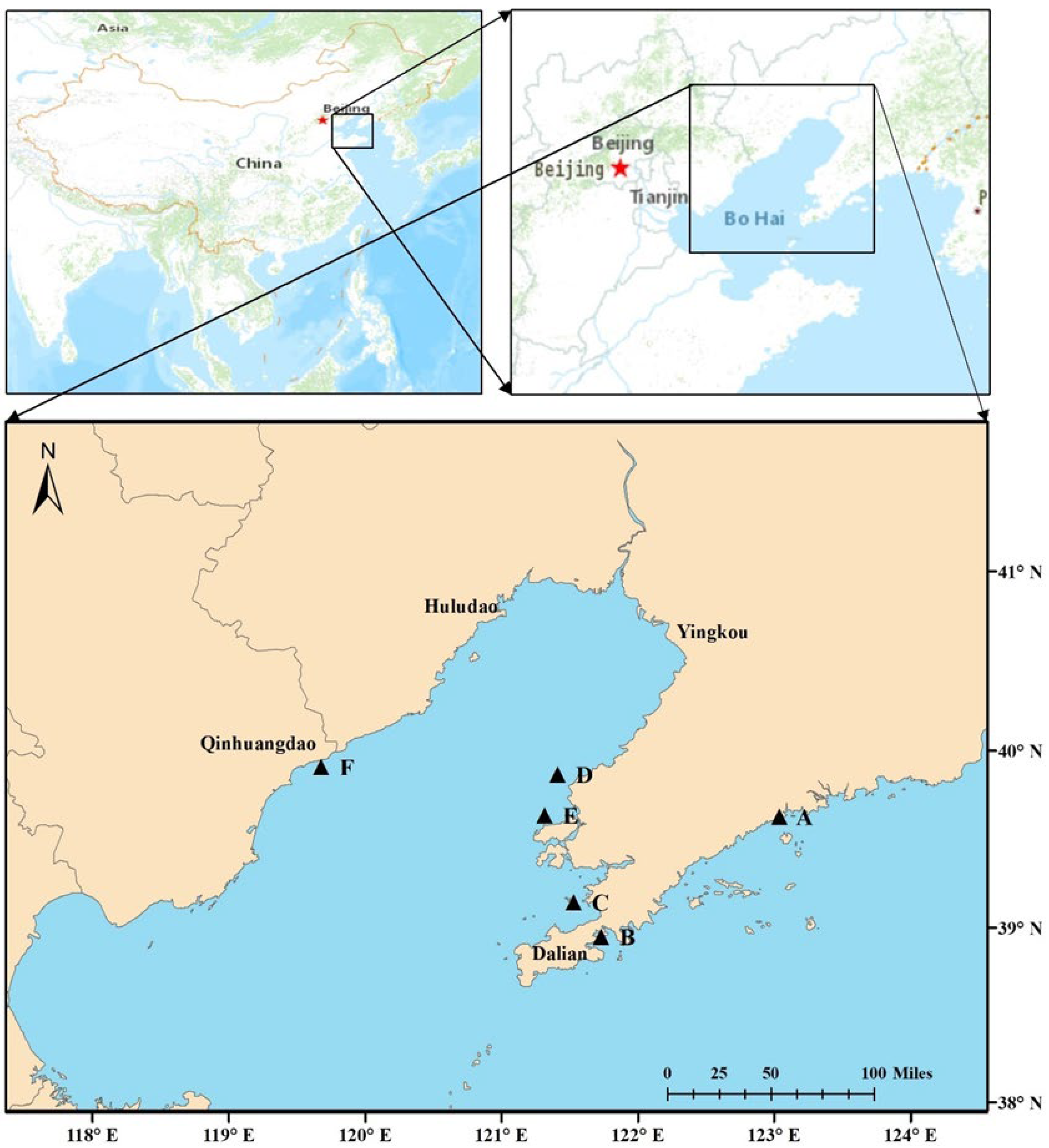
2.1. Field Sampling and Sample Preparation
2.2. Cloning of Full-Length cDNA of CYP4
2.3. Sequence Analysis
2.4. Transcriptional Expression of CYP4 Gene in S. sculata
2.5. Determination of PAHs in Sediments
2.6. Statistical Analysis
3. Results
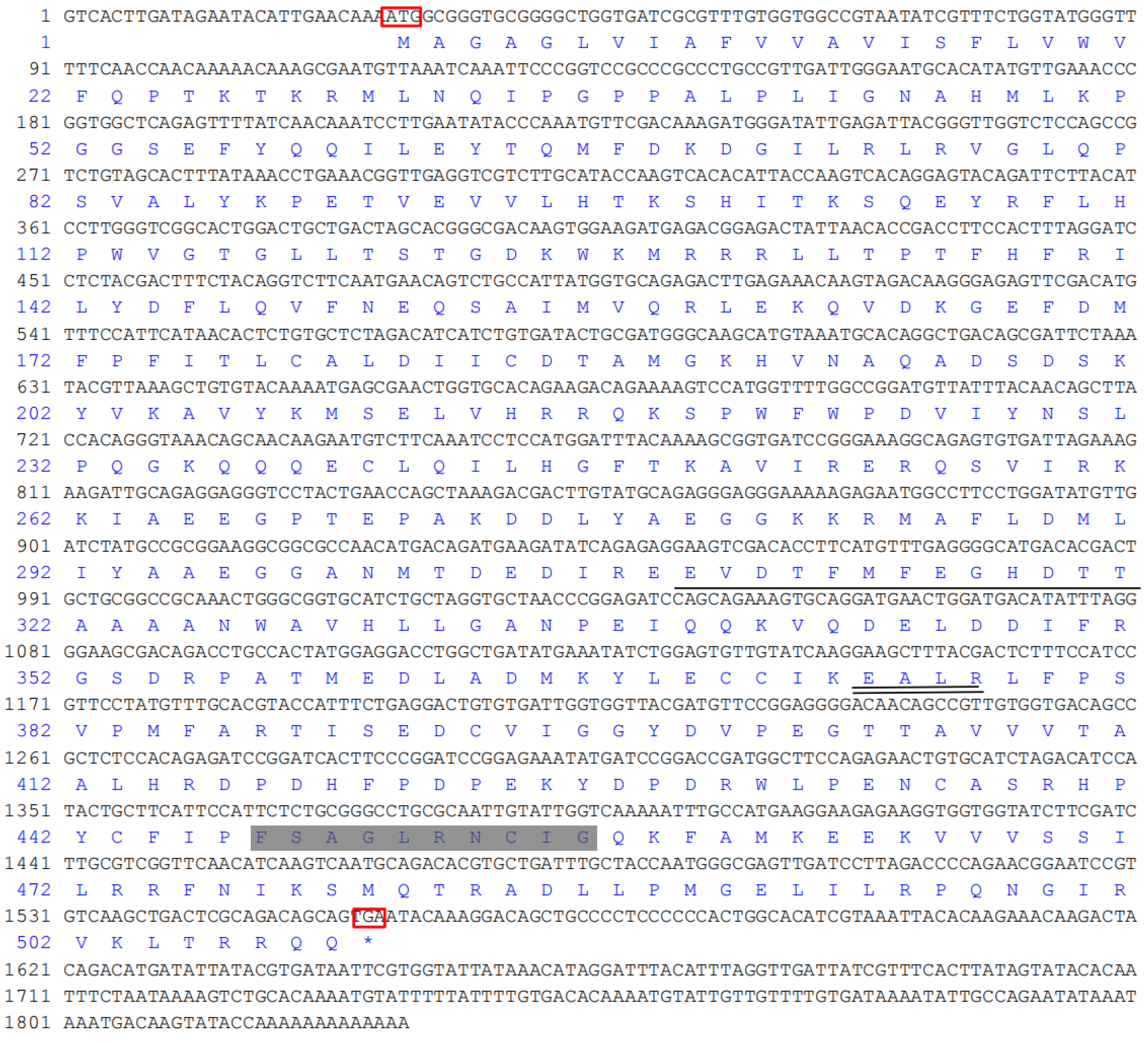
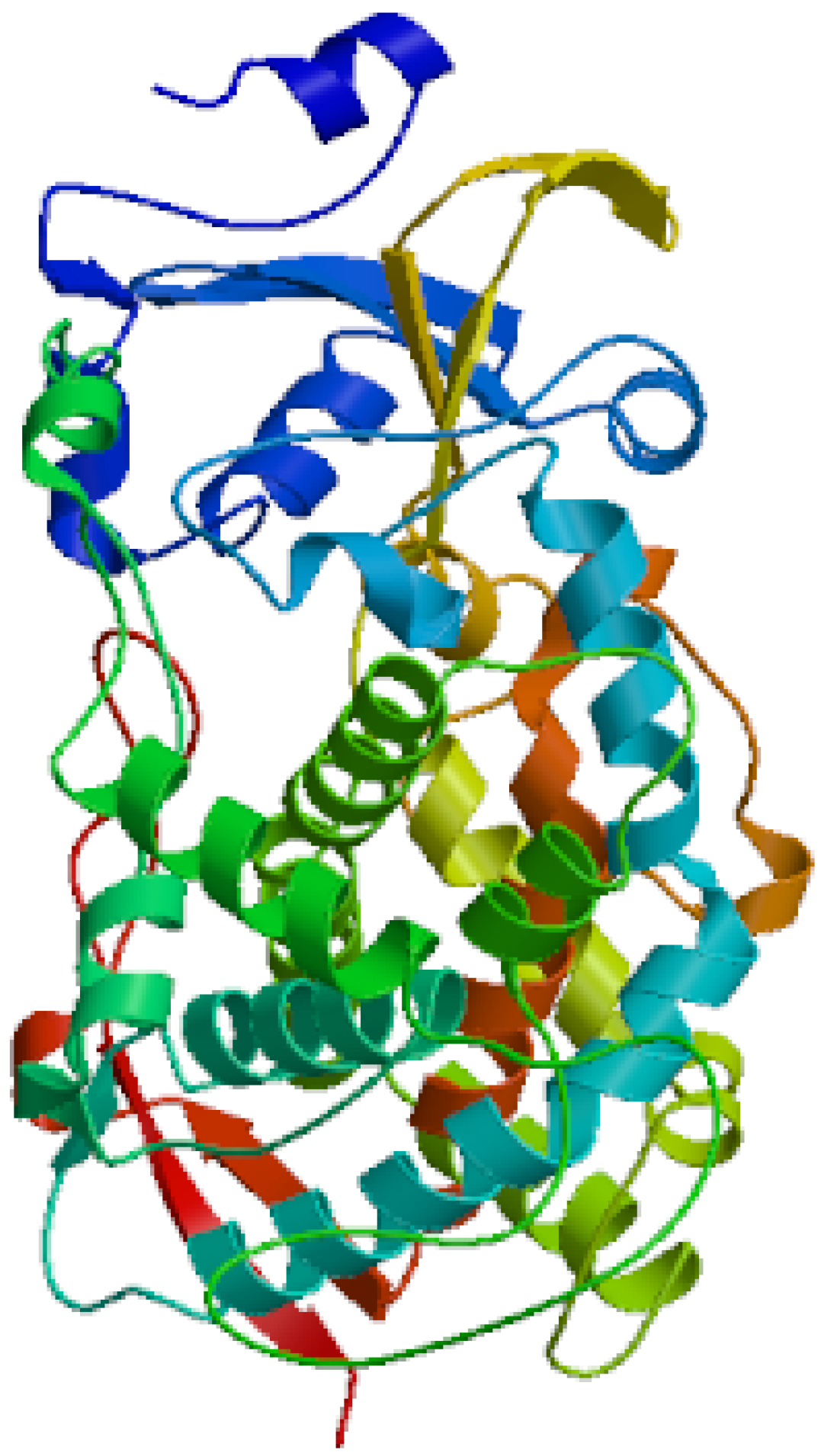
3.1. Complete Sequence and Analysis of CYP4 cDNA of S. scutate
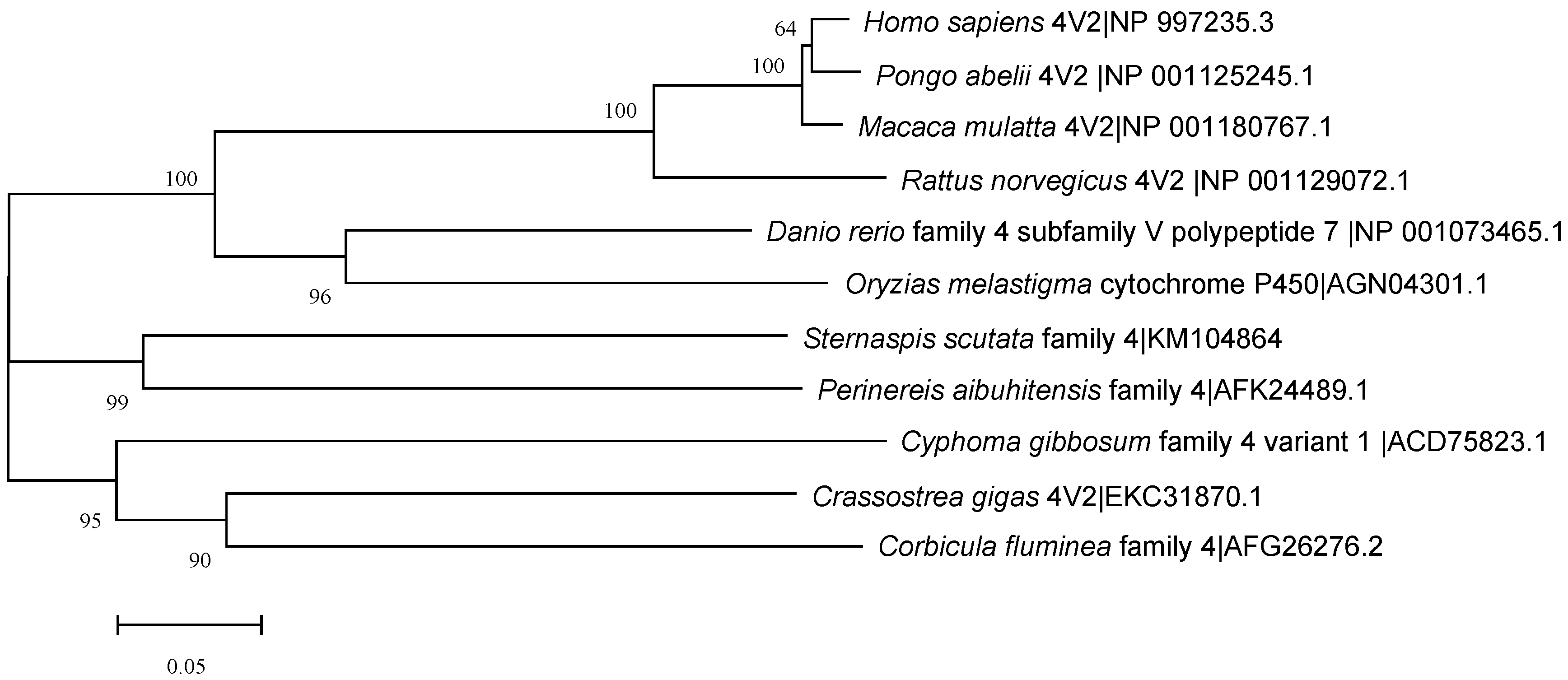
3.2. Similarity Comparison Constructs Evolutionary Tree
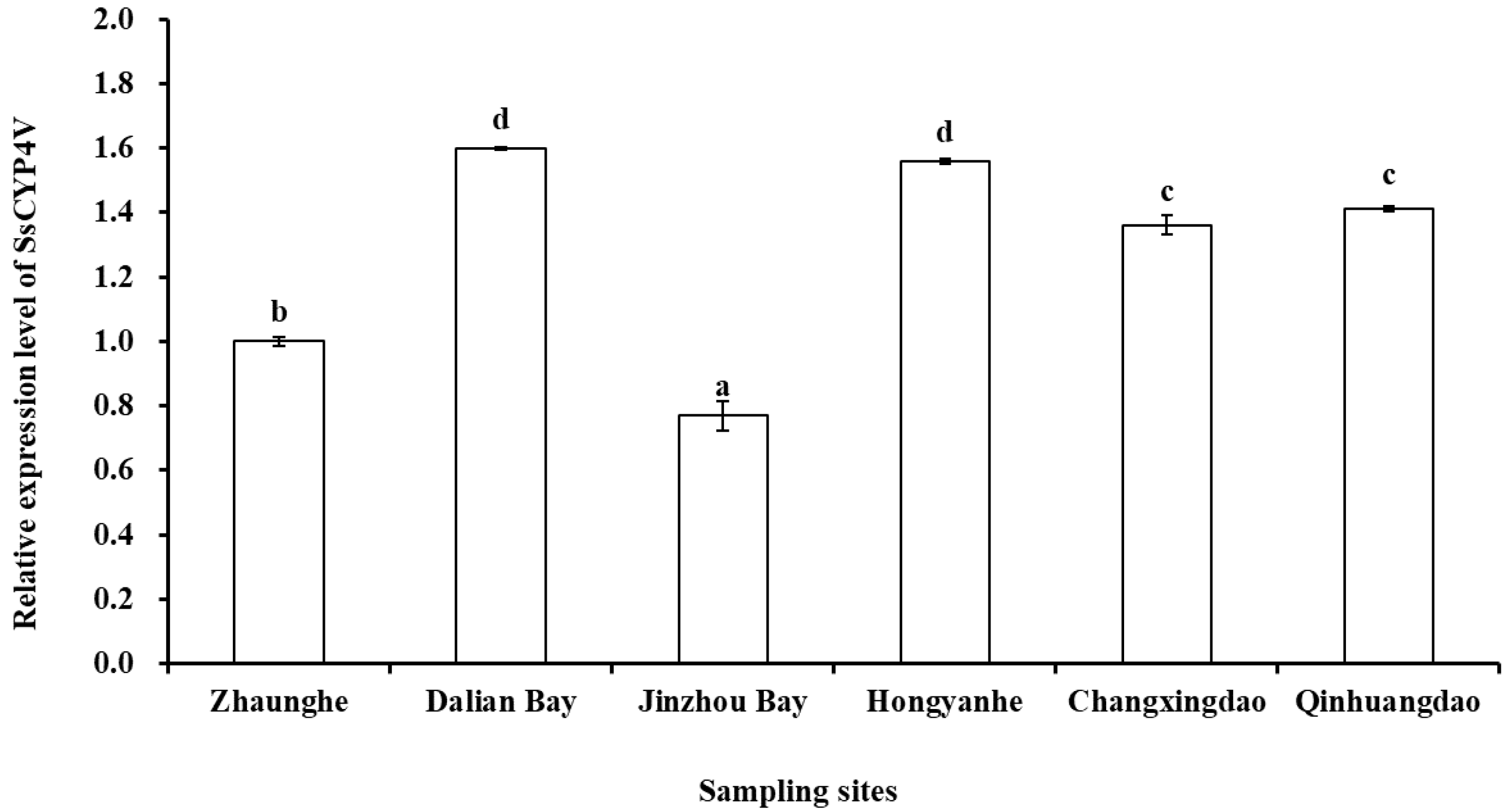
3.3. Expression Level of SsCYP4V in the Field
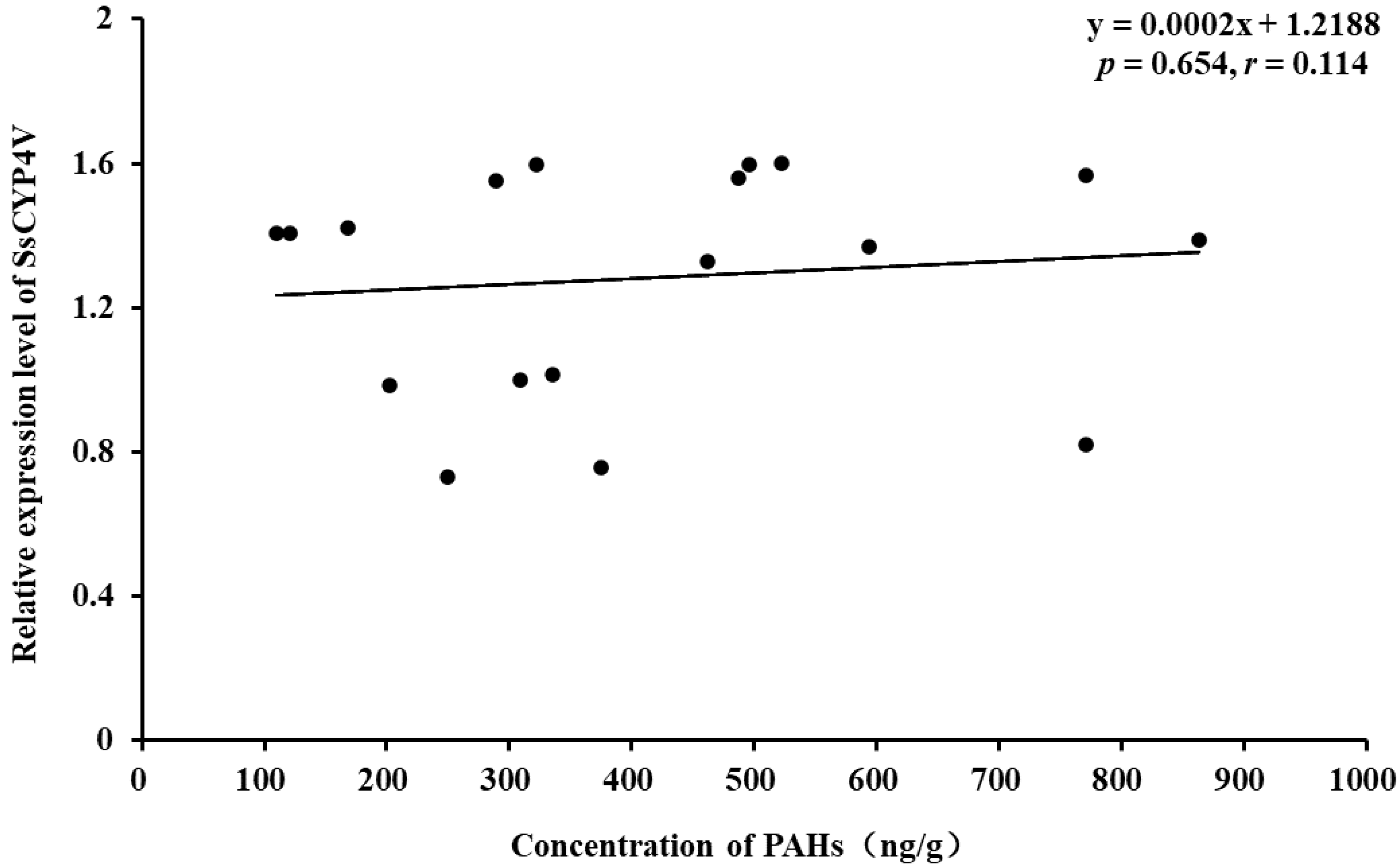
3.4. PAHs in Sediments and the Correlation with Expression of SsCYP4V
4. Discussion
4.1. Sequence and Evolutionary Tree Analysis of SsCYP4V Gene
4.2. PAHs at Different Sampling Sites
4.3. Correlation between PAHs and SsCYP4V Expression in Field Sediments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, B.L.; Sun, R.P.; Yang, D.J. Studies on Nereidae in the Coastal Waters of China; China Ocean Press: Beijing, China, 1981. [Google Scholar]
- Yang, D.J.; Sun, R.P. Polychaete Annelids in the Coastal Waters of China; Agricultural Publishing House: Beijing, China, 1988. [Google Scholar]
- Jørgensen, A.; Giessing, A.M.; Rasmussen, L.J.; Andersen, O. Biotransformation of polycyclic aromatic hydrocarbons in marine polychaetes. Mar. Environ. Res. 2008, 65, 171–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoddard, E.G.; Nag, S.; Martin, J.; Tyrrell, K.J.; Gibbins, T.; Anderson, K.A.; Shukla, A.K.; Corley, R.; Wright, A.T.; Smith, J.N. Exposure to an Environmental Mixture of Polycyclic Aromatic Hydrocarbons Induces Hepatic Cytochrome P450 Enzymes in Mice. Chem. Res. Toxicol. 2021, 34, 2145–2156. [Google Scholar] [CrossRef] [PubMed]
- Sharifian, S.; Homaei, A.; Kamrani, E.; Etzerodt, T.; Patel, S. New insights on the marine cytochrome P450 enzymes and their biotechnological importance. Int. J. Biol. Macromol. 2020, 142, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, L.; Lei, Y.; Wang, Y.; Yang, D.; Zhou, Y.; Yuan, X. Identification of a novel CYP4V gene in the polychaete Perinereis aibuhitensis: Transcriptional comparison with a CYP4B gene exposed to PAHs. Environ. Sci. Pollut. Res. 2022, 29, 47527–47538. [Google Scholar] [CrossRef]
- Won, E.-J.; Rhee, J.-S.; Shin, K.-H.; Jung, J.-H.; Shim, W.J.; Lee, Y.-M.; Lee, J.-S. Expression of three novel cytochrome P450 (CYP) and antioxidative genes from the polychaete, Perinereis nuntia exposed to water accommodated fraction (WAF) of Iranian crude oil and Benzo[α]pyrene. Mar. Environ. Res. 2013, 90, 75–84. [Google Scholar] [CrossRef]
- Li, B.; Bisgaard, H.C.; Forbes, V.E. Identification and expression of two novel cytochrome P450 genes, belonging to CYP4 and a new CYP331 family, in the polychaete Capitella capitata sp.I. Biochem. Biophys. Res. Commun. 2004, 325, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Chen, B.; Qiu, X.; Lin, K.; Yu, X. Three novel cytochrome P450 genes identified in the marine polychaete Perinereis nuntia and their transcriptional response to xenobiotics. Aquat. Toxicol. 2013, 134–135, 11–22. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Yang, D.; Zhao, H.; Wang, L.; Yuan, X. CYP4 mRNA expression in marine polychaete Perinereis aibuhitensis in response to petroleum hydrocarbon and deltamethrin. Mar. Pollut. Bull. 2012, 64, 1782–1788. [Google Scholar] [CrossRef]
- Cai, W.Q.; Meng, W.; Liu, L.S.; Zhu, Y.Z.; Zhou, J. Long-term trends of the dominant macrozoobenthos in Bohai Bay. Acta Sci. Circumstantiae 2013, 33, 2332–2340. [Google Scholar]
- Reish, D.J.; Gerlinger, T.V. A review of the toxicological studies with polychaetous annelids. Bull. Mar. Sci. 1997, 60, 584–607. [Google Scholar]
- Yuan, X.; Zhao, H.; Wang, Y.; Wang, L.; Li, D.; Zhang, A.; Yang, X.; Ma, X.; Yang, D.; Zhou, Y. Expression profile of a novel glutathione S-transferase gene in the marine polychaete Perinereis aibuhitensis in short-term responses to phenanthrene, fluoranthene, and benzo[α]pyrene. Mar. Pollut. Bull. 2021, 169, 112552. [Google Scholar] [CrossRef] [PubMed]
- Ai, W.N.; Wu, R.; Diao, X.L. Development and primary application of double-standard curves method of a real-time fluorescent quantitative PCR assay for detection of PRNP. Chin. Vet. Sci. 2013, 43, 1240–1245. [Google Scholar]
- Yuan, X.; Yang, X.; Zhang, A.; Ma, X.; Gao, H.; Na, G.; Zong, H.; Liu, G.; Sun, Y. Distribution, potential sources and ecological risks of two persistent organic pollutants in the intertidal sediment at the Shuangtaizi Estuary, Bohai Sea of China. Mar. Pollut. Bull. 2017, 114, 419–427. [Google Scholar] [CrossRef]
- Feng, S.; Li, Y.; Zhang, R.; Zhang, Q.; Wang, W. Origin of metabolites diversity and selectivity of P450 catalyzed benzo[a]pyrene metabolic activation. J. Hazard. Mater. 2022, 435, 129008. [Google Scholar] [CrossRef] [PubMed]
- Rewitz, K.; Kjellerup, C.; Jørgensen, A.; Petersen, C.; Andersen, O. Identification of two Nereis virens (Annelida: Polychaeta) cytochromes P450 and induction by xenobiotics. Comp. Biochem. Physiol. Part C Toxicol. Pharm. 2004, 138, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Penry, D.L.; Weston, D.P. Digestive determinants of benz[a]pyrene and phenanthrene bioaccumulation by a deposit-feeding polychaete. Environ. Toxicol. Chem. 1998, 17, 2254–2265. [Google Scholar] [CrossRef]
- Nelson, D.; Koymans, L.; Kamataki, T.; Stegeman, J.J.; Feyereisen, R.; Waxman, D.; Waterman, M.R.; Gotoh, O.; Coon, M.J.; Estabrook, R.W.; et al. P450 superfamily: Update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics 1996, 6, 1–42. [Google Scholar] [CrossRef]
- Dai, C.; Han, Y.; Duan, Y.; Lai, X.; Fu, R.; Liu, S.; Leong, K.H.; Tu, Y.; Zhou, L. Review on the contamination and remediation of polycyclic aromatic hydrocarbons (PAHs) in coastal soil and sediments. Environ. Res. 2021, 205, 112423. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, H.; Lang, Y.; Li, Z. Pollution status of PAHs in surface sediments from different marginal seas along China Mainland: A quantitative evaluation on a national scale. Environ. Pollut. 2020, 263, 114431. [Google Scholar] [CrossRef]
- Hong, W.-J.; Jia, H.; Li, Y.-F.; Sun, Y.; Liu, X.; Wang, L. Polycyclic aromatic hydrocarbons (PAHs) and alkylated PAHs in the coastal seawater, surface sediment and oyster from Dalian, Northeast China. Ecotoxicol. Environ. Saf. 2016, 128, 11–20. [Google Scholar] [CrossRef]
- Lin, F.; Han, B.; Ding, Y.; Li, Q.; Gao, W.; Zheng, L. Distribution characteristics, sources, and ecological risk assessment of polycyclic aromatic hydrocarbons in sediments from the Qinhuangdao coastal wetland, China. Mar. Pollut. Bull. 2018, 127, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, A.; Rasmussen, L.J.; Andersen, O. Characterisation of two novel CYP4 genes from the marine polychaete Nereis virens and their involvement in pyrene hydroxylase activity. Biochem. Biophys. Res. Commun. 2005, 336, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Y.; Yuan, X.T.; Zhang, S.L.; Yang, D.Z.; Guo, H.; Zhou, Y.B. Single and joint toxic effects of benzo(a)pyrene and cadmium on development of three-setiger juvenile of polychaete Perinereis aibuhitensis Grube. Mar. Environ. Sci. 2011, 30, 333–336. [Google Scholar]
- Koenig, S.; Fernández, P.; Solé, M. Differences in cytochrome P450 enzyme activities between fish and crustacea: Relationship with the bioaccumulation patterns of polychlorobiphenyls (PCBs). Aquat. Toxicol. 2012, 108, 11–17. [Google Scholar] [CrossRef]

| Application | Primer Name | Primer Sequence (5′-3′) |
|---|---|---|
| Degenerate primer | F1 | GAYACITTYATGTTYGARGGNCAYGAYAC |
| R1 | GCDATYTTYTGNCCDATRCARTT | |
| For 3′RACE PCR | RACE F2 | GACTCTTTCCATCCGTTCCTAT |
| RACE R2 | TGATTGGTGGTTACGATGTTCC | |
| For 5′RACE PCR | RACE F3 | GAGAATGGAATGAAGCAGTATG |
| RACE R3 | GGAACATCGTAACCACCAATCA | |
| For real-time PCR | Actin-F1 | ACTGTTGTATGTGGTCTCGTGGA |
| Actin-R1 | ACTCTGCTATGTCGCCTTGGA | |
| CYP-F4 | TGTGTGATTGGTGGTTACGATG | |
| CYP-R4 | GAATGGAATGAAGCAGTATGGATG |
| Content (ng/g) | Zhaunghe | Dalian Bay | Jinzhou Bay | Hongyanhe | Changxingdao | Qinhuangdao |
|---|---|---|---|---|---|---|
| Naphthalene (Nap) | 40.5 ± 10.0 | 37.5 ± 12.0 | 19.7 ± 4.2 | 43.5 ± 14.9 | 42.3 ± 14.9 | 13.7 ± 1.8 |
| Acenaphthylene (Ace) | 2.1 ± 0.1 | 4.3 ± 0.8 | 3.2 ± 0.7 | 2.2 ± 0.4 | 2.6 ± 0.2 | 1.4 ± 0.3 |
| Acenaphthene (Acp) | 4.4 ± 0.3 | 7.9 ± 2.1 | 6.1 ± 1.2 | 4.3 ± 1.0 | 5.4 ± 0.7 | 4.0 ± 0.5 |
| Fluoranthene (FlT) | 28.0 ± 2.1 | 44.9 ± 12.2 | 38.9 ± 8.7 | 27.3 ± 7.3 | 31.6 ± 2.5 | 7.3 ± 0.5 |
| Phenanthrene (Phe) | 64.9 ± 5.0 | 96.5 ± 19.9 | 89.9 ± 5.6 | 70.1 ± 6.2 | 95.2 ± 8.0 | 21.7 ± 0.7 |
| Anthracene (Ant) | 1.2 ± 0.1 | 3.5 ± 0.8 | 2.6 ± 0.5 | 1.5 ± 0.3 | 1.2 ± 0.2 | 2.2 ± 0.2 |
| Fluorene (Flu) | 20.7 ± 0.8 | 39.0 ± 2.9 | 34.0 ± 5.2 | 32.2 ± 6.8 | 25.2 ± 2.9 | 11.6 ± 1.0 |
| Pyrene (Pyr) | 14.9 ± 0.8 | 30.1 ± 2.5 | 25.5 ± 4.3 | 21.2 ± 4.5 | 16.7 ± 2.1 | 9.4 ± 1.3 |
| Benzo[a]anthracene (BaA) | 5.7 ± 1.8 | 11.2 ± 1.9 | 14.7 ± 9.5 | 14.3 ± 5.4 | 16.2 ± 4.3 | 2.7 ± 0.8 |
| Chrysene (Chr) | 21.9 ± 5.3 | 33.8 ± 8.1 | 48.2 ± 2.7 | 77.0 ± 43.2 | 132.0 ± 48 | 9.3 ± 2.9 |
| Benzo[b]fluoranthene (BbF) | 23.1 ± 5.2 | 35.6 ± 8.5 | 48.4 ± 2.6 | 71.9 ± 40.3 | 103.3 ± 28 | 8.6 ± 1.4 |
| Benzo[k]fluoranthene (BkF) | 12.0 ± 3.5 | 19.7 ± 3.9 | 28.7 ± 2.0 | 35.0 ± 15.7 | 55.3 ± 22.6 | 5.0 ± 1.3 |
| Benzo[a]pyrene (BaP) | 4.0 ± 1.1 | 10.2 ± 0.6 | 7.9 ± 4.8 | 7.2 ± 2.7 | 5.3 ± 1.7 | 3.6 ± 0.6 |
| Indeno [1,2,3-cd]pyrene (Ipy) | 16.3 ± 4.0 | 29.1 ± 5.0 | 41.2 ± 2.7 | 46.7 ± 22.3 | 46.9 ± 9.8 | 7.9 ± 1.7 |
| Dibenzo[a,h]anthracene (DbA) | 8.7 ± 2.2 | 17.0 ± 4.7 | 20.8 ± 1.3 | 24.7 ± 11.6 | 27.2 ± 6.3 | 13.6 ± 6.2 |
| Benzo[g,hi]perylene (BPE) | 13.9 ± 3.1 | 26.3 ± 4.0 | 35.5 ± 21.7 | 36.6 ± 14.1 | 33.5 ± 6.2 | 11.1 ± 1.04 |
| Total | 282 ± 41 ab | 447 ± 63 ab | 465 ± 157 ab | 516 ± 140 ab | 640 ± 118 b | 133 ± 18 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Qi, A.; Wang, L.; Shan, E.; Li, D.; Yang, X.; Zhang, A. A Novel CYP4 Gene Identified in the Polychaete Sternaspis scutata and Its Transcriptional Levels along the Coasts of the Liaodong Peninsula. Water 2022, 14, 3489. https://doi.org/10.3390/w14213489
Yu Z, Qi A, Wang L, Shan E, Li D, Yang X, Zhang A. A Novel CYP4 Gene Identified in the Polychaete Sternaspis scutata and Its Transcriptional Levels along the Coasts of the Liaodong Peninsula. Water. 2022; 14(21):3489. https://doi.org/10.3390/w14213489
Chicago/Turabian StyleYu, Zhenglin, Aimin Qi, Lili Wang, Encui Shan, Dongmei Li, Xiaolong Yang, and Anguo Zhang. 2022. "A Novel CYP4 Gene Identified in the Polychaete Sternaspis scutata and Its Transcriptional Levels along the Coasts of the Liaodong Peninsula" Water 14, no. 21: 3489. https://doi.org/10.3390/w14213489





