Nanocomposite Zinc Oxide-Based Photocatalysts: Recent Developments in Their Use for the Treatment of Dye-Polluted Wastewater
Abstract
:1. Introduction
| Dye Class | Characteristics | Pollution Contributor |
|---|---|---|
| Acidic | Water-soluble anionic compounds | Colour, organic acids, unfixed dyes. |
| Basic | Water-soluble used in weakly bright acidic dye baths | Not applicable. |
| Direct | Water-soluble, anionic compounds used without mordant | Colour, salts, surfactants, unfixed dyes, retarding agents; finish; diluents. |
| Dispersive | Insoluble in water | Colour; organic acids; carriers; levelling agents; phosphates; defoamers; lubricants; dispersants; delustrants; diluents. |
| Reactive | Water-soluble anionic dye | Colour; salt; alkali; unfixed dye;surfactants; defoamer; diluents; finish. |
| Sulphur | Organic compounds containing sulphur/sulphide | Colour; alkali; oxidising agent; reducing agent; unfixed dye. |
| Vat | Chemically complex and water-insoluble | Colour; alkali; oxidising agents; reducing agents. |
- (i)
- Doping with metals and non-metals;
- (ii)
- Coupling with other semiconductors;
- (iii)
- Surface deposition of conducting metals;
- (iv)
- Coupling with carbon materials.
2. Synthetic Strategies for the Preparation of ZnO Nanocomposites for Improved Photocatalytic Activity
2.1. Sol-Gel Method
2.2. Chemical Precipitation/Co-Precipitation
- Impurities that may precipitate with the product;
- Time-consuming processes;
- Not applicable to uncharged species;
- Lack of batch-to-batch reproducibility;
- The difficulty encountered with handling components that precipitate at different rates.
2.3. Chemical Precipitation/Co-Precipitation Incorporating Other Techniques
2.4. Hydrothermal Methods
2.5. Microwave Synthesis Methods
2.6. Other Relevant Methods
| S/N | Fabrication Method | Catalyst/Type/Dosage | Catalytic Application | Catalytic Activity | Enhanced Factors | Ref. |
|---|---|---|---|---|---|---|
| 1 | Solvothermal | BiOCl/ZnO (50 mg) | RhB (10 ppm) | 100% in 15 min | Charge separation by heterojunction. | [61] |
| 2 | Thermal oxidation, sulfidation & hydrothermal | CuO/CuS/ZnO (1 cm × 2 cm foam) | RhB (5 ppm) | 93.20% in 160 min | p–n junction, reduced charge recombination. | [62] |
| 3 | Pulsed laser ablation/ Photodeposition | ZnO/Au/Pd (0.5 mg) | MB (5.0 × 10−5 M) | 97% in 180 min | Synergistic effect between the ZnO, Au and Pd metals. | [63] |
| 4 | Fungal-secreted enzymes and proteins/sol-gel process | CuO/ZnO/binary oxide (40 mg) | MB (10 ppm) | 97.00% in 85 min | Increased ratio of ZnO, increases particle size, improves efficiency. | [105] |
| 5 | Vegetable waste extracts as potential structure-directing agents | ZnO–CuO (25 mg) | MB (0.001 M) | 95.60% in 120 min | Nanosization & p–n heterojunctions allowing better e−/h+ separation. | [106] |
| 6 | High-energy ball milling | Ni co-doped Al-ZnO (50 mg) | MO & CR (10 ppm) | 100% MO in 30 min | Enhanced charge separation and visible light response. | [107] |
| 7 | Surfactant-assisted hydrothermal method | ZnO and g-C3N4 (1 g) | MB & RhB (10 ppm) | 97% MB in 50 min | Enhanced charge separation and visible light response. | [108] |
| 8 | One-pot recrystallisation | ZnO–SWCNT (130 mg) | MB (7.9 × 10−4 M) | 100% in 120 min | Chemical bonding promotes light absorption and reduced charge recombination. | [109] |
| 9 | Jet nebuliser spray pyrolysis | ZnO/g-C3N4/Ag/thin film | MB & MG (1 × 10−5 M) | 96% & 99% in 90 min | Reduced band gap & reduced charge recombination. | [110] |
| 10 | Solvent-free synthesis | ZnS-ZnO/graphene (10 mg) | MB & MO (1 × 10−5 M) | 99% in 90 min & 97.5% in 160 min | Reduced band gap, good charge transfer & reduced charge recombination. | [111] |
| 11 | Parallel flow precipitation | Fe-ZnO (50 mg) | RhB (10 ppm) | 84% in 120 min | Higher specific surface area & charge separation efficiency. | [112] |
| 12 | Low-temperature precipitation | Chl-Cu/ZnO (30 mg) | RhB (60 ppm) | 99% in 120 min | The synergy between chlorophyll and Cu improved visible light response. | [113] |
3. Characterisation Techniques and Structural Analysis
3.1. General Structure of ZnO
3.2. Classification of ZnO Nanostructures
3.3. Electronic Structure of ZnO
3.4. Structural Analysis and Characterisation
4. Techniques for Improving the Performance of ZnO Materials in Catalysis
4.1. Doping with Metals
4.2. Coupling of Semiconductors
4.3. Coupling with Carbon Materials
4.4. Immobilised Photocatalysts
5. Further Details on Applications of the ZnO Nanomaterials in Photocatalysis
5.1. Graphitic Carbon Nitride (gC3N4) and Organic Doped Materials
5.2. Heterojunction Semiconductor and Metal-Doped Materials
5.3. Graphene Oxide, Mesoporous and Polymeric Material-Modified Catalysts
6. Conclusions and Prospects
- Despite the numerous reports on ZnO nanoparticles with different morphologies, many desirable enhancements to important properties (such as the crystallinity and sphericity of the particles) are required for improved photocatalytic performance.
- More photocatalysis research should use pollutants of concern instead of just model compounds. In recent works, dyes were used as models for organic pollutants in photocatalytic degradation studies. However, many dyes (containing known chromophores) are easier to degrade than persistent organic compounds such as pesticides, pharmaceuticals, and organ disruptors.
- A more fundamental understanding of the mineralisation pathways at the interface between the photocatalyst and the contaminant is still desired.
- Operational problems need to be addressed. These include the poor recovery of photocatalysts during post-treatment work-up and its impact on the photoactivity of the recycled catalyst. The problem also extends to catalysts dispersed as powders and the reduction in the activity of immobilised/supported photocatalysts on subsequent runs.
- Finally, there is a need for theoretical tools in developing new photocatalysts. Meta-analytic studies will cut down on waste by trial and error, which is common in many of today’s research endeavours.
Author Contributions
Funding
Conflicts of Interest
References
- Rajasulochana, P.; Preethy, V. Comparison on the efficiency of various techniques in the treatment of waste and sewage water–A comprehensive review. Resour.-Effic. Technol. 2016, 2, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Markham, A.C. A Brief History of Pollution; Routledge: London, UK, 2019. [Google Scholar]
- Zeng, G.; Chen, M.; Zeng, Z. Risks of neonicotinoid pesticides. Science 2013, 340, 1403. [Google Scholar] [CrossRef] [PubMed]
- Değermenci, G.D.; Değermenci, N.; Ayvaoğlu, V.; Durmaz, E.; Çakır, D.; Akan, E. Adsorption of reactive dyes on lignocellulosic waste; characterization, equilibrium, kinetic and thermodynamic studies. J. Clean. Prod. 2019, 225, 1220–1229. [Google Scholar] [CrossRef]
- Banaei, A.; Samadi, S.; Karimi, S.; Vojoudi, H.; Pourbasheer, E.; Badiei, A. Synthesis of silica gel modified with 2, 2′-(hexane-1, 6-diylbis (oxy)) dibenzaldehyde as a new adsorbent for the removal of Reactive Yellow 84 and Reactive Blue 19 dyes from aqueous solutions: Equilibrium and thermodynamic studies. Powder Technol. 2017, 319, 60–70. [Google Scholar] [CrossRef]
- Moussavi, G.; Mahmoudi, M. Removal of azo and anthraquinone reactive dyes from industrial wastewaters using MgO nanoparticles. J. Hazard. Mater. 2009, 168, 806–812. [Google Scholar] [CrossRef]
- Shimada, C.; Kano, K.; Sasaki, Y.F.; Sato, I.; Tsuda, S. Differential colon DNA damage induced by azo food additives between rats and mice. J. Toxicol. Sci. 2010, 35, 547–554. [Google Scholar] [CrossRef] [Green Version]
- Walthall, W.K.; Stark, J.D. The acute and chronic toxicity of two xanthene dyes, fluorescein sodium salt and phloxine B, to Daphnia Pulex. Environ. Pollut. 1999, 104, 207–215. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Attour, A.; Ben Grich, N.; Mouldi Tlili, M.; Ben Amor, M.; Lapicque, F.; Leclerc, J.P. Intensification of phosphate removal using electrocoagulation treatment by continuous pH adjustment and optimal electrode connection mode. Desalination Water Treat. 2016, 57, 13255–13262. [Google Scholar] [CrossRef]
- Tang, X.; Zheng, H.; Teng, H.; Sun, Y.; Guo, J.; Xie, W.; Yang, Q.; Chen, W. Chemical coagulation process for the removal of heavy metals from water: A review. Desalination Water Treat. 2016, 57, 1733–1748. [Google Scholar] [CrossRef]
- G Gallego-Urrea, J.A.; Hammes, J.; Cornelis, G.; Hassellöv, M. Coagulation and sedimentation of gold nanoparticles and illite in model natural waters: Influence of initial particle concentration. NanoImpact 2016, 3, 67–74. [Google Scholar] [CrossRef]
- Charles, J.; Bradu, C.; Morin-Crini, N.; Sancey, B.; Winterton, P.; Torri, G.; Badot, P.M.; Crini, G. Pollutant removal from industrial discharge water using individual and combined effects of adsorption and ion-exchange processes: Chemical abatement. J. Saudi Chem. Soc. 2016, 20, 185–194. [Google Scholar] [CrossRef]
- Wang, D.K.; Elma, M.; Motuzas, J.; Hou, W.-C.; Xie, F.; Zhang, X. Rational design and synthesis of molecular-sieving, photocatalytic, hollow fiber membranes for advanced water treatment applications. J. Membr. Sci. 2017, 524, 163–173. [Google Scholar] [CrossRef] [Green Version]
- Pype, M.-L.; Lawrence, M.G.; Keller, J.; Gernjak, W. Reverse osmosis integrity monitoring in water reuse: The challenge to verify virus removal—A review. Water Res. 2016, 98, 384–395. [Google Scholar] [CrossRef]
- Dickhout, J.M.; Moreno, J.; Biesheuvel, P.; Boels, L.; Lammertink, R.G.; de Vos, W.M. Produced water treatment by membranes: A review from a colloidal perspective. J. Colloid Interface Sci. 2017, 487, 523–534. [Google Scholar] [CrossRef]
- Subramani, A.; Jacangelo, J.G. Emerging desalination technologies for water treatment: A critical review. Water Res. 2015, 75, 164–187. [Google Scholar] [CrossRef]
- Munoz, I.; Rodriguez, A.; Rosal, R.; Fernandez-Alba, A.R. Life cycle assessment of urban wastewater reuse with ozonation as tertiary treatment: A focus on toxicity-related impacts. Sci. Total Environ. 2009, 407, 1245–1256. [Google Scholar] [CrossRef]
- Miranda, A.C.; Lepretti, M.; Rizzo, L.; Caputo, I.; Vaiano, V.; Sacco, O.; Lopes, W.S.; Sannino, D. Surface water disinfection by chlorination and advanced oxidation processes: Inactivation of an antibiotic resistant E. coli strain and cytotoxicity evaluation. Sci. Total Environ. 2016, 554, 1–6. [Google Scholar] [CrossRef]
- Taka, A.L.; Pillay, K.; Mbianda, X.Y. Nanosponge cyclodextrin polyurethanes and their modification with nanomaterials for the removal of pollutants from waste water: A review. Carbohydr. Polym. 2017, 159, 94–107. [Google Scholar] [CrossRef]
- Muhd Julkapli, N.; Bagheri, S.; Bee Abd Hamid, S. Recent advances in heterogeneous photocatalytic decolorization of synthetic dyes. Sci. World J. 2014, 2014, 692307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srikanth, B.; Goutham, R.; Badri Narayan, R.; Ramprasath, A.; Gopinath, K.P.; Sankaranarayanan, A.R. Recent advancements in supporting materials for immobilised photocatalytic applications in waste water treatment. J. Environ. Manag. 2017, 200, 60–78. [Google Scholar] [CrossRef]
- Sleiman, M.; Vildozo, D.; Ferronato, C.; Chovelon, J.M. Photocatalytic degradation of azo dye Metanil Yellow: Optimization and kinetic modelling using a chemometric approach. Appl. Catal. B 2007, 77, 1–11. [Google Scholar] [CrossRef]
- Whipple, D.T.; Kenis, P.J.A. Prospects of CO2 Utilization via Direct Heterogeneous Electrochemical Reduction. J. Phys. Chem. Lett. 2010, 1, 3451–3458. [Google Scholar] [CrossRef]
- Rochkind, M.; Pasternak, S.; Paz, Y. Using Dyes for Evaluating Photocatalytic Properties: A Critical Review. Molecules 2015, 20, 88–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinodgopal, K.; Bedja, I.; Hotchandani, S.; Kamat, P.V. A Photocatalytic Approach for the Reductive Decolorization of Textile Azo Dyes in Colloidal Semiconductor Suspensions. Langmuir 1994, 10, 1767–1771. [Google Scholar] [CrossRef]
- Shu, H.M.; Xie, J.M.; Xu, H.; Li, H.M.; Gu, Z.; Sun, G.S.; Xu, Y.G. Structural characterization and photocatalytic activity of NiO/AgNbO3. J. Alloys Compd. 2010, 496, 633–637. [Google Scholar] [CrossRef]
- Shaham-Waldmann, N.; Paz, Y. Away from TiO2: A critical minireview on the developing of new photocatalysts for degradation of contaminants in water. Mater. Sci. Emicond. Process. 2016, 42, 72–80. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, Q.; Wu, A.; Jiang, M.; Liang, Z.; Jiang, B.; Fu, H. Cost-effective large-scale synthesis of ZnO photocatalyst with excellent performance for dye photodegradation. Chem. Commun. 2012, 48, 2858–2860. [Google Scholar] [CrossRef]
- Chang, C.J.; Chu, K.W.; Hsu, M.H.; Chen, C.Y. Ni-doped ZnS decorated graphene composites with enhanced photocatalytic hydrogen-production performance. Int. J. Hydrogen Energy 2015, 40, 14498–14506. [Google Scholar] [CrossRef]
- Ruíz-Santoyo, V.; Marañon-Ruiz, V.F.; Romero-Toledo, R.; González Vargas, O.A.; Pérez-Larios, A. Photocatalytic degradation of Rhodamine B and methylene orange using TiO2-ZrO2 as nanocomposite. Catalysts 2021, 11, 1035. [Google Scholar] [CrossRef]
- Zeng, Z.; Xu, Y.; Zhang, Z.; Gao, Z.; Luo, M.; Yin, Z.; Zhang, C.; Xu, J.; Huang, B.; Luo, F.; et al. Rare-earth-containing perovskite nanomaterials: Design, synthesis, properties and applications. Chem. Soc. Rev. 2020, 49, 1109–1143. [Google Scholar] [CrossRef]
- Li, Z.; Meng, X.; Zhang, Z. Recent development on MoS2-based photocatalysis: A review. J. Photochem. Photobiol. C 2018, 35, 39–55. [Google Scholar] [CrossRef]
- Sayama, K.; Hayashi, H.; Arai, T.; Yanagida, M.; Gunji, T.; Sugihara, H. Highly active WO3 semiconductor photocatalyst prepared from amorphous peroxo-tungstic acid for the degradation of various organic compounds. Appl. Catal. B 2010, 94, 150–157. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J.G. CdS/Graphene Nanocomposite Photocatalysts. Adv. Energy Mater. 2015, 5, 1500010. [Google Scholar] [CrossRef]
- Mahadik, M.; Shinde, S.; Mohite, V.; Kumbhar, S.; Rajpure, K.; Moholkar, A.; Kim, J.; Bhosale, C. Photoelectrocatalytic oxidation of Rhodamine B with sprayed α-Fe2O3 photocatalyst. Mater. Express 2013, 3, 247–255. [Google Scholar] [CrossRef]
- Periyat, P.; Pillai, S.C.; McCormack, D.E.; Colreavy, J.; Hinder, S.J. Improved high-temperature stability and sun-light-driven photocatalytic activity of sulfur-doped anatase TiO2. J. Phys. Chem. C 2008, 112, 7644–7652. [Google Scholar] [CrossRef] [Green Version]
- Afzaal, M.; Malik, M.A.; O’Brien, P. Preparation of zinc containing materials. New J. Chem 2007, 31, 2029–2040. [Google Scholar] [CrossRef]
- Sun, B.; Sirringhaus, H. Solution-processed zinc oxide field-effect transistors based on self-assembly of colloidal nanorods. Nano Lett. 2005, 5, 2408–2413. [Google Scholar] [CrossRef]
- Ellmer, K.; Klein, A.; Rech, B. Transparent Conductive Zinc Oxide: Basics and Applications in Thin Film Solar Cells; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Lee, C.R.; Lee, H.W.; Song, J.S.; Kim, W.W.; Park, S. Synthesis and Ag recovery of nanosized ZnO powder by solution combustion process for photocatalytic applications. J. Mater. Synth. Process. 2001, 9, 281–286. [Google Scholar] [CrossRef]
- Dodd, A.C.; McKinley, A.J.; Saunders, M.; Tsuzuki, T. Effect of particle size on the photocatalytic activity of nanoparticulate zinc oxide. J. Nanopart. Res. 2006, 8, 43–51. [Google Scholar] [CrossRef]
- Klingshirn, C. ZnO: Material, physics and applications. Chemphyschem 2007, 8, 782–803. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Fenoll, J.; Ruiz, E.; Hellin, P.; Flores, P.; Navarro, S. Heterogeneous photocatalytic oxidation of cyprodinil and fludioxonil in leaching water under solar irradiation. Chemosphere 2011, 85, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lu, W.; Yao, Y.; Xu, M. Highly efficient decomposition of organic dyes by aqueous-fiber phase transfer and in situ catalytic oxidation using fiber-supported cobalt phthalocyanine. Environ. Sci. Technol. 2007, 41, 6240–6245. [Google Scholar] [CrossRef]
- Lucilha, A.C.; Bonancêa, C.E.; Barreto, W.J.; Takashima, K. Adsorption of the diazo dye Direct Red 23 onto a zinc oxide surface: A spectroscopic study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2010, 75, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Wang, T.H.; Zhao, J.C. Enhanced photocatalytic activity of ZnO nanotetrapods. Appl. Phys. Lett. 2005, 87, 083105:1–083105:4. [Google Scholar] [CrossRef]
- Bauer, C.; Boschloo, G.; Mukhtar, E.; Hagfeldt, A. Electron injection and recombination in Ru(dcbpy)2(NCS)2 sensitized nanostructured ZnO. J. Phys. Chem. B 2001, 105, 5585–5588. [Google Scholar] [CrossRef]
- Fattakhova-Rohlfing, D.; Zaleska, A.; Bein, T. Three-dimensional titanium dioxide nanomaterials. Chem. Rev. 2014, 11, 9487–9558. [Google Scholar] [CrossRef]
- Blencowe, D.K.; Morby, A.P. Zn (II) metabolism in prokaryotes. FEMS Microbiol. Rev. 2003, 27, 291–311. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sust. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, S.; Liu, Z.; Wang, C.; Mao, Z. Studies on the enhanced photocatalytic hydrogen evolution over Pt/PEG-modified TiO2 photocatalysts. Int. J. Hydrogen Energy 2008, 33, 1112–1117. [Google Scholar] [CrossRef]
- Macak, J.M.; Sirotna, K.; Schmuki, P. Self-organized porous titanium oxide prepared in Na2SO4/NaF electrolytes. Electrochim. Acta 2005, 50, 3679–3684. [Google Scholar] [CrossRef]
- Zhu, P.F.; Chen, Y.J.; Duan, M.; Liu, M.; Zou, P.; Zhou, M. Enhanced visible photocatalytic activity of Fe-Cu-ZnO/graphene oxide photocatalysts for the degradation of organic dyes. Can. J. Chem. Eng. 2018, 96, 1479–1488. [Google Scholar] [CrossRef]
- Raj, R.B.; Umadevi, M.; Parimaladevi, R. Enhanced photocatalytic degradation of textile dyeing wastewater under UV and visible light using ZnO/MgO nanocomposites as a novel photocatalyst. Part. Sci. Technol. 2020, 38, 812–820. [Google Scholar] [CrossRef]
- Das, D.; Nandi, P. Ternary ZnCdSO composite photocatalyst for efficient dye degradation under visible light retaining Z-scheme of migration pathways for the photogenerated charge carriers. Sol. Energy Mater. Sol. Cells 2020, 217, 110674. [Google Scholar] [CrossRef]
- Sabri, M.; Habibi-Yangjeh, A.; Ghosh, S. Novel ZnO/CuBi2O4 heterostructures for persulfate-assisted photocatalytic degradation of dye contaminants under visible light. J. Photochem. Photobiol. A 2020, 391, 112397:1–112397:11. [Google Scholar] [CrossRef]
- Vignesh, S.; Suganthi, S.; Sundar, J.K.; Raj, V.; Devi, P.R.I. Highly efficient visible light photocatalytic and antibacterial performance of PVP capped Cd:Ag: ZnO photocatalyst nanocomposites. Appl. Surf. Sci. 2019, 479, 914–929. [Google Scholar] [CrossRef]
- Chang, J.-Q.; Zhong, Y.; Hu, C.-H.; Luo, J.-L.; Wang, P.-G. Study on highly efficient BiOCl/ZnO pn heterojunction: Synthesis, characterization and visible-light-excited photocatalytic activity. J. Mol. Struct. 2019, 1183, 209–216. [Google Scholar] [CrossRef]
- Cao, F.; Pan, Z.H.; Ji, X.H. Enhanced photocatalytic activity of a pine-branch-like ternary CuO/CuS/ZnO heterostructure under visible light irradiation. New J. Chem. 2019, 43, 11342–11347. [Google Scholar] [CrossRef]
- Lee, S.J.; Jung, H.J.; Koutavarapu, R.; Lee, S.H.; Arumugam, M.; Kim, J.H.; Choi, M.Y. ZnO supported Au/Pd bimetallic nanocomposites for plasmon improved photocatalytic activity for methylene blue degradation under visible light irradiation. Appl. Surf. Sci. 2019, 496, 143665. [Google Scholar] [CrossRef]
- Ouyang, W.; Zhu, J. Catalyst-free synthesis of macro-scale ZnO nanonail arrays on Si substrate by simple physical vapor deposition. Mater. Lett. 2008, 62, 2557–2560. [Google Scholar] [CrossRef]
- Wang, C.Z.; Chen, Z.; Hu, H.Q.; Zhang, D. Effect of the oxygen pressure on the microstructure and optical properties of ZnO films prepared by laser molecular beam epitaxy. Phys. B Condens. Matter 2009, 404, 4075–4082. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Modirshahla, N.; Ghazalian, E. Synthesis of ZnO nanoparticles at different conditions: A comparison of photocatalytic activity. Dig. J. Nanomater. Biostructures 2011, 6, 467–474. [Google Scholar]
- Zhou, K.; Zhang, Q.; Shi, Y.; Jiang, S.; Hu, Y.; Gui, Z. A facile method for preparation ZnO with different morphology and their optical property. J. Alloys Compd. 2013, 577, 389–394. [Google Scholar] [CrossRef]
- Tsay, C.-Y.; Fan, K.-S.; Lei, C.-M. Synthesis and characterization of sol–gel derived gallium-doped zinc oxide thin films. J. Alloys Compd. 2012, 512, 216–222. [Google Scholar] [CrossRef]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, K.; Zubair, N.; Ikram, S.; Khan, Z.U.; Khalid, H. Synthesis and characterization of ZnO nanostructures with varying morphology. Bull. Mater. Sci. 2017, 40, 459–466. [Google Scholar] [CrossRef] [Green Version]
- Gupta, J.; Barick, K.; Bahadur, D. Defect mediated photocatalytic activity in shape-controlled ZnO nanostructures. J. Alloys Compd. 2011, 509, 6725–6730. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, L.; Yin, J.; Su, H.; Liao, C.; Yan, C. Control of ZnO Morphology via a Simple Solution Route. Chem. Mater. 2002, 14, 4172–4177. [Google Scholar] [CrossRef]
- Ramakrishna, G.; Ghosh, H.N. Effect of particle size on the reactivity of quantum size ZnO nanoparticles and charge-transfer dynamics with adsorbed catechols. Langmuir 2003, 19, 3006–3012. [Google Scholar] [CrossRef]
- Selvin, S.S.P.; Radhika, N.; Borang, O.; Lydia, I.S.; Merlin, J.P. Visible light driven photodegradation of Rhodamine B using cysteine capped ZnO/GO nanocomposite as photocatalyst. J. Mater. Sci. Mater. Electron. 2017, 28, 6722–6730. [Google Scholar] [CrossRef]
- Oppong, S.O.B.; Anku, W.W.; Opoku, F.; Shukla, S.K.; Govender, P.P. Photodegradation of Eosin Yellow Dye in Water under Simulated Solar Light Irradiation Using La-Doped ZnO Nanostructure Decorated on Graphene Oxide as an Advanced Photocatalyst. ChemistrySelect 2018, 3, 1180–1188. [Google Scholar] [CrossRef]
- Kalisamy, P.; Lallimathi, M.; Suryamathi, M.; Palanivel, B.; Venkatachalam, M. ZnO-embedded S-doped gC3N4 heterojunction: Mediator-free Z-scheme mechanism for enhanced charge separation and photocatalytic degradation. RSC Adv. 2020, 10, 28365–28375. [Google Scholar] [CrossRef]
- Shemeena, M.; Binitha, N.N. Visible light active ZnO-g-C3N4 photocatalyst for dye pollutant degradation. Mater.-Proc. 2020, 25, 107–110. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, X.; Liu, X.; Yang, X.; Yang, Y. A ternary magnetic recyclable ZnO/Fe3O4/gC3N4 composite photocatalyst for efficient photodegradation of monoazo dye. Nanoscale Res. Lett 2019, 14, 147. [Google Scholar] [CrossRef]
- Abdel-Aziz, R.; Ahmed, M.A.; Abdel-Messih, M.F. A novel UV and visible-light-driven photocatalyst AgIO4/ZnO nanoparticles with highly enhanced photocatalytic performance for removal of rhodamine B and indigo carmine dyes. J. Photochem. Photobiol. C 2020, 389, 112245. [Google Scholar] [CrossRef]
- Liu, S.-H.; Wei, Y.-S.; Lu, J.-S. Visible-light-driven photodegradation of sulfamethoxazole and methylene blue by Cu2O/rGO photocatalysts. Chemosphere 2016, 154, 118–123. [Google Scholar] [CrossRef]
- Moradi, M.; Haghighi, M.; Allahyari, S. Precipitation dispersion of Ag–ZnO nanocatalyst over functionalized multiwall carbon nanotube used in degradation of Acid Orange from wastewater. Process. Saf. Environ. Prot. 2017, 107, 414–427. [Google Scholar] [CrossRef]
- Praveen, R.; Chandreshia, C.B.; Ramaraj, R. Silicate sol-gel matrix stabilized ZnO-Ag nanocomposites materials and their environmental remediation applications. J. Environ. Chem. Eng. 2018, 6, 3702–3708. [Google Scholar] [CrossRef]
- Sarmah, K.; Roy, U.K.; Maji, T.K.; Pratihar, S. Role of Metal Exchange toward the Morphology and Photocatalytic Activity of Cu/Ag/Au-Doped ZnO: A Study with a Zinc-Sodium Acetate Complex as the Precursor. ACS Appl. Nano Mater. 2018, 1, 2049–2056. [Google Scholar] [CrossRef]
- Zarrabi, M.; Haghighi, M.; Alizadeh, R.; Mahboob, S. Solar-light-driven photodegradation of organic dyes on sono-dispersed ZnO nanoparticles over graphene oxide: Sono vs. conventional catalyst design. Sep. Purif. Technol. 2019, 211, 738–752. [Google Scholar] [CrossRef]
- Saleem, H.; Haneef, M.; Abbasi, H.Y. Synthesis route of reduced graphene oxide via thermal reduction of chemically exfoliated graphene oxide. Mater. Chem. Phys. 2018, 204, 1–7. [Google Scholar] [CrossRef]
- Bharathi, P.; Harish, S.; Archana, J.; Navaneethan, M.; Ponnusamy, S.; Muthamizhchelvan, C.; Shimomura, M.; Hayakawa, Y. Enhanced charge transfer and separation of hierarchical CuO/ZnO composites: The synergistic effect of photocatalysis for the mineralization of organic pollutant in water. Appl. Surf. Sci. 2019, 484, 884–891. [Google Scholar] [CrossRef]
- Santos, P.B.; Santos, J.J.; Correa, C.C.; Corio, P.; Andrade, G.F.S. Plasmonic photodegradation of textile dye Reactive Black 5 under visible light: A vibrational and electronic study. J. Photochem. Photobiol. A 2019, 371, 159–165. [Google Scholar] [CrossRef]
- Das, A.; Wary, R.R.; Nair, R.G. Cu modified ZnO nanoflakes: An efficient visible light-driven photocatalyst and a promising photoanode for dye sensitized solar cell (DSSC). Solid State Sci. 2020, 104, 106290. [Google Scholar] [CrossRef]
- Peter, C.N.; Anku, W.W.; Sharma, R.; Joshi, G.M.; Shukla, S.K.; Govender, P.P. N-doped ZnO/graphene oxide: A photostable photocatalyst for improved mineralization and photodegradation of organic dye under visible light. Ionics 2019, 25, 327–339. [Google Scholar] [CrossRef]
- Selvaraj, S.; Mohan, M.K.; Navaneethan, M.; Ponnusamy, S.; Muthamizhchelvan, C. Synthesis and photocatalytic activity of Gd doped ZnO nanoparticles for enhanced degradation of methylene blue under visible light. Mater. Sci. Semicond. Process. 2019, 103, 104622. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Mahlambi, M.M.; Ngila, C.J.; Mamba, B.B. Recent developments in environmental photocatalytic degradation of organic pollutants: The case of titanium dioxide nanoparticles—A review. J. Nanomater. 2015, 2015, 790173. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.Y.; Mei, J.Y.; Yi, S.S.; Guan, X.X. Constructing of Z-scheme 3D g-C3N4-ZnO@graphene aerogel heterojunctions for high-efficient adsorption and photodegradation of organic pollutants. Appl. Surf. Sci. 2019, 492, 808–817. [Google Scholar] [CrossRef]
- Zhang, B.; Li, M.; Wang, X.; Zhao, Y.; Wang, H.; Song, H. Pompon-like structured gC3N 4/ZnO composites and their application in visible light photocatalysis. Res. Chem. Intermed. 2018, 44, 6895–6906. [Google Scholar] [CrossRef]
- Gaurav, A.; Beura, R.; Kumar, J.S.; Thangadurai, P. Study on the effect of copper ion doping in zinc oxide nanomaterials for photocatalytic applications. Mater. Chem. Phys. 2019, 230, 162–171. [Google Scholar] [CrossRef]
- Venugopal, G.; Thangavel, S.; Vasudevan, V.; Zoltan, K. Efficient visible-light piezophototronic activity of ZnO-Ag8S hybrid for degradation of organic dye molecule. J. Phys. Chem. Solids 2020, 143, 109473. [Google Scholar] [CrossRef]
- Karunakaran, C.; JebaSing, I.; Vinayagamoorthy, P. Synthesis of Superparamagnetic ZnFe2O4-Core/Ag-Deposited ZnO-Shell Nanodiscs for Application as Visible Light Photocatalyst. J. Nanosci. Nanotechnol. 2019, 19, 4064–4071. [Google Scholar] [CrossRef]
- Srinivasan, N.; Anbuchezhiyan, M.; Harish, S.; Ponnusamy, S. Hydrothermal synthesis of C doped ZnO nanoparticles coupled with BiVO4 and their photocatalytic performance under the visible light irradiation. Appl. Surf. Sci 2019, 494, 771–782. [Google Scholar] [CrossRef]
- Tofa, T.S.; Ye, F.; Kunjali, K.L.; Dutta, J. Enhanced Visible Light Photodegradation of Microplastic Fragments with Plasmonic Platinum/Zinc Oxide Nanorod Photocatalysts. Catalysts 2019, 9, 819. [Google Scholar] [CrossRef] [Green Version]
- Bharti, D.B.; Bharati, A.V. Photocatalytic degradation of Alizarin Red dye under visible light using ZnO & CdO nanomaterial. Optik 2018, 160, 371–379. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, W.Z.; Yao, L.Z.; Wang, J.; Liang, Y.J.; Fu, J.L. Insights into charge transfer and solar light photocatalytic activity induced by the synergistic effect of defect state and plasmon in Au nanoparticle-decorated hierarchical 3D porous ZnO microspheres. Appl. Surf. Sci. 2019, 494, 959–968. [Google Scholar] [CrossRef]
- Manjunatha, C.; Abhishek, B.; Shivaraj, B.; Ashoka, S.; Shashank, M.; Nagaraju, G. Engineering the Mx Zn1− x O (M= Al3+, Fe3+, Cr3+) nanoparticles for visible light-assisted catalytic mineralization of methylene blue dye using Taguchi design. Chem. Zvesti 2020, 74, 2719–2731. [Google Scholar] [CrossRef]
- Saad, A.M.; Abukhadra, M.R.; Ahmed, S.A.K.; Elzanaty, A.M.; Mady, A.H.; Betiha, M.A.; Shim, J.J.; Rabie, A.M. Photocatalytic degradation of malachite green dye using chitosan supported ZnO and Ce-ZnO nano-flowers under visible light. J. Environ. Manag. 2020, 258, 110043. [Google Scholar] [CrossRef] [PubMed]
- González-Casamachin, D.A.; De la Rosa, J.R.; Lucio-Ortiz, C.J.; De Rio, D.A.D.H.; Martínez-Vargas, D.X.; Flores-Escamilla, G.A.; Guzman, N.E.D.; Ovando-Medina, V.M.; Moctezuma-Velazquez, E. Visible-light photocatalytic degradation of acid violet 7 dye in a continuous annular reactor using ZnO/PPy photocatalyst: Synthesis, characterization, mass transfer effect evaluation and kinetic analysis. Chem. Eng. J. 2019, 373, 325–337. [Google Scholar] [CrossRef]
- Fouda, A.; Salem, S.S.; Wassel, A.R.; Hamza, M.F.; Shaheen, T.I. Optimization of green biosynthesized visible light active CuO/ZnO nano-photocatalysts for the degradation of organic methylene blue dye. Heliyon 2020, 6, e04896-1. [Google Scholar] [CrossRef]
- Ullah, H.; Mushtaq, L.; Ullah, Z.; Fazal, A.; Khan, A.M. Effect of vegetable waste extract on microstructure, morphology, and photocatalytic efficiency of ZnO-CuO nanocomposites. Inorg. Nano-Met. Chem. 2021, 51, 963–975. [Google Scholar] [CrossRef]
- Reddy, I.N.; Reddy, C.V.; Shim, J.; Akkinepally, B.; Cho, M.Y.; Yoo, K.; Kim, D. Excellent visible-light-driven photocatalyst of (Al, Ni) co-doped ZnO structures for organic dye degradation. Catal. Today 2020, 340, 277–285. [Google Scholar] [CrossRef]
- Prabhu, S.; Pudukudy, M.; Harish, S.; Navaneethan, M.; Sohila, S.; Murugesan, K.; Ramesh, R. Facile construction of djembe-like ZnO and its composite with g-C3N4 as a visible-light-driven heterojunction photocatalyst for the degradation of organic dyes. Mater. Sci. Semicond. Process. 2020, 106, 104754. [Google Scholar] [CrossRef]
- Sapkota, K.P.; Lee, I.; Hanif, M.A.; Islam, M.A.; Hahn, J.R. Solar-light-driven efficient ZnO–single-walled carbon nanotube photocatalyst for the degradation of a persistent water pollutant organic dye. Catalysts 2019, 9, 498. [Google Scholar] [CrossRef] [Green Version]
- Ravichandran, K.; Sindhuja, E. Fabrication of cost-effective g-C3N4 +Ag activated ZnO photocatalyst in thin-film form for enhanced visible light-responsive dye degradation. Mater. Chem. Phys. 2019, 221, 203–215. [Google Scholar] [CrossRef]
- Lonkar, S.P.; Pillai, V.V.; Alhassan, S.M. Facile and scalable production of heterostructured ZnS-ZnO/Graphene nano-photocatalysts for environmental remediation. Sci. Rep. 2018, 8, 13401. [Google Scholar] [CrossRef]
- Meng, Q.M.; Lu, Q.L.; Wang, L.X.; Wang, J. Fe-doped ZnO synthesized by parallel flow precipitation process for improving photocatalytic activity. In IOP Conference Series: Materials Science and Engineering, Proceedings of the 2nd International Conference on New Material and Chemical Industry (NMCI2017), Sanya, China, 18–20 November 2017; IOP Publishing Ltd.: Bristol, UK, 2018; Volume 292, p. 012065. [Google Scholar] [CrossRef]
- Worathitanon, C.; Jangyubol, K.; Ruengrung, P.; Donphai, W.; Klysubun, W.; Chanlek, N.; Prasitchoke, P.; Chareonpanich, M. High-performance visible-light responsive Chl-Cu/ZnO catalysts for photodegradation of rhodamine B. Appl. Catal. B 2019, 241, 359–366. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.; Doğan, S.; Avrutin, V.C.S.J.; Cho, S.J.; Morkoç, A.H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 11. [Google Scholar] [CrossRef] [Green Version]
- Manzoor, U.; Kim, D.K. Size control of ZnO nanostructures formed in different temperature zones by varying Ar flow rate with tunable optical properties. Phys. E Low Dimens. Syst. Nanostruct. 2009, 41, 500–505. [Google Scholar] [CrossRef]
- Ginley, D.S.; Perkins, J.D. Transparent conductors. In Handbook of Transparent Conductors; Springer: Boston, MA, USA, 2011; pp. 1–25. [Google Scholar]
- Claflin, B.; Look, D.C.; Park, S.-J.; Cantwell, G. Persistent n-type photoconductivity in p-type ZnO. J. Cryst. Growth 2006, 287, 16–22. [Google Scholar] [CrossRef]
- Yang, C.; Kwack, Y.; Kim, S.H.; An, T.K.; Hong, K.; Nam, S.; Park, M.; Choi, W.S.; Park, C.E. Ambipolar thin-film transistors and an inverter based on pentacene/self-assembled monolayer modified ZnO hybrid structures for balanced hole and electron mobilities. Org. Electron. 2011, 12, 411–418. [Google Scholar] [CrossRef]
- Tsukazaki, A.; Ohtomo, A.; Onuma, T.; Ohtani, M.; Makino, T.; Sumiya, M.; Ohtani, K.; Chichibu, S.F.; Fuke, S.; Segawa, Y.; et al. Repeated temperature modulation epitaxy for p-type doping and light-emitting diode based on ZnO. Nat. Mater. 2005, 4, 42–46. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [Green Version]
- Shanmugam, V.; Jeyaperumal, K.S. Investigations of visible-light-driven Sn and Cu doped ZnO hybrid nanoparticles for photocatalytic performance and antibacterial activity. Appl. Surf. Sci. 2018, 449, 617–630. [Google Scholar] [CrossRef]
- Al-Fori, M.; Dobretsov, S.; Myint, M.T.Z.; Dutta, J. Antifouling properties of zinc oxide nanorod coatings. Biofouling 2014, 30, 871–882. [Google Scholar] [CrossRef]
- Gharoy Ahangar, E.; Abbaspour-Fard, M.H.; Shahtahmassebi, N.; Khojastehpour, M.; Maddahi, P. Preparation and characterization of PVA/ZnO nanocomposite. J. Food Process. Preserv. 2015, 39, 1442–1451. [Google Scholar] [CrossRef]
- Kuo, W.; Ho, P. Solar photocatalytic decolourization of methylene blue in water. Chemosphere 2001, 45, 77–83. [Google Scholar] [CrossRef]
- Khan, M.T.; Chatterjee, D.; Bala, M. Photocatalytic reduction of N2 to NH3 sensitized by the [RuIII-ethylenediaminetetraacetate-2, 2′-bipyridyl]−complex in a Pt-TiO2 semiconductor particulate system. J. Photochem. Photobiol. A 1992, 67, 349–352. [Google Scholar] [CrossRef]
- Borjigin, B.; Ding, L.; Li, H.; Wang, X. A solar light-induced photo-thermal catalytic decontamination of gaseous benzene by using Ag/Ag3PO4/CeO2 heterojunction. Chem. Eng. J. 2020, 402, 126070. [Google Scholar] [CrossRef]
- Deng, Q.; Tang, H.; Liu, G.; Song, X.; Xu, G.; Li, Q.; Ng, D.H.; Wang, G. The fabrication and photocatalytic performances of flower-like Ag nanoparticles/ZnO nanosheets-assembled microspheres. Appl. Surf. Sci. 2015, 331, 50–57. [Google Scholar] [CrossRef]
- Subramanian, V.; Wolf, E.; Kamat, P.V. Semiconductor−metal composite nanostructures. To what extent do metal nanoparticles improve the photocatalytic activity of TiO2 films? J. Phys. Chem. B 2001, 105, 11439–11446. [Google Scholar] [CrossRef]
- Li, Y.-F.; Zhang, M.; Guo, D.-L.; He, F.-X.; Li, Y.-Z.; Wang, A.-J. Facile solvothermal synthesis of BiOCl/ZnO heterostructures with enhanced photocatalytic activity. J. Nanomater. 2014, 2014, 347061. [Google Scholar] [CrossRef] [Green Version]
- Nenavathu, B.P.; Kandula, S.; Verma, S. Visible-light-driven photocatalytic degradation of safranin-T dye using functionalized graphene oxide nanosheet (FGS)/ZnO nanocomposites. RSC Adv. 2018, 8, 19659–19667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahlambi, M.M.; Mishra, A.K.; Mishra, S.B.; Krause, R.W.; Mamba, B.B.; Raichur, A.M. Synthesis and characterization of carbon-covered alumina (CCA) supported TiO2 nanocatalysts with enhanced visible-light photodegradation of Rhodamine B. In Nanotechnology for Sustainable Development; Springer: Berlin/Heidelberg, Germany, 2012; pp. 89–99. [Google Scholar] [CrossRef]
- Velanganni, S.; Manikandan, A.; Prince, J.J.; Mohan, C.N.; Thiruneelakandan, R. Nanostructured ZnO coated Bi2S3 thin films: Enhanced photocatalytic degradation of methylene blue dye. Phys. B Condens. Matter 2018, 545, 383–389. [Google Scholar] [CrossRef]
- Baradaran, M.; Ghodsi, F.E. Highly efficient visible photocatalytic degradation of MB organic dye by heteromorphic ZnO/AZO/ZnO nanocatalysts: Effect of AZO thickness. J. Sol-Gel Sci. Technol. 2019, 92, 25–39. [Google Scholar] [CrossRef]
- Tsai, C.-E.; Yeh, S.-M.; Chen, C.-H.; Lin, H.-N. Flexible photocatalytic paper with Cu2O and Ag nanoparticle-decorated ZnO nanorods for visible light photodegradation of organic dye. Nanoscale Res. Lett. 2019, 14, 204. [Google Scholar] [CrossRef]
- Wu, N. Plasmonic metal–semiconductor photocatalysts and photoelectrochemical cells: A review. Nanoscale 2018, 10, 2679–2696. [Google Scholar] [CrossRef]
- Anwer, H.; Mahmood, A.; Lee, J.; Kim, K.-H.; Park, J.-W.; Yip, A.C.K. Photocatalysts for degradation of dyes in industrial effluents: Opportunities and challenges. Nano Res. 2019, 12, 955–972. [Google Scholar] [CrossRef]
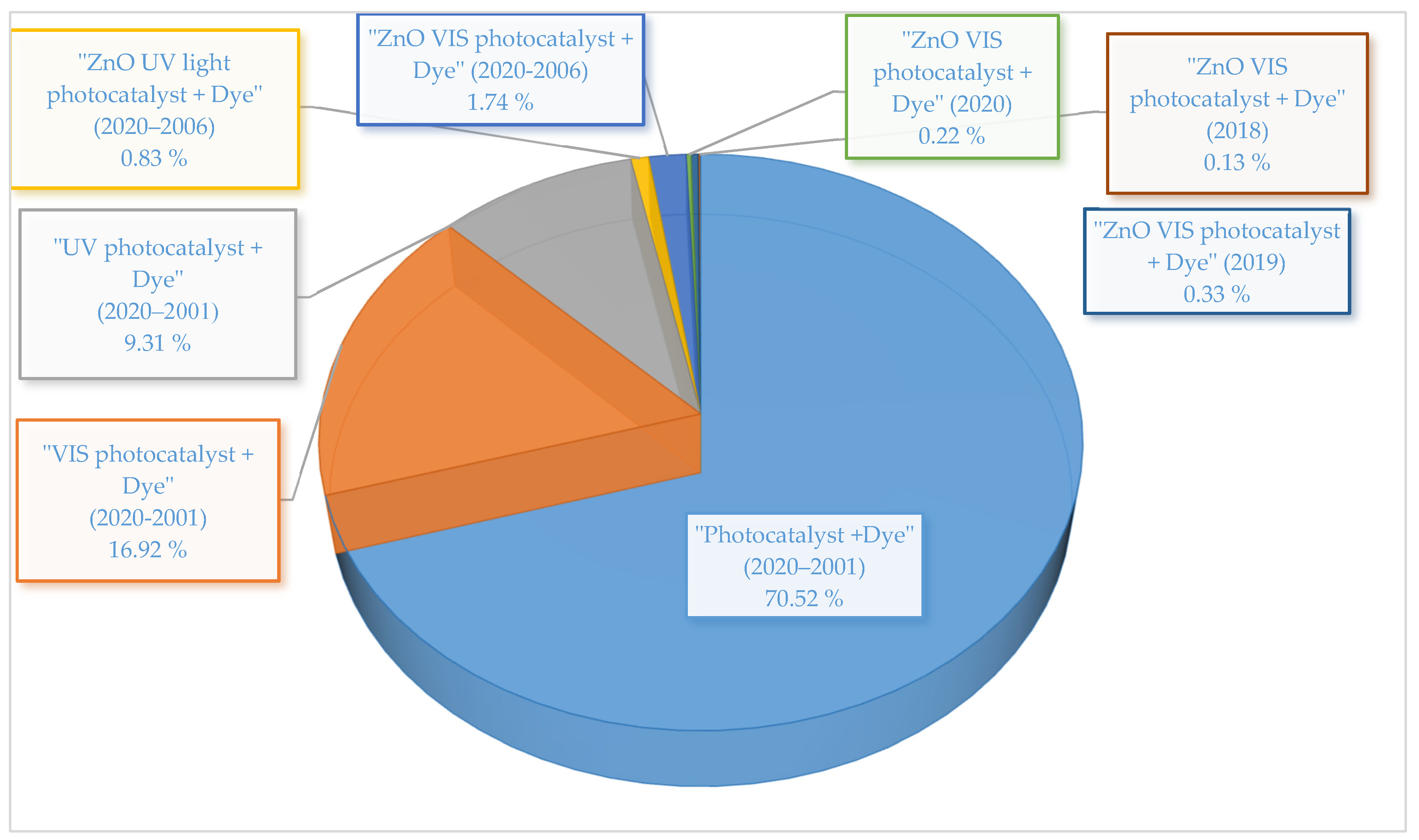

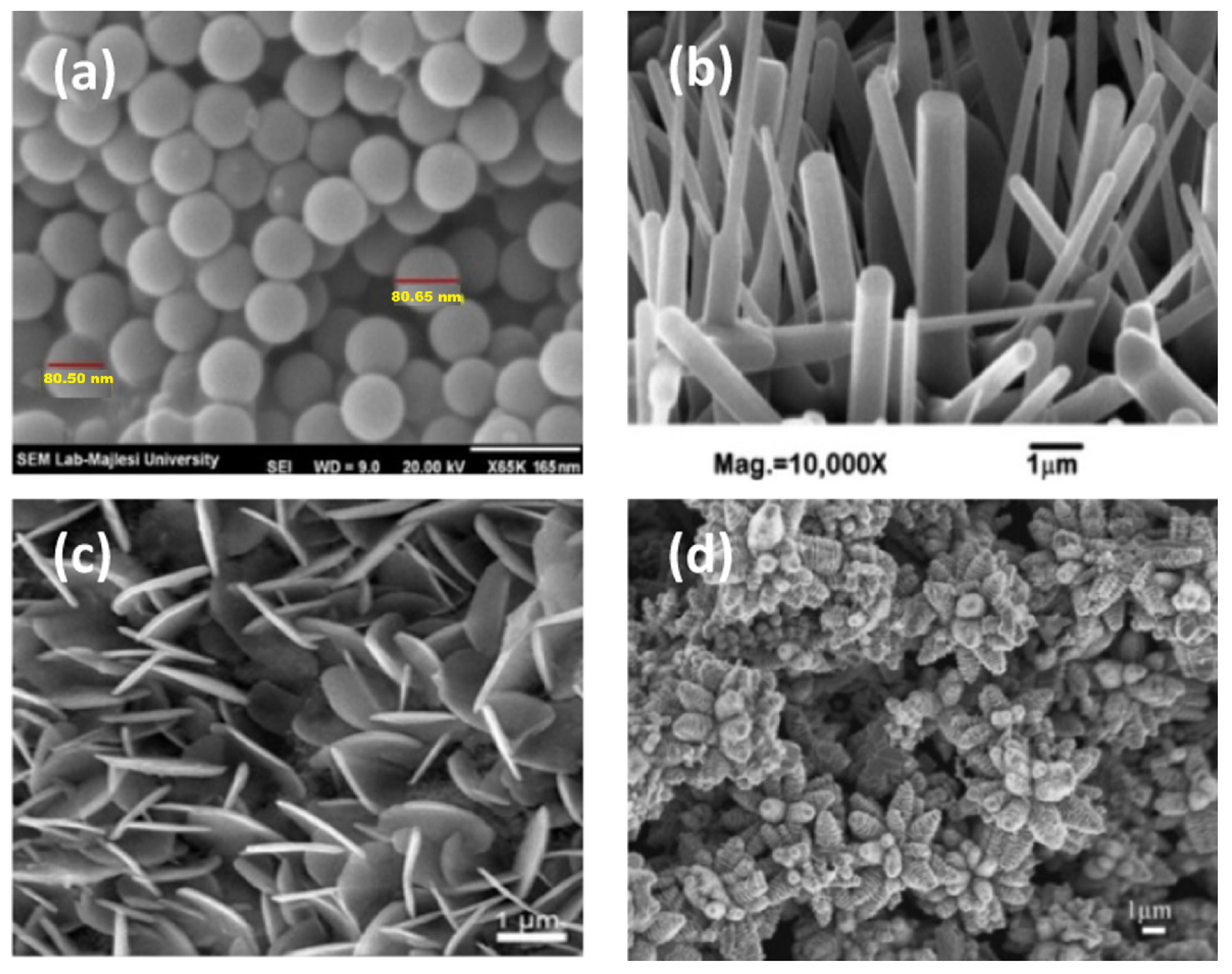
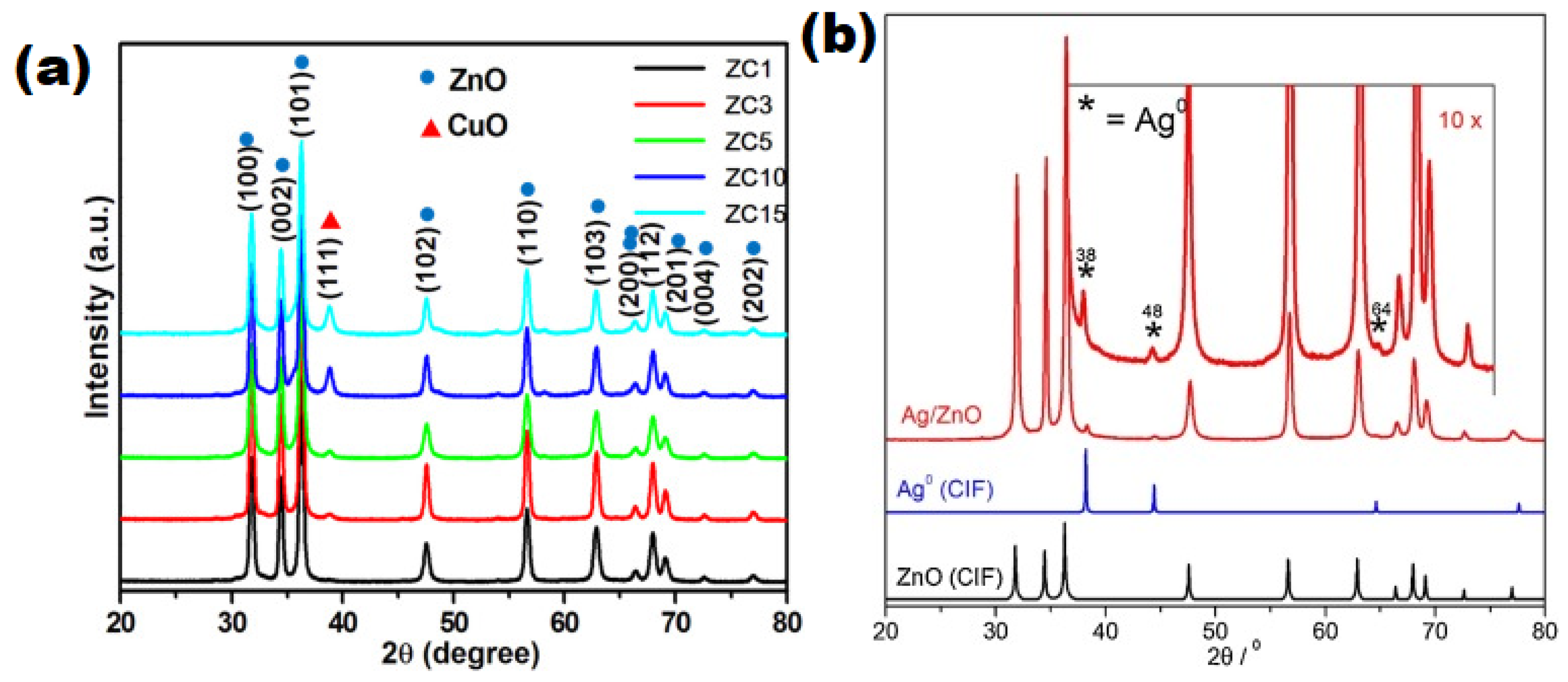


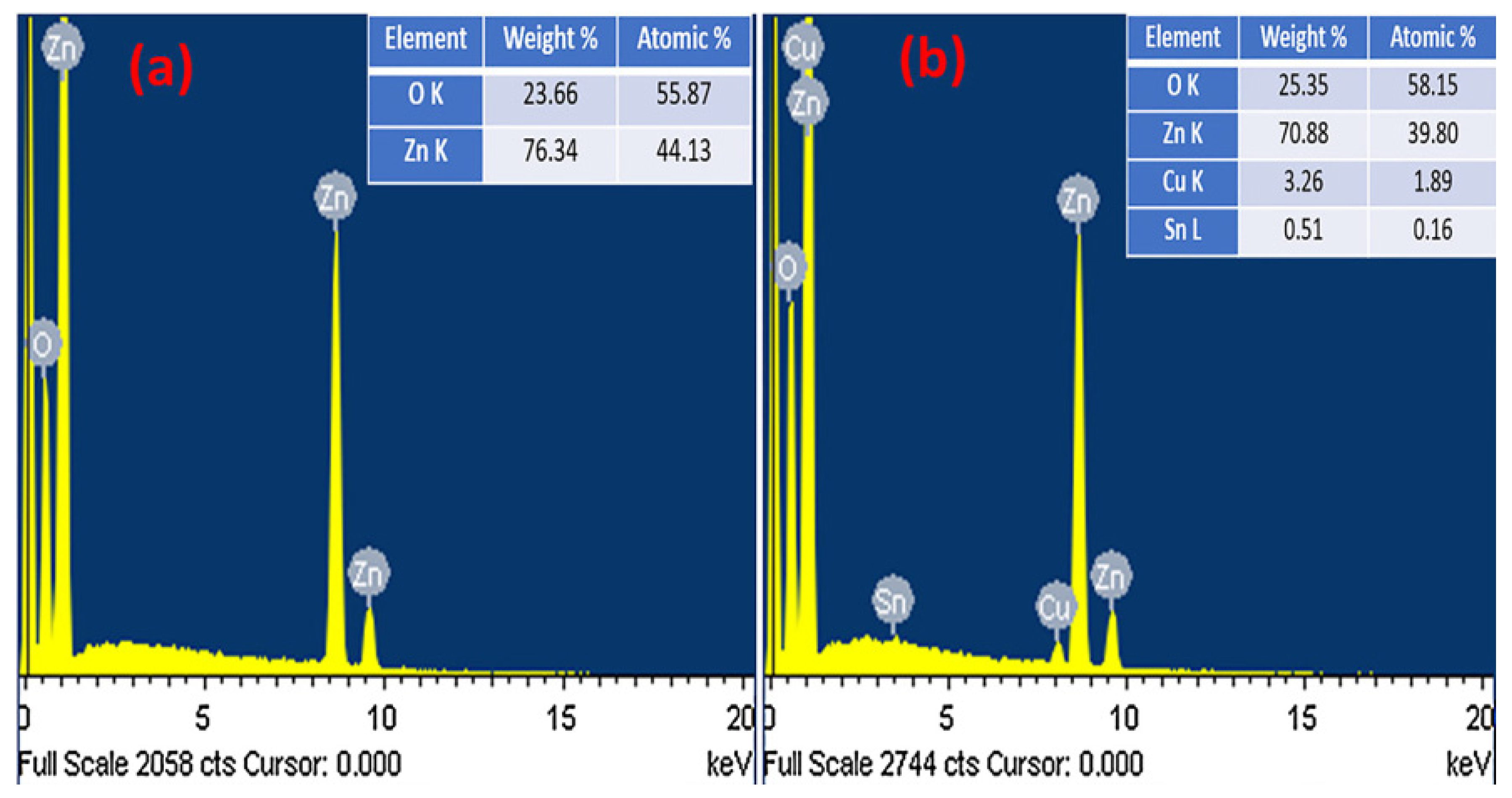







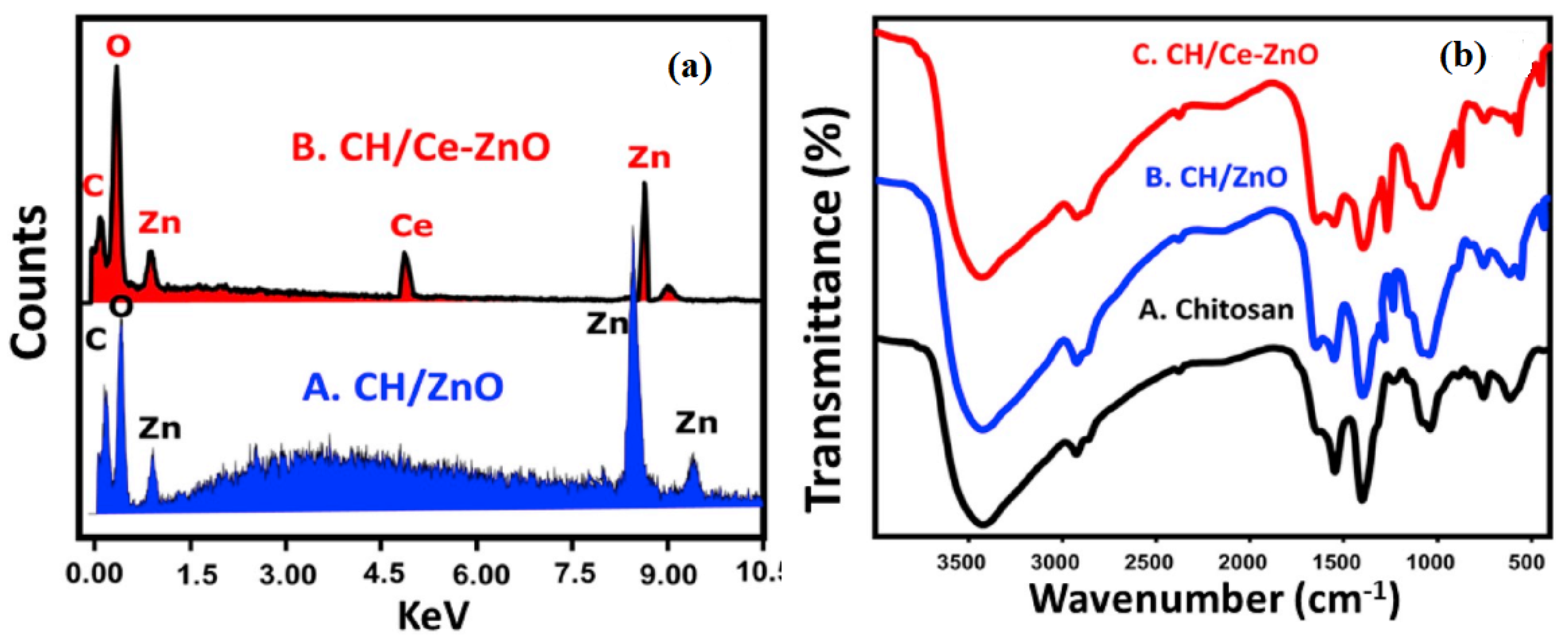

| S/N | ZnO Properties | Ref. |
|---|---|---|
| 1 | Higher efficient photocatalytic activity compared to TiO2. | [47,48,49] |
| 2 | High electron mobility and lifespan. | [50] |
| 3 | ZnO possesses relatively lower production costs and easy fabrication into a variety of nanostructures that include nanowires, nanoribbons, nanobelts, nanocombs, nanospheres, nanofibers and nanotetrapods. | [51] |
| 4 | There is a general reduction of the recombination of electrons and holes by the surface states as well as their high surface-to-volume ratio, ZnO nanotetrapods showed enhanced photocatalytic activities. | [49] |
| 5 | Zn based compounds are biocompatible with the functions of the human body. | [52] |
| Precursor | Reaction Conditions | Morphology of Product(s) | Composite Photocatalyst/Dosage | Catalytic Application | Catalytic Activity | Enhancements | Ref. |
|---|---|---|---|---|---|---|---|
| Zinc acetate dehydrate, cadmium chloride monohydrate and sodium sulphide nonahydrate | Precipitation and calcination | Platelets-like hexagonal wurtzite ZnO, hexagonal CdS and cubic CdO phases. | ZnO–CdS–CdO (50 mg) | Methylene blue and rhodamine B (2 × 10−5 M) | 95% in 120 min. | Reduced band gap, improved. photocatalytic efficiency. | [58] |
| Zinc nitrate hexahydrate, copper(II) nitate trihydrate | Co-precipitation | Flake like shaped wurtzite Cu/ZnO. | Cu/ZnO/Dopant (500 mg/L) | Methylene blue (0.015 M) | 4.7 times better than ZnO under solar irradiation. | Reduced charge carrier Recombination. | [88] |
| Zinc acetate dehydrate, urea and graphite flakes | Precipitation/wet chemical | Hexagonal wurtzite structure of N-ZnO densely packed on the surface of GO nanosheets | N-doped ZnO/GO (50 mg) | Brilliant green (20 ppm) | 100% in 90 min. | Reduced band gap & reduced charge recombination. | [89] |
| Zinc acetate di-hydrate and gadolinium(III) nitrate hexahydrate | Co-precipitation | Spherical shaped crystalline wurtzite Gd-ZnO | Gd-ZnO (25 mg) | Methylene blue (10 ppm) | 93% in 90 min. | Reduced charge recombination. | [90] |
| Fabrication Method | Composite Photocatalyst/Dosage | Catalytic Application | Catalytic Activity | Enhanced Factors | Ref. |
|---|---|---|---|---|---|
| Hydrothermal | ZnO/CuBi2O4 + potassium persulfate/100 mg | MO, RhB & CR ((1.0 × 10−5 M)) | 75% RhB in 210 min | Larger surface area, heterojunction formation. | [59] |
| Hydrothermal | Cu-ZnO (50 mg) | MO (0.02 mM) | 99.50% in 150 min | Good charge transfer & reduced charge recombination. | [95] |
| Hydrothermal, UV-C photoreduction | ZnO-Pt (1 × 1 cm thin film) | MB (10 µM) | 90 min | Reduced charge recombination. | [99] |
| Microwave-assisted hydrothermal | ZnO, CdO (10 mg) | Alizarin Red (10 ppm) | 92% & 80% in 75 min | Surface & morphology. | [100] |
| Hydrothermal | Au-ZnO (60 mg) | MB (10 ppm) | 100% in 75 min | Good charge transfer & reduced charge recombination. | [101] |
| Technique/Method | Parameters Determined |
|---|---|
| Powder XRD | Crystal structure, composition, crystallite size. |
| XAS (EXAFS, XANES) | X-ray absorption coefficient (element-specific)—oxidation state of species, inter-atomic distances. |
| XPS | Electronic structure, elemental constituents, chemical states, ligand binding (surface-chemistry). |
| SEM-HRSEM, T-SEM-EDX | Morphology, dispersion of nanoparticles in cells and other matrices/supports, oxidation state. |
| Electron backscatter diffraction | Crystal configuration, grain texture, defects, shapes, and distortion. |
| Atomic force microscopy | Morphology in 3D mode, nanoparticle morphology, and examination–elemental composition. |
| High-resolution TEM (HRTEM) | TEM information and the crystal structure of single particles. Study defects differentiate between monocrystalline, polycrystalline, and amorphous nanoparticles. |
| Electron diffraction | Crystal structure, lattice parameters, study order-disorder transformation. |
| EELS (EELS-STEM) | The qualitative oxidation state of atoms, chemical environment, bulk plasmon resonance. |
| Aberration-corrected (STEM, TEM) | Nanoparticle clusters, homogeneity and phase segregation. |
| Electron tomography | The actual 3D image down to the atomic scale. |
| Scanning TEM | Morphology, structure, elemental constituent and hetero-interfaces. |
| FTIR | Surface chemical state. |
| Low energy ion scattering | Thickness and chemical constituents of nanoparticles. |
| Secondary ion mass spectrometry | Chemical information (surface-chemistry), molecular coordination and configuration, surface structure. |
| Brunauer–Emmett–Teller | Surface area, pore size. |
| Liquid TEM | Study growth mechanism (in real-time), single particle motion, and superlattice formation. |
| ICP-MS | Elemental constituents and nanoparticle concentration. |
| Ferromagnetic resonance | Nanoparticle size, homogeneity, shape, crystallographic defects, surface constituents, M values, magnetic anisotropic constant. |
| Transmission electron microscopy (TEM) | Nanoparticle shape and aggregation state. |
| Thermogravimetric analysis | Thermal stability. |
| UV-Vis | Absorption characteristics and hints on nanoparticle shape. |
| Photoluminescence spectroscopy | Optical profile– relation to crystal structural properties and constituents. |
| Dynamic light scattering | Detection of aggregation. |
| Nanoparticle tracking analysis and Differential scanning calorimetry | nanoparticle size and homogeneity. |
| S/N | Material (Dosage) | Dye (Conc.) | Efficiency | Affected Material Properties | Ref. |
|---|---|---|---|---|---|
| 1 | Fe-Cu-ZnO/GO (1 g) | Dark green Dye (50 ppm) | 99% in 90 min | Pollutant removal efficiency by enhanced adsorption due to Cu & Fe doping & large specific surface area due GO, efficient charge separation. | [56] |
| 2 | ZnO/MgO (100 mg) | Textile dyeing (50 mL) | 65% in 120 min | Small particle size, reduced band gap. | [57] |
| 3 | Cd:Ag:ZnO: PVP (30 mg) | MB (20 ppm) | 99% in 120 min | Reduced band gap, good charge transfer and reduced charge recombination | [60] |
| 4 | BiOCl/ZnO (50 mg) | RhB (10 ppm) | 100% in 15 min | charge separation by heterojunction. | [61] |
| 5 | La-ZnO-GO (100 mg) | EY (20 ppm) | 100% in 210 min | Pollutant removal efficiency by enhanced enhanced VIS response due to La doping & large specific surface area due GO, efficient charge separation. | [75] |
| 6 | ZnO-embedded S-doped g-C3N4 (50 mg) | MB and RhB (10 ppm) | 93% 80 min | Enhanced visible light response and charge separation behaviour. | [76] |
| 7 | ZnO/g-C3N4 (25 mg) | Congo red (10 ppm) | 100% in 120 min | Reduced band gap. | [77] |
| 8 | ZnO/Fe3O4/g-C3N4 (10 mg) | MO, AYR, & OG (30 ppm) | 98%, 98%, & 83% in 150 min respectively | Charge separation efficiency by heterojunction. | [78] |
| 9 | AgIO4/ZnO (100 mg). | RhB (2 × 10−5 M) & IC (5 × 10−5 M) dyes | 81% of RhB & 98% of IC in 120 min | Reduction in band gap | [79] |
| 10 | TPDT/(ZnO-Ag) 20 mg | MB, Cr(iv) (1.5 × 10−5 M) | 100% in 60 min | Charge separation efficiency by heterojunction | [82] |
| 11 | Cu/Ag/Au-loaded ZnO (20 mg) | Various phenolic dyes (100 ppm) | 100% in 180 min | Band gap dependent on metal-exchange capacity, doped metals improves VIS response & reduced charge recombination. | [83] |
| 12 | ZnO/GO (100 mg) | Ciprofloxacin MB, MG, EY, (25 ppm) | 100%, 100%, 98%, 87% in 180 min | Increase active sites due to GO, reduced charge recombination, reduced band gap all of which improve photocatalytic performance | [84] |
| 13 | CuO/ZnO (50 mg) | MB (10 ppm) | 97% in 25 min | p–n junction, reduced charge recombination | [86] |
| 14 | Ag/ZnO (500 mg) | RB5 (1.0 × 10−5 M) | 72% in 780 min | Plasmonic effect | [87] |
| 15 | g-C3N4-ZnO@graphene (5 mg) | RhB, MV, MO (20 ppm) | 83% 150 min | Pollutant removal efficiency by synergy btw adsorption & photocatalysis, charge separation by heterojunction. | [93] |
| 16 | g-C3N4/ZnO (50 mg) | RhB | 92% in 120 min | good charge transfer & reduced charge recombination. | [94] |
| 17 | Cu-ZnO (50 mg) | MO (0.02 mM) | 100% in 150 min | good charge transfer & reduced charge recombination | [95] |
| 18 | ZnO-Ag8S (1 × 1 cm2 thin film) | RhB (1 × 10−3 M) | 7.3 times better than ZnO | electron trapping which hindered the charge carrier recombination | [96] |
| 19 | ZnFe2O4/Ag-ZnO (50 mg) | RhB (10 ppm) | 100% in 300 min | good charge transfer & reduced charge recombination | [97] |
| 20 | C-ZnO/BiVO4 (75 mg) | MB (10 ppm) | 100% in 50 min | Doping, heterojunction, surface defect traps charges control recombination | [98] |
| 21 | Ce–ZnO/Chitosan (20 mg) | MG (5 ppm) | 87% in 90 min | Reduction in band gap | [103] |
| 22 | ZnO/polypyrrole (50 mg) | AV 7 (5 ppm) | 64% in 360 min | band gap indeterminable, but high VIS shift. | [104] |
| 23 | ZnO and g-C3N4 (1 g) | MB & RhB (10 ppm) | 97% MB in 50 min | Enhanced charge separation and visible light response. | [108] |
| 24 | ZnO/g-C3N4/Ag (Thin film) | MB & MG (1×10−5 M) | 96% & 99% in 90 min | Reduced band gap & reduced charge recombination. | [110] |
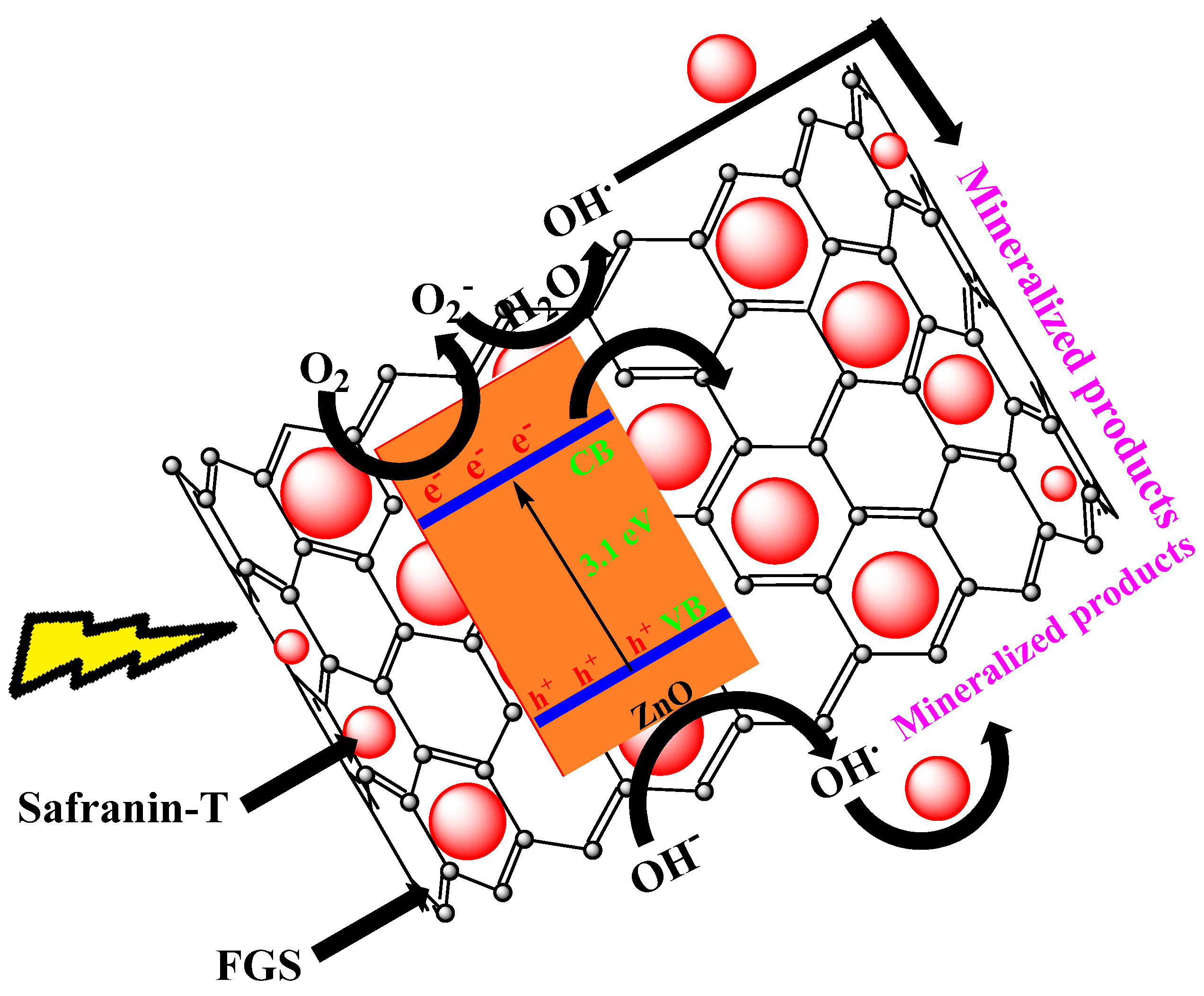
| 25 | FGS/ZnO (20 mg). | Safranin T (2.2 × 10−4 M) | 95% in 90 min | Dye removal efficiency by enhanced adsorption due to FGS & efficient charge separation | [130] |
| 26 | Bi2S3/ZnO (Thin film) | MB (20 ppm) | 92% in 200 min | Charge separation efficiency by heterojunction | [132] |
| 27 | ZnO/AZO/ZnO (Thin film) | MB (30 mg) | 95% in 180 min | Smaller crystallite size, surface defect, results in efficient separation of e/h pairs. | [133] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Folawewo, A.D.; Bala, M.D. Nanocomposite Zinc Oxide-Based Photocatalysts: Recent Developments in Their Use for the Treatment of Dye-Polluted Wastewater. Water 2022, 14, 3899. https://doi.org/10.3390/w14233899
Folawewo AD, Bala MD. Nanocomposite Zinc Oxide-Based Photocatalysts: Recent Developments in Their Use for the Treatment of Dye-Polluted Wastewater. Water. 2022; 14(23):3899. https://doi.org/10.3390/w14233899
Chicago/Turabian StyleFolawewo, Abayomi D., and Muhammad D. Bala. 2022. "Nanocomposite Zinc Oxide-Based Photocatalysts: Recent Developments in Their Use for the Treatment of Dye-Polluted Wastewater" Water 14, no. 23: 3899. https://doi.org/10.3390/w14233899
APA StyleFolawewo, A. D., & Bala, M. D. (2022). Nanocomposite Zinc Oxide-Based Photocatalysts: Recent Developments in Their Use for the Treatment of Dye-Polluted Wastewater. Water, 14(23), 3899. https://doi.org/10.3390/w14233899







