Preparation of Hydrogels Based Radix Isatidis Residue Grafted with Acrylic Acid and Acrylamide for the Removal of Heavy Metals
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Pretreatment of Radix Isatidis Residue (RIR)
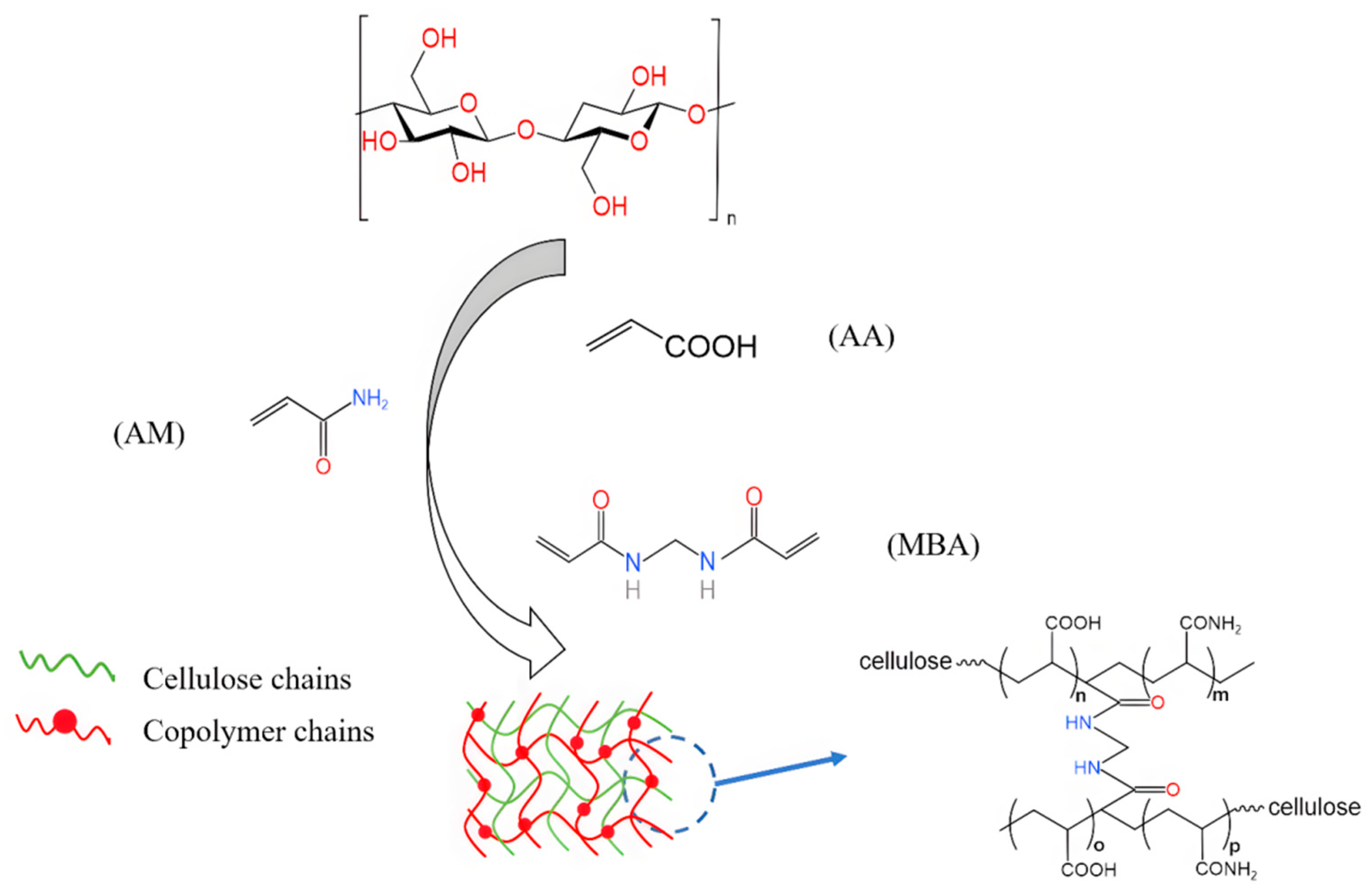
2.3. Preparation of Hydrogels
2.4. Characterization
2.5. Swelling Experiments
2.6. Adsorption Experiments
3. Results and Discussion
3.1. Structure Characterization and Analysis of Hydrogels
3.1.1. Photos of RIR/AA-co-AM Hydrogel
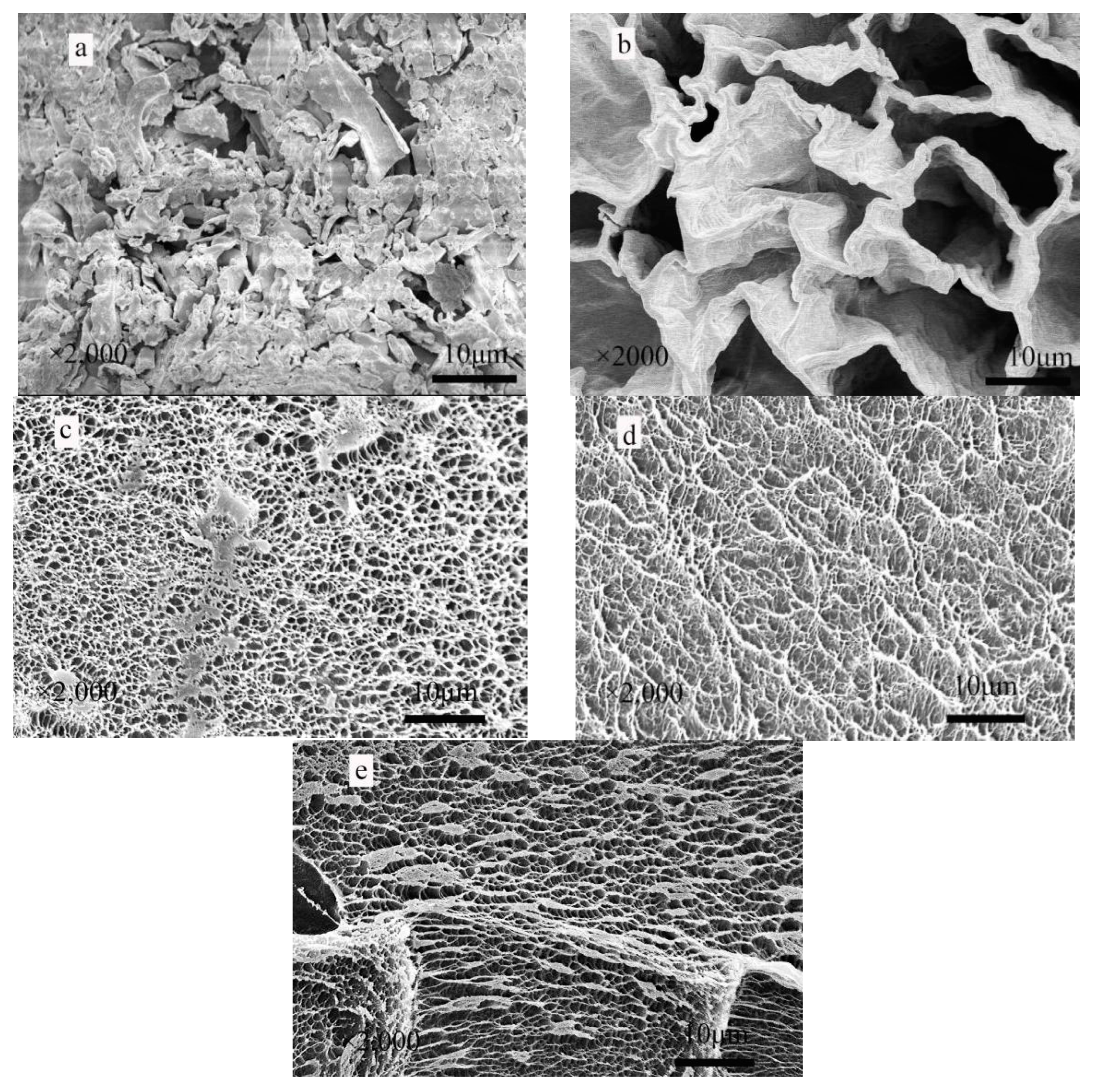
3.1.2. SEM
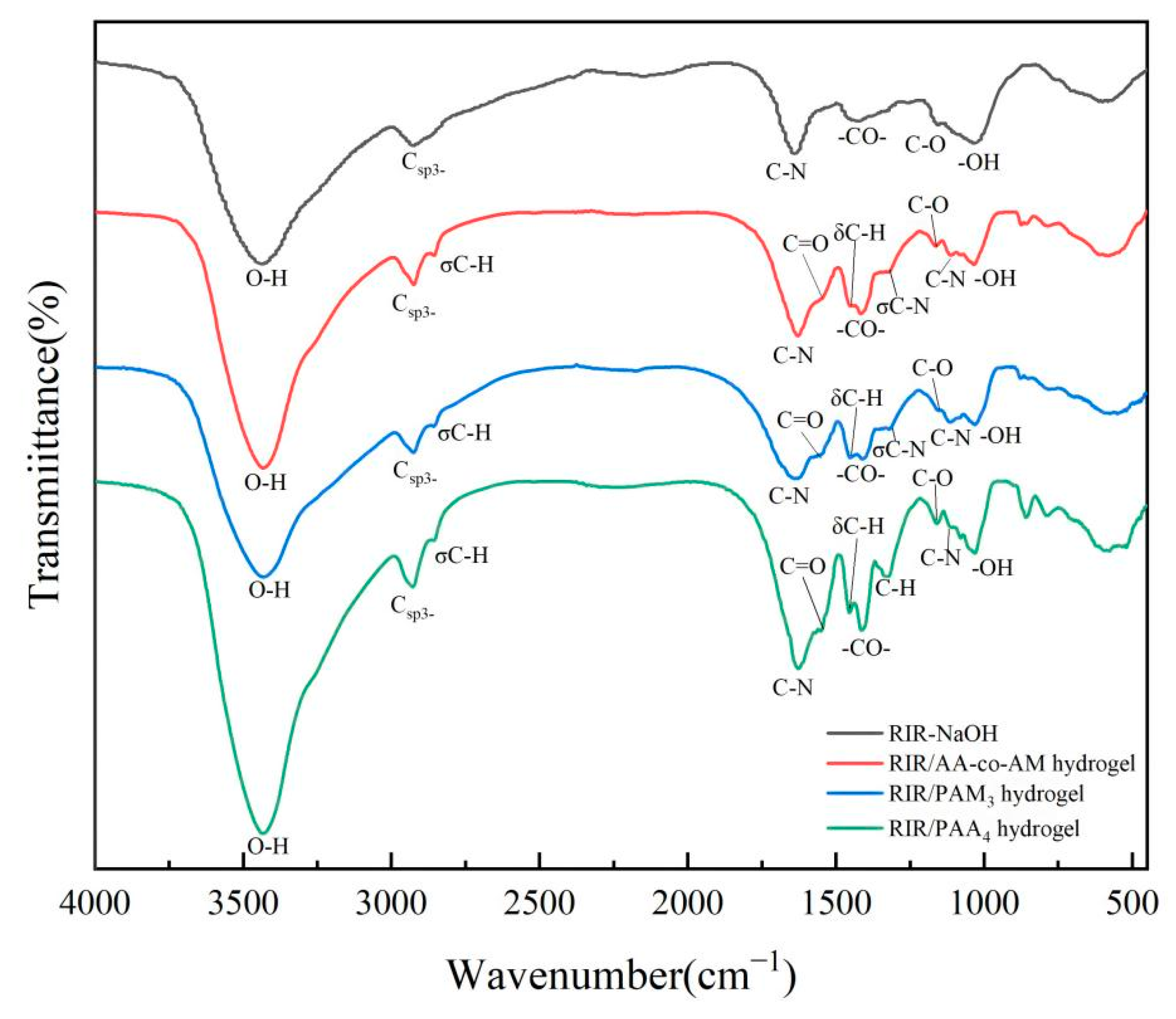
3.1.3. FTIR Analysis
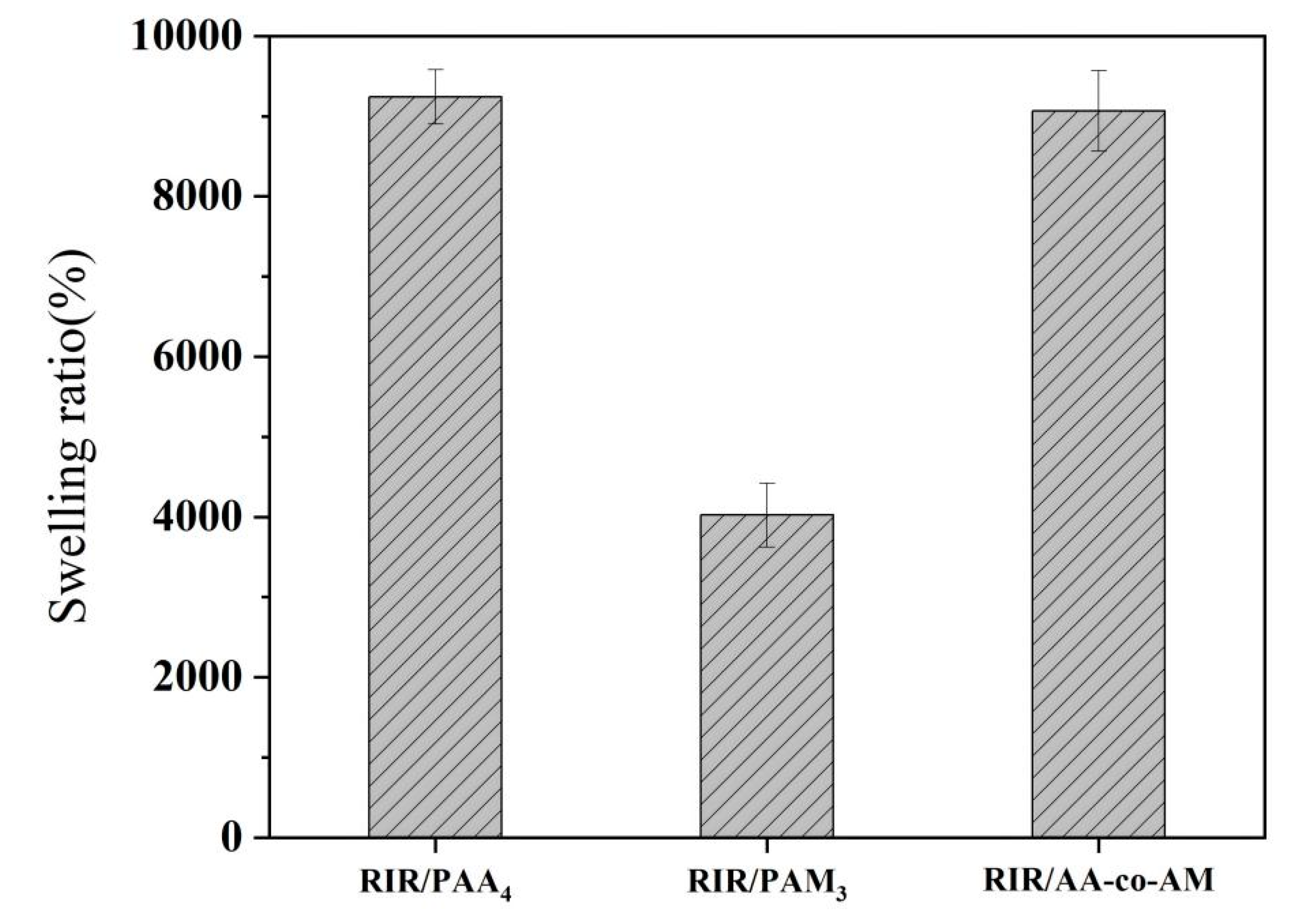
3.2. Swelling Ratio of Hydrogels
3.3. Adsorption of RIR/AA-co-AM
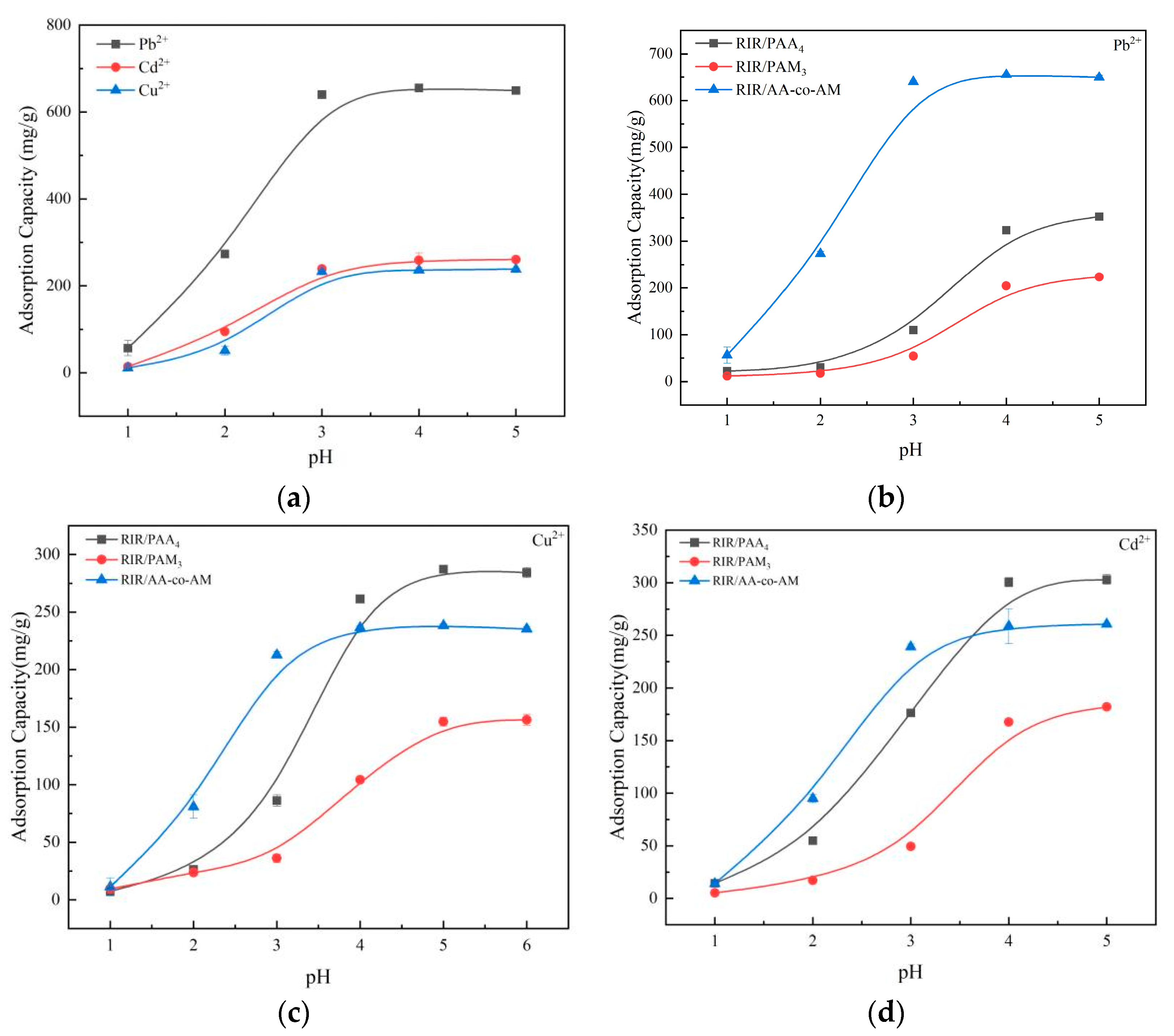
3.3.1. Effect of pH on Adsorption
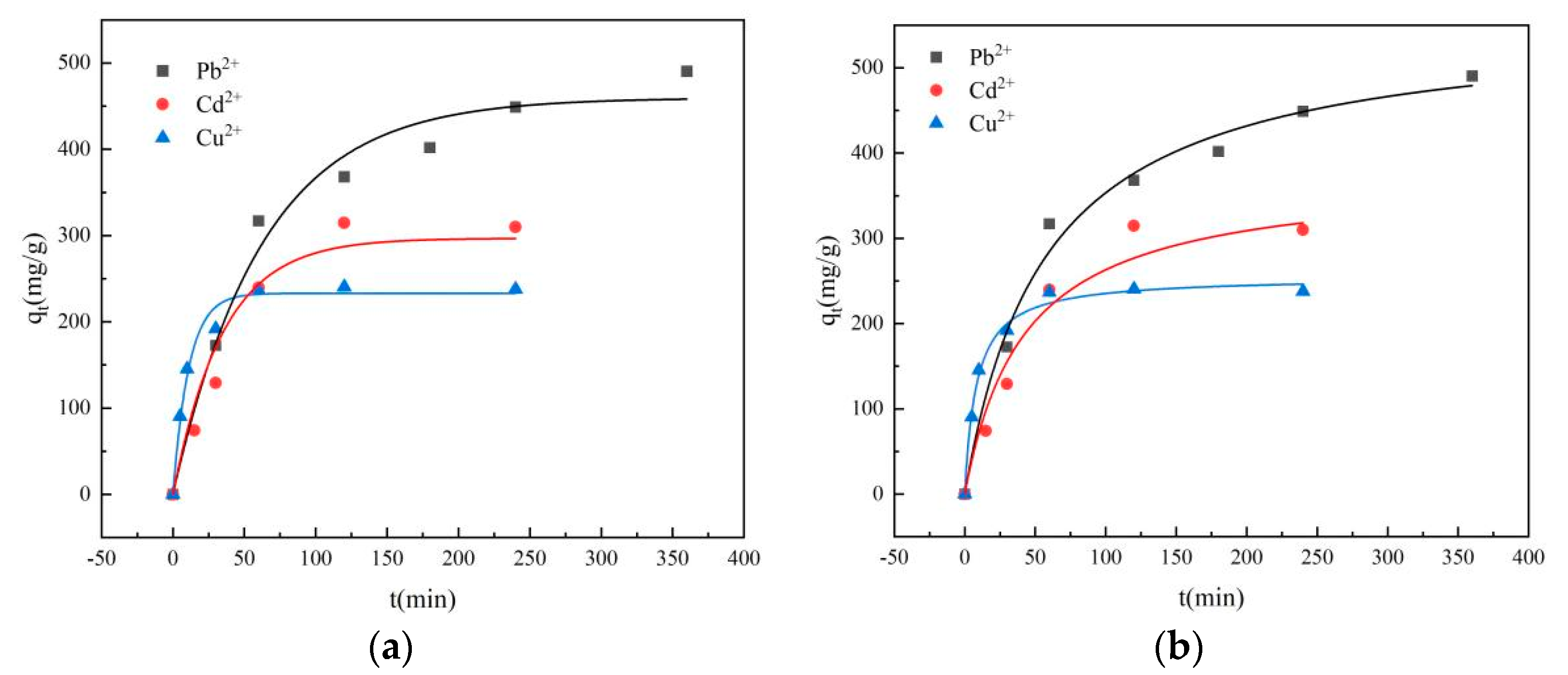
3.3.2. Effect of Contact Time on Adsorption
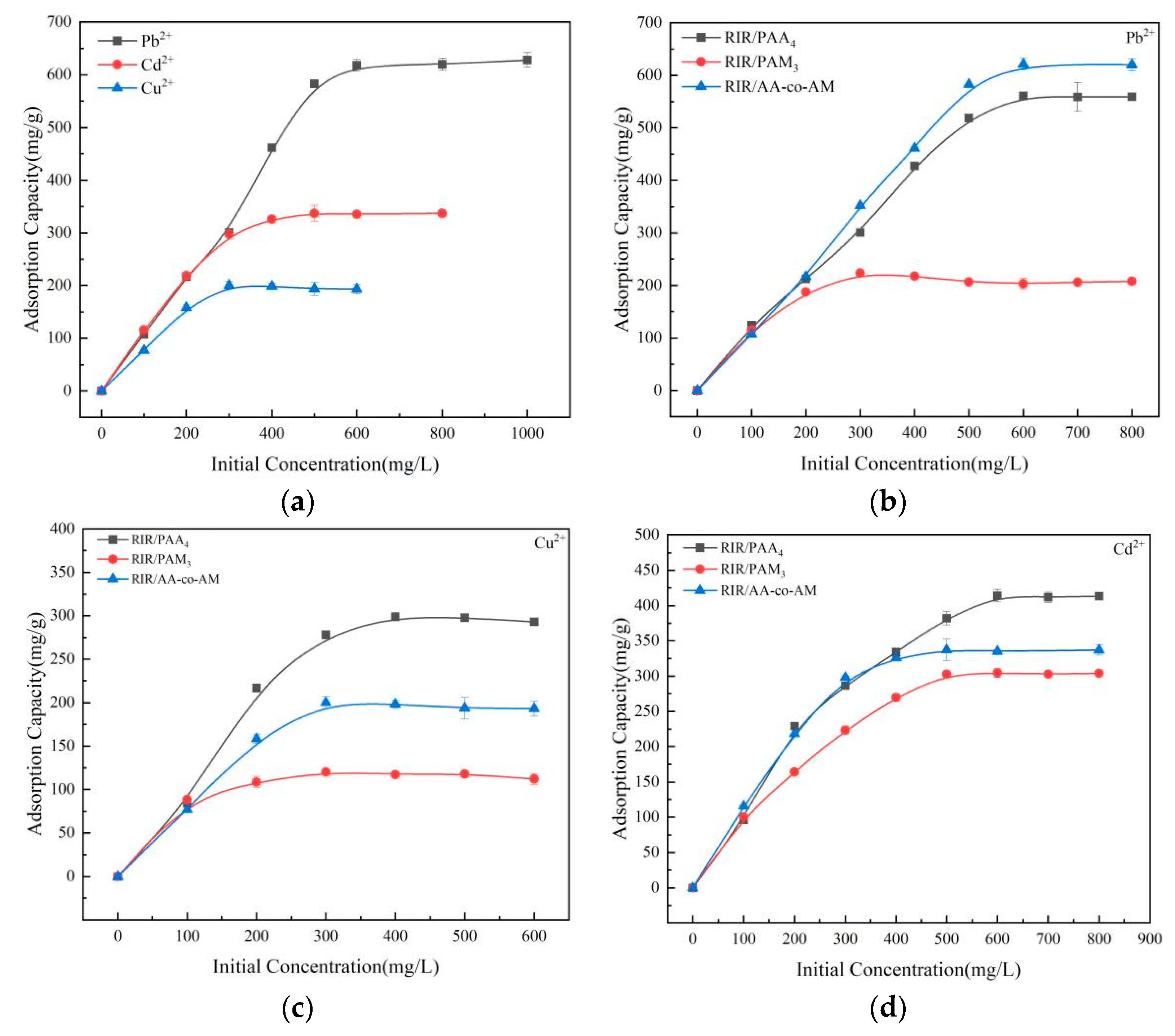
3.3.3. Effect of Initial Ion Concentration on Adsorption
3.3.4. Adsorption Kinetics
3.3.5. Adsorption Isotherm
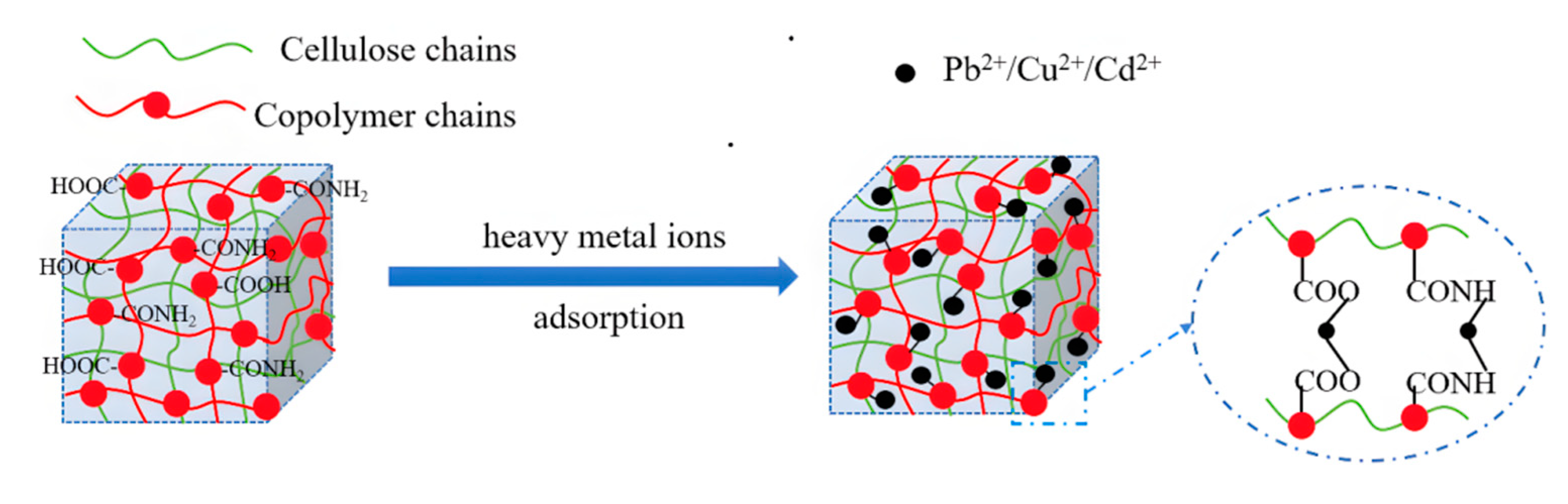
3.4. Adsorption Mechanism of Hydrogel
4. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Richardson, J.R.; Fitsanakis, V.; Westerink RH, S.; Kanthasamy, A.G. Neurotoxicity of pesticides. Acta Neuropathol. 2019, 138, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Chandrabose, G.; Dey, A.; Gaur, S.S.; Pitchaimuthu, S.; Jagadeesan, H.; Braithwaite, N.S.J.; Selvaraj, V.; Kumar, V.; Krishnamurthy, S. Removal and degradation of mixed dye pollutants by integrated adsorption-photocatalysis technique using 2-D MoS2/TiO2 nanocomposite. Chemosphere 2021, 279, 130467–130478. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Qu, Z.; Wang, J.; Cao, L.; Han, Q. Microalgal bioremediation of heavy metal pollution in water: Recent advances, challenges, and prospects. Chemosphere 2022, 286, 131870. [Google Scholar] [CrossRef]
- Komijani, M.; Shamabadi, N.S.; Shahin, K.; Eghbalpour, F.; Tahsili, M.R.; Bahram, M. Heavy metal pollution promotes antibiotic resistance potential in the aquatic environment. Environ. Pollut. 2021, 274, 116569. [Google Scholar] [CrossRef]
- Li, X.; Shen, H.; Zhao, Y.; Cao, W.; Hu, C.; Sun, C. Distribution and Potential Ecological Risk of Heavy Metals in Water, Sediments, and Aquatic Macrophytes: A Case Study of the Junction of Four Rivers in Linyi City, China. Int. J. Environ. Res. Public Health 2019, 16, 2861. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Liu, Y.; Shen, J.; Zhang, S.; Liu, X.; Chen, X.; Ma, Y.; Ren, S.; Fang, G.; Li, S.; et al. Simultaneous removal of Pb2+, Cu2+ and Cd2+ ions from wastewater using hierarchical porous polyacrylic acid grafted with lignin. J. Hazard. Mater. 2020, 392, 122208. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, B.; Wang, Q.; Di, J.; Miao, S.; Yu, J. Amino-Functionalized Porous Nanofibrous Membranes for Simultaneous Removal of Oil and Heavy-Metal Ions from Wastewater. ACS Appl. Mater. Interfaces 2018, 11, 1672–1679. [Google Scholar] [CrossRef]
- Zhu, L.; Ji, J.; Wang, S.; Xu, C.; Yang, K.; Xu, M. Removal of Pb(II) from wastewater using Al2O3-NaA zeolite composite hollow fiber membranes synthesized from solid waste coal fly ash. Chemosphere 2018, 206, 278–284. [Google Scholar] [CrossRef]
- Rehman, M.-U.; Rehman, W.; Waseem, M.; Hussain, S.; Haq, S.; Rehman, M.A. Adsorption mechanism of Pb2+ ions by Fe3O4, SnO2, and TiO2 nanoparticles. Environ. Sci. Pollut. Res. 2019, 26, 19968–19981. [Google Scholar] [CrossRef]
- Joseph, L.; Jun, B.M.; Flora, J.R.V.; Park, C.M.; Yoon, Y. Removal of heavy metals from water sources in the developing world using low-cost materials: A review. Chemosphere 2019, 229, 142–159. [Google Scholar] [CrossRef]
- Ali, I.; Peng, C.; Lin, D.; Saroj, D.P.; Naz, I.; Khan, Z.M.; Sultan, M.; Ali, M. Encapsulated green magnetic nanoparticles for the removal of toxic Pb2+ and Cd2+ from water: Development, characterization and application. J. Environ. Manag. 2018, 234, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Wang, N.; Wang, S. Blood lead level of outpatient children in Anqing from 2015 to 2018. Chin. J. Sch. Health 2021, 42, 1548–1551. [Google Scholar] [CrossRef]
- Darban, Z.; Shahabuddin, S.; Gaur, R.; Ahmad, I.; Sridewi, N. Hydrogel-Based Adsorbent Material for the Effective Removal of Heavy Metals from Wastewater: A Comprehensive Review. Gels 2022, 8, 263. [Google Scholar] [CrossRef] [PubMed]
- Saxena, G.; Purchase, D.; Mulla, S.I.; Saratale, G.D.; Bharagava, R.N. Phytoremediation of Heavy Metal-Contaminated Sites: Eco-environmental Concerns, Field Studies, Sustainability Issues, and Future Prospects. In Reviews of Environmental Contamination and Toxicology; Springer: Cham, Switzerland, 2019; Volume 249, pp. 71–131. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Jiang, H.; Lin, Z.; Xu, S.; Xie, J.; Zhang, A. Preparation of acrylamide/acrylic acid cellulose hydrogels for the adsorption of heavy metal ions. Carbohydr. Polym. 2019, 224, 115022. [Google Scholar] [CrossRef]
- Yin, X.C.; Zhang, N.D.; Du, M.X.; Zhu, H.; Ke, T. Preparation of bio-absorbents by modifying licorice residue via chemical methods and removal of copper ions from wastewater. Water Sci. Technol. 2021, 84, 3528–3540. [Google Scholar] [CrossRef]
- Zhan, Y.; Guan, X.; Ren, E.; Lin, S.; Lan, J. Fabrication of zeolitic imidazolate framework-8 functional polyacrylonitrile nanofibrous mats for dye removal. J. Polym. Res. 2019, 26, 145. [Google Scholar] [CrossRef]
- Liu, X.; Tian, J.; Li, Y.; Sun, N.; Mi, S.; Xie, Y.; Chen, Z. Enhanced dyes adsorption from wastewater via Fe3O4 nanoparticles functionalized activated carbon. J. Hazard. Mater. 2019, 373, 397–407. [Google Scholar] [CrossRef]
- Hua, J.; Meng, R.; Wang, T.; Gao, H.; Luo, Z.; Jin, Y.; Liu, L.; Yao, J. Highly Porous Cellulose Microbeads and their Adsorption for Methylene Blue. Fibers Polym. 2019, 20, 794–803. [Google Scholar] [CrossRef]
- Yang, S.C.; Liao, Y.; Karthikeyan, K.G.; Pan, X.J. Mesoporous cellulose-chitosan composite hydrogel fabricated via the co-dissolution-regeneration process as biosorbent of heavy metals. Environ. Pollut. 2021, 286, 117324–117333. [Google Scholar] [CrossRef]
- Yan, R.R.; Gong, J.S.; Su, C.; Liu, Y.L.; Qian, J.Y.; Xu, Z.H.; Shi, J.S. Preparation and applications of keratin biomaterials from natural keratin wastes. Appl. Microbiol. Biotechnol. 2022, 106, 2349–2366. [Google Scholar] [CrossRef]
- Yin, X.C.; Li, F.Y.; He, Y.F.; Wang, Y.; Wang, R.M. Study on effective extraction of chicken feather keratins and their films for controlling drug release. Biomater. Sci. 2013, 1, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.M.; Wu, S.W. The Application of Nature Absorbents for Heavy Metals Uptake from Contaminated Water; Chongqing Technol Business University (NatSciEd): Chongqing, China, 2005; pp. 537–540. [Google Scholar]
- Shi, Y.Z.; Yin, X.C.; Si, G.H.; Zhang, N.D.; Du, M.X.; Wang, X.H. Bio-adsorbent preparation based on Chinese radix isatidis residue for Pb(II) removal. Water Pract. Technol. 2020, 15, 1202–1212. [Google Scholar] [CrossRef]
- Huang, Y.; Meng, F.; Liu, R.; Yu, Y.; Yu, W. Morphology and supramolecular structure characterization of cellulose isolated from heat-treated moso bamboo. Cellulose 2019, 26, 7067–7078. [Google Scholar] [CrossRef]
- Wittmar AS, M.; Baumert, D.; Ulbricht, M. Cotton as Precursor for the Preparation of Porous Cellulose Adsorbers. Macromol. Mater. Eng. 2021, 306, 2000778. [Google Scholar] [CrossRef]
- Qin, Q.; Guo, R.; Lin, S.; Jiang, S.; Lan, J.; Lai, X.; Cui, C.; Xiao, H.; Zhang, Y. Waste cotton fiber/Bi2WO6 composite film for dye removal. Cellulose 2019, 26, 3909–3922. [Google Scholar] [CrossRef]
- Liu, Q.; He, W.Q.; Aguedo, M.; Xia, X.; Bai, W.B.; Dong, Y.Y.; Song, J.Q.; Richel, A.; Goffin, D. Microwave-assisted alkali hydrolysis for cellulose isolation from wheat straw: Influence of reaction conditions and non-thermal effects of microwave. Carbohydr. Polym. 2020, 253, 117170–117199. [Google Scholar] [CrossRef]
- Meez, E.; Rahdar, A.; Kyzas, G. Sawdust for the Removal of Heavy Metals from Water: A Review. Molecules 2021, 26, 4318. [Google Scholar] [CrossRef]
- Qamouche, K.; Chetaine, A.; El Yahyaoui, A.; Moussaif, A.; Fröhlich, P.; Bertau, M.; Haneklaus, N. Uranium and other heavy metal sorption from Moroccan phosphoric acid with argan nutshell sawdust. Miner. Eng. 2021, 171, 107085. [Google Scholar] [CrossRef]
- Phitsuwan, P.; Sakka, K.; Ratanakhanokchai, K. Structural changes and enzymatic response of Napier grass (Pennisetum purpureum) stem induced by alkaline pretreatment. Bioresour. Technol. 2016, 218, 247–256. [Google Scholar] [CrossRef]
- Huang, L.J.; Lee, W.J.; Chen, Y.C. Bio-Based Hydrogel and Aerogel Composites Prepared by Combining Cellulose Solutions and Waterborne Polyurethane. Polymers 2022, 14, 204. [Google Scholar] [CrossRef]
- Liu, G.; Huang, Y.; Xu, L. Biochar from Chinese herb residues as adsorbent for toxic metals removal. IOP Conf. Ser. Earth Environ. Sci. 2017, 61, 12147. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Xia, L.H.; Zhang, Z.H.; Zu, Y.G. Present situation and development trend of eco-utilization of residue production in plant extraction. Mod. Chem. Ind. 2008, 28, 14–17. [Google Scholar] [CrossRef]
- Guo, F.; Dong, Y.; Dong, L.; Jing, Y. An innovative example of herb residues recycling by gasification in a fluidized bed. Waste Manag. 2013, 33, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhou, T. Biosorption of methylene blue from wastewater by an extraction residue of Salvia miltiorrhiza Bge. Bioresour. Technol. 2016, 219, 330–337. [Google Scholar] [CrossRef]
- Feng, N.; Zhang, F. Untreated Chinese ephedra residue as biosorbents for the removal of Pb2+ ions from aqueous solutions. Procedia Environ. Sci. 2013, 18, 794–799. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.T.; Du, C.F.; Wu, Z.H.; Zhang, L.H. Relationship of the cellulose and lignin contents in biomass to the structure and RB-19 adsorption behavior on activated carbon. New J. Chem. 2018, 42, 16493–16502. [Google Scholar] [CrossRef]
- Teow, Y.H.; Kam, L.M.; Mohammad, A.W. Synthesis of cellulose hydrogel for copper (II) ions adsorption. J. Environ. Chem. Eng. 2018, 6, 4588–4597. [Google Scholar] [CrossRef]
- Shalla, A.H.; Yaseen, Z.; Bhat, M.A.; Rangreez, T.A.; Maswal, M. Recent review for removal of metal ions by hydrogels. Sep. Sci. Technol. 2019, 54, 89–100. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Y.; Ding, M.; Fan, X.; Hu, J.; Chen, Y.; Li, J.; Li, Z.; Liu, W. A 4arm-PEG macromolecule crosslinked chitosan hydrogels as antibacterial wound dressing. Carbohydr. Polym. 2022, 277, 118871. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Lu, J.; Ding, M.; Chen, Y. Synthesis and properties of Poly(vinyl alcohol) hydrogels with high strength and toughness. Polym. Test. 2022, 108, 107516. [Google Scholar] [CrossRef]
- Ozay, O.; Ekici, S.; Baran, Y.; Kubilay, S.; Aktas, N.; Sahiner, N. Utilization of magnetic hydrogels in the separation of toxic metal ions from aqueous environments. Desalination 2010, 260, 57–64. [Google Scholar] [CrossRef]
- Jang, S.H.; Jeong, Y.G.; Gil Min, B.; Lyoo, W.S.; Lee, S.C. Preparation and lead ion removal property of hydroxyapatite/polyacrylamide composite hydrogels. J. Hazard. Mater. 2008, 159, 294–299. [Google Scholar] [CrossRef]
- Dai, L.; Cheng, T.; Xi, X.; Nie, S.; Ke, H.; Liu, Y.; Tong, S.; Chen, Z. A versatile TOCN/CGG self-assembling hydrogel for integrated wastewater treatment. Cellulose 2020, 27, 915–925. [Google Scholar] [CrossRef]
- Godiya, C.B.; Cheng, X.; Li, D.; Chen, Z.; Lu, X. Carboxymethyl cellulose/polyacrylamide composite hydrogel for cascaded treatment/reuse of heavy metal ions in wastewater. J. Hazard. Mater. 2019, 364, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Ozay, O.; Ekici, S.; Baran, Y.; Aktas, N.; Sahiner, N. Removal of toxic metal ions with magnetic hydrogels. Water Res. 2009, 43, 4403–4411. [Google Scholar] [CrossRef] [PubMed]
- Maijan, P.; Junlapong, K.; Arayaphan, J.; Khaokong, C.; Chantarak, S. Synthesis and characterization of highly elastic superabsorbent natural rubber/polyacrylamide hydrogel. Polym. Degrad. Stab. 2021, 186, 109499. [Google Scholar] [CrossRef]
- Zainal, S.H.; Mohd, N.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of cellulose-based hydrogel: A review. J. Mater. Res. Technol. 2021, 10, 935–952. [Google Scholar] [CrossRef]
- Akter, M.; Bhattacharjee, M.; Dhar, A.K.; Rahman, F.B.A.; Haque, S.; Rashid, T.U.; Kabir, S.M.F. Cellulose-Based Hydrogels for Wastewater Treatment: A Concise Review. Gels 2021, 7, 30. [Google Scholar] [CrossRef]
- Javed, R.; Shah, L.A.; Sayed, M.; Khan, M.S. Uptake of heavy metal ions from aqueous media by hydrogels and the irconversion to nanoparticles for generation of a catalyst system: Two-fold application study. RSC Adv. 2018, 8, 14787–14797. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Barghi, A. Synthesis of poly(AN)/poly(AA-co-AM) hydrogel nanocomposite with electrical conductivity and antibacterial properties. Polym. Compos. 2018, 40, 2724–2733. [Google Scholar] [CrossRef]
- Huang, S.; Wang, X.; Shen, J.; Wu, R.; Zhao, H.; Wang, Y.; Wang, Y.; Xia, Y. Surface functionalization of cellulose nanocrystals with polymeric ionic liquids during phase transfer. Carbohydr. Polym. 2017, 157, 1426–1433. [Google Scholar] [CrossRef]
- Chen, H.; Shao, J. Analysis of Polyaeryamide by Infrared Spectroscopy. Anal. Instrum. 2011, 3, 36–40. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, S.; Chen, Z.; Wang, M.; Cao, J.; Wang, R. Preparation and Characterization of Superabsorbent Polymers Based on Sawdust. Polymers 2019, 11, 1891. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.E.H. Removal of heavy metals from model wastewater by using carboxymehyl cellulose/2-acrylamido-2-methyl propane sulfonic acid hydrogels. J. Appl. Polym. Sci. 2012, 123, 763–769. [Google Scholar] [CrossRef]
- Ge, D.; Yuan, H.; Xiao, J.; Zhu, N. Insight into the enhanced sludge dewaterability by tannic acid conditioning and pH regulation. Sci. Total Environ. 2019, 679, 298–306. [Google Scholar] [CrossRef]
- Bai, B.; Bai, F.; Li, X.; Nie, Q.; Jia, X.; Wu, H. The remediation efficiency of heavy metal pollutants in water by industrial red mud particle waste. Environ. Technol. Innov. 2022, 28, 102944. [Google Scholar] [CrossRef]
- Peng, X.; Zheng, J.; Liu, Q.; Hu, Q.; Sun, X.; Li, J.; Liu, W.; Lin, Z. Efficient removal of iron from red gypsum via synergistic regulation of gypsum phase transformation and iron speciation. Sci. Total Environ. 2021, 791, 148319. [Google Scholar] [CrossRef]
- Badsha, M.A.; Khan, M.; Wu, B.; Kumar, A.; Lo, I.M.C. Role of surface functional groups of hydrogels in metal adsorption: From performance to mechanism. Hazard. Mater. 2021, 408, 124463. [Google Scholar] [CrossRef]
- Badsha, M.A.; Lo, I.M. An innovative pH-independent magnetically separable hydrogel for the removal of Cu(II) and Ni(II) ions from electroplating wastewater. Hazard. Mater. 2020, 381, 121000. [Google Scholar] [CrossRef]
- Milosavljević, N.B.; Ristić, M.Đ.; Perić-Grujić, A.A.; Filipović, J.M.; Štrbac, S.B.; Rakočević, Z.L.; Kalagasidis Krušić, M.T. Hydrogel based on chitosan, itaconic acid and methacrylic acid as adsorbent of Cd2+ ions from aqueous solution. Chem. Eng. J. 2010, 165, 554–562. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Dawodu, F.A.; Adebowale, K.O. Mechanism on the sorption of heavy metals from binary-solution by a low cost montmorillonite and its desorption potential. Alex. Eng. J. 2015, 54, 757–767. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.; Ghafar, N.A.; Ngadi, N.; Razmi, F.A.; Inuwa, I.M.; Mat, R.; Amin, N.A.S. Effective removal of anionic textile dyes using adsorbent synthesized from coffee waste. Sci. Rep. 2020, 10, 2928–2940. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhou, S.; Zhang, L.; You, T.; Xu, F. Adsorption of heavy metals by graphene oxide/cellulose hydrogel prepared from NaOH/urea aqueous solution. Materials 2016, 9, 582. [Google Scholar] [CrossRef]
- Abdelwahab, H.E.; Hassan, S.Y.; Mostafa, M.A.; El Sadek, M.M. Synthesis and characterization of glutamic-chitosan hydrogel for copper and nickel removal from wastewater. Molecules 2016, 21, 684. [Google Scholar] [CrossRef] [Green Version]
- Kabir, S.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.R.; Ali, A.; Islam, M. Cellulose-based hydrogel materials: Chemistry, properties and their prospective applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Chen, Q.; Zhao, H.; Dang, J.; Jin, R.; Zhao, W.; Li, Y. Wheat straws and corn straws as adsorbents for the removal of Cr(VI) and Cr(III) from aqueous solution: Kinetics, isotherm, and mechanism. ACS Omega 2020, 5, 6003–6009. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, J.; Zheng, L.; Dang, Z.; Zhang, L. Classical theory and electron-scale view of exceptional Cd adsorption onto mesoporous cellulose biochar via experimental analysis coupled with DFT calculations. Chem. Eng. J. 2018, 350, 1000–1009. [Google Scholar] [CrossRef]
- Nongbe, M.C.; Bretel, G.; Ekou, T.; Ekou, L.; Yao, B.K.; Le Grognec, E.; Felpin, F.X. Cellulose paper grafted with polyamines as powerful adsorbent for heavy metals. Cellulose 2018, 25, 4043–4055. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Naffrechoux, E. Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon: Part, I. Two-parameter models and equations allowing determination of thermodynamic parameters. J. Hazard. Mater. 2007, 147, 381–394. [Google Scholar] [CrossRef]
- Song, R.Z.; Chen, Y.F.; Pan, H.S.; Zeng, M.Z. Graft Copolymerization of Acrylic Acid onto Superfine Cellulose. Cellul. Sci. Technol. 2001, 4, 11–15+20. [Google Scholar] [CrossRef]
- Sinha, V.; Chakma, S. Advances in the preparation of hydrogel for wastewater treatment: A concise review. J. Environ. Chem. Eng. 2019, 7, 103295. [Google Scholar] [CrossRef]
- Kong, W.; Yue, Q.; Li, Q.; Gao, B. Adsorption of Cd2+ on GO/PAA hydrogel and preliminary recycle to GO/PAA-CdS as efficient photocatalyst. Sci. Total Environ. 2019, 668, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Zhao., Z.; Huang, Y.; Wu, Y.; Li, S.; Yin, H.; Wang, J. α-ketoglutaric acid modified chitosan/polyacrylamide semi-interpenetrating polymer network hydrogel for removal of heavy metal ions. Colloids Surf. Physicochem. Eng. Asp. 2021, 628, 127262. [Google Scholar] [CrossRef]




| Pseudo-First-Order | Pseudo-Second-Order | |||||
|---|---|---|---|---|---|---|
| k1 | qe1 | R2 | k2 | qe2 | R2 | |
| Pb(II) | 0.016 | 459.60 | 0.9747 | 0.00003 | 555.17 | 0.9867 |
| Cd(II) | 0.029 | 296.87 | 0.9583 | 0.00005 | 374.23 | 0.9627 |
| Cu(II) | 0.09 | 233.11 | 0.9800 | 0.00005 | 254.8 | 0.9910 |
| Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|
| qm | KL | RL | R2 | n | KF | R2 | |
| Pb(II) | 689.65 | 0.0244 | 0.0639 | 0.9745 | 2.695 | 80.654 | 0.5151 |
| Cd(II) | 346.02 | 0.0879 | 0.0222 | 0.9997 | 4.196 | 90.021 | 0.8328 |
| Cu(II) | 213.68 | 0.0292 | 0.1025 | 0.9828 | 3.085 | 31.521 | 0.6722 |
| Adsorbent | Adsorption Capacity (mg/g) | References | ||
|---|---|---|---|---|
| Pb2+ | Cd2+ | Cu2+ | ||
| RIR/AA-co-AM | 655.38 | 337.16 | 242.79 | Present study |
| LR-NaOH | - | - | 43.65 | [16] |
| MCC-g-poly(AA-co-AM) | 393.28 | 289.97 | 157.51 | [7] |
| SR–PAA | 422.69 | 160.75 | - | [52] |
| Ch/IA/MAA | - | 285.7 | - | [62] |
| GO/PAA | - | 316.4 | - | [74] |
| KCTS/PAM | 61.41 | - | 72.39 | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, X.; Zhu, H.; Ke, T.; Gu, Y.; Wang, H.; Xu, P. Preparation of Hydrogels Based Radix Isatidis Residue Grafted with Acrylic Acid and Acrylamide for the Removal of Heavy Metals. Water 2022, 14, 3811. https://doi.org/10.3390/w14233811
Yin X, Zhu H, Ke T, Gu Y, Wang H, Xu P. Preparation of Hydrogels Based Radix Isatidis Residue Grafted with Acrylic Acid and Acrylamide for the Removal of Heavy Metals. Water. 2022; 14(23):3811. https://doi.org/10.3390/w14233811
Chicago/Turabian StyleYin, Xiaochun, Hai Zhu, Ting Ke, Yonge Gu, Huiyao Wang, and Pei Xu. 2022. "Preparation of Hydrogels Based Radix Isatidis Residue Grafted with Acrylic Acid and Acrylamide for the Removal of Heavy Metals" Water 14, no. 23: 3811. https://doi.org/10.3390/w14233811
APA StyleYin, X., Zhu, H., Ke, T., Gu, Y., Wang, H., & Xu, P. (2022). Preparation of Hydrogels Based Radix Isatidis Residue Grafted with Acrylic Acid and Acrylamide for the Removal of Heavy Metals. Water, 14(23), 3811. https://doi.org/10.3390/w14233811










