Abstract
Recently, in situ YSI EXO2 phycocyanin fluorescence probes have been widely deployed as a means to determine cyanobacterial abundance in drinking water sources, yet few studies have evaluated the effects of natural organic matter (NOM) and the ambient water temperature on the probe readings. In this study, Suwannee River NOM was added to laboratory cultivated cyanobacterial species to test the performance of the phycocyanin probe. The impact of temperature on phycocyanin fluorescence was evaluated by monitoring the laboratory cultivated cyanobacterial species and extracted phycocyanin pigment. Additionally, in situ phycocyanin fluorescence of the field samples from the water intake of a drinking water treatment plant (DWTP) in 2018 were compared with grab sample laboratory taxonomic analyses. We found: (1) the presence of Suwannee River NOM leads to the decrease in cell-bound cyanobacterial phycocyanin readings; (2) increasing ambient water temperature reduces dissolved and cell-bound cyanobacterial phycocyanin readings; (3) field study phycocyanin probe readings significantly correlated with the total cyanobacterial biovolume (R = 0.73, p < 0.1), and the relationship depends on the biovolume of dominant cyanobacterial species; (4) phycocyanin probe readings have a strong positive correlation with the natural light intensities; and (5) probe users should be fully aware of the sources of interferences when interpreting the results and apply the other physical-chemical parameters data simultaneously generated by the fluorometry to improve the probe’s measurements.
1. Introduction
Cyanobacteria-related illnesses are a rising concern with regards to the quality of drinking water sources. Certain cyanobacterial species can biosynthesize different types of cyanotoxins which pose risks to human and animal health. Cyanobacteria can also produce nuisance taste and odor (T&O) compounds in distributed water, which may reduce water aesthetic quality, and does not meet customer expectation [1]. It is expected that the frequency, duration, and density of cyanobacterial blooms will increase in the near future, due to global climate change and human activities [2,3,4,5,6,7]. To assess the risk posed to drinking water supplies, and to ensure that treatment is adjusted to respond to varying levels of cyanobacteria present, quick detection of cyanobacterial biomass is of critical importance for water utilities.
The technologies used to monitor cyanobacteria can be classified into three main categories: microscopic enumeration and identification; molecular-based methods; and pigment concentration measurements. The counting of taxonomic microscopic cells is time-consuming, expensive, and requires an experienced experimenter [8,9,10,11]. The results are prone to significant error (up to 40%) because of varying cell morphology, aggregation, presence of the preservation solution, and analyst bias and experience [10,12,13,14,15,16]. Considering molecular-based methods, PCR, Real-Time PCR, and high-throughput sequencing can greatly improve the ability to identify the microbial community composition, determine the functional genes, and trace the activity of the cyanobacterial cell genes [11,15,17,18,19,20,21,22,23,24,25,26,27]. However, molecular-based methods are complex, high-cost, and require skillful analysts [11,18]. Moreover, bias could be caused by an incomplete gene bank library, primer selection, amplification, and genomic DNA base composition [25,28,29]. These methods restrict the number of samples that can be analyzed, undermine online assessment, and limit the ability to reflect the cyanobacterial biomass fluctuation. Cyanobacteria can also be monitored by pigment quantification. Phycocyanin is a unique phycobilisome pigment of freshwater cyanobacteria, and has strong fluorescence signatures. The excitation wavelength of the phycocyanin is between 610 and 630 nm, and the emission wavelength is between 600 and 700 nm [30,31,32]. Numerous studies have suggested using fluorescence phycocyanin probes as an online tool for cyanobacterial monitoring due to its operational simplicity, high detection frequency, and low cost [33,34,35].
It is important to determine sources of uncertainties in order to use phycocyanin probe readings to estimate cyanobacterial levels in water sources. Correlations have been reported between the phycocyanin fluorescence probe readings and the extracted phycocyanin concentration, total taxonomic cell counts, and total cyanobacterial biovolume (Table S1). In general, phycocyanin probe readings are well correlated with the laboratory-cultured unicellular species, and less promising with the colonial species and mixed species. Field samples are more likely to be subject to interferences. The reference method to which the probe readings are compared is also important. From Table S1, phycocyanin probe readings are better correlated with biovolume, compared to taxonomic cell counts. Various factors can influence the accuracy of phycocyanin fluorescence analysis (Table S2). Considerable investigations have been reported into different cells’ growth phases, sizes, and geometries, which can result in deviation in the phycocyanin readings [33,36,37,38,39,40,41]. Sources of interference relate to several potential environmental factors, which interfere with the probe’s capacity to precisely measure phycocyanin pigment content. Such interferences include cell prior light exposure [10,39,41,42,43,44], the presence of chlorophyll-a [9,10,45,46], and water turbidity [41,44,47,48].
The variation in natural organic matter (NOM) content and water temperature are other critical factors that can influence the accuracy of in situ phycocyanin probe monitoring. For this reason, some fluorometer manufacturers specify their operating conditions. For instance, Turner Cyanofluor corrects the interference from dissolved organic carbon (DOC) using a filtrate blank [49]. In the actual experimental situation, recent research has shown a significant impact of NOM on phycocyanin probe response to cyanobacterial cells, but the different water matrix, types of NOM investigated, type and presence of cyanobacterial cells, and type of probe investigated were limited [37,50,51]. Furthermore, DOC concentration in the study of Bertone et al. [50] was measured by a YSI EXO2 fDOM sensor, which is not as reliable as direct DOC measurements and does not provide information on the adsorption spectra of the NOM. Temperature was also shown to influence phycocyanin probe readings. Some probe manufacturers incorporate or propose a temperature compensation, and these factors vary between manufacturers and devices. Recent investigations of the impact of temperature on several probe devices showed that corrective factors vary between devices [39,52]. To the best of our knowledge, this is the first to report on the impact of different ambient water temperatures on the YSI EXO2 phycocyanin probe.
Evaluating the impact of NOM and temperature on the phycocyanin probe measurements is important because climate change and the increased frequency of extreme events in natural water are likely to result in higher temperatures and NOM levels in freshwater [53,54]. Moreover, in the application of field in situ measurement, it is challenging to maintain a constant water temperature, and the probe is generally deployed in a wide range of temperatures.
The main objective of the work was to validate the use of an in situ phycocyanin fluorescence probe for cyanobacteria detection in the intake water of a drinking water treatment plant (DWTP). The specific objectives of this study were to investigate: (1) the impact of Suwannee River NOM in ultrapure and lake water on the monitoring of cyanobacterial phycocyanin readings and investigate the mechanism of interference; (2) the impact of temperatures from 6 to 33 °C in ultrapure water on phycocyanin readings on dissolved and cell-bound phycocyanin; (3) relationships between cyanobacterial biovolume and phycocyanin probe measurements; (4) the applicability for the measurement of cyanobacteria within the DWTP by using the fluorescence probe.
2. Materials and Methods
2.1. Cell Culture
Microcystis aeruginosa strain CPCC 300, referred to Microcystis, and Dolichospermum sp. (formerly known as Anabaena sp.) strain CPCC 544, referred to Dolichospermum, were selected in this study. Microcystis and Dolichospermum are common unicellular, and filamentous cyanobacterial species, respectively [55]. Axenic cultures were cultivated in 9 L flasks with BG-11 medium [36]. The temperature of the culture room was 20 ± 2 °C. A cool fluorescence light at an intensity of 70 μmol/S.m2 radiated to the room 12 h a day. Air diffusers were implemented in the cyanobacterial cell cultures to provide sufficient oxygen and mixing conditions. Cultures were harvested at the stationary growth phase.
2.2. Microscopic Taxonomic Cell Counts
Cultures were centrifuged by Sorvall Legend RT at 10,000× g and 4 °C for 15 min to separate the cells from the medium and extracellular organic matter [56]. The supernatant was discarded, and the pelleted cells were re-suspended in 5 mL of ultrapure water (Millipore Pty Ltd., Burlington, MA, USA) by vortexing for 20 s. An inverted microscopic (Olympus IX71) at 20× was used for taxonomic cell counts in a Sedgwick-Rafter counting chamber. The final counting result was derived from the cell number of the known area to 1 mL of sample. The cell sample was then diluted with ultrapure water or lake water (mentioned below) to low (2000 cells/mL), medium (20,000 cells/mL), and high (100,000 cells/mL) cell concentrations according to the microscopic cell counts results, as described by Zamyadi et al. [57].
2.3. Fluorometer Measurements
A YSI EXO2 probe (YSI, Yellow Springs, OH, USA), which featured a phycocyanin/chlorophyll-a sensor, conductivity/temperature sensor, dissolved oxygen (DO) sensor, turbidity sensor, and pH sensor, was used in this study. A self-cleaning wiper was applied to the probe. The probe converts the light emitted from the phycocyanin pigments first into ratio fluorescent unit (RFU), and then to the micrograms per liter phycocyanin pigment concentration (µg/L) via an onboard default conversion factor. Several studies have shown better correlation with the phycocyanin RFU content in comparison with µg/L [41,58,59,60]. Probe phycocyanin optics has the excitation wavelength of 590 ± 15 nm, and emission wavelength of 685 ± 20 nm [61]. The probe was calibrated according to the manufacturer’s user manual [61]. Negative phycocyanin RFU readings were observed from the monitoring of ultrapure water. Thus, we calculated the limit of blank (LoB) using Equation (1) [62], and the phycocyanin values that were lower than LoB were adjusted as 0 for all measurements.
where meanblank and SDblank are the mean and the standard deviation of the measurement of ultrapure water.
LoB= meanblank + 1.645 (SDblank)
All measurements were conducted using the probe calibration cap with a black bottom to eliminate light influence. The probe and its sensors were cleaned with ultrapure water before each sample. The probe data were recorded once the readings were stabilized. The readings were recorded for 10 min at a 0.25 s interval.
2.4. NOM Addition
To test the interference of NOM on the fluorescence signal of the cyanobacterial cells, assays were performed by using the solution made from Suwannee River NOM (2R101N, International Humic Substances Society, Saint Paul, MN, USA; SRNOM). SRNOM is measured based on the DOC content in this study. The DOC concentrations of the sample were measured using a total organic carbon analyzer (Sievers Analytical Instruments, Boulder, CO, USA), after sample filtration with the pre-rinsed 0.45 μm membrane (Supor 45 μm, PES PALL, Port Washington, NY, USA). In the set of assays, four concentrations of DOC (0, 0.7, 1.7, 11 mg/L) of SRNOM were added in sequence to a range of concentrations of cyanobacterial cells (Microcystis and Dolichospermum, at 0, 2000, 20,000, and 100,000 cells/mL). Experiments were assessed on two matrixes: ultrapure water and lake water (DOC of 6.31 mg/L). Lake water was collected in May 2019 from Lake Champlain (Quebec, QC, Canada).
2.5. Water Temperature Effect
To evaluate the interference of the water temperature with cyanobacterial phycocyanin fluorescence signal, Microcystis was spiked into the different temperatures of ultrapure water. Initially, 500 mL ultrapure water samples were placed into containers with temperatures of 6, 12, 33 °C, and room temperature (21 °C). Then, a Microcystis cell sample was spiked into each of the different water temperature reactors to reach the cell concentration of 2000, 20,000, and 100,000 cells/mL. In addition, the 1 mg/L C-phycocyanin extract (P2172, Sigma-Aldrich, St. Louis, MO, USA) was dissolved into the above-mentioned ultrapure water with different temperatures.
2.6. Fluorescence Excitation-Emission Matrix Spectra Analysis
The fluorescence spectra of ultrapure water containing Microcystis (100,000 cells/mL) with and without SRNOM were measured using fluorescence excitation-emission matrix (EEM) spectra by a fluorescence spectrophotometer (Agilent, Cary Eclipse, Santa Clara, CA, USA). Both excitation spectra and emission spectra were scanned from the wavelength of 200 to 750 nm using a 1 cm quartz cuvette for each sample. The increments of the excitation and the emission spectra were 5 and 2 nm, respectively.
2.7. Field Sampling
Field sampling was conducted from Bedford DWTP from July to August 2018. Bedford DWTP draws water from Lake Champlain in southern Quebec. Cyanobacterial bloom has occurred in the Bedford DWTP during the summer and fall over the past decade [8,35,63]. In the DWTP, the data was recorded every 2 h, each round for 30 min at 5 min intervals, by a YSI EXO2 phycocyanin probe.
2.8. Data Analysis
A non-parametric Kruskal–Wallis test was used to determine if there were significant variations between the set of NOM and temperature essays. Dunn’s post hoc test was used to detect significant differences between each treatment. The level of significance was 0.05.
EEM data processing followed the method reported in Stedmon and Bro [64]. In brief, the raw spectra data were subtracted from the blank spectra with ultrapure water. Rayleigh scatter and Raman scatter were removed to eliminate the light scattering. Data manipulations were performed using MATLAB software (MathWorks, Natick, MA, USA) and the DOMFlour toolbox.
The correlation scatter plots between the phycocyanin readings and total cyanobacterial biovolume (and cell counts) were performed with the ggscatter R package with the Pearson correlation coefficient.
The correlations between the environmental parameters and phycocyanin readings were conducted using the R function corrplot.
3. Results and Discussion
3.1. Addition of NOM
3.1.1. Impact of NOM on Phycocyanin Probe Performance
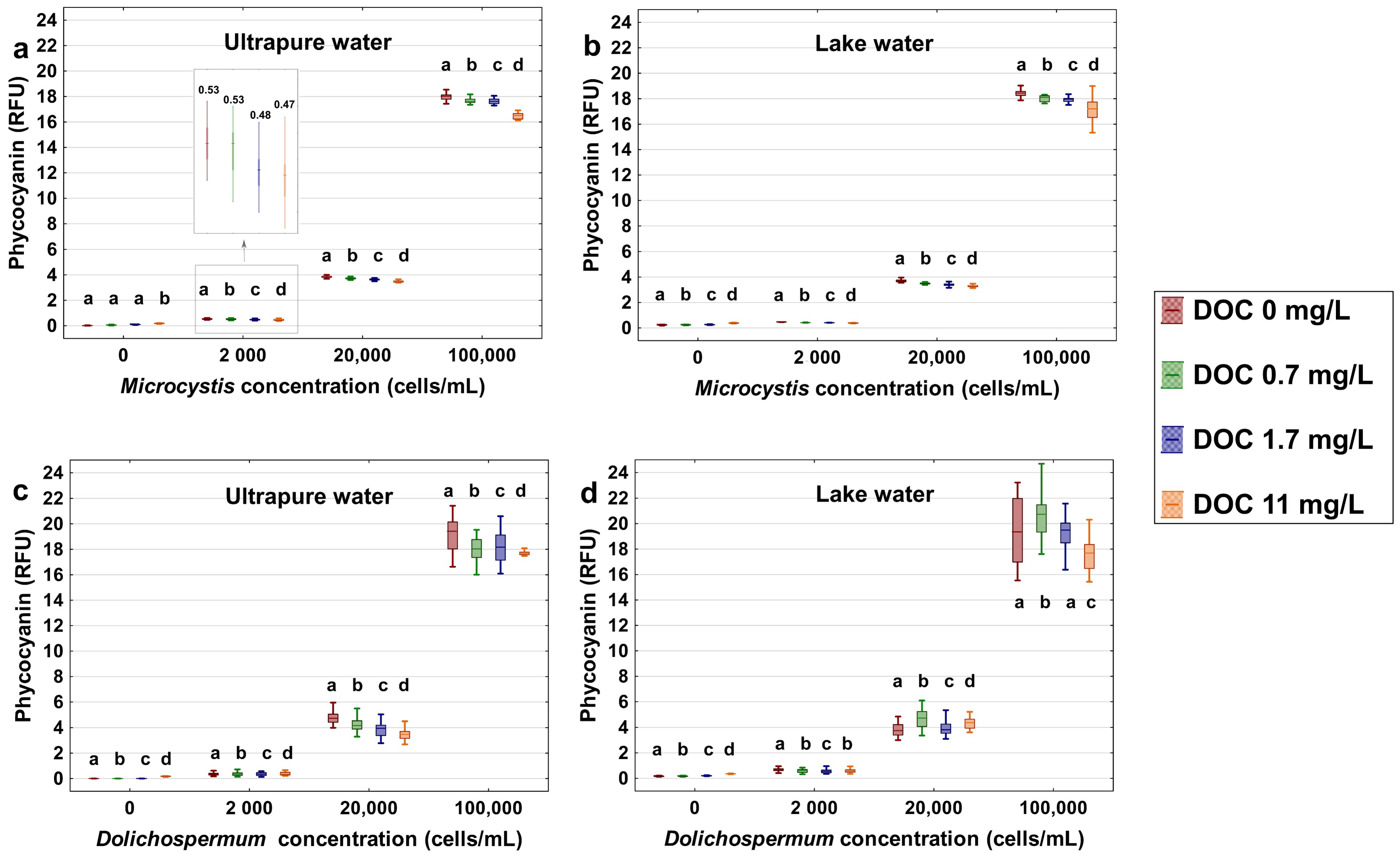
To investigate the extent of potential interferences by SRNOM on cell-induced fluorescence, the fluctuation of phycocyanin readings as a function of dosage and water matrix are shown in Figure 1. The results show that the level of phycocyanin readings decrease in the presence of cells after the addition of SRNOM in both ultrapure water and lake water, and statistical significant results are indicated by the letters a, b, c, d bearing on the boxplots (Figure 1). The addition of 11 mg/L of DOC of SRNOM in the high cell concentration (100,000 cells/mL) decreases the median phycocyanin readings by up to 1.7 RFU (1.2 to 1.7 RFU) for both Microcystis and Dolichospermum suspended in ultrapure water and lake water. The decrease in phycocyanin signal corresponds to a significant underestimation of 6.5–8.8% for high cyanobacterial cell concentrations. Although significant, this underestimation is modest for cell concentrations of 2000 and 20,000 cells/mL. The SRNOM interference is most pertinent when both cell concentration and the SRNOM contents are high.
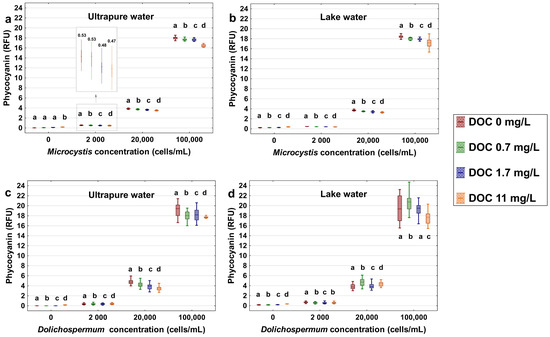
Figure 1.
Phycocyanin readings (RFU) by using the YSI EXO2 probe of 0 (no cells added), 2000, 20,000, and 100,000 cells/mL of cyanobacterial species with spike 0, 0.7, 1.7, 11 mg/L dissolved organic carbon (DOC) of Suwannee River NOM (SRNOM): (a,b) Microcystis aeruginosa strain CPCC 300 suspended in 500 mL ultrapure water and Lake Champlain water; (c,d) Dolichospermum sp. strain CPCC 544 suspended in 500 mL ultrapure water and Lake Champlain water. The probe measured each of the sample at 250 ms interval for 10 min. The bottom and top of each box represent the 25th–75th percentiles, respectively. The whiskers represent the minimum and maximum values. The line within each box corresponds to the median value. Different letters represent significant differences between treatment comparisons (Kruskal–Wallis followed by Dunn’s post hoc test, p ≤ 0.05).
In comparison with phycocyanin readings in the water matrix without the cells, the addition of 11 mg/L DOC of SRNOM causes an increase of the fluorescence signal of 0.2 RFU in ultrapure water and lake water (Figure 1). In lake water, a background fluorescence reading of 0.2 RFU is also noted for DOC of 6.31 mg/L (Figure 1b,d). These findings are in agreement with a prior study reporting a 0.8 RFU phycocyanin signal increase in a commercial humic acid solution (DOC of 10 mg/L) [51].
It is notable that the phycocyanin fluorescence signal of Dolichospermum suspensions (Figure 1c,d) is more dispersed as compared to readings from Microcystis (Figure 1a,b). Similar observations of greater variability in the fluorescence signals for the Dolichospermum were also reported by Kring et al. [65], as compared to unicellular Microcystis aeruginosa. These authors attributed their results to the lack of species-specific calibration (fingerprint) of the probes and the filamentous nature of Dolichospermum. Additionally, the cell-induced phycocyanin fluorescence in the lake matrix is even more dispersed than the tests that were conducted in ultrapure water for both Microcystis and Dolichospermum. In lake water, the addition of SRNOM in the presence of NOM increases fluorescence signal for Dolichospermum at low DOC (1.7 mg/L), suggesting an interaction between the cells and the NOM. Optical measurements in natural water can be influenced by the presence of the suspended particles in the natural water, which can lead to the scattering and absorption of the fluorescence [10,66].
3.1.2. Fluorescence EEM Studies
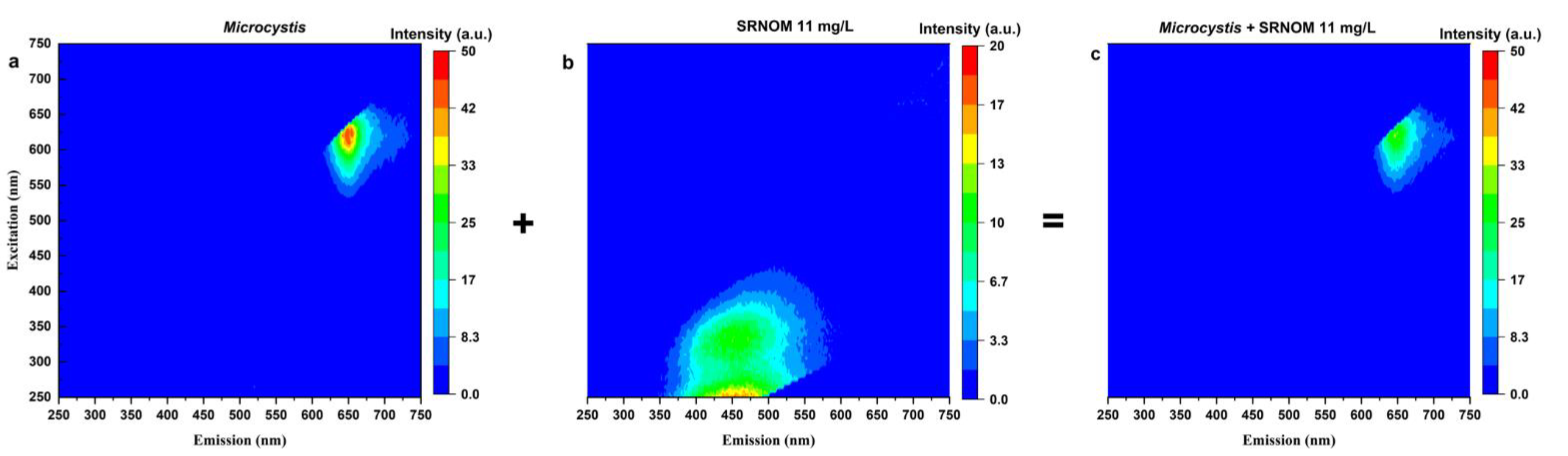
Fluorescence EEM spectroscopy is used to investigate the fluctuation of the Microcystis fluorescence by the addition of SRNOM. The excitation-emission results of ultrapure water in the presence of Microcystis cells (with and without SRNOM) are presented in Figure 2. Figure 2 demonstrates that the peak location is not changed by adding 11 mg/L DOC of SRNOM in the 100,000 cells/mL Microcystis samples. Both EEM spectra show the phycocyanin fluorescence measured at excitation wavelength of 625 nm, and emission wavelength of 650 nm, exactly matched the phycocyanin optical window. Strikingly, the addition of SRNOM decreases the fluorescence intensity by up to 33%, reflecting the decrease of fluorescence signal in Figure 1.
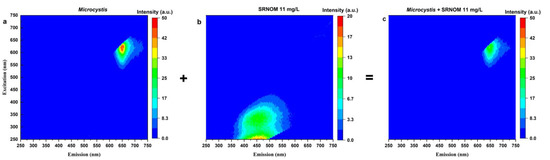
Figure 2.
Excitation-emission matrix for (a) 100,000 cells/L Microcystis aeruginosa CPCC 300 suspension in ultrapure water, (b) 11 mg/L DOC of SRNOM, (c) 11 mg/L DOC of SRNOM added to 100,000 cells/L Microcystis aeruginosa CPCC 300 suspension in ultrapure water. Note the Microcystis aeruginosa intensity is twice as high as that of SRNOM.
SRNOM exhibits fluorescence maximum at an excitation wavelength of 250 nm and emission wavelengths ranging from 430 to 480 nm (Figure 2b), which aligns with the wavelength reported in Yoo and Lee [67]. Cyanobacterial phycocyanin fluorescence signatures occur at high wavelength range (excitation and emission wavelength of 625 and 650 nm, respectively; Figure 2a). Comparison between the wavelengths of SRNOM and cyanobacterial phycocyanin indicates that their fluorescence is unlikely to overlap, precluding the interference from these compounds, provided quenching effects are accounted for. Similar investigations into whether the presence of NOM affects phycocyanin fluorescence readings have been undertaken by Korak et al. [37] by analyzing the EEMs. These authors found that DOM from river water (DOC of 2.65 mg/L) has no fluorescence interference with the extracted 1 mg/L phycocyanin pigment; however, with the Microcystis aeruginosa intracellular organic matter (IOM), the phycocyanin fluorescence intensity decreased by more than an order of magnitude. In addition to decreased intensity of Microcystis aeruginosa IOM, new fluorescence behavior was noted from the tests of other two species (Oscillatoria sp. and Lyngbya sp.) [37]. The authors attribute this to the quenching of cell IOM phycobiliprotein fluorescence [37]. Furthermore, Korak et al. [37] demonstrated that the decrease in phycocyanin fluorescence monitored in a DWTP could be used as a good indicator for the IOM release. However, our study found that the variation in NOM level could also cause a decline in fluorescence intensity of cyanobacterial cells (cell-bound phycocyanin). Thus, the previously found phycocyanin reduction may not be due to the release of IOM for a system that contains NOM.
The magnitude of the interference caused by NOM also depends on both the number of cells and the aromaticity of NOM. Bertone et al. [50] found that concentrated DOM from lake water (SUVA254 of 4.21 L/(mg.m)) results in underestimation of up to 14% of the phycocyanin signal measured by a YSI EXO2 probe (with 8,000,000 cells/mL Raphidiopsis raciborskii). The authors also reported that, the more aromatic and humic the DOM (higher SUVA254), the lower the measured phycocyanin signal. However, the concentrations of DOM were not specified in this study [50]. In our study, both cell concentrations (100,000 cells/mL) and SUVA254 value (SRNOM is 3.6 L/(mg.m)) were lower than in Bertone et al. [50]. As a result, lower suppression of phycocyanin was expected to be observed. Our observations complement the findings from these studies and consolidate the fact that the source of NOM may determine the extent of the impact on the phycocyanin fluorescence signal.
To quantify the importance of NOM interference, important points to consider include: the concentration of NOM added, aromaticity of NOM (SUVA254), the ratio of added NOM to the number of cells, and prior exposure of the cells to NOM for the testing of the interference. Firstly, it is difficult to identify the concentration of NOM in some studies, as different DOC measurement methods are used. Bertone et al. [50] used an fDOM sensor to measure DOC concentration but did not report on the concentration. fDOM sensors may not be as reliable as direct DOC measurements, as fDOM sensors need to be validated for the interference from ambient temperature, turbidity, and suspended particles [9,66,68]. Secondly, as shown in Figure 1, the species present (Microcystis aeruginosa and Dolichospermum sp.) and the background of DOC in the test water define the extent and the trends for fluorescence quenching. Thirdly, the interference caused by the addition of NOM is not impacted by the ratio of the added NOM to the number of cells, which varies from 0.11 to 5.5 µg DOC/cell.
The use of a phycocyanin probe to detect cyanobacteria in the presence of NOM will likely result in an underestimation of cyanobacteria. High concentrations of NOM (DOC of 11 mg/L) may lead to significant underestimation of bloom densities that require prompt response from managers. However, Figure 1 shows that significant false negative readings are associated with considerable NOM levels. An interference in the low probe signals of less than 1 RFU would not result in any change in terms of cyanobacteria surveillance and management solutions. Moreover, in a real-world application, it is unlikely that a sudden increase in NOM extent will occur in the source water. According to our field sample results, DOC concentration ranges from 5.3 to 7.9 mg/L, provided that the NOM-induced interference appears negligible. The calibration to a standard DOC solution may reduce the interference by the presence of NOM in the background water.
3.2. Varying Water Matrix Temperature
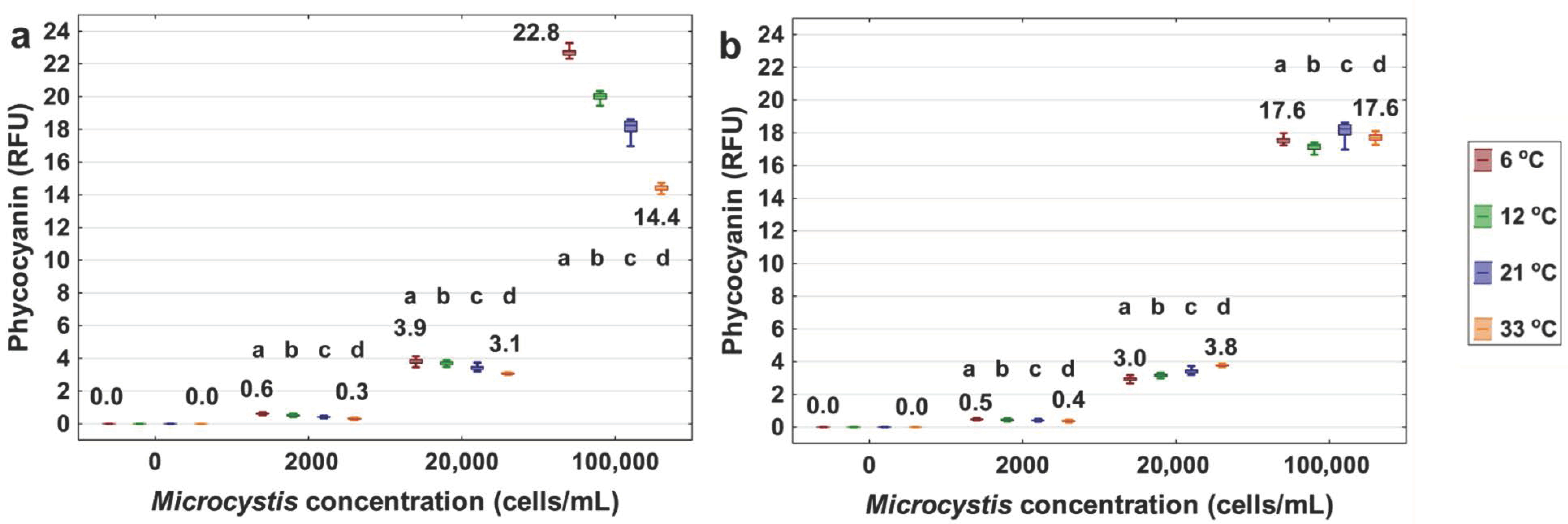
The phycocyanin readings of Microcystis were measured at different ultrapure water temperatures. Figure 3a shows that the level of phycocyanin readings decreases at higher water temperatures. Kruskal–Wallis tests show a statistically significant effect in all the sets of assays. Dunn’s post hoc tests reveal significant differences between each assay. For the high Microcystis density (100,000 cell/mL), the phycocyanin readings decrease by 8.4 RFU when water temperature increases from 6 to 33 °C. The decrease in phycocyanin signal corresponds to a significant underestimation of 36.8% for the 100,000 cells/mL cyanobacterial cell concentration. The underestimation is 50.0% for 2000 cells/mL and 20.5% for 20,000 cells/mL of Microcystis.
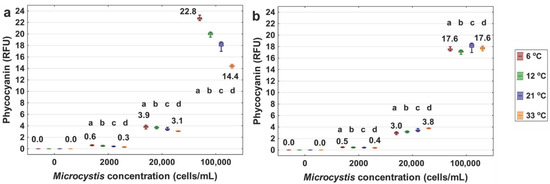
Figure 3.
Phycocyanin readings (RFU) with the YSI EXO2 probe of the 0, 2000, 20,000 and 100,000 cells/mL of Microcystis aeruginosa strain CPCC 300 suspended in 6, 12, 21, 33 °C of ultrapure water. The probe measured each of the samples at a 250 ms interval for 10 min (a). In (b) the temperature interference was removed by adjusting the raw data to a reference temperature of 21 °C using Equation (3). The bottom and top of each box represent the 25th–75th percentiles, respectively. The whiskers represent the minimum and maximum values. The line within each box corresponds to the median value. Different letters represent significant differences between treatment comparisons (Kruskal–Wallis followed by Dunn’s post hoc test, p ≤ 0.05).
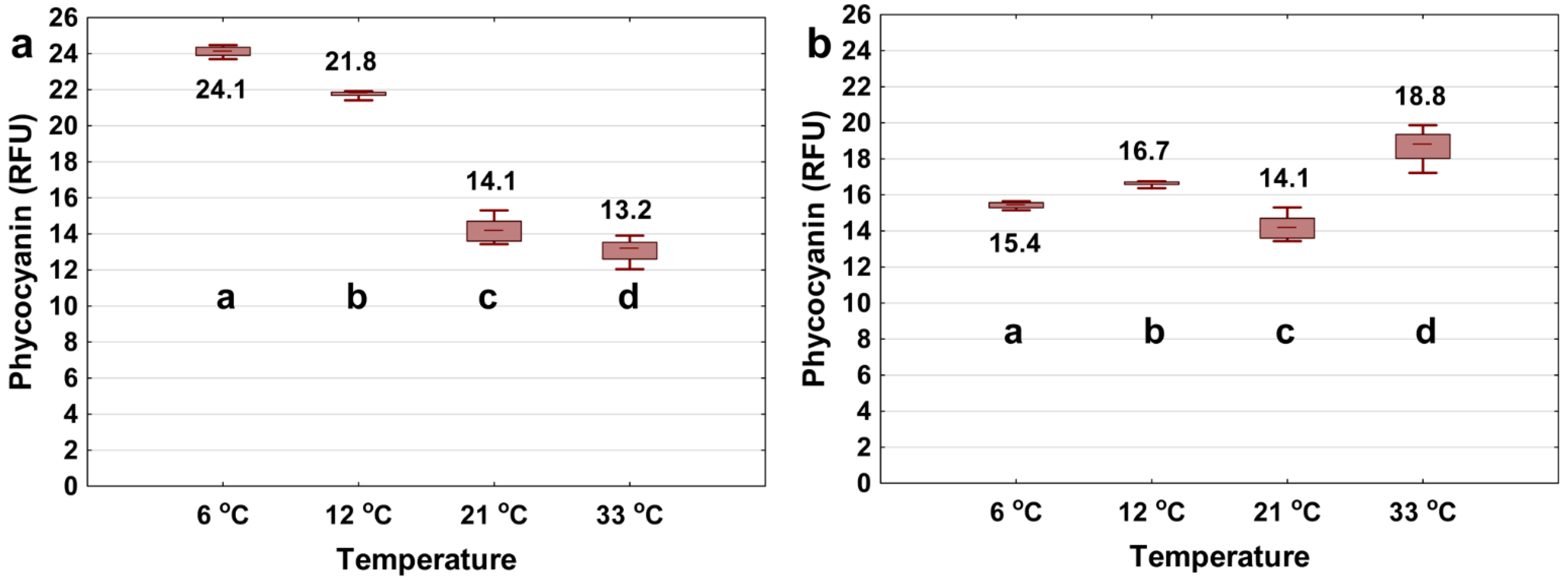
It is important to know whether this phenomenon is due to the physiological state of the cells or the radiative/non-radiative decay of the excited fluorophore. To understand the phenomenon, 1 mg/L phycocyanin was dissolved in ultrapure water and phycocyanin signals were measured at different temperatures (same condition as with Microcystis cells added). Figure 4a shows that the phycocyanin signal decreases with increasing water temperature, which confirms the trends shown in Figure 3a. Temperature may impact the excited fluorophore energy decay pathways. In other words, the dependence of the phycocyanin probe signal on the temperature variation is due to the radiative decay of the excited fluorophore rather than the physiological state of the cells [69]. In the latter case, fluorescence readings of the phycocyanin extract are expected to be the same as the variation in the ambient water temperature. So and Dong [69] explained that, when phycocyanin is excited at a specific wavelength, it releases energy either as radiative decay (fluorescence emission) or as nonradiative decay (heat); the temperature variation can shift the energy releasing pathway, so that as temperature increases, the nonradiative decay increases, which in turn reduces the fluorescence intensity.
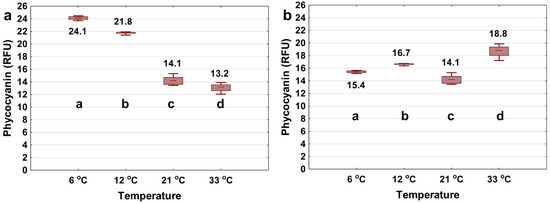
Figure 4.
Phycocyanin readings (RFU) using the YSI EXO2 probe of 1 mg/L extract phycocyanin pigment in 6, 12, 21, 33 °C of ultrapure water. All samples were tested over the period of 10 min. The probe measured each of the sample at a 250 ms interval for 10 min (a). In (b) the temperature interference was removed by adjusting the raw data to a reference temperature of 21 °C using Equation (3). The bottom and top of each box represent the 25th–75th percentiles, respectively. The whiskers represent the minimum and maximum values. The line within each box corresponds to the median value. Different letters represent significant differences between treatment comparisons (Kruskal–Wallis followed by Dunn’s post hoc test, p ≤ 0.05).
Few studies have quantified temperature interference on phycocyanin florescence readings. Watras et al. [52] proposed a detailed demonstration in which the impact of temperature on a Turner Cyclops-7 probe can be compensated by adjusting raw phycocyanin fluorescence data to a reference temperature. The authors report a linear correlation (Equation (2)) with temperature for extracted phycocyanin pigment and phycocyanin from lake water, and an exponential correlation (Equation (3)) for laboratory-cultured cyanobacteria Synechococcus leopoliensis. We inferred that the algorithms derived from Watras et al. [52] could be also applied to Microcystis aeruginosa and YSI EXO2 probe. Thus, we applied Equations (2) and (3) to assess its ability to compensate fluorescence readings with the YSI EXO2 probe, using the 21 °C test as the reference temperature.
where stands for reference florescence (RFU), stands for measured florescence (RFU), stands for reference temperature (°C), stands for measured temperature (°C), (between −0.018 and −0.037), and (between −0.017 and −0.030) represents the temperature coefficient [52].
As shown in Figure 3, after fitting with the exponential algorithm (Equation (3)), the measured phycocyanin difference dropped from 50.0% to 20.0% for the 2000 cells/mL, slightly increased from 20.5% to 26.7% for the 20,000 cells/mL, and dropped from 36.8% to 0% for the 100,000 cells/mL of Microcystis, for temperatures ranging from 6 to 33 °C. For the dissolved extract phycocyanin in ultrapure water, the difference dropped from 45.2% to 22.1% (Figure 4). In addition, we applied the linear algorithm (Equation (2)) to the Microcystis (Figure S1) and extracted phycocyanin (Figure S2) fluorescence readings. Results show that the exponential model was most efficient in reducing temperature interference for both Microcystis cells and extracted phycocyanin.
Watras et al. [52] observed that the adjusted readings of cyanobacterial cells at other temperatures are higher than the readings at the reference temperature. The difference in fluorescence increased with the difference between the reference temperature and the tested temperature (approximately 4 to 33 °C). The deviations were more prominent at high cell densities. In our study, the corrected readings show different trends depending on cell densities: an under/over estimation at 20,000 cells/mL, and a systematic but small underestimation at 100,000 cells/mL (Figure 3b). Hodges et al. [39] investigated two units of the same Turner Cyclops-7 phycocyanin probe as used by Watras et al. [52]. On both units, he did not report significant differences in readings for a narrower temperature range (13.8, 17, and 23.5 °C) using laboratory-cultured Microcystis aeruginosa (181,000 to 828,000 cells/mL). By comparison, phycocyanin readings at 4 °C were higher than those at other adjusted temperatures for the other unit tested. The authors also assessed Eureka Manta II and TriLux probes, but no significant differences were found among all the temperatures tested (4 to 23.5 °C). Prior work and this study show that phycocyanin probe readings can be significantly affected by temperature in the range of their typical application (6–33 °C). Clearly, some models appear to have incorporated correction algorithms (Eureka Manta II and TriLux), while others, such as the model tested in this study, do not. When deploying and interpreting the probe readings, users should be fully aware of the probe features.
According to our controlled validation testing, the use of uncorrected temperature readings can lead to an overestimation (up to 26.0%) and underestimation (20.4%), compared to the readings at the reference temperature (21 °C) when the temperature ranges from 6 to 33 °C. The reliability of phycocyanin probe measurements is subject to the temperature variation through time. During the cyanobacteria monitoring, if the probe is deployed, and the temperature varies during the deployment period, the phycocyanin values should be interpreted carefully considering the temperature variation. The significance of the difference depends on the application; in particular, refer to the alert levels used for cyanobacteria management plans. According to McQuaid et al. [33], probe reading 1.7 RFU represents WHO alert level 1 (published in 1999), and probe reading 10.1 RFU represents WHO alert level 2. The use of the probe tested for temperatures between 6 and 33 °C could lead to false detection of cyanobacteria at around 6000 cells/mL if 1.7 RFU is applied as a guideline. If a higher threshold is used, for instance, 10.1 RFU, the cyanobacteria at 34,000 cells/mL need to be carefully checked. However, during our field cyanobacteria sampling, the intake water temperature ranged from 22.9 to 28.2 °C, which is the typical temperature during bloom season. Such a narrower temperature range indicates that the estimation error appears to be reduced, compared with the results in our study.
Finally, it is important to consider that laboratory cultured cyanobacterial cells, such as Microcystis, are usually unicellular, whereas they may aggregate and form colonies in natural blooms. Therefore, when applying the results from the laboratory fluorescence test to the in situ monitoring, these discrepancies and complexities need to be considered. Readings in the laboratory-cultured cyanobacterial cells may not represent real bloom conditions, and the monitoring frameworks should be validated using real bloom assemblages. Even for the temperature-corrected readings, other factors such as the different genera present, single species versus mixed blooms, and the presence of NOM may impact the temperature deviation of the phycocyanin readings. Modification of existing correction algorisms to incorporate other external conditions, such as cyanobacterial cell species and status, probe character, reference temperature chosen, and water quality, require further investigation.
3.3. Validation of Probes with Field Samples
3.3.1. Relationships between Cyanobacterial Biovolume and Phycocyanin Probe Measurements
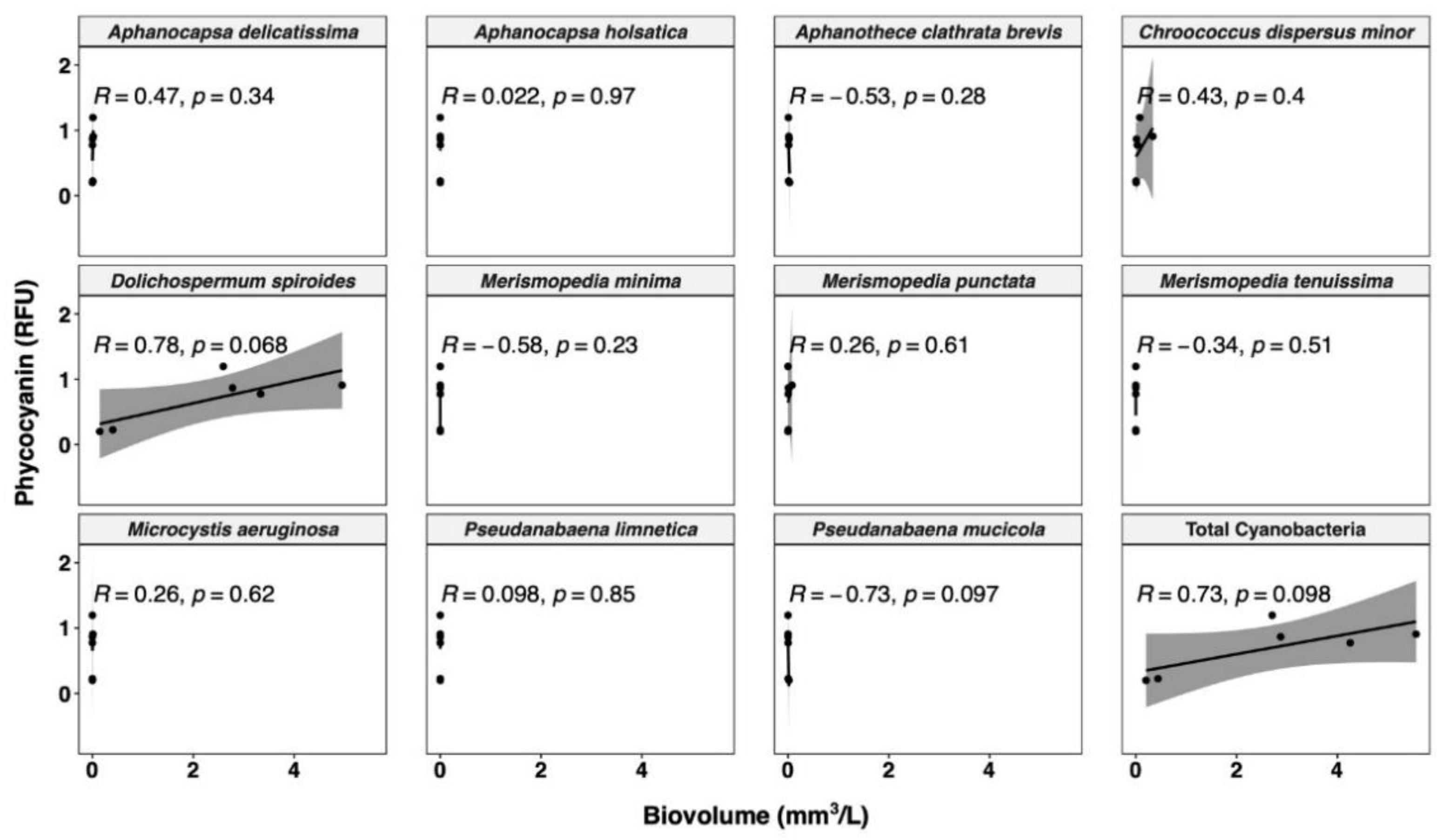
Phycocyanin fluorescence probe readings were validated with taxonomic cell counts results on Bedford intake water from 24 July to 26 August 2018. The values of the phycocyanin in RFU from the YSI EXO2 probes are plotted against the main cyanobacterial species biovolume and total cyanobacterial biovolume in Figure 5. A significant relationship was observed between phycocyanin and total cyanobacterial biovolume (R = 0.73, p = 0.098). Correlations have been extensively reported in the literature between the phycocyanin fluorescence probe readings and the extracted phycocyanin concentration, total cyanobacterial biovolume, and total cyanobacterial cell counts (Table S1). Phycocyanin probe readings are generally well adjusted with the laboratory-cultured unicellular species [39,40,41,45,50,60,70]. However, the correlation is less promising with the colonial species [39], mixed cultured species [70], and field-collected species [31,33,35,37,39,40,42,47,58,60,71,72,73,74,75]. A variety of factors can affect probe measurement for environmental samples. Differences in phycocyanin content in cyanobacteria have been reported in the literature. For example, the phycocyanin quota may vary due to different growth phases [38,50,76,77]. Ziegmann et al. [76] revealed higher phycocyanin content in the decay phase by analyzing the EEM spectra of Microcystis aeruginosa. Such findings are consistent with those of Bertone et al. [50], who used the same probe model as in our study (YSI EXO2), and reported increasing phycocyanin content over time (day 7–35) for Microcystis sp. However, Bertone et al. [50] noted inconsistent phycocyanin content in different growth stages depending on different cyanobacterial species, as the authors also found decreasing phycocyanin content over time (day 7–35) for Aphanocapsa sp. and Sphaerospermopsis sp. These results differ from those reported by Chang et al. [38] for Microcystis aeruginosa, Dolichospermum circinalis, and Planktothricoides raciborskii using a Turner Designs Model 10 probe. Interference with probe readings can be also due to the spatial dispersion of the cyanobacteria. Cyanobacterial agglomeration can vary in time and be composed of various species. The biovolumes of such species span a wide range, which leads to the inaccuracy of the estimation [33,78]. When cyanobacterial species aggregate (such as Microcystis), the fluorescence emission and excitation may not penetrate the whole colony of a few hundred millimeters, leading to the underestimation of the phycocyanin concentration [38,79]. These are all attributes that differ greatly between environmental samples and may explain the weaker correlation compared to the more controlled laboratory-cultured samples.
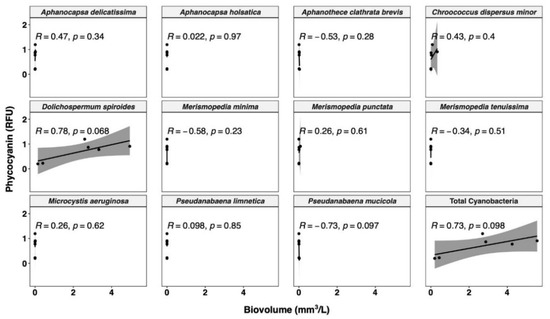
Figure 5.
Relationship between phycocyanin probe readings and cell biovolume for different cyanobacterial species. Shaded area around line represents 95% confidence interval.
The cyanobacterial community composition has a considerable influence on the phycocyanin measurements. The R value of the best-fit line between biovolume and phycocyanin for each species ranges from −0.73 to 0.78 (Figure 5). Only with high biovolume species are high R values observed, as expected, since a low count or biovolume is not expected to correlate with a global fluorescence reading. The strongest relationship occurs in the species Dolichospermum spiroides, which accounts for 93.5% of the total cyanobacterial biovolume. Such a wide range may be due to the difference in cell size and phycocyanin cell quota between species [70,77], which can be validated by comparing the phycocyanin signal to cell counts of each species.
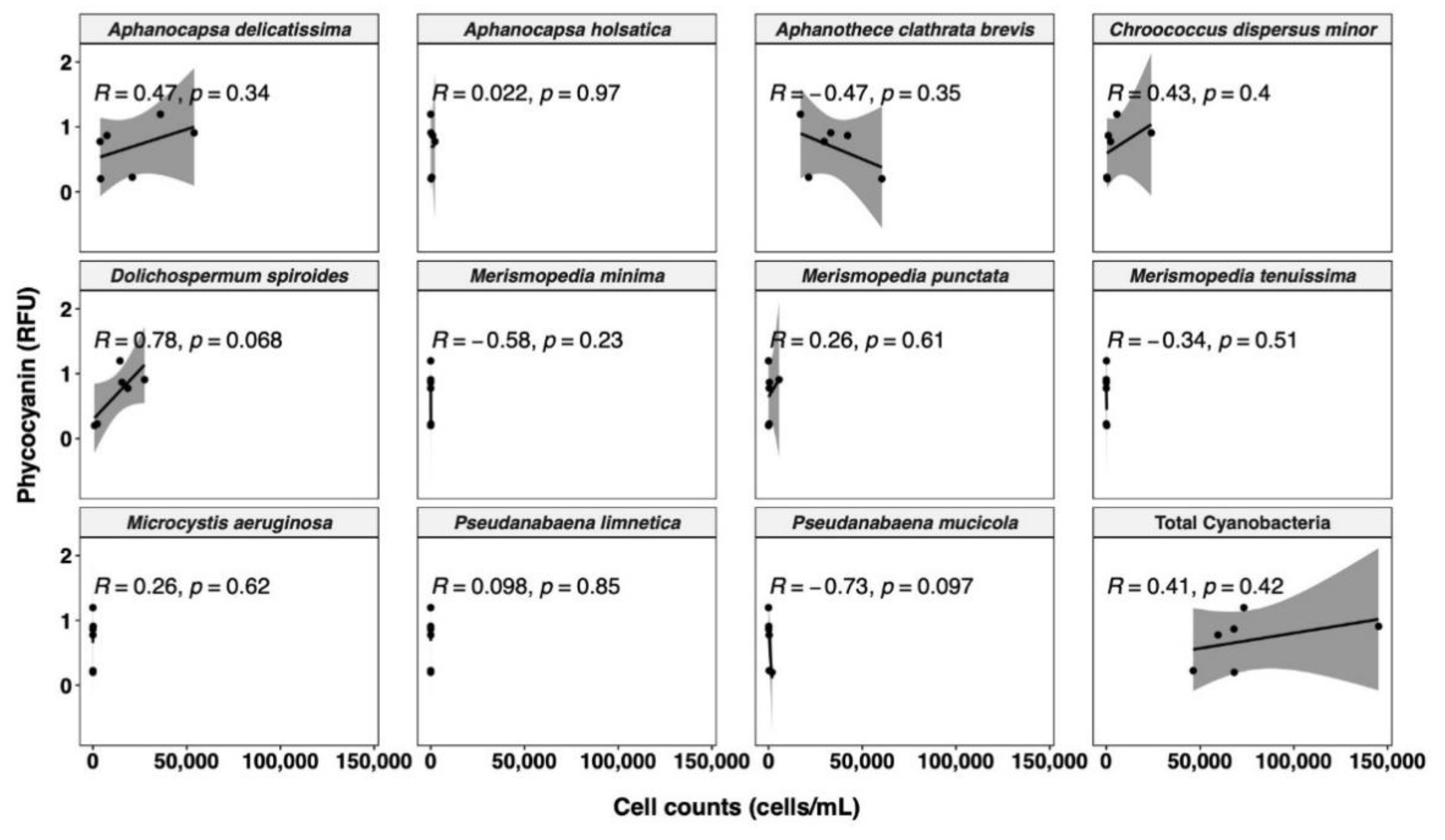
In terms of cell counts, the samples exhibit more diverse cyanobacterial communities (Figure 6). The most abundant species is Aphanothece clathrate brevis (33.1%), followed by Aphanocapsa delicatissima (31.6%), and Dolichospermum spiroides (19.0%). The different patterns between the cell counts and cell biovolume may be due to the different cell size [70]. For instance, in our study, Dolichospermum spiroides has the highest biovolume of 179.6 µm3/cell. This contrasted sharply with the most abundant Aphanothece clathrate brevis and Aphanocapsa delicatissima, with very low cell biovolumes of 0.5 and 0.3 µm3/cell, respectively. Cell phycocyanin quota has been demonstrated to be proportionally related to cell volume, so species with larger cell sizes have more phycocyanin per cell [44]. Furthermore, much weaker correlations are observed between phycocyanin RFU and total cyanobacterial cell counts (R = 0.41, Figure 6) than for total cyanobacterial biovolume (R = 0.73, Figure 5), in line with previous studies [33,41,70,78,80]. Rousso et al. [77] assessed four cyanobacterial species using the same probe as in our study, and reported less variability in phycocyanin fluorescence per unit biomass (or per unit biovolume) among species than per cell. Therefore, conversion of phycocyanin RFU to biovolume may result in a reduced bias than conversion to cell counts. Moreover, the WHO alert level has recently updated its cyanobacterial monitoring framework. Compared with the WHO guidance level published in 1999 [81], the recently published WHO alert level incorporates cyanobacterial cell biovolume, instead of taxonomic cell counts, as an indicator, due to the substantial variation in the cell size [16]. Our findings, together with those of earlier research, suggest that phycocyanin per cell varies greatly between species, primarily due to pigment concentration varying with cell size. Biovolume is a better indicator of phycocyanin fluorescence than cell counts. Nevertheless, phycocyanin per biovolume can still differ greatly between species, as observed by Thomson-Laing et al. [40], which showed more than 100-fold phycocyanin fluorescence per biovolume differences between the studied 12 species. Overall, for improved speciation and biomass correlation, in situ phycocyanin probe readings require more validation with robust methods.
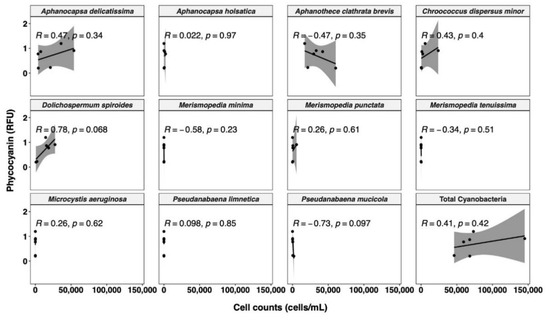
Figure 6.
Relationship between phycocyanin probe readings and cell counts for different cyanobacterial species. Shaded area around the line represents the 95% confidence interval.
In addition, the timing of the phycocyanin fluorescence probe measurements and the grab sample collection is an important factor to consider. Samples for taxonomic cell counts were collected at a fixed time using a short flushing of about 2 min followed by the collection of 1 L. At the Bedford DWTP, the probe recorded data for 30 min every 2 h with 5 min intervals, as multiple flows were measured. We performed the comparison of the grab samples with the probe data by averaging the set of six data points taken at the time closest to the collection of the grab sample. Both were taken from the same raw water sampling line. This means that the samples were not truly paired in terms of volume collected and exact time of collection. As cyanobacterial biomass may vary rapidly [59,82], these differences in sample volume and timing may be significant.
3.3.2. Comparison of Field Phycocyanin Measurements and Other Water Quality Parameters
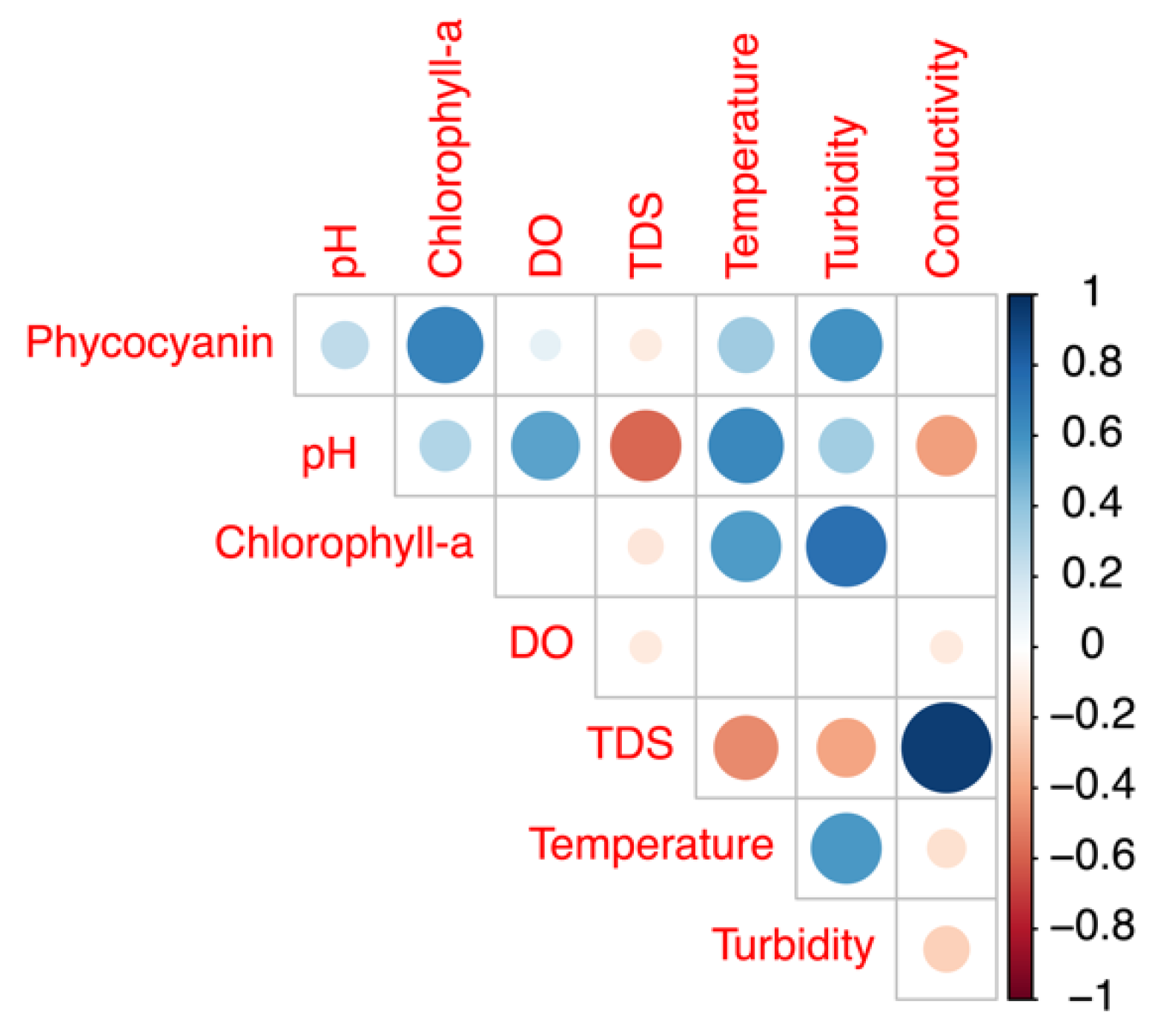
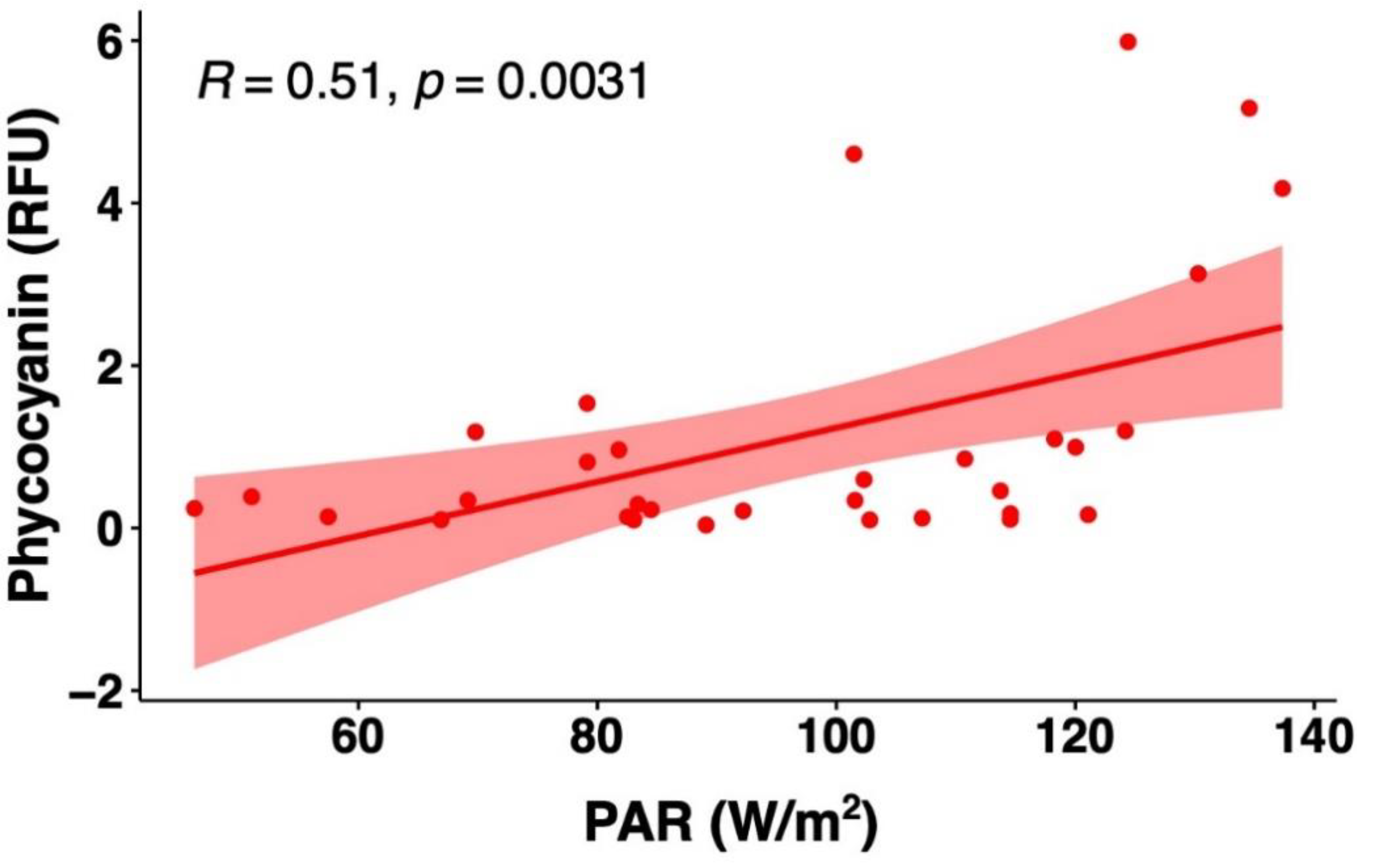
The potential impact of several other water quality attributes measured by the YSI EXO2 probe on the phycocyanin readings was also considered. The concentration ranges and mean values of the intake water quality for Bedford for the period from 24 July to 26 August 2018 are summarized in Table 1. Phycocyanin, chlorophyll-a, and other physicochemical parameters of the intake water correlations are illustrated in Figure 7. Since DOC results were obtained on certain days’ grab samples, such results were not compared with the hourly phycocyanin probe readings. Phycocyanin was found to be positively correlated with chlorophyll-a (R = 0.67, p ≤ 0.01), turbidity (R = 0.60, p ≤ 0.01), temperature (R = 0.35, p ≤ 0.01), pH (R = 0.25, p ≤ 0.01), and DO (R = 0.10, p ≤ 0.01); negatively corrected with total dissolved solids (TDS) (R = 0.11, p ≤ 0.01); and no significant correlation was found with conductivity (R = 0.00, p = 0.89). In addition, we obtained daily total photosynthetically available radiation (PAR) (W/m2) from NASA POWER open source, and we found a positive correlation between phycocyanin readings and PAR (R = 0.51, p ≤ 0.01).

Table 1.
Physicochemical variables for the raw water of Bedford DWTP during July and August of 2018.
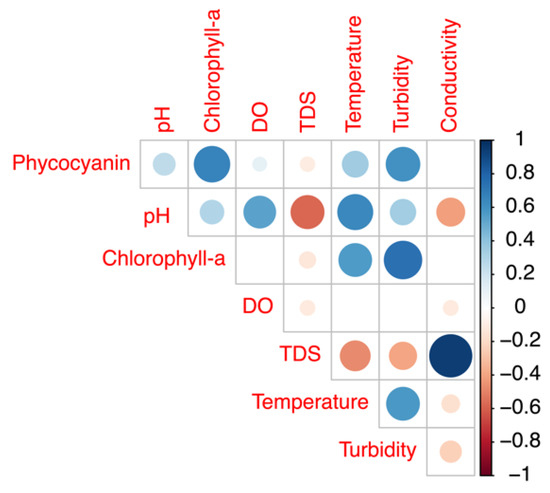
Figure 7.
Correlation plot of physicochemical variables for the raw water of Bedford drinking water treatment plant (DWTP) during July and August of 2018.
Cyanobacteria contain both phycocyanin and chlorophyll-a pigment [16]. Eukaryotic phytoplankton, which contribute chlorophyll-a but not phycocyanin pigment, would have also been present at the time of our experiments. In addition, phytoplankton cells may lead to increased turbidity measurements. Thus, phycocyanin readings are expected to be positively correlated with chlorophyll-a and turbidity. Previous studies have reported inaccurate phycocyanin measurement due to the presence of eukaryotic phytoplankton, ranging from 200% underestimation to 405% overestimation for different species and fluorometers applied [10,33,38,45]. In our study, the impact of eukaryotic phytoplankton is unlikely to be large, as the highest chlorophyll-a level is 4.5 RFU, and the mean value is 1.3 RFU, indicating only a small total eukaryotic phytoplankton biomass was present during our monitoring period. The reminder of that portion of the chlorophyll-a would have been from cyanobacteria.
Investigations also suggest that light intensity can affect phycocyanin fluorescence measurements [83]. The growth of cyanobacteria depends on the amount of light available and used. Theoretically, low light intensity may stimulate phycocyanin production, because cells accumulate more pigments to capture more light as energy; conversely, when the light intensity is high, some species have the potential to degrade phycocyanin in order to prevent damage to the cells [10,79,84]. The outcomes are variable depending on the light source, exposure time, measuring instrument, and reference cyanobacterial species used. In our study, we found positive relationships between in situ phycocyanin readings and natural light intensity (Figure 8). Such an outcome is also observed in other studies. Ma et al. [85] revealed that higher artificial light intensity (90 μmol m−2s−1) resulted in an increase in phycocyanin content in Nostoc sphaeroides. Brient et al. [44] used a TriOS probe, whereby dissolved phycocyanin solution and Planktothrix agardhii culture were exposed to various intensities of artificial light (0–150 μmol m−2s−1), and the authors showed that fluorescence increased with increasing light intensity. Hodges [79] investigated the phycocyanin fluorescence of Aphanizomenon sp. under natural light and dark treatments, and the authors reported mixed trends. The fluorescence measured from the Manta and one of two Turner probes with light treatment were significantly higher compared to the dark treatments; whereas the phycocyanin readings were not modified using the other Turner probe and the YSI probe [79]. Rousso et al. [42] and Misumi et al. [43] observed a reduction in phycocyanin fluorescence for cyanobacteria cultures exposed to artificial light, attributing the suppression to non-photochemical quenching. To the best of our knowledge, this is the first study to report on the variation in phycocyanin fluorescence readings in environmental water under natural light intensities. Previous research on light interference have predominantly studied species-specific phycocyanin fluorescence responses from monocultures, which may not cover a broad range to clearly identify the variation in phycocyanin content reported throughout the range of species and morphologies. In our study, the daily total PAR ranged from 46.3 to 137.3 W/m2, which are equivalent to around 98 to 287 μmol m−2s−1, assuming 45% of total solar irradiation is photosynthetically active [86]. When the light intensity is high, the cyanobacteria photosynthetic rate is rapid, and, consequently, the carbon dioxide will decrease and DO will increase [87,88], leading to the increase in cyanobacterial biomass [89]. Such a contribution may explain the positive correlation between phycocyanin, and pH and DO.
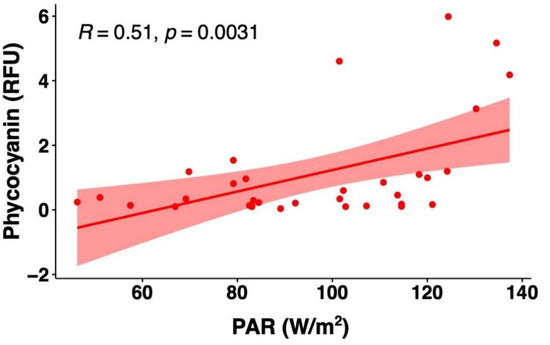
Figure 8.
Correlations between photosynthetically available radiation (PAR) and phycocyanin probe readings for the raw water of Bedford DWTP during July and August of 2018. Shaded area around the line represents the 95% confidence interval.
Phycocyanin fluorescence probes are equipped to concurrently measure other quality parameters, including water temperature, DO, pH, turbidity, and specific conductivity. Such synchronous measurements may be used to improve phycocyanin estimation accuracy. Few studies have assessed the bias in phycocyanin readings by synchronously measured water quality data. Bowling et al. [47] deployed a YSI 6600 V2 probe in a river in Australia during November 2008 to May 2009, and the authors found similar positive trends between phycocyanin and chlorophyll-a (R2 = 0.077, p < 0.05), turbidity (R2 = 0.07, p < 0.05), and pH (R2 = 0.04, p < 0.05). Although Bowling et al. [47] found a weak positive correlation between phycocyanin readings and conductivity (R2 = 0.05, p < 0.05), this study did not. Besides the above-mentioned study, there are limited studies on the relationship between water quality attributes and phycocyanin probe readings. Most studies have focused on the effect of environmental factors on the formation of cyanobacteria. For example, Rome et al. [90] and Guo et al. [91] found that bloom occurrence is associated with higher water temperature (above 25 °C), elevated pH (7 to 9), and super-saturated DO. Zhao and Huang [92] indicated that nitrate concentration and air temperature are positively correlated with bloom occurrence, whereas wind speed, relative humidity, and conductivity are negatively correlated with bloom occurrence. Ndong et al. [93] applied a YSI 6600 V2-4 probe in the same water body as in our study (Missisquoi Bay), and the authors showed the maximum probability of 68% that the transport of cells to the plant intake were associated with the water temperature and wind that drive lake hydrodynamics and water column mixing. Although these researchers have shown the factors related to the physical presence of cells at a given location, the current study also demonstrates that the factors influencing the phycocyanin probe readings must be considered simultaneously. Interestingly, it is reported that water temperatures act as a major controlling factor in cyanobacterial growth [90,92,94,95,96]. Our field study also showed a positive correlation (R = 0.35, p ≤ 0.01) between water temperature and phycocyanin readings. However, our laboratory tests with a fixed number of cyanobacterial cells revealed that phycocyanin readings decrease with elevated temperature. Thus, the improvement in the accuracy of phycocyanin readings using water temperature may partially stem from the inherent interactions between the temperature and cyanobacterial growth.
In order to develop a comprehensive model to estimate the cyanobacteria, future work might employ other available commercial probes and different cyanobacterial species to better understand the impacts of varied attributes. When designing or applying management criteria based on phycocyanin, it is critical to acquire preliminary data on the general community composition, dominant cyanobacterial species, and other possible environmental conditions that may impact readings. It may be feasible to conduct a series of experiments and incorporate the results into algorithms to reduce the errors. Fluorometers cannot effectively provide reliable estimation of cyanobacteria without proper compensation of the effect discussed above, especially in environments where physical-chemical attributes vary, and with more complicated species.
4. Conclusions
- SRNOM can quench and interfere with the reliability of the cell-bound phycocyanin readings. High SRNOM content in the water matrix can lead to an underestimation of cyanobacterial quantification measured by a phycocyanin probe, especially for high cyanobacterial cell concentrations.
- The concentration of NOM added, the aromaticity of NOM, the species present, and the background DOC in the test water define the extent and the trends for fluorescence quenching.
- In the case of a source with high naturally occurring NOM, DWTP managers need to assess the interference caused by NOM, and the phycocyanin RFU-based thresholds can be adjusted accordingly.
- Phycocyanin probe readings can be significantly affected by temperature in the range of their typical application (6–33 °C). Applying the correction algorithms can yield high success rates to decrease the interference. The relative importance of such interference varies between commercially available probes.
- The correlation between probe readings and taxonomic cell counts is unreliable, and only biovolume-based estimations are a relevant option for management purposes.
- A simultaneous measurement of the other physical-chemical parameters may help increase the accuracy of phycocyanin readings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w14223749/s1, Table S1: Correlations between the in situ phycocyanin fluorescence probe readings and microscopic cell counts, biovolume estimation, and extracted pigment concentration; Table S2: Main sources of interference associated with the in situ phycocyanin fluorescence probe; Figure S1: Phycocyanin readings (RFU) with the YSI EXO2 probe of the 0, 2000, 20,000, and 100,000 cells/mL of Microcystis aeruginosa strain CPCC 300 suspended in 6, 12, 21, 33 °C of ultrapure water. The probe measured each of the sample at a 250 ms interval for 10 min. The temperature interference was removed by adjusting the raw data to a reference temperature of 21 °C using Equation (2). The bottom and top of each box represent the 25th–75th percentiles, respectively. The whiskers represent the minimum and maximum values. The line within each box corresponds to the median value. Different letters represent significant differences between treatment comparisons (Kruskal–Wallis followed by Dunn’s post hoc test, p ≤ 0.05); Figure S2: Phycocyanin readings (RFU) using the YSI EXO2 probe of 1 mg/L of extracted phycocyanin pigment in the 6, 12, 21, 33 °C of ultrapure water. All samples were tested over the period of 10 min. The probe measured each of the samples at a 250 ms interval for 10 min. The temperature interference was removed by adjusting the raw data to a reference temperature of 21 °C using Equation (2). The bottom and top of each box represent the 25th–75th percentiles, respectively. The whiskers represent the minimum and maximum values. The line within each box corresponds to the median value. Different letters represent significant differences between treatment comparisons (Kruskal–Wallis followed by Dunn’s post hoc test, p ≤ 0.05).
Author Contributions
Conceptualization, L.M., S.M., A.Z., S.D. and M.P.; methodology, L.M., S.M., J.F.G.M., A.Z., S.D. and M.P.; software, L.M. and J.F.G.M.; validation, L.M., J.F.G.M., A.Z., S.D. and M.P.; formal analysis, L.M., S.M. and J.F.G.M.; investigation, L.M., J.F.G.M., A.Z., S.D. and M.P.; resources, L.M., A.Z., S.D. and M.P.; data curation, L.M. and J.F.G.M.; writing—original draft preparation, L.M.; writing—review and editing, L.M., S.M., S.D. and M.P.; visualization, L.M., S.M., J.F.G.M., A.Z., S.D. and M.P.; supervision, A.Z., S.D. and M.P.; project administration, A.Z., S.D. and M.P.; funding acquisition, S.D. and M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Algal Blooms, Treatment, Risk Assessment, Prediction, and Prevention through Genomics Project (ATRAPP) with the financial support of Genome Canada and Génome Québec, grant number Genome Canada/NOA 10512.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors greatly acknowledge the financial support of Algal Blooms, Treat- ment, Risk Assessment, Prediction, and Prevention through Genomics (ATRAPP) (Genome Canada and Génome Québec). In addition, the authors sincerely thank the staff at NSERC Industrial Chair on Drinking Water at Polytechnique Montréal, GRIL lab, Dana F. Simon (Université de Montréal), Irina Moukhina (Université du Québec à Montréal), and the studied plant’s operators for their important contributions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baures, E.; Thomas, O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef] [PubMed]
- Grattan, L.M.; Holobaugh, S.; Morris, J.G. Harmful algal blooms and public health. Harmful Algae 2016, 57, 2–8. [Google Scholar] [CrossRef]
- Alex, E.J. Is the future blue-green? A review of the current model predictions of how climate change could affect pelagic freshwater cyanobacteria. Water Res. 2012, 46, 1364–1371. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef]
- Beaulieu, M.; Pick, F.; Gregory-Eaves, I. Nutrients and water temperature are significant predictors of cyanobacterial biomass in a 1147 lakes data set. Limnol. Oceanogr. 2013, 58, 1736–1746. [Google Scholar] [CrossRef]
- Richardson, J.; Feuchtmayr, H.; Miller, C.; Hunter, P.D.; Maberly, S.C.; Carvalho, L. Response of cyanobacteria and phytoplankton abundance to warming, extreme rainfall events and nutrient enrichment. Glob. Change Biol. 2019, 25, 3365–3380. [Google Scholar] [CrossRef]
- Zamyadi, A.; McQuaid, N.; Prévost, M.; Dorner, S. Monitoring of potentially toxic cyanobacteria using an online multi-probe in drinking water sources. J. Environ. Monit. 2012, 14, 579–588. [Google Scholar] [CrossRef]
- Bertone, E.; Burford, M.A.; Hamilton, D.P. Fluorescence probes for real-time remote cyanobacteria monitoring: A review of challenges and opportunities. Water Res. 2018, 141, 152–162. [Google Scholar] [CrossRef]
- Zamyadi, A.; Choo, F.; Newcombe, G.; Stuetz, R.; Henderson, R.K. A review of monitoring technologies for real-time management of cyanobacteria: Recent advances and future direction. TrAC Trends Anal. Chem. 2016, 85, 83–96. [Google Scholar] [CrossRef]
- Lou, I.; Han, B.; Zhang, W. Advances in Monitoring and Modelling Algal Blooms in Freshwater Reservoirs: General principles and a Case Study of Macau; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Hawkins, P.R.; Holliday, J.; Kathuria, A.; Bowling, L. Change in cyanobacterial biovolume due to preservation by Lugol’s Iodine. Harmful Algae 2005, 4, 1033–1043. [Google Scholar] [CrossRef]
- Newcombe, G. International Guidance Manual for the Management of Toxic Cyanobacteria; Global Water Research Coalition and Water Quality Research Australia: London, UK, 2009; p. 44. [Google Scholar]
- America Water Works Association (AWWA). Algae Source to Treatment. Manual of Water Supply Practices - M57, 1st ed.; America Water Works Association (AWWA): Denver, CO, USA, 2010; p. 481. [Google Scholar]
- Sanseverino, I.; Conduto António, D.; Loos, R.; Lettieri, T. Cyanotoxins: Methods and Approaches for Their Analysis and Detection; EUR 28624; European Commission: Brussels, Belgium, 2017; p. 70. [Google Scholar]
- Chorus, I.; Welker, M. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; Taylor & Francis: Abingdon, UK, 2021; p. 859. [Google Scholar]
- Serrano-Silva, N.; Calderón-Ezquerro, M.C. Metagenomic survey of bacterial diversity in the atmosphere of Mexico City using different sampling methods. Environ. Pollut. 2018, 235, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Singh, S.; Ahn, C.-Y.; Oh, H.-M.; Asthana, R.K. Monitoring approaches for a toxic cyanobacterial Bloom. Environ. Sci. Technol. 2013, 47, 8999–9013. [Google Scholar] [CrossRef]
- Romanis, C.S.; Pearson, L.A.; Neilan, B.A. Cyanobacterial blooms in wastewater treatment facilities: Significance and emerging monitoring strategies. J. Microbiol. Methods 2021, 180, 106123. [Google Scholar] [CrossRef] [PubMed]
- John, N.; Koehler, A.V.; Ansell, B.R.E.; Baker, L.; Crosbie, N.D.; Jex, A.R. An improved method for PCR-based detection and routine monitoring of geosmin-producing cyanobacterial blooms. Water Res. 2018, 136, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Te, S.H.; Chen, E.Y.; Gin, K.Y.-H. Comparison of quantitative PCR and droplet digital PCR multiplex assays for two genera of bloom-forming cyanobacteria, Cylindrospermopsis and Microcystis. Appl. Environ. Microbiol. 2015, 81, 5203–5211. [Google Scholar] [CrossRef]
- Chiu, Y.T.; Chen, Y.H.; Wang, T.S.; Yen, H.K.; Lin, T.F. A qPCR-based tool to diagnose the presence of harmful cyanobacteria and cyanotoxins in drinking water sources. Int. J. Environ. Res. Public Health 2017, 14, 547. [Google Scholar] [CrossRef]
- Tromas, N.; Fortin, N.; Bedrani, L.; Terrat, Y.; Cardoso, P.; Bird, D.; Greer, C.W.; Shapiro, B.J. Characterising and predicting cyanobacterial blooms in an 8-year amplicon sequencing time course. ISME J. 2017, 11, 1746–1763. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, J.; Wang, X.; Yang, Y.; Chen, N.; Lu, Z.; Ge, Q.; Jiang, R.; Zhang, X.; Yang, Y.; et al. Responses of cyanobacterial aggregate microbial communities to algal blooms. Water Res. 2021, 196, 117014. [Google Scholar] [CrossRef]
- Moradinejad, S.; Trigui, H.; Guerra Maldonado, J.F.; Shapiro, J.; Terrat, Y.; Zamyadi, A.; Dorner, S.; Prévost, M. Diversity assessment of toxic cyanobacterial blooms during oxidation. J. Toxins 2020, 12, 728. [Google Scholar] [CrossRef]
- Zamyadi, A.; Romanis, C.; Mills, T.; Neilan, B.; Choo, F.; Coral, L.A.; Gale, D.; Newcombe, G.; Crosbie, N.; Stuetz, R.; et al. Diagnosing water treatment critical control points for cyanobacterial removal: Exploring benefits of combined microscopy, next-generation sequencing, and cell integrity methods. Water Res. 2019, 152, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Jalili, F.; Trigui, H.; Guerra Maldonado, J.F.; Dorner, S.; Zamyadi, A.; Shapiro, B.J.; Terrat, Y.; Fortin, N.; Sauvé, S.; Prévost, M. Can cyanobacterial diversity in the source predict the diversity in sludge and the risk of toxin release in a drinking water treatment plant? Toxins 2021, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Bharti, R.; Grimm, D.G. Current challenges and best-practice protocols for microbiome analysis. Brief Bioinform 2021, 22, 178–193. [Google Scholar] [CrossRef]
- Tan, B.; Ng, C.; Nshimyimana, J.; Loh, L.-L.; Gin, K.; Thompson, J. Next-generation sequencing (NGS) for assessment of microbial water quality: Current progress, challenges, and future opportunities. Front. Microbiol. 2015, 6, 1027. [Google Scholar] [CrossRef] [PubMed]
- Bryant, D.A. The cyanobacterial photosynthetic apparatus: Comparisosn to those of higher plants and photosynthetic bacteria. Can. Bull. Fish. Aquat. Sci. 1986, 214, 423–500. [Google Scholar]
- Carr, N.G.; Whitton, B.A. The Biology of Cyanobacteria; University of California Press: Berkeley, CA, USA, 1982; p. 688. [Google Scholar]
- Prescott, L.M.; Harley, J.P.; Klein, D.A. Microbiology, 6th ed.; The McGraw Hill Companies Inc.: New York, NY, USA, 2005; p. 1152. [Google Scholar]
- McQuaid, N.; Zamyadi, A.; Prévost, M.; Bird, D.F.; Dorner, S. Use of in vivo phycocyanin fluorescence to monitor potential microcystin-producing cyanobacterial biovolume in a drinking water source. J. Environ. Monit. 2011, 13, 455–463. [Google Scholar] [CrossRef]
- Pazouki, P.; Dorner, S.; Bouchard, R.; Zamyadi, A. Applying an online multi-probe to monitor the potentially toxic cyanobacteria in Lake Erie. In Proceedings of the 29th Eastern Canadian Symposium on Water Quality Research, Montréal, QC, Canada, 17 October 2014. [Google Scholar]
- Zamyadi, A.; Dorner, S.; Ndong, M.; Bolduc, A.; Bastien, C.; Prévost, M. Application of in vivo measurements for the management of cyanobacterial cell breakthrough into drinking water treatment plants. Environ. Sci. Process. Impacts 2014, 16, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.l.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Korak, J.A.; Wert, E.C.; Rosario-Ortiz, F.L. Evaluating fluorescence spectroscopy as a tool to characterize cyanobacteria intracellular organic matter upon simulated release and oxidation in natural water. Water Res. 2015, 68, 432–443. [Google Scholar] [CrossRef]
- Chang, D.-W.; Hobson, P.; Burch, M.; Lin, T.-F. Measurement of cyanobacteria using in-vivo fluoroscopy-Effect of cyanobacterial species, pigments, and colonies. Water Res. 2012, 46, 5037–5048. [Google Scholar] [CrossRef]
- Hodges, C.M.; Wood, S.A.; Puddick, J.; McBride, C.G.; Hamilton, D.P. Sensor manufacturer, temperature, and cyanobacteria morphology affect phycocyanin fluorescence measurements. Environ. Sci. Pollut. Res. 2018, 25, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Thomson-Laing, G.; Puddick, J.; Wood, S.A. Predicting cyanobacterial biovolumes from phycocyanin fluorescence using a handheld fluorometer in the field. Harmful Algae 2020, 97, 101869. [Google Scholar] [CrossRef] [PubMed]
- Zamyadi, A.; McQuaid, N.; Dorner, S.; Bird, D.F.; Burch, M.; Baker, P.; Hobson, P.; Prévost, M. Cyanobacterial detection using in vivo fluorescence probes: Managing interferences for improved decision-making. J. Am. Water Work. Assoc. 2012, 104, E466–E479. [Google Scholar] [CrossRef]
- Rousso, B.Z.; Bertone, E.; Stewart, R.A.; Rinke, K.; Hamilton, D.P. Light-induced fluorescence quenching leads to errors in sensor measurements of phytoplankton chlorophyll and phycocyanin. Water Res. 2021, 198, 117133. [Google Scholar] [CrossRef] [PubMed]
- Misumi, M.; Katoh, H.; Tomo, T.; Sonoike, K. Relationship between photochemical quenching and non-photochemical quenching in six species of cyanobacteria reveals species difference in redox state and species commonality in energy dissipation. Plant Cell Physiol. 2016, 57, 1510–1517. [Google Scholar] [CrossRef]
- Brient, L.; Lengronne, M.; Bertrand, E.; Rolland, D.; Sipel, A.; Steinmann, D.; Baudin, I.; Legeas, M.; Le Rouzic, B.; Bormans, M. A phycocyanin probe as a tool for monitoring cyanobacteria in freshwater bodies. J. Environ. Monit. 2008, 10, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Choo, F.; Zamyadi, A.; Newton, K.; Newcombe, G.; Bowling, L.; Stuetzb, R.; Henderson, R.K. Performance evaluation of in situ fluorometers for real-time cyanobacterial monitoring. H2Open J. 2018, 1, 26–46. [Google Scholar] [CrossRef]
- Choo, F.; Zamyadi, A.; Stuetz, R.M.; Newcombe, G.; Newton, K.; Henderson, R.K. Enhanced real-time cyanobacterial fluorescence monitoring through chlorophyll-a interference compensation corrections. Water Res. 2019, 148, 86–96. [Google Scholar] [CrossRef]
- Bowling, L.C.; Merrick, C.; Swann, J.; Green, D.; Smith, G.; Neilan, B.A. Effects of hydrology and river management on the distribution, abundance and persistence of cyanobacterial blooms in the Murray River, Australia. Harmful Algae 2013, 30, 27–36. [Google Scholar] [CrossRef]
- Symes, E.; Van Ogtrop, F. Determining the Efficacy of a Submersible in situ Fluorometric Device for Cyanobacteria Monitoring Coalesced with Total Suspended Solids Characteristic of Lowland Reservoirs. River Res. Appl. 2016, 32, 1632–1641. [Google Scholar] [CrossRef]
- Turner Designs. CyanoFluor Handheld HAB Indicator User Manual; Turner Designs: San Jose, CA, USA, 14 March 2017; p. 19. [Google Scholar]
- Bertone, E.; Chuang, A.; Burford, M.A.; Hamilton, D.P. In-situ fluorescence monitoring of cyanobacteria: Laboratory-based quantification of species-specific measurement accuracy. Harmful Algae 2019, 87, 101625. [Google Scholar] [CrossRef] [PubMed]
- Courtois, S.; Steinmann, D.; Cajon, A.; van der Linden, L. Continuous monitoring of cyanobacterial blooms: Benefits and conditions for using fluorescence probes. Rev. Des Sci. Eau/J. Water Sci. 2017, 30, 149–155. [Google Scholar] [CrossRef][Green Version]
- Watras, C.J.; Morrison, K.A.; Rubsam, J.L.; Hanson, P.C.; Watras, A.J.; LaLiberte, G.D.; Milewski, P. A temperature compensation method for chlorophyll and phycocyanin fluorescence sensors in freshwater. Limnol Ocean.-Meth 2017, 15, 642–652. [Google Scholar] [CrossRef]
- Pachauri, R.K. Intergovernmental Panel on Climate Change (IPPC). Working Group Impacts. In Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2015. [Google Scholar]
- Wauthy, M.; Rautio, M.; Christoffersen, K.S.; Forsström, L.; Laurion, I.; Mariash, H.L.; Peura, S.; Vincent, W.F. Increasing dominance of terrigenous organic matter in circumpolar freshwaters due to permafrost thaw. Limnol. Oceanogr. Lett. 2018, 3, 186–198. [Google Scholar] [CrossRef]
- Winter, J.G.; DeSellas, A.M.; Fletcher, R.; Heintsch, L.; Morley, A.; Nakamoto, L.; Utsumi, K. Algal blooms in Ontario, Canada: Increases in reports since 1994. Lake Reserv. Manag. 2011, 27, 107–114. [Google Scholar] [CrossRef]
- Qu, F.; Liang, H.; Wang, Z.; Wang, H.; Yu, H.; Li, G. Ultrafiltration membrane fouling by extracellular organic matters (EOM) of Microcystis aeruginosa in stationary phase: Influences of interfacial characteristics of foulants and fouling mechanisms. Water Res. 2012, 46, 1490–1500. [Google Scholar] [CrossRef]
- Zamyadi, A.; Coral, L.A.; Barbeau, B.; Dorner, S.; Lapolli, F.R.; Prévost, M. Fate of toxic cyanobacterial genera from natural bloom events during ozonation. Water Res. 2015, 73, 204–215. [Google Scholar] [CrossRef]
- Bowling, L.C.; Zamyadi, A.; Henderson, R.K. Assessment of in situ fluorometry to measure cyanobacterial presence in water bodies with diverse cyanobacterial populations. Water Res. 2016, 105, 22–33. [Google Scholar] [CrossRef]
- Genzoli, L.; Kann, J. Evaluation of Phycocyanin Probes as a Monitoring Tool for Toxigenic Cyanobacteria in the Klamath River below Iron Gate Dam; Aquatic Ecosystems LLC: The Woodlands, TX, USA, November 2016. [Google Scholar]
- Bastien, C.; Cardin, R.; Veilleux, E.; Deblois, C.; Warren, A.; Laurion, I. Performance evaluation of phycocyanin probes for the monitoring of cyanobacteria. J. Environ. Monit. 2011, 13, 110–118. [Google Scholar] [CrossRef]
- YSI Incorporated. EXO User Manual; YSI Incorporated: Yellow Springs, OH, USA, 2019. [Google Scholar]
- Armbruster, D.A.; Pry, T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008, 29 (Suppl. 1), S49–S52. [Google Scholar]
- Bowling, L.C.; Blais, S.; Sinotte, M. Heterogeneous spatial and temporal cyanobacterial distributions in Missisquoi Bay, Lake Champlain: An analysis of a 9 year data set. J. Great Lakes Res. 2015, 41, 164–179. [Google Scholar] [CrossRef]
- Stedmon, C.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial: Fluorescence-PARAFAC analysis of DOM. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Kring, S.; Figary, S.E.; Boyer, G.; Watson, S.; Twiss, M. Rapid in situ measures of phytoplankton communities using the bbe FluoroProbe: Evaluation of spectral calibration, instrument intercompatibility, and performance range. Can. J. Fish. Aquat. Sci. 2014, 71, 1087–1095. [Google Scholar] [CrossRef]
- De Oliveira, F.G.; Bertone, E.; Stewart, A.R.; Awad, J.; Holland, A.; O’Halloran, K.; Bird, S. Multi-parameter compensation method for accurate in situ fluorescent dissolved organic matter monitoring and properties characterization. Water 2018, 10, 1146. [Google Scholar] [CrossRef]
- Yoo, S.M.; Lee, S.Y. Optical biosensors for the detection of pathogenic microorganisms. Trends Biotechnol. 2016, 34, 7–25. [Google Scholar] [CrossRef]
- Downing, B.D.; Pellerin, B.A.; Bergamaschi, B.A.; Saraceno, J.F.; Kraus, T.E.C. Seeing the light: The effects of particles, dissolved materials, and temperature on in situ measurements of DOM fluorescence in rivers and streams. Limnol. Oceanogr. Methods 2012, 10, 767–775. [Google Scholar] [CrossRef]
- So, P.T.C.; Dong, C.Y. Fluorescence Spectrophotometry; eLS: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Kong, Y.; Lou, I.; Zhang, Y.; Lou, C.U.; Mok, K.M. Using an online phycocyanin fluorescence probe for rapid monitoring of cyanobacteria in Macau freshwater reservoir. Hydrobiologia 2014, 741, 33–49. [Google Scholar] [CrossRef]
- Almuhtaram, H.; Cui, Y.; Zamyadi, A.; Hofmann, R. Cyanotoxins and cyanobacteria cell accumulations in drinking water treatment plants with a low risk of bloom formation at the source. Toxins 2018, 10, 430. [Google Scholar] [CrossRef]
- Zamyadi, A.; MacLeod, S.; Fan, Y.; McQuaid, N.; Dorner, S.; Sauvé, S.; Prévost, M. Toxic cyanobacterial breakthrough and accumulation in a drinking water plant: A monitoring and treatment challenge. Water Res. 2012, 46, 1511–1523. [Google Scholar] [CrossRef]
- Cotterill, V.; Hamilton, D.P.; Puddick, J.; Suren, A.; Wood, S.A. Phycocyanin sensors as an early warning system for cyanobacteria blooms concentrations: A case study in the Rotorua lakes. N. Z. J. Mar. Freshw. Res. 2019, 53, 555–570. [Google Scholar] [CrossRef]
- Song, K.; Li, L.; Tedesco, L.P.; Clercin, N.; Hall, R.; Li, S.; Shi, K.; Liu, D.; Sun, Y. Remote estimation of phycocyanin (PC) for inland waters coupled with YSI PC fluorescence probe. Environ. Sci. Pollut. Res. 2013, 20, 5330–5340. [Google Scholar] [CrossRef] [PubMed]
- Zamyadi, A.; Henderson, R.K.; Stuetz, R.; Newcombe, G.; Newtown, K.; Gladman, B. Cyanobacterial management in full-scale water treatment and recycling processes: Reactive dosing following intensive monitoring. Environ. Sci. Water Res. Technol. 2016, 2, 362–375. [Google Scholar] [CrossRef]
- Ziegmann, M.; Abert, M.; Muller, M.; Frimmel, F.H. Use of fluorescence fingerprints for the estimation of bloom formation and toxin production of Microcystis aeruginosa. Water Res. 2010, 44, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Rousso, B.Z.; Bertone, E.; Stewart, R.; Aguiar, A.; Chuang, A.; Hamilton, D.P.; Burford, M.A. Chlorophyll and phycocyanin in-situ fluorescence in mixed cyanobacterial species assemblages: Effects of morphology, cell size and growth phase. Water Res. 2022, 212, 118127. [Google Scholar] [CrossRef]
- Kasinak, J.-M.E.; Holt, B.M.; Chislock, M.F.; Wilson, A.E. Benchtop fluorometry of phycocyanin as a rapid approach for estimating cyanobacterial biovolume. J. Plankton Res. 2015, 37, 248–257. [Google Scholar] [CrossRef]
- Hodges, C.M. A Validation Study of Phycocyanin Sensors for Monitoring Cyanobacteria in Cultures and Field Samples. Master’s Thesis, University of Waikato, Hamilton, New Zealand, 2016. [Google Scholar]
- Macário, I.P.E.; Castro, B.B.; Nunes, M.I.S.; Antunes, S.C.; Pizarro, C.; Coelho, C.; Gonçalves, F.; de Figueiredo, D.R. New insights towards the establishment of phycocyanin concentration thresholds considering species-specific variability of bloom-forming cyanobacteria. Hydrobiologia 2015, 757, 155–165. [Google Scholar] [CrossRef]
- Chorus, I.; Bartram, J. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring, and Management; WHO: London, UK; New York, NY, USA, 1999. [Google Scholar]
- Miller, T.R.; Beversdorf, L.; Chaston, S.D.; McMahon, K.D. Spatiotemporal molecular analysis of cyanobacteria blooms reveals Microcystis—Aphanizomenon interactions. PLoS ONE 2013, 8, e74933. [Google Scholar] [CrossRef]
- Donkor, V.; Häder, D.-P. Effects of ultraviolet irradiation on photosynthetic pigments in some filamentous cyanobacteria. Aquat. Microb. Ecol. 1996, 11, 143–149. [Google Scholar] [CrossRef]
- Mulders, K.J.M.; Lamers, P.P.; Martens, D.E.; Wijffels, R.H. Phototrophic pigment production with microalgae: Biological constraints and opportunities. J. Phycol. 2014, 50, 229–242. [Google Scholar] [CrossRef]
- Ma, R.; Fan, L.; Bi, Y.; Hu, Z. Effects of light intensity and quality on phycobiliprotein accumulation in the cyanobacterium Nostoc sphaeroides Kützing. Biotechnol. Lett. 2015, 37, 1663–1669. [Google Scholar] [CrossRef]
- Thimijan, R.; Heins, R. Photometric, radiometric, and quantum light units of measure: A review of procedures for interconversion. Hortic. Sci. 1983, 18, 818–822. [Google Scholar] [CrossRef]
- Dervaux, J.; Mejean, A.; Brunet, P. Irreversible Collective Migration of Cyanobacteria in Eutrophic Conditions. PLoS ONE 2015, 10, e0120906. [Google Scholar] [CrossRef] [PubMed]
- Aparicio Medrano, E.; Uittenbogaard, R.E.; van de Wiel, B.J.H.; Dionisio Pires, L.M.; Clercx, H.J.H. An alternative explanation for cyanobacterial scum formation and persistence by oxygenic photosynthesis. Harmful Algae 2016, 60, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Hu, C.; Visser, P.M.; Ma, R. Diurnal changes of cyanobacteria blooms in Taihu Lake as derived from GOCI observations. Limnol. Oceanogr. 2018, 63, 1711–1726. [Google Scholar] [CrossRef]
- Rome, M.; Beighley, R.E.; Faber, T. Sensor-based detection of algal blooms for public health advisories and long-term monitoring. Sci. Total Environ. 2021, 767, 144984. [Google Scholar] [CrossRef]
- Guo, D.; Robinson, C.; Herrera, J.E. Mechanism of dissolution of minium (Pb3O4) in water under depleting chlorine conditions. Corros. Sci. 2016, 103, 42–49. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, W. Models for identifying significant environmental factors associated with cyanobacterial bloom occurrence and for predicting cyanobacterial blooms. J. Great Lakes Res. 2014, 40, 265–273. [Google Scholar] [CrossRef]
- Ndong, M.; Bird, D.; Nguyen-Quang, T.; de Boutray, M.L.; Zamyadi, A.; Vincon-Leite, B.; Lemaire, B.J.; Prevost, M.; Dorner, S. Estimating the risk of cyanobacterial occurrence using an index integrating meteorological factors: Application to drinking water production. Water Res. 2014, 56, 98–108. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Newcombe, G.; House, J.; Ho, L.; Baker, P.; Burch, M. Management Strategies for Cyanobacteria (Blue-Green Algae): A Guide for Water Utilities; The Cooperative Research Centre for Water Quality and Treatment: Adelaïde, South Australia, June 2010; p. 112. [Google Scholar]
- Gao, J.; Zhu, J.; Wang, M.; Dong, W. Dominance and Growth Factors of Pseudanabaena sp. in Drinking Water Source Reservoirs, Southern China. Sustainability 2018, 10, 3936. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).