Reactive and Hydraulic Behavior of Granular Mixtures Composed of Zero Valent Iron
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thakur, A.K.; Vithanage, M.; Das, D.B.; Kumar, M. A review on design, material selection, mechanism, and modelling of permeable reactive barrier for community-scale groundwater treatment. Environ. Technol. Innov. 2020, 19, 100917. [Google Scholar] [CrossRef]
- Elder, C.R.; Benson, C.H. Performance and economic comparison of PRB types in heterogeneous aquifers. Environ. Geotech. 2018, 6, 214–224. [Google Scholar] [CrossRef]
- Moraci, N.; Bilardi, S.; Calabrò, P.S. Critical aspects related to Fe0 and Fe0/pumice PRB design. Environ. Geotech. 2016, 3, 114–124. [Google Scholar] [CrossRef]
- Faisal, A.A.H.; Sulaymon, A.H.; Khaliefa, Q.M. A review of permeable reactive barrier as passive sustainable technology for groundwater remediation. Int. J. Environ. Sci. Technol. 2018, 15, 1123–1138. [Google Scholar] [CrossRef]
- Vogan, J.L.; Focht, R.M.; Clark, D.K.; Graham, S.L. Performance evaluation of a permeable reactive barrier for remediation of dissolved chlorinated solvents in groundwater. J. Hazard. Mater. 1999, 68, 97–108. [Google Scholar] [CrossRef]
- Phillips, D.H.; Van Nooten, T.; Bastiaens, L.; Russell, M.I.; Dickson, K.; Plant, S.; Ahad, J.M.E.; Newton, T.; Elliot, T.; Kalin, R.M. Ten Year Performance Evaluation of a Field-Scale Zero-Valent Iron Permeable Reactive Barrier Installed to Remediate Trichloroethene Contaminated Groundwater. Environ. Sci. Technol. 2010, 44, 3861–3869. [Google Scholar] [CrossRef]
- Gandhi, S.; Oh, B.-T.T.; Schnoor, J.L.; Alvarez, P.J.J. Degradation of TCE, Cr(VI), sulfate, and nitrate mixtures by granular iron in flow-through columns under different microbial conditions. Water Res. 2002, 36, 1973–1982. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J. Reduction of nitrate by zero valent iron (ZVI)-based materials: A review. Sci. Total Environ. 2019, 671, 388–403. [Google Scholar] [CrossRef]
- Guan, Q.; Li, F.; Chen, X.; Tian, C.; Liu, C.; Liu, D. Assessment of the use of a zero-valent iron permeable reactive barrier for nitrate removal from groundwater in the alluvial plain of the Dagu River, China. Environ. Earth Sci. 2019, 78, 244. [Google Scholar] [CrossRef]
- Lee, K.J.; Lee, Y.; Yoon, J.; Kamala-Kannan, S.; Park, S.M.; Oh, B.T. Assessment of zero-valent iron as a permeable reactive barrier for long-term removal of arsenic compounds from synthetic water. Environ. Technol. 2009, 30, 1425–1434. [Google Scholar] [CrossRef]
- Statham, T.M.; Stark, S.C.; Snape, I.; Stevens, G.W.; Mumford, K.A. A permeable reactive barrier (PRB) media sequence for the remediation of heavy metal and hydrocarbon contaminated water: A field assessment at Casey Station, Antarctica. Chemosphere 2016, 147, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, R.D.; Smyth, D.J.A.; Blowes, D.W.; Spink, L.E.; Wilkin, R.T.; Jewett, D.G.; Weisener, C.J. Treatment of Arsenic, Heavy Metals, and Acidity Using a Mixed ZVI-Compost PRB. Environ. Sci. Technol. 2009, 43, 1970–1976. [Google Scholar] [CrossRef] [PubMed]
- Cao, V.; Ndé-Tchoupé, A.I.; Hu, R.; Gwenzi, W.; Noubactep, C. The mechanism of contaminant removal in Fe(0)/H2O systems: The burden of a poor literature review. Chemosphere 2021, 280, 130614. [Google Scholar] [CrossRef]
- Hu, R.; Gwenzi, W.; Sipowo-Tala, V.R.; Noubactep, C. Water Treatment Using Metallic Iron: A Tutorial Review. Processes 2019, 7, 622. [Google Scholar] [CrossRef] [Green Version]
- Hu, R.; Noubactep, C. Redirecting Research on Fe0 for Environmental Remediation: The Search for Synergy. Int. J. Environ. Res. Public Health 2019, 16, 4465. [Google Scholar] [CrossRef] [Green Version]
- Hu, R.; Yang, H.; Tao, R.; Cui, X.; Xiao, M.; Amoah, B.K.; Cao, V.; Lufingo, M.; Soppa-Sangue, N.P.; Ndé-Tchoupé, A.I.; et al. Metallic Iron for Environmental Remediation: Starting an Overdue Progress in Knowledge. Water 2020, 12, 641. [Google Scholar] [CrossRef] [Green Version]
- Moraci, N.; Ielo, D.; Bilardi, S.; Calabrò, P.S. Modelling long-term hydraulic conductivity behaviour of zero valent iron column tests for permeable reactive barrier design. Can. Geotech. J. 2016, 53, 946–961. [Google Scholar] [CrossRef]
- Henderson, A.D.; Demond, A.H. Impact of Solids Formation and Gas Production on the Permeability of ZVI PRBs. J. Environ. Eng. 2011, 137, 689–696. [Google Scholar] [CrossRef]
- Guan, X.; Sun, Y.; Qin, H.; Li, J.; Lo, I.M.C.; He, D.; Dong, H. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: The development in zero-valent iron technology in the last two decades (1994–2014). Water Res. 2015, 75, 224–248. [Google Scholar] [CrossRef]
- Noubactep, C. Relevant Reducing Agents in Remediation Fe0/H2O Systems. CLEAN—Soil Air Water 2013, 41, 493–502. [Google Scholar] [CrossRef]
- Hu, R.; Cui, X.; Gwenzi, W.; Wu, S.; Noubactep, C. Fe0/H2O Systems for Environmental Remediation: The Scientific History and Future Research Directions. Water 2018, 10, 1739. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Hu, R.; Ruppert, H.; Noubactep, C. Modeling porosity loss in Fe0-based permeable reactive barriers with Faraday’s law. Sci. Rep. 2021, 11, 16998. [Google Scholar] [CrossRef] [PubMed]
- Bilardi, S.; Calabrò, P.S.; Caré, S.; Moraci, N.; Noubactep, C. Effect of pumice and sand on the sustainability of granular iron beds for the aqueous removal of CuII, NiII, and ZnII. Clean—Soil Air Water 2013, 41, 835–843. [Google Scholar] [CrossRef] [Green Version]
- Henderson, A.D.; Demond, A.H. Long-Term Performance of Zero-Valent Iron Permeable Reactive Barriers: A Critical Review. Environ. Eng. Sci. 2007, 24, 401–423. [Google Scholar] [CrossRef] [Green Version]
- Liang, L.; Moline, G.R.; Kamolpornwijit, W.; West, O.R. Influence of hydrogeochemical processes on zero-valent iron reactive barrier performance: A field investigation. J. Contam. Hydrol. 2005, 80, 71–91. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Lee, T.R.; Sexton, M.R.; Acree, S.D.; Puls, R.W.; Blowes, D.W.; Kalinowski, C.; Tilton, J.M.; Woods, L.L. Geochemical and Isotope Study of Trichloroethene Degradation in a Zero-Valent Iron Permeable Reactive Barrier: A Twenty-Two-Year Performance Evaluation. Environ. Sci. Technol. 2019, 53, 296–306. [Google Scholar] [CrossRef]
- Mackenzie, P.D.; Horney, D.P.; Sivavec, T.M. Mineral precipitation and porosity losses in granular iron columns. J. Hazard. Mater. 1999, 68, 1–17. [Google Scholar] [CrossRef]
- Jun, D.; Yongsheng, Z.; Weihong, Z.; Mei, H. Laboratory study on sequenced permeable reactive barrier remediation for landfill leachate-contaminated groundwater. J. Hazard. Mater. 2009, 161, 224–230. [Google Scholar] [CrossRef]
- Klausen, J.; Ranke, J.; Schwarzenbach, R.P. Influence of solution composition and column aging on the reduction of nitroaromatic compounds by zero-valent iron. Chemosphere 2001, 44, 511–517. [Google Scholar] [CrossRef]
- Liu, T.; Lo, I.M.C. Influences of Humic Acid on Cr(VI) Removal by Zero-Valent Iron From Groundwater with Various Constituents: Implication for Long-Term PRB Performance. Water Air Soil Pollut. 2011, 216, 473–483. [Google Scholar] [CrossRef]
- Moraci, N.; Bilardi, S.; Calabrò, P.S. Design of permeable reactive barriers for remediation of groundwater contaminated by heavy metals. Riv. Ital. di Geotec. 2015, 49, 59–86. [Google Scholar]
- Bilardi, S.; Calabrò, P.S.; Moraci, N.; Madaffari, M.G.; Ranjbar, E. A comparison between Fe0 /pumice and Fe0 /lapillus mixtures in permeable reactive barriers. Environ. Geotech. 2020, 7, 524–539. [Google Scholar] [CrossRef]
- Ruhl, A.S.; Jekel, M. Degassing, gas retention and release in Fe(0) permeable reactive barriers. J. Contam. Hydrol. 2014, 159, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Benson, C.H. Evaluation of five strategies to limit the impact of fouling in permeable reactive barriers. J. Hazard. Mater. 2010, 181, 170–180. [Google Scholar] [CrossRef]
- Bilardi, S.; Amos, R.T.; Blowes, D.W.; Calabrò, P.S.; Moraci, N. Reactive Transport Modeling of ZVI Column Experiments for Nickel Remediation. Groundw. Monit. Remediat. 2013, 33, 97–104. [Google Scholar] [CrossRef]
- Bilardi, S.; Calabrò, P.S.; Moraci, N. The removal efficiency and long-term hydraulic behaviour of zero valent iron/lapillus mixtures for the simultaneous removal of Cu2+, Ni2+ and Zn2+. Sci. Total Environ. 2019, 675, 490–500. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Bilardi, S.; Moraci, N. Advancements in the use of filtration materials for the removal of heavy metals from multicontaminated solutions. Curr. Opin. Environ. Sci. Health 2021, 20, 100241. [Google Scholar] [CrossRef]
- Noubactep, C. Should the term ‘metallic iron’ appear in the title of a research paper? Chemosphere 2022, 287, 132314. [Google Scholar] [CrossRef]
- Cao, V.; Yang, H.; Ndé-Tchoupé, A.I.; Hu, R.; Gwenzi, W.; Noubactep, C. Tracing the Scientific History of Fe0-Based Environmental Remediation Prior to the Advent of Permeable Reactive Barriers. Processes 2020, 8, 977. [Google Scholar] [CrossRef]
- Bilardi, S.; Calabró, P.S.; Moraci, N. Simultaneous removal of CU II, NI II and ZN II by a granular mixture of zero-valent iron and pumice in column systems. Desalin. Water Treat. 2015, 55, 767–776. [Google Scholar] [CrossRef]
- Madaffari, M.G.; Bilardi, S.; Calabrò, P.S.; Moraci, N. Nickel removal by zero valent iron/lapillus mixtures in column systems. Soils Found. 2017, 57, 745–759. [Google Scholar] [CrossRef]
- Moraci, N.; Bilardi, S.; Mandaglio, M.C. Factors affecting geotextile filter long-term behaviour and their relevance in design. Geosynth. Int. 2022, 29, 19–42. [Google Scholar] [CrossRef]
- Noubactep, C. Research on metallic iron for environmental remediation: Stopping growing sloppy science. Chemosphere 2016, 153, 528–530. [Google Scholar] [CrossRef] [PubMed]
- Moraci, N.; Bilardi, S.; Calabrò, P.S. Fe0/pumice mixtures: From laboratory tests to permeable reactive barrier design. Environ. Geotech. 2017, 4, 245–256. [Google Scholar] [CrossRef]
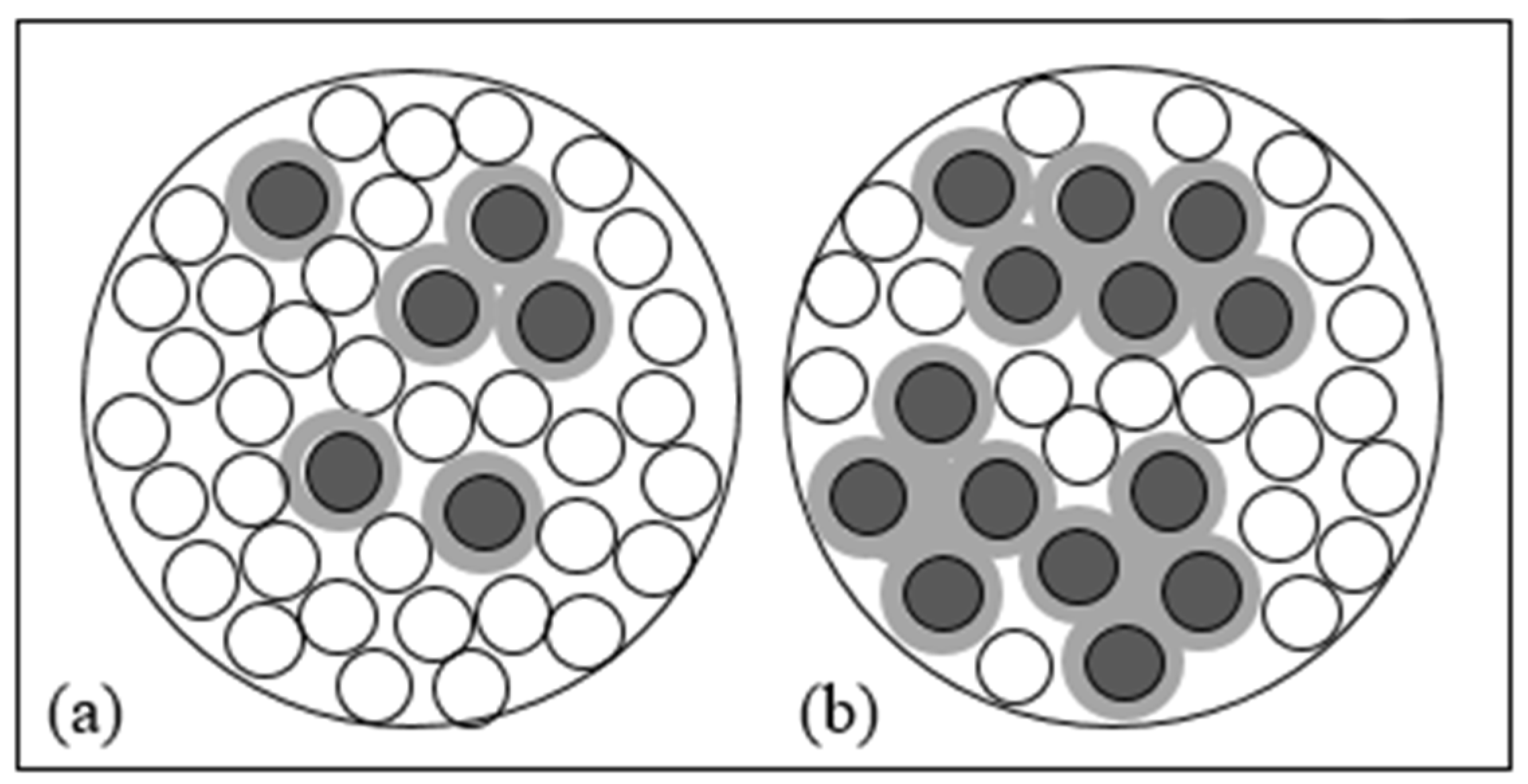

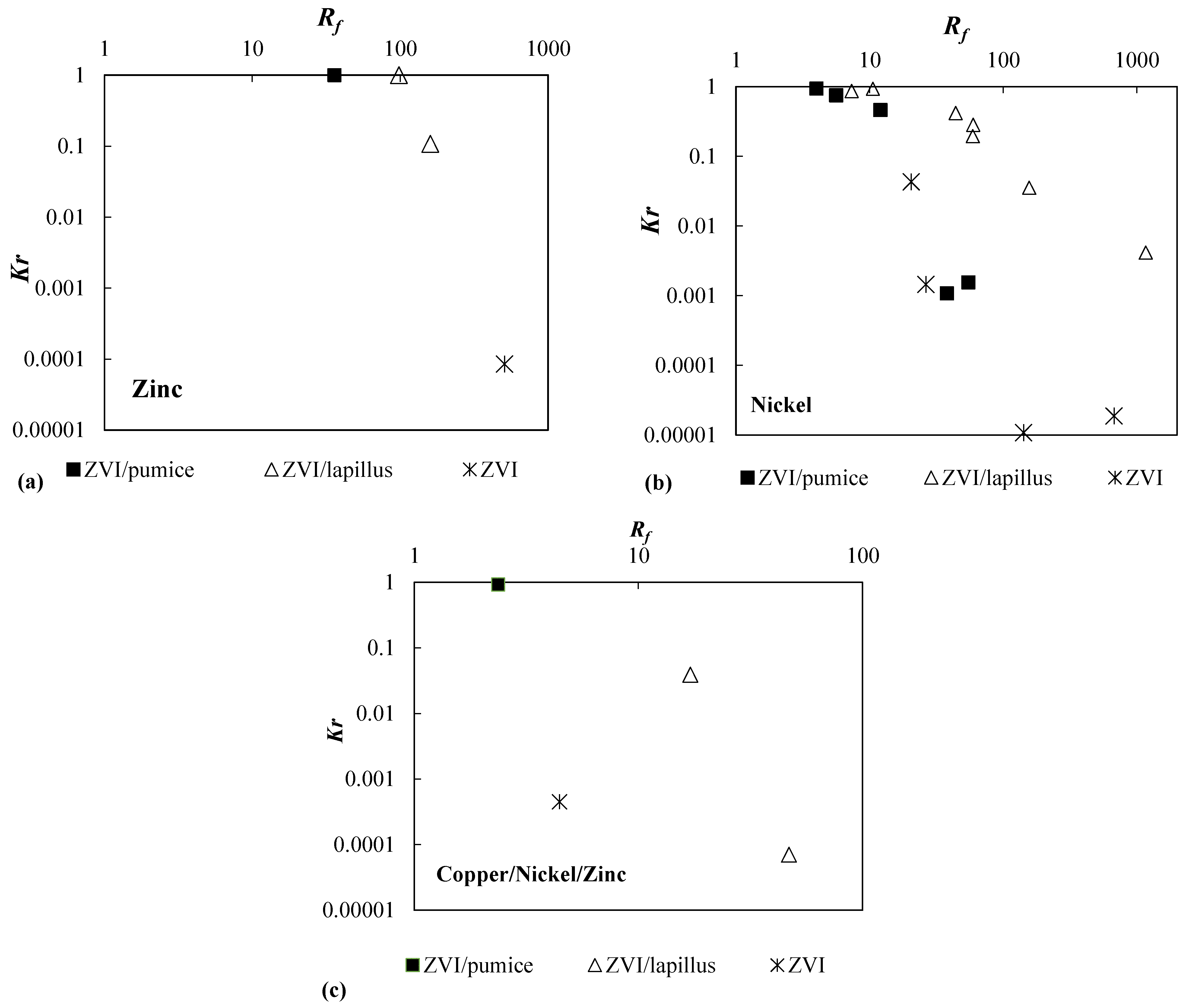
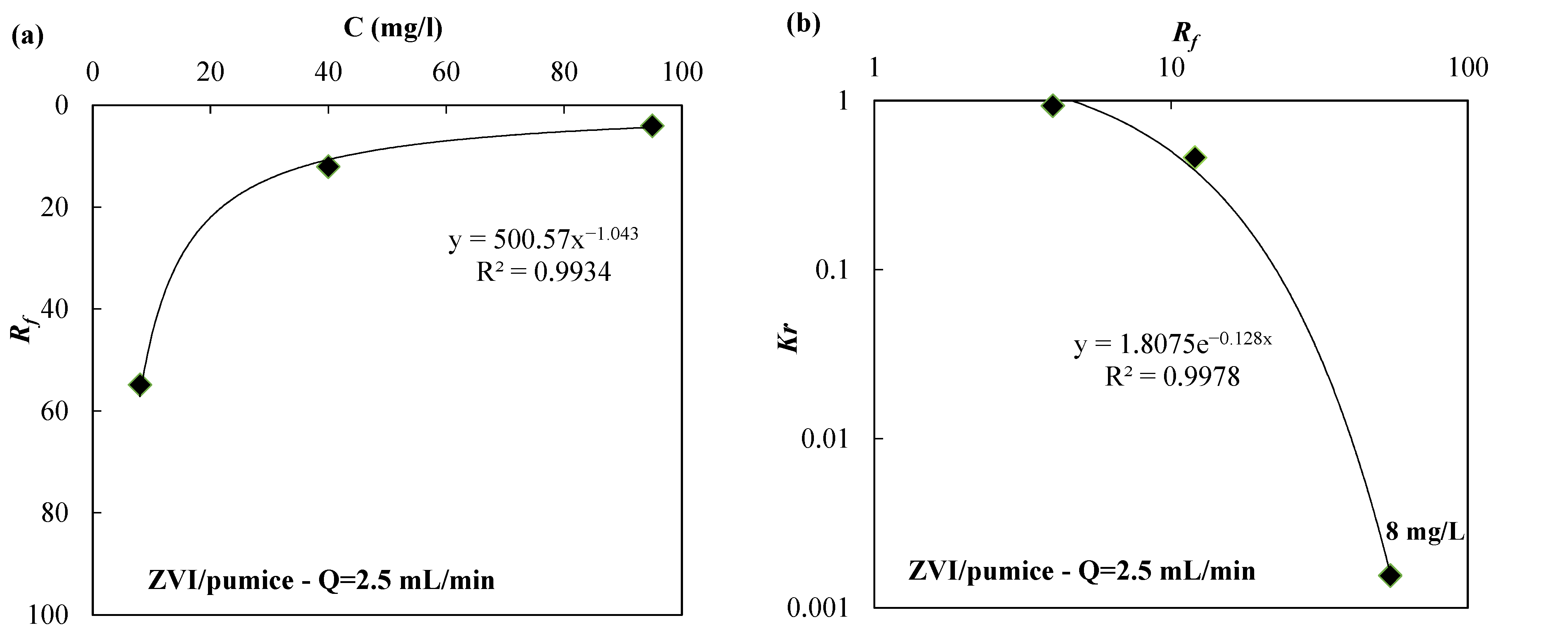

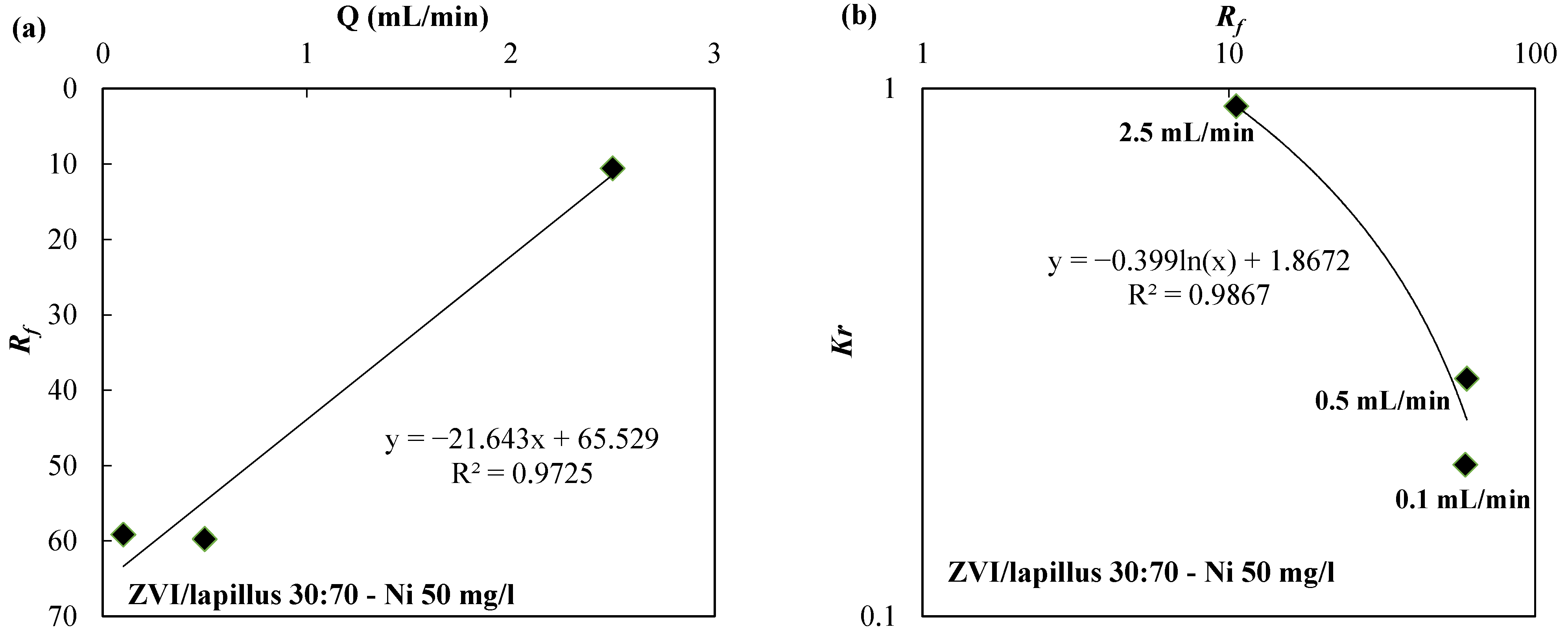
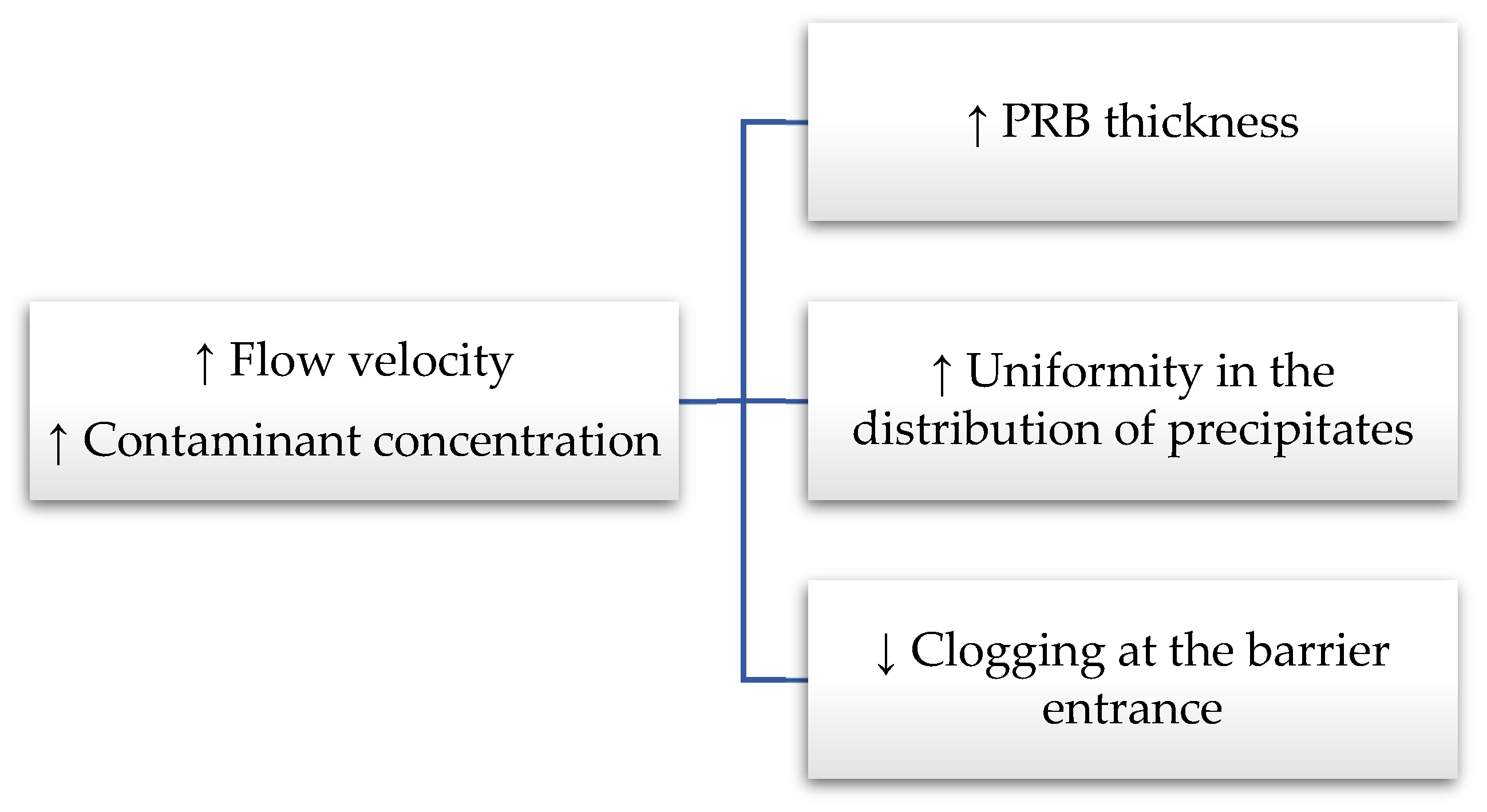
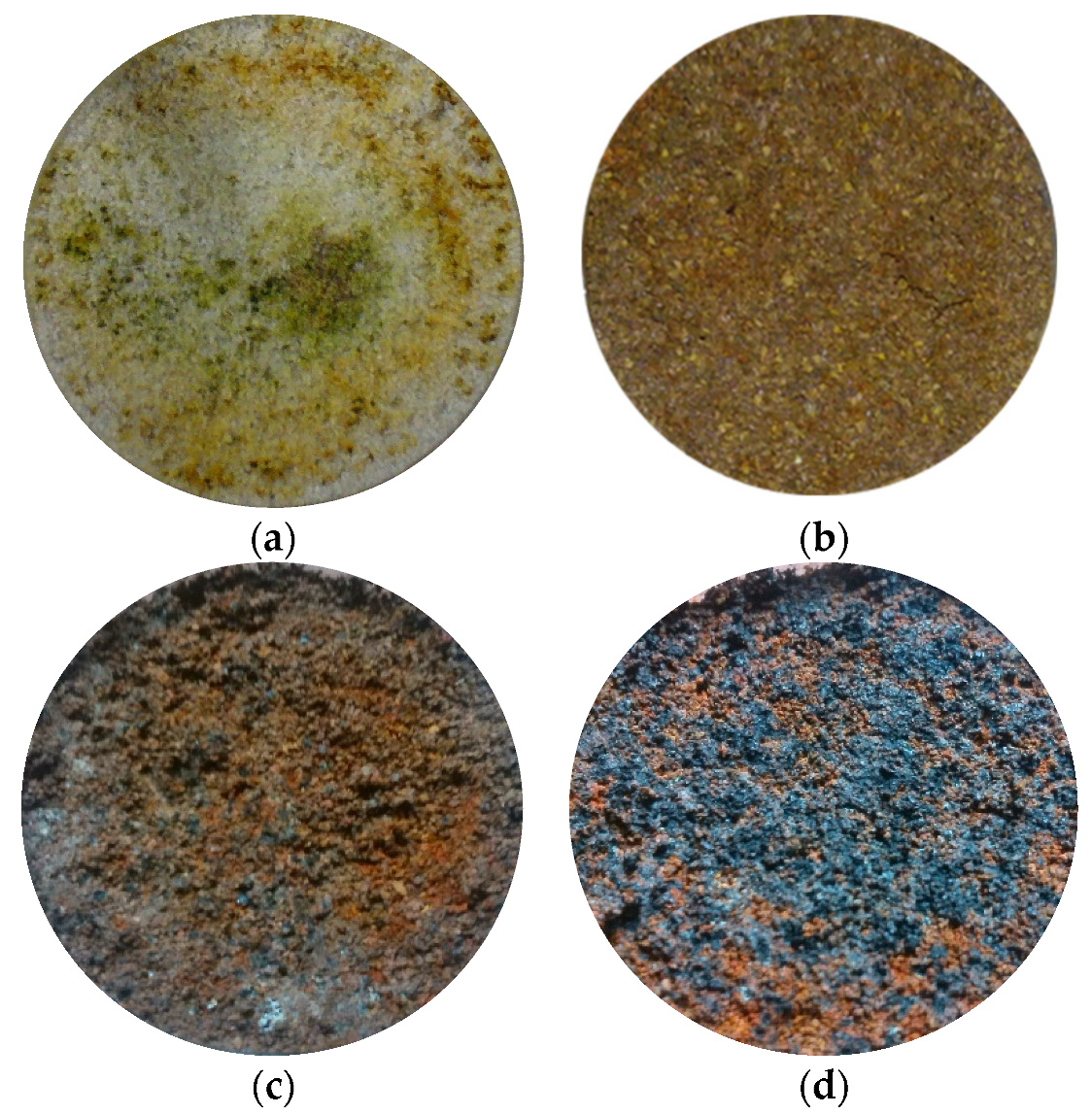
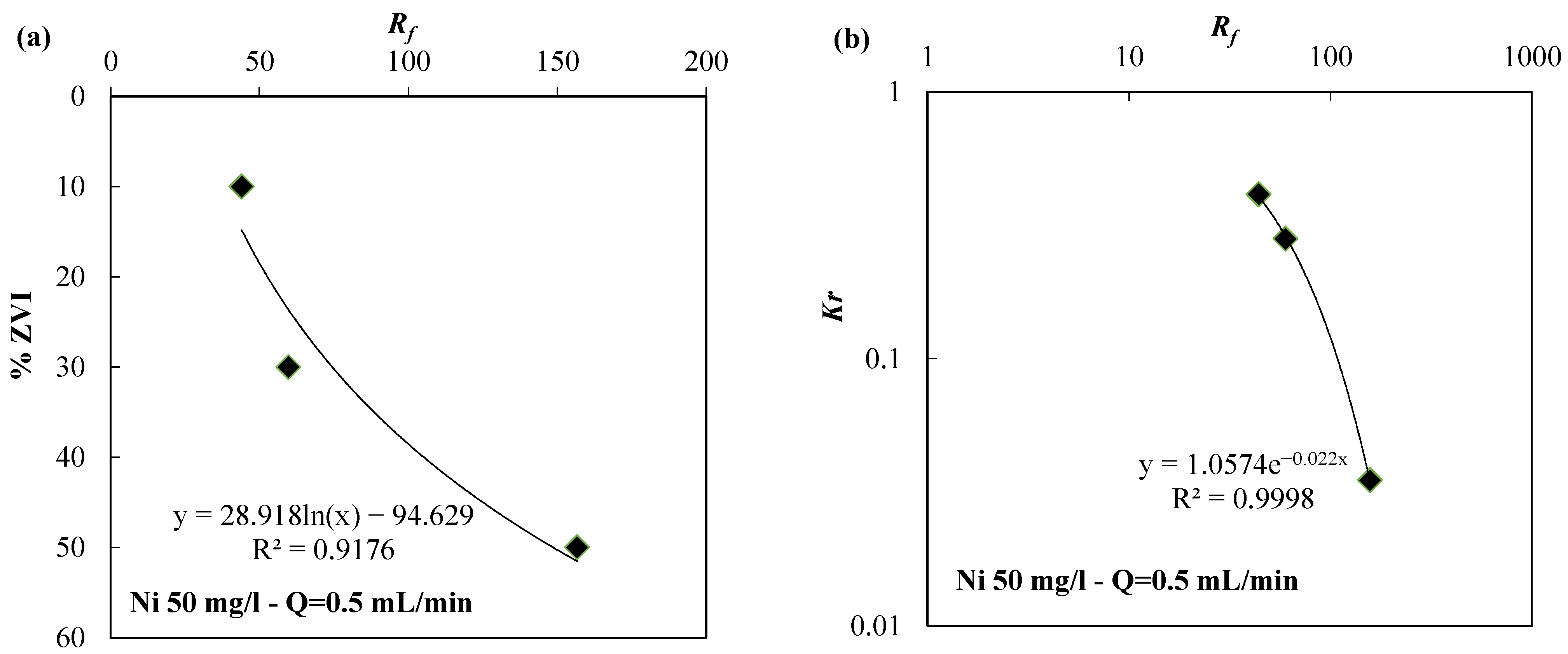

| Reactive Medium | Contaminant- Concentration [mg/L] | Q [mL/min] | L [cm] | Test Duration [days] | References |
|---|---|---|---|---|---|
| ZVI/pumice 30:70 | Zn-50 | 0.5 | 50 | 87 | [40] |
| ZVI/lapillus 30:70 | Zn-50 | 0.5 | 50 | 108 | [32] |
| ZVI/lapillus 50:50 | Zn-50 | 0.5 | 50 | 394 | New test |
| ZVI | Zn-50 | 0.5 | 3 | 84 | [40] |
| ZVI/pumice 30:70 | Ni-95 | 0.1 | 50 | 104 | [31] |
| ZVI/pumice 30:70 | Ni-95 | 2.5 | 50 | 27 | |
| ZVI | Ni-95 | 2.5 | 22.5 | 52 | |
| ZVI/pumice 30:70 | Ni-40 | 0.1 | 50 | 541 | |
| ZVI/pumice 30:70 | Ni-40 | 2.5 | 50 | 35 | |
| ZVI | Ni-40 | 0.1 | 22.5 | 375 | |
| ZVI | Ni-40 | 2.5 | 22.5 | 32 | |
| ZVI/pumice 30:70 | Ni-8 | 2.5 | 50 | 87 | |
| ZVI | Ni-8 | 2.5 | 50 | 18 | |
| ZVI/pumice 30:70 | Cu-500/Ni-50/Zn-50 | 0.5 | 50 | 87 | [40] |
| ZVI | Cu-500/Ni-50/Zn-50 | 0.5 | 3 | 25 | |
| ZVI/lapillus 30:70 | Cu-500/Ni-50/Zn-50 | 0.5 | 50 | 192 | [36] |
| ZVI/lapillus 50:50 | Cu-500/Ni-50/Zn-50 | 0.5 | 50 | 140 | |
| ZVI/lapillus 10:90 | Ni-50 | 0.5 | 50 | 216 | [41] |
| ZVI/lapillus 30:70 | Ni-50 | 0.5 | 50 | 250 | |
| ZVI/lapillus 50:50 | Ni-50 | 0.5 | 50 | 222 | |
| ZVI/lapillus 30:70 | Ni-50 | 0.1 | 50 | 1223 | |
| ZVI/lapillus 30:70 | Ni-50 | 2.5 | 50 | 35 | |
| ZVI/lapillus 30:70 | Ni-10 | 0.5 | 50 | 502 | |
| ZVI/lapillus 30:70 | Ni-100 | 0.5 | 50 | 120 |
| Reactive Medium | Concentration [mg/L] | Q [mL/min] | Rf | Kr | |
|---|---|---|---|---|---|
| Zn | ZVI/pumice 30:70 | 50 | 0.5 | 35.86 | 1 |
| ZVI/lapillus 30:70 | 50 | 0.5 | 97.95 | 1 | |
| ZVI/lapillus 50:50 | 50 | 0.5 | 159.51 | 1.08 × 10−1 | |
| ZVI | 50 | 0.5 | 506.67 | 8.52 × 10−5 | |
| Ni | ZVI/pumice 30:70 | 95 | 0.1 | 5.61 | 7.45 × 10−1 |
| ZVI/pumice 30:70 | 95 | 2.5 | 4.00 | 9.32 × 10−1 | |
| ZVI | 95 | 2.5 | 20.53 | 4.29 × 10−2 | |
| ZVI/pumice 30:70 | 40 | 0.1 | 37.96 | 1.08 × 10−3 | |
| ZVI/pumice 30:70 | 40 | 2.5 | 12.05 | 4.59 × 10−1 | |
| ZVI | 40 | 0.1 | 142.12 | 1.07 × 10−5 | |
| ZVI | 40 | 2.5 | 26.43 | 1.45 × 10−3 | |
| ZVI/pumice 30:70 | 8 | 2.5 | 54.84 | 1.55 × 10−3 | |
| ZVI | 8 | 2.5 | 676.99 | 1.86 × 10−5 | |
| ZVI/lapillus 10:90 | 50 | 0.5 | 44.03 | 4.13 × 10−1 | |
| ZVI/lapillus 30:70 | 50 | 0.5 | 59.74 | 2.82 × 10−1 | |
| ZVI/lapillus 50:50 | 50 | 0.5 | 156.67 | 3.51 × 10−2 | |
| ZVI/lapillus 30:70 | 50 | 0.1 | 59.16 | 1.94 × 10−1 | |
| ZVI/lapillus 30:70 | 50 | 2.5 | 10.58 | 9.27 × 10−1 | |
| ZVI/lapillus 30:70 | 10 | 0.5 | 1171.18 | 4.13 × 10−3 | |
| ZVI/lapillus 30:70 | 100 | 0.5 | 7.34 | 8.57 × 10−1 | |
| Cu | ZVI/pumice 30:70 | 500 | 0.5 | 11.46 | 9.19 × 10−1 |
| Ni | 50 | 9.24 | |||
| Zn | 50 | 2.37 | |||
| Cu | ZVI | 500 | 0.5 | - | 4.51 × 10−4 |
| Ni | 50 | 4.44 | |||
| Zn | 50 | 30.55 | |||
| Cu | ZVI/lapillus 30:70 | 500 | 0.5 | 706.66 | 3.92 × 10−2 |
| Ni | 50 | 17.04 | |||
| Zn | 50 | 42.26 | |||
| Cu | ZVI/lapillus 50:50 | 500 | 0.5 | - | 7.00 × 10−5 |
| Ni | 50 | 46.98 | |||
| Zn | 50 | 92.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilardi, S.; Calabrò, P.S.; Moraci, N. Reactive and Hydraulic Behavior of Granular Mixtures Composed of Zero Valent Iron. Water 2022, 14, 3613. https://doi.org/10.3390/w14223613
Bilardi S, Calabrò PS, Moraci N. Reactive and Hydraulic Behavior of Granular Mixtures Composed of Zero Valent Iron. Water. 2022; 14(22):3613. https://doi.org/10.3390/w14223613
Chicago/Turabian StyleBilardi, Stefania, Paolo S. Calabrò, and Nicola Moraci. 2022. "Reactive and Hydraulic Behavior of Granular Mixtures Composed of Zero Valent Iron" Water 14, no. 22: 3613. https://doi.org/10.3390/w14223613
APA StyleBilardi, S., Calabrò, P. S., & Moraci, N. (2022). Reactive and Hydraulic Behavior of Granular Mixtures Composed of Zero Valent Iron. Water, 14(22), 3613. https://doi.org/10.3390/w14223613








