Abstract
Hydroponics is a soilless cultivation technique in which plants are grown in a nutrient solution typically made from mineral fertilizers. This alternative to soil farming can be advantageous in terms of nutrient and water use efficiency, plant pest management, and space use. However, developing methods to produce nutrient solutions based on local organic materials is crucial to include hydroponics within a perspective of sustainability. They would also allow hydroponics to be developed in any context, even in remote areas or regions that do not have access to commercial fertilizers. This emerging organic form of hydroponics, which can be qualified as “bioponics”, typically recycles organic waste into a nutrient-rich solution that can be used for plant growth. Many methods have been developed and tested in the past three decades, leading to greatly heterogenous results in terms of plant yield and quality. This review describes the main organic materials used to produce nutrient solutions and characterizes and categorizes the different types of methods. Four main categories emerged: a “tea”-type method, an aerobic microbial degradation method, an anaerobic digestion method, and a combined anaerobic-aerobic degradation method. The advantages and drawbacks of each technique are discussed, as well as potential lines of improvement. This aims at better understanding the links between agronomic results and the main biochemical processes involved during the production, as well as discussing the most suitable method for certain plants and/or contexts.
1. Introduction
Hydroponics can be defined as a method for growing plants in the absence of soil, in which plant roots extract essential nutrients from a nutrient solution, i.e., nutrient-enriched water [1]. In comparison to traditional soil farming, this technique shows advantages in nutrient and water use efficiency as well as in plant pest and disease management [2,3,4]. The method can also be space-efficient when performed vertically and allows production all year around in climate-controlled greenhouses [4,5,6]. This technique is particularly interesting in geographical zones with limited access to land, and/or in the presence of soils that are polluted, degraded, infertile, or unsuitable for agriculture. Consequently, hydroponics appears well suited to urban areas or areas facing pedoclimatic conditions not conducive to traditional farming [3,4,7]. It could represent part of the answer to several challenges faced by agriculture today and in the near future, e.g., reduced surfaces of arable land, limited access to water, and climate change. However, hydroponics typically uses mineral fertilizers to create the nutrient solution. This can question its relevance and hinder its development worldwide as producing and/or extracting mineral fertilizers poses multiple problems. Focusing on the three major nutrients required in large quantities for plant growth–nitrogen (N), phosphorous (P) and potassium (K)—several issues can be pointed out. The manufacturing of ammonia (NH3), from which 97% of mineral N fertilizers derive, is based on a highly energy-demanding process (36.9 GJ/t of NH3 on average) that uses gas and other hydrocarbons for feedstock and fuel [8]. Derived from NH3, the production of nitric acid at the origin of N fertilizers widely used in hydroponics generates large emissions of nitrous oxide (N2O), which has a global warming potential 298 times greater than that of CO2 [8]. On the other hand, P and K mineral fertilizers mostly rely on the mining of rock phosphate and potash, respectively, whose extraction requires more and more energy in view of their increasing scarcity [9]. Exploiting these resources not only contributes to land degradation, water contamination, excessive energy consumption and air pollution, but they are also highly inequitably distributed around the world [8]. Morocco and Western Sahara alone hold around ¾ of the remaining world rock phosphate reserves [10]. The global reserves have been assessed various times over the past decades and have been extensively debated [11,12,13,14,15]. While some authors estimate commercial reserves to be depleted in the next 200–400 years, other scientists estimate this limit will be reached in the next 50–100 years, depending on the existing reserves, quality, global demand and mining rate [9,13,15,16].
The dependence of mineral fertilizers on fossil fuels and their unequal distribution worldwide makes their financial cost sensitive to global crises. The historical price peaks reached by mineral fertilizers in 2022 reinforce the idea that alternatives must be found [17]. Moreover, they are often inaccessible in developing countries or in more remote areas, due to their lack of availability on the local market and/or their high prices [7,18,19].
The development of alternatives to mineral fertilizers for hydroponics is crucial for this technique to be included in a perspective of sustainability and meet the challenges of agriculture and climate change worldwide. The use of liquid organic fertilizers derived from organic waste and residues as a substitute for mineral fertilizers is of growing interest. This alternative method to conventional hydroponics can be found under the terms of organic hydroponics or “bioponics”, standing for biological hydroponics [20]. Several studies have shown positive effects on plant disease mitigation and crop produce quality, notably with higher health-promoting compounds and/or lower nitrate levels in leafy vegetables [2,21,22,23,24,25,26]. Nitrates accumulated in vegetables are often associated with harmful impacts on human health, such as methemoglobinemia and higher cancer risk [27]. Moreover, the use of nutrient-rich organic waste and effluents as fertilizers in closed-loop bioponics can represent an interesting way of managing these materials, which can be sources of important ecosystem alterations if they accumulate in water and soil, e.g., eutrophication, toxicity in aquatic organisms, soil acidification [2,28,29,30,31]. From this perspective, bioponics can act as a nutrient recycling process while decreasing the demand for synthetic mineral fertilizers.
Liquid organic fertilizers are typically solutions concentrated in nutrients of organic origin, diluted with water and added to the hydroponic systems [32]. Essential nutrients present in organic materials are entrapped in organic molecules that plant roots cannot take up as such. They have to go through biochemical processes of decomposition and mineralization, carried out by a multitude of microorganisms in order to release their nutrients in a mineral form readily available to plants [33]. Therefore, bioponics presents several challenges as it involves a living organic environment that conventional hydroponics do not have to deal with. The management of physicochemical parameters such as pH and electroconductivity can differ and be more complex than in conventional hydroponics [32,34]. Furthermore, the major challenge is to provide a satisfactory nutrient solution for plants, containing all the essential nutrients in adequate quantities, and free of phytotoxic substances [2,35]. Several liquid fertilizers for bioponics are already on the market and produced industrially [32,36,37,38,39]. However, the biochemical processes involved in their manufacturing and the original organic materials from which they are derived are not explicit. Various studies have explored methods of producing nutrient solutions from organic sources and compared them with their efficiency on plants [21,34,40,41]. These studies are of interest, as they show that one can implement bioponics in any context, using local organic waste. However, the production methods are very diverse. They include the use of various sources of organic materials and various conditions of mineralization, also leading to greatly heterogeneous results on plants. Moreover, the biochemical processes involved in these methods are not always clear and identified, so it is difficult to choose an appropriate and efficient method adapted to the context and desired crop. The objectives of this review are to (1) characterize the different types of organic materials that are or could be used for bioponics, (2) characterize and categorize the different methods for producing organic nutrient solutions found in the literature in terms of biochemical processes involved, and (3) relate the different methods to their results in terms of nutrient solution production and effectiveness on plants. The resulting overview of the various existing methods and their efficiency provides an understanding of these methods from a biochemical point of view, and a discussion of their advantages and drawbacks in different contexts.
2. Sources of Organic Fertilizers for Bioponics
Organic materials from various sources have been used for producing bioponic solutions, ranging from animal manure [23,34,35,42,43,44], compost [5,22,45], algae [46,47,48] to agro-industrial [21,49] and household waste [40]. The role of these materials is to meet plant needs by providing adequate amounts of essential nutrients. However, the nutrient composition can vary greatly among organic fertilizers. Likewise, crops have diverse nutrient needs depending on the plant species and variety, the growth stage or the part of the plant to be harvested [1]. For instance, leafy plants have a high N demand in comparison to fruit plants, which require more K, P and calcium (Ca) [1]. For this reason, characterizing the different types of organic fertilizers currently or potentially used in bioponics would make it possible to wisely choose the organic materials to be used.
Organic fertilizers can come from (1) agricultural waste (e.g., animal manure, slaughterhouse waste, composting and vermicomposting, plant residues) (2) agro-industrial waste, (3) household waste, or (4) algae [50,51,52]. These materials can be characterized with different parameters to assess their quality as a fertilizer. The quantities of nutrients contained in the materials can be used to assess their capacity to meet plant needs. In the case of the macronutrients N and P, their relative mineral/organic form ratio provides the quantity of nutrients directly available for the plant—the mineral fraction—and the quantity of nutrients that will first need to be mineralized in order to be taken up—the organic fraction. Meanwhile, the carbon-to-nitrogen ratio (C/N) is a relevant indicator of the efficiency and speed of the organic portion to be mineralized. Table 1 presents the C/N ratios and the amounts of N, P and K (in % dry matter) of different organic fertilizers found in the literature. The proportions of N and P in mineral form are specified. K can only be found in a mineral form.
Animal manure represents the main source of organic fertilizer used in agriculture worldwide [51,52]. It usually contains high levels of N, P and K readily available or rapidly available for plants [52,53]. The actual amounts of nutrients and their form—organic or mineral—depend on numerous factors, such as the animal species, the state of the animal itself (age, lactation), the diet, as well the conditions in which the manure was stored [53,54]. Manures from forage-based diets (cattle, goat, sheep and horse manure; Table 1) often have a lower mineral content than manures from concentrate-based diets (swine and poultry manure; Table 1) [51,54]. Their higher N content also explains their lower C/N ratio, i.e., 4–18 for poultry droppings and 10–14 for swine manure vs. 9–65 for forage-based manures. These low-C/N manures tend to mineralize faster than the manures from forage-fed animals and can be advantageous for bioponics. Focusing on N, urine usually contains most of the N, mostly in the form of urea [54]. Thanks to the widespread urease enzyme, urea tends to mineralize into ammonium (NH4+) very quickly in nature [55]. Poultry excreta, which are a mixture of feces and urine, contain high levels of inorganic minerals and soluble hydrolyzable organic compounds that mineralize rapidly in the environment [52]. Human urine can also be used as a liquid fertilizer after separation from solid excreta [56,57]. Using these types of materials for bioponics can result in high levels of readily or rapidly available N for plants, requiring a low to no mineralization process. Besides NH4+, inorganic N in the form of nitrate (NO3−) is also present in animal manures, but to a much lesser extent [54]. As for P, animal manures often contain more than 50% of total P in an inorganic form [54,58,59]. Poultry and swine manures especially often contain high inorganic P levels as a result of their feed, i.e., up to about 90% of total P in the form of inorganic P for both swine and poultry manures (Table 1). Finally, the K content ranges between 0.2 and 7.8% dry matter (DM) for all animal manures (Table 1), with a bioavailability rate of almost 100% [54,60].
Slaughterhouse waste products are also used as organic fertilizers, more particularly as a source of N [61]. Blood meal—dried powdered blood—can contain around 12% of N (Table 1). Similarly, bone meal—dried powdered by-products of cattle slaughterhouses—contain 4.1–4.2% of N, but also and most importantly high inorganic P (8.7–23.5%) and Ca contents (Table 1). Nutrients in those powders are predominantly in their inorganic forms and represent a source of rapidly available minerals for bioponics.
Besides, composted materials can have relatively high levels of bioavailable nutrients because mineralization is at a more or less advanced stage. However, the actual nutrient content greatly depends on factors such as the original material composition and oxygenation [54,61]. Vermicomposting tends to result in very stable compost, with interesting N and P contents (0.5–3.5% N; 0.1–4.7% P, Table 1) as well as a high content in humic substances that are potential plant-growth-promoting compounds [51].
Plant residues contain nutrients mostly in their organic form [61]. They can also contain undegradable compounds, such as lignin or cellulose-like molecules. Therefore, their C/N ratio is often much higher than those of animal waste products or compost and requires a long period of degradation and mineralization before effectively fertilizing plants. Their exact nutrient content depends on several factors such as the plant species and variety, the part of the plant that is being used, or the developmental stage of the plant [55]. Leguminous plants usually contain more N and P than cereals [55] (1–3.1% N and 0.4–0.8% P for soybean residues vs. 0.4–0.8% N and 0.1–0.4% P for wheat residues) (Table 1).
Algae have also been used for many years in coastal regions as organic fertilizer, representing a good source of nutrients in organic form (0.3–17.5% N, 0.1–4.5% P, 0.1–8.5% K; Table 1), plant growth regulators such as gibberellin, auxin and cytokinin-like substances, and vitamins and amino acids for plants [50,62,63,64,65]. Notably, marine macroalgae or seaweeds, are used as biostimulants, including in hydroponics, usually in the form of compost made with other organic waste, dried powder and/or liquid extracts [46,47,48,50,63,64,65,66].
Besides, residual organic residues from agro-industrial manufacturing processes, such as cottonseed or canola seed meal, molasses or corn steep liquor, i.e., by-products of oil extraction from cotton seeds and rapeseed, of sugar production and of corn-wet-milling, respectively, can have relatively high N, P or K contents [21,51,67,68,69]. Many of these organic residues are already in a powder or liquid form and could be used in bioponics. Several studies have shown encouraging results with bioponically grown plants using corn-steep liquor and molasses [21,41,70]. Cottonseed, canola meal or corn steep liquor can represent a good source of N (4.5–7.4% DM, 5.6–6.6% DM and 3.4% DM, respectively), while molasses is reported to be a good source of K (0.5–3.8% DM) (Table 1) [21,67,68,71]. However, most of the N and P nutrients in these materials are in an organic form and thus require an intense mineralization process.
Finally, household waste can have very different compositions and degradability levels depending on the residues (0.9–5.8% N, 0.4–0.1% P, 0.8–0.1% K, with a C/N ratio of 7–27). These residues can be milled and dried and then ground to be used in bioponics [40].

Table 1.
Nutrient content (N, P, K) and C/N ratio of commonly used organic fertilizers. TN, total nitrogen; TP, total phosphorus; TK, total potassium; DM, dry matter.
Table 1.
Nutrient content (N, P, K) and C/N ratio of commonly used organic fertilizers. TN, total nitrogen; TP, total phosphorus; TK, total potassium; DM, dry matter.
| Organic Source | N Content | P Content | TK (% DM) | C/N Ratio | References | ||
|---|---|---|---|---|---|---|---|
| TN (% DM) | Inorganic N (%TN) | TP (% DM) | Inorganic P (% TP) | ||||
| Animal manure | |||||||
| Cattle | 0.2–5.3 | 9.0–72.0 | 0.1–1.5 | 48.0–75.0 | 0.2–6.2 | 16.0–31.0 | [51,53,54,55,72,73,74,75,76,77,78] |
| Equine/horse | 1.4–3.9 | 5.8 | 0.6–1.2 | 45.0–49.0 | 1.2–4.2 | 19.0–25.0 | [51,73,74,76] |
| Sheep | 1.2–2.9 | 20.0–20.8 | 0.6–1.1 | 48.0–49.0 | 1.4–3.4 | 9.0–29.0 | [51,74,75,79] |
| Goat | 0.7–3.6 | 38.2 | 0.5–2.7 | 36.0–47.0 | 0.6–5.9 | 16.0–65.1 | [73,74,76,80] |
| Swine | 0.4–7.5 | 34.0–41.9 | 0.1–4.9 | 18.0–92.0 | 0.2–7.8 | 10.0–14.0 | [51,53,54,55,59,73,74,81] |
| Poultry | 1.2–6.1 | 32.6–48.0 | 0.4–3.6 | 25.0–90.0 | 0.8–3.1 | 4.0–18.0 | [53,55,59,73,74,75,77,78,81,82,83] |
| Slaughterhouse waste | |||||||
| Blood meal | 11.8–15.8 | <1 | 0.1–0.7 | n.a. 1 | 0.1–0.7 | 3.0–5.0 | [51,78,84,85] |
| Bone meal | 4.1–4.2 | n.a. | 8.7–23.5 | n.a. | 1.6 | 4.0–7.0 | [51,78,86,87] |
| Compost | 0.7–3.1 | 5.0–29.0 | 0.2–2 | 15.0–84.0 | 0.8–2.4 | 11.3–64.0 | [51,59,75] |
| Vermicompost | 0.5–3.5 | 2.7–37.0 | 0.1–4.7 | n.a. | 0.2–2.1 | 7.1–36.7 | [51,88,89,90,91,92] |
| Agro-industrial waste | |||||||
| Corn steep liquor | 3.4 | 2.5–8.2 | 1.5 | n.a. | 2.7 | n.a. | [21,93] |
| Cottonseed meal | 4.5–7.4 | <1 | 1.2–2.8 | n.a. | 1.7–2 | 8.0 | [51,53,67,68] |
| Canola meal | 5.6–6.6 | <1 | 2.8 | n.a. | 1.2 | 8.0 | [67,68] |
| Molasses | 0.4–2 | n.a. | 1.7 ± 0.7 | n.a. | 0.5–3.8 | n.a. | [69,71,94] |
| Plant residues | |||||||
| Soybean residue | 1.0–3.1 | n.a. | 0.4–0.8 | n.a. | 1.5–0.8 | 12.3–30.0 | [55,82,95] |
| Wheat residue | 0.4–0.8 | n.a. | 0.1–0.4 | n.a. | 1.2–3.8 | 84.0–124.0 | [95,96,97,98] |
| Leaf litter residue | 0.5–1 | n.a. | 0.4–0.8 | n.a. | 0.0–1.0 | 40.0–80.0 | [51,76,77] |
| Algae | 0.3–17.5 | n.a. | 0.1–4.5 | n.a. | 0.1–8.5 | n.a. | [99,100,101] |
| Household waste | 0.9–5.8 | 1.4 | 0.4–1.0 | n.a. | 0.8–1 | 7.0–27.0 | [40,51,75,82] |
1 Note: n.a., not available.
The commonly used organic sources have diverse and varied characteristics. Therefore, the choice of organic fertilizers to be used for bioponics should be made based on their nutritional composition and degradability. Highly degradable materials rich in nutrients in a mineral form will be determined because they can provide nutrients directly or make them quickly available to plants [21,34,102]. A combination of different organic materials can also be made to provide complete fertilization, in accordance with specific plant needs. Moreover, the choice of organic materials should also and above all consider their accessibility in the local context. The materials should be easily and locally findable, otherwise, the relevance and ecological advantages associated with bioponics will be lost.
3. Methods Used for Producing Organic-Based Nutrient Solutions
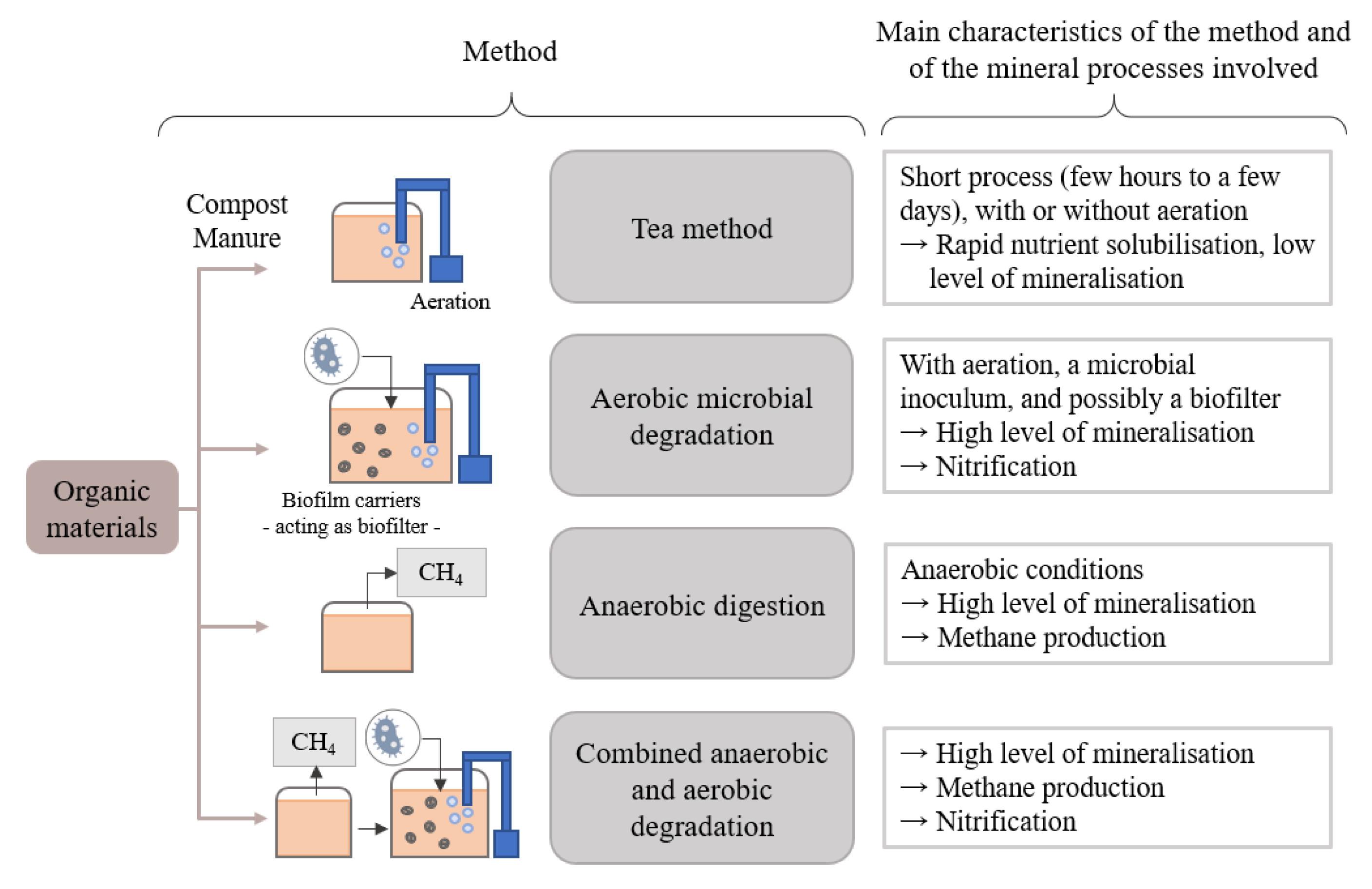
The different methods developed to produce organic-based nutrient solutions can be characterized and grouped according to the main biochemical processes they involve. Four main categories have been identified: (1) the so-called “tea” method, (2) the aerobic microbial degradation method, (3) the anaerobic digestion method, and (4) the combined anaerobic-aerobic degradation method, as illustrated in Figure 1.
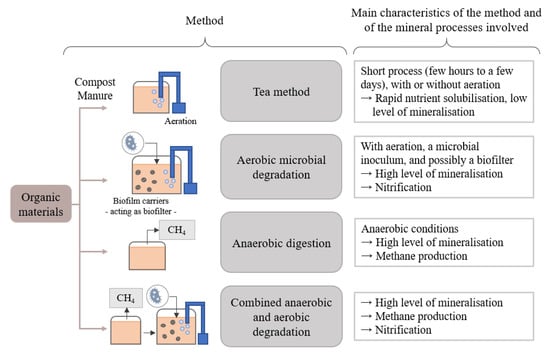
Figure 1.
Summary diagram of tea, aerobic microbial degradation, anaerobic digestion and combined anaerobic–aerobic degradation methods for the production of organic nutrient solution.
Table 2 presents the composition of the nutrient solutions used on plants and found in the various studies when the data were provided. These solutions are also compared with a “classic” nutrient solution used in conventional hydroponics.

Table 2.
Chemical and nutrient properties of organic nutrient solutions used on plants (in mg/L). EC, electro-conductivity; N, nitrogen; N-NO3−, nitrate-nitrogen, N-NH4+, ammonium-nitrogen, N-NO2−, nitrite-nitrogen; P, phosphorus; K, potassium; Ca, calcium; Mg, magnesium.
3.1. The “Tea” Method
3.1.1. Definition and Principles
Several studies are focused on a “tea” method, also referred to as the “extract” method, to make organic nutrient solutions [5,23,34,35,42,45,102,107]. Teas can be defined as brewed water extracts of compost or animal manure, qualified as “compost tea” or “manure tea”, and commonly applied on cultivated soil [107,108,109,110,111]. Historically, compost teas have been used for centuries by suspending a porous bag of compost in water for up to 14 days, resulting in a nutritious extract for plants [108,109,112]. Then, this “passive” or “non-aerated compost tea” was replaced by a more recent technique where the tea is brewed for a shorter period (a few hours to a few days), and often oxygenated with air pumps [108,109]. This aerated compost tea supposedly contains the microorganisms initially present in the compost and is typically used in organic farming as a substitute for chemical fertilizers and pesticides [107,108,109]. Positive impacts on root stimulation, vegetative growth, soil microbial activity and disease suppression have been observed when sprayed on soils [109,110,113,114,115,116,117,118]. Some of these effects are linked to the presence of biologically active metabolites such as humic acids and plant growth regulators present in the organic materials, in addition to the provision of readily bioavailable nutrients [107].
3.1.2. Use of the “Tea” Method in Bioponic Cultivation
Animal Manure Tea
The method consists of “bathing” animal manure in a volume of aerated or non-aerated water for one to seven days. Once filtered, this tea is added to the water of hydroponic systems at a certain concentration. Tikasz et al. (2019) [34] showed that the use of a bioponics solution based on turkey manure tea (50 g/L tea, 2-day tea production process) resulted in healthy lettuce and kale plants, and even significantly greater dry biomass than in conventional hydroponics in the case of lettuce [34]. However, cow and chicken manure teas resulted in lower yields (less than 50% of the yield of the control). Those nutrient solutions contained lower levels of NO3− (<4 mg/L N-NO3−), in comparison to the turkey-manure tea solution (11.7 mg/L N-NO3−) (Table 2). More particularly, the chicken manure solution resulted in the lowest biomass, most likely due to ammonium toxicity (284.6 mg/L of N-NH4+) (Table 2). In Charoenpakdee et al.’s (2014) [102] study, the use of three teas made from bat, cattle or pig manure was tested on bioponically grown lettuce (dilution ratio 1:3; 1-day tea production process) [102]. The bat manure tea resulted in a yield almost as high as when the commercial mineral solution was used (95% of the total fresh weight of the commercial solution result), while the cattle and pig teas performed poorly (35% and 32% of the fresh weight of the lettuces grown in commercial solution, respectively). Conversely, in Atkins and Nikols’ (2004) [35] study, similar growth rates were obtained for lettuces grown with a cattle manure-based nutrient solution and lettuces grown with the conventional nutrient solution [35]. In El-Shinawi et al. (1999) [23], low lettuce yields were obtained using cattle manure tea (~25% of the fresh weight of the control), while chicken manure tea performed well (lettuces reached 92% of the fresh weight of the commercial control) [23]. Additionally, a very low NO3− content was measured in the leaves, which is a quality sign because NO3− accumulation in leafy greens can lead to a health hazard in case of excessive intake [119]. N was mostly in the form of NH4+ in all manure teas. Likewise, in Mowa et al.’s (2015) [42] study, the use of teas made from different types of manure were used on bioponically grown Swiss chard (dilution 59 g/L, 7-day tea production process) [42]. Conventional hydroponics performed best, but among the organic solutions, the composted goat manure tea resulted in the highest yield (~72% of the fresh biomass of the control), while the goat manure, chicken manure and cow manure teas did not perform as satisfactorily (~40%, 38% and 42% of the control, respectively). Table 3 summarizes the main bioponics studies mentioned above, with the organic materials and tea method used to produce the nutrient solution, as well as the type of hydroponic system and cultivated plant.

Table 3.
Main organic materials and method of tea for the production of nutrient solution used in different types of bioponics.
Compost Tea
Using the same method as for manure tea, Leudtke (2010) [45] showed that a compost tea-based nutrient solution resulted in lower yields than conventional hydroponics on Wisconsin Fast Plants™ (Brassica rapa) (~68% of the total dry weight of the plants fertilized with the commercial chemical solution) [45]. In comparison to the usually recommended mineral concentrations in conventional hydroponics (Table 2), the compost tea solutions had a good N-NH4+/N-NO3− ratio (1:3), but relatively low macronutrient contents (Table 2). An excessive level of sodium (Na) was also noticed. This unbalanced nutrient composition was also shown by Arancon et al. (2012) [107] and Caballero et al. (2009) [110], who investigated the nutrient composition of compost teas. In comparison to what is usually recommended in conventional hydroponics, most of the extracts showed too high levels of NH4+ (32–237 mg/L of N-NH4+) and too high levels of heavy metals such as copper (Cu) and zinc (Zn) [110]. A tea made from food waste-based vermicompost showed too low macronutrient content (<2 mg/L of total N, 15 mg/L of K and 11.9 mg/L of Mg) [107].
Several studies have thus explored the use of compost teas as additives and/or partial substitutes for chemical fertilizers rather than as sole fertilizers in hydroponics. Gimenez et al. (2020) [22] observed that the growth and quality of baby leaf red lettuce were improved, with a significantly lower leaf NO3− content and higher health-promoting shoot components such as flavonoids, phenols and antioxidant capacity [22]. Root length and the number of fine roots were also increased in the compost tea-supplemented solution in comparison to the inorganic treatment. Besides, the compost tea mitigated the incidence of the plant pathogen Pythium irregulare on the plants. Several hypotheses were advanced to explain this: a healthier root system potentially caused by radicular growth substances such as auxin-like components originating from the compost and/or the presence of antibiotic-like substances or microorganisms. Considering the sole compost tea nutrient composition, low tea concentrations of N (13.6 mg/L), K (26.9 mg/L) and nutrients such as Ca were obtained. Similar observations were made by Arancon et al.’s (2019) [5] study, in which the vermicompost tea had too low nutrient concentrations to meet the plant needs as the sole fertilizer (1.3 mg/L of N, 2.4 mg/L of P, 45.4 mg/L of K) (Table 2). However, yields were significantly improved when vermicompost tea was added to supplement mineral nutrient solutions concentrated at only 25% or 50% of the full-rate recommended solutions for lettuce, and at 50% for tomato, in comparison to the tea-free modalities. These positive effects were attributed to the presence of trace quantities of phytohormones and humic acids.
3.1.3. Drawbacks of the Method
Unbalanced Nutrient Composition and N-NH4+/N-NO3− Ratio
The heterogeneity and poor yields associated with the use of teas in bioponics are most likely due to an unbalanced nutrient composition. In some cases, N levels were too low for optimal plant growth [5,22,23,42,45,102] and it was mainly in the form of NH4+ rather than NO3−. As opposed to NO3−, NH4+ is toxic to most higher plants when present as the sole N source, leading to decreased plant growth, leaf chlorosis, a low root/shoot ratio and oxidative stress [120,121,122,123,124,125]. These symptoms have been associated with too high energy consumption by plants caused by NH4+ cycling, carbon scarcity explained by the reduction in carbon skeletons induced by excess NH4+ assimilation, cation deficiency and extracellular acidification caused by NH4+ uptake [120]. For these reasons, NO3− is typically provided as the major form of N in hydroponics, with a maximum recommended N-NH4+ concentration of 30 mg/L in the nutrient solution for a recommended N-NO3− concentration of 80–150 mg/L (Table 2) [106]. Other sources suggest that the N-NH4+:N-NO3− ratio should not exceed 25:75 for the optimal growth of most plants [124,126,127,128,129]. The tea process is relatively short—a few hours to a few days—we can expect most of the inorganic nutrient content to correspond to the already soluble inorganic nutrient content of the primary organic materials, via rapid abiotic solubilization [130,131,132]. Part of the soluble organic compounds can be mineralized and added mineral nutrients to the teas, but no significant mineralization is expected to occur over such a short period of time [130,131]. Likewise, if most of the N in the materials is in the form of NH4+-NH3, tea N will also most likely be predominantly in this form, as no significant nitrification (oxidation of NH4+ into nitrite (NO2−) and then into NO3− by nitrifying bacteria [72]) is expected to occur in such a short time.
The manure nutrient content depends on various factors, as previously mentioned. The storage duration and conditions of the manures can explain some variations in tea composition, even for manure from a given animal species. Tikasz et al. (2019) [34] and Mowa et al. (2015) [42] used manures that had different storage periods varying from 1 to 12 months. During this period, more or less advanced mineralization and nitrification processes might have taken place, potentially explaining the differences in NO3− and NH4+ concentrations. Therefore, the—intentional or unintentional—“composting” of manure can be a significant factor in manure-derived tea composition [133]. For instance, the chicken manure-based vermicompost tea prepared by Arancon et al. (2012) [107] contained an optimal level of N-NO3− (137.9 mg/L) and a low level of N-NH4+ (0.6 mg/L) (Table 2). This contrasts with the non-composted chicken manure tea developed by Tikasz et al. (2019) [34], which contained 0.6 mg/L of N-NO3− and 284.6 mg/L of N-NH4+ (Table 2).
Phytotoxicity of Organic Compounds
The presence of phytotoxic organic compounds might be another factor behind the poor yields in bioponics. Teas can indeed contain relatively high levels of organic compounds that can inhibit plant growth, originating from primary organic materials [130,131]. Garland and Mackowiak 1990 [134], Garland et al., 1993 [130], Mackowiak et al., 1996 [131] and Finger and Strayer [135] investigated the ability to grow food bioponically in space habitats with nutrient solutions derived from food waste. The direct use of organic “leachates” (2-hour aerated water extraction of dried crop residues) produced a solution that contained enough inorganic nutrients for plant growth. However, the extracts also contained high levels of dissolved organic compounds that inhibited plant growth because of (1) the presence of organic phytotoxic compounds, and (2) a high biological oxygen demand (BOD) in the root zone caused by too much microbial activity, which consumed the dissolved oxygen and asphyxiated the plant roots [131,132]. The excessive development of a microbial biofilm on the root surface and system components also led to an increased potential for denitrification and represented a potential nutrient sink able to absorb, entrap and precipitate inorganic material [135]. Similar conclusions were drawn by Carballo et al. (2009) [110], who observed high phytotoxicity in teas made from unstable composts, as they contained high organic acid levels that over-acidified the teas and induced decomposition within the solutions. Thus, the presence of labile organic matter in bioponic solutions is detrimental to plant growth.
3.1.4. Discussion on the Method
The tea method could produce organic nutrient solutions for short life cycle crops, such as leafy greens, very easily and rapidly from accessible and inexpensive material. However, results are contrasted, and some nutrient solutions resulted in yields 50% lower than in conventional hydroponics [23,34,102]. Therefore, this method shows great heterogeneity, even when teas are made from the same type of organic material. As no important mineralization and/or nitrification is expected to occur within such a short brewing time, the quality of the tea highly relies on the quality of the primary material, which itself depends on numerous factors. Therefore, the material should be stable and have a high mineral content and a large proportion of mineral N in the form of NO3− rather than NH4+ to avoid phytotoxic organic compounds, a high BOD, and an unbalanced nutrient composition. The quality of the primary organic waste products should thus be assessed beforehand. Besides, using teas as an additional or partial substitute to mineral fertilizers could be of real interest as they are a source of plant-growth-promoting microorganisms and additional nutrients [5,22]. A pre-composting stage carried out before maceration can greatly improve their quality [42,107]. Along the same line, an in-depth degradation and mineralization of the organic materials and/or the tea could address these issues.
3.2. Aerobic Degradation Method with Microbial Processing
3.2.1. Principles and the Method Developed by Shinohara et al. (2011) [21]
In aerobic conditions, the oxidation of organic matter into minerals is mainly driven by heterotrophic microorganisms’ respiration [136]. The oxidation of NH4− into NO3− can also occur, thanks to nitrifying microorganisms. Aerobic mineralization can be achieved by injecting air into organic materials-containing water, via air pumps or sufficient water circulation [136].
The idea of extensive aerobic microbial pre-processing of organic extracts before incorporating them in the hydroponic systems was first studied in the 1990s by the same searchers who explored bioponics in space habitats [130,131,132,134,135,137] after these authors observed the shortcomings of using raw organic material extracts for plant growth. The process consisted of the loading of dried crop residues in an aerobic bioreactor with continuous stirring, in which the pH and dissolved oxygen (DO) were controlled [135]. The treatment reduced the amount of labile organic compounds, which eliminated the growth-inhibiting effect of those compounds on various crops [131,132]. However, the ability of the bioreactor to oxidize more recalcitrant organic materials and perform nitrification was rather limited [132].
A decade later, Shinohara et al. (2011) [21] addressed these issues by developing an innovative method to produce a well-balanced nutrient solution, using soil microorganisms that mineralized organic compounds and efficiently oxidized NH4− into NO3− in aerobic conditions. By being able to culture these microorganisms both during the nutrient solution preparation and during plant cultivation in the hydroponic systems, achieving a mean 98% efficiency in N-NO3− generation from organic N, they were able to add the organic fertilizer directly in the hydroponic systems during crop cultivation without causing any phytotoxicity. More specifically, the production of this microbial bioponic solution consists of first adding 1 g/L of an inoculum containing organic matter mineralizing and nitrifying microorganisms to an aerated volume of water at 25 °C. Organic materials, i.e., a fish-based organic fertilizer (1–1.5 g/L), are then gradually added and aeration is maintained until all mineral N is present in the form of NO3− (12 to 170 days). Then, plants are added to the hydroponic system, and small amounts of organic fertilizers are added during cultivation. Different sources of inoculum were tested—soil, compost and sea water—and compost was considered the best one [21,49]. In addition to the fish-based fertilizer, other organic materials in liquid or powder form were tested, such as corn steep liquor, fish flour, rice bran or fermented poultry manure [21]. By observing that organic materials with high C/N ratios did not generate NO3−, these authors suggested that organic fertilizers with a C/N ratio below 11 should be used for this method, as they would avoid the N starvation of the nitrifying bacteria. On komatsuna (Brassica rapa), the organic nutrient solution performed similarly to the chemical fertilizer, even resulting in produce of better nutritional quality, i.e., a significant decrease in the leaf NO3− content [21]. With this method, NO3− was generated and absorbed gradually during plant growth, while it was likely to be rapidly taken up by the plants and stored in the tissues in conventional hydroponics. In tomatoes, the yield and quality (ascorbic acid content) of the fruit obtained with the organic fertilizer were not significantly different from the ones obtained with a chemical solution. In addition to aspects related to yield and product quality, this method has also showed advantages in terms of soil-borne and air-bone disease control. Trials on susceptibility to bacterial wilt diseases were performed on tomato and showed that the phytopathogenic bacterium Ralstonia solanacearum was inhibited when the organic nutrient solution was used instead of chemical fertilizer [138]. The exact causes of the suppressive effects were unclear, but they likely originated from the biofilm that developed on the roots of the bioponic plants, as well as from components produced by microbes in the organic solution, with potential antibiotic effects. Systemic resistance to Botrytis cinerea, which causes grey mold, was also induced in lettuce and cucumber when applying the method [139].
3.2.2. Use of Aerobic Degradation for Bioponic Cultivation
Kawamura-Aoyama et al. (2014) [40] used dried and ground solid food waste as an organic fertilizer in bioponics, requiring microbial pre-processing for 2 months to generate NO3−. On lettuce, the organic solution resulted in similar yields in comparison to chemical fertilizer. The efficiency of inorganic N recovery was systematically improved when the nutrient solution was filtrated, reinforcing the idea that undegraded organic compounds negatively impact plant growth [40]. In Kano et al.’s (2021) [70] study, the growth and yield of bok choy (Brassica rapa var. chinensis) under corn steep liquor organic fertilization and conventional hydroponics were similar in summer cultivation when the same amount of total N was used. In Mowa et al.’s (2018) [140] study that used goat manure, 10 g/L of compost was needed to provide sufficient beneficial microorganisms, and adding small amounts of manure (0.25 g/L/day of dried ground manure) at the initial stage of microbial mineralization supported the establishment of the microbial ecosystem in comparison to large amounts of manure [140]. Goat manure-derived nutrient solution proved to be a good alternative to chemical fertilizers for tomatoes: the fruits were of commercially acceptable weight and of better quality than under chemical fertilization [24], i.e., higher lycopene content, which has health benefits. This study also served as a comparison to the previously described tea method developed by Mowa et al. (2015) [42], in which goat manure tea resulted in a poor yield. The goat manure aerobic mineralization process even surpassed that of tea made from composted goat manure, which in turn gave better results than non-composted manure [42].
3.2.3. A Parallel with Aquaponics
The idea of mineralizing organic materials directly within the hydroponic system during plant cultivation [21,40], is in agreement with the principles of aquaponics. Aquaponics aims at remediating aquaculture effluents by combining aquaculture with hydroponics in a recirculating system where fish effluents are mineralized by beneficial microorganisms, including nitrifying bacteria, to provide nutrients for plant growth and clear the water of toxic elements for fish [141]. The development of microbial communities is promoted by the presence of a biofilter—a well-oxygenated compartment in which a large surface area allows colonization by microorganisms [142]. Aquaponics can thus be seen as a variant of bioponics, with fish effluents as the organic fertilizer.
Applying the operating procedure of aquaponics, Wongkiew et al. (2021) [43] used chicken manure directly in the hydroponic systems during plant cultivation, thanks to the presence of a biofilter. The latter consisted of three layers of filter pads to filtrate the solution and provide a large surface area and dry chicken manure in a filter bag was placed in-between the layers. As for Shinohara et al. (2011) [21], liquid compost (20 mL/L) was added to the hydroponic system prior to the start of the experiment to activate mineralization and nitrification. The biodegradation of chicken manure continued until the end of plant cultivation. As a result, the system effectively produced lettuces and resulted in an N use efficiency of 35–42% and a P use efficiency of 7–8% [43]. The microbial communities in the biofilter and in the plant roots showed a unique composition in comparison to aquaponic systems and comprised a variety of plant-growth-promoting bacteria able to increase P and N availability through solubilization and mineralization.
Table 4 summarizes the main studies mentioned above, with the organic materials and aerobic degradation method used.

Table 4.
Main organic materials and method of aerobic degradation for the production of nutrient solution used in different types of bioponics.
3.2.4. Discussion on the Method
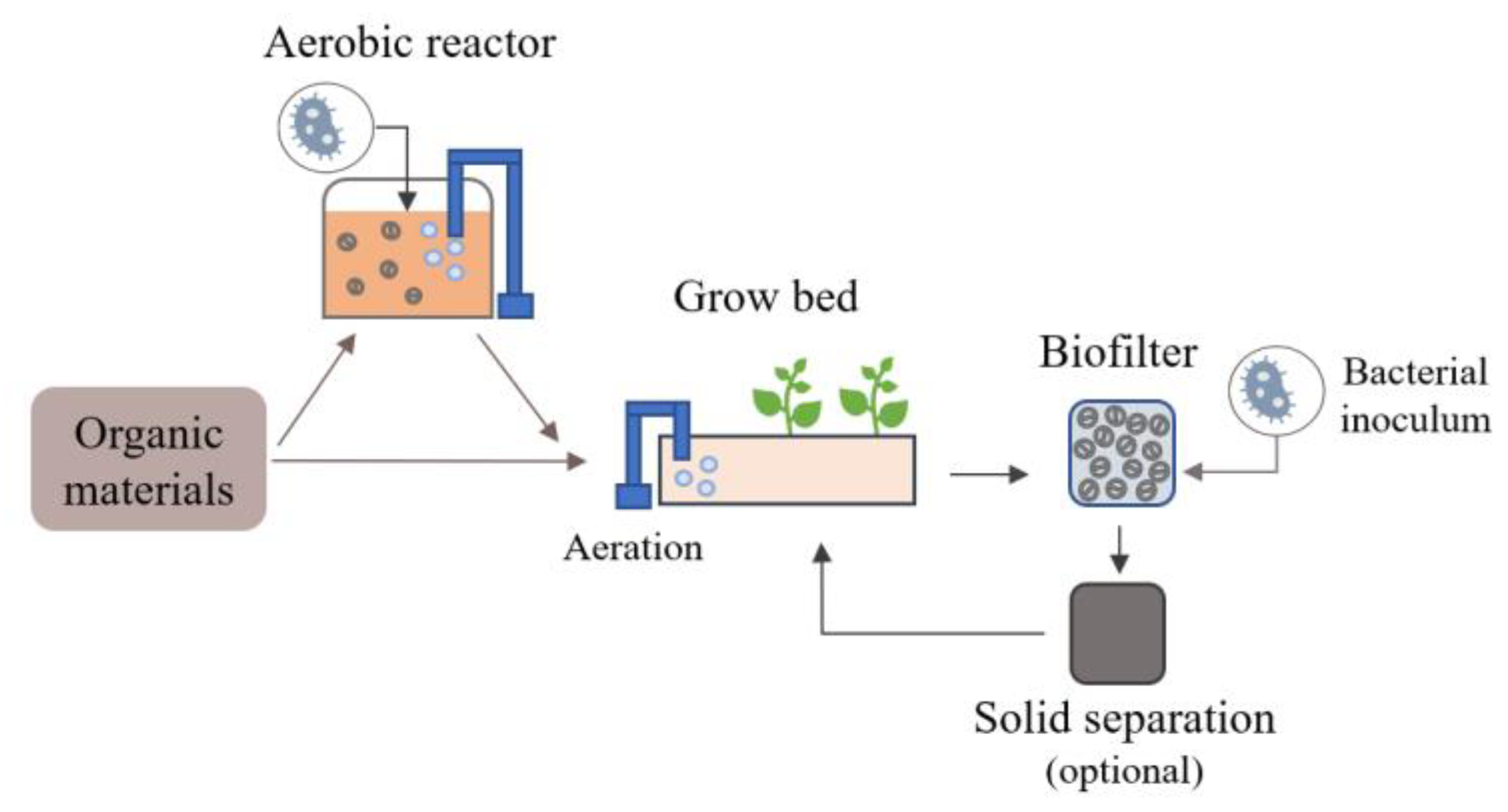
Aerobic degradation of organic materials appears to be very promising for the development of bioponics. This method produces NO3−, i.e., the preferential form for N uptake by plants, thanks to the presence of nitrifying bacteria that transform NH4+ into NO3− in aerobic conditions [72]. These bacteria naturally develop in environments where organic matter is degraded in the presence of oxygen, e.g., during organic nutrient solution production under good oxygenation using air pumps. However, natural inoculation and the development of these bacteria may take a long time depending on the environmental conditions. The external supply of these bacteria can thus be added to the nutrient solution through materials such as compost, soil, seawater or activated sludge [21,40,43,49,140]. Then, the nutrient solution conditions can be adapted to approach the optimal conditions for these nitrifiers, estimated to be a pH of around 7–8, a temperature of 20–30 °C, a minimum DO of 5 mg/L and a limitation in the dissolved organic compounds [31,72,141,142,143,144,145,146]. Mineralization can also continue in the hydroponic systems, as illustrated in Figure 2, so that organic fertilizers can be added during plant cultivation and thus better meet the plant’s needs [21,40,103]. In this case, mineralization can be enhanced by adding a biofilter [43]. The potential of using microbubbles and nanobubbles could also be explored, notably for deep-water culture (DWC) systems, as it can improve oxygenation and in turn nitrification compared to the macrobubbles generated by classic air pumps [31,147]. It can also favor root oxygenation and limit pathogen development by favoring the activities of beneficial organisms [31,148,149]. Moreover, the integration of a solid removal compartment such as a mechanical filter or a sedimentation tank (Figure 2) could also improve the nitrification efficiency by limiting residual organic compounds and, in turn, excessive heterotrophic activities [31]. This should be further explored in bioponics.
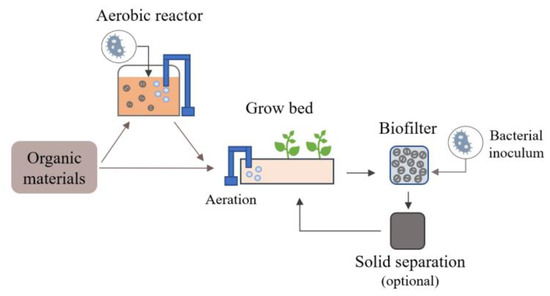
Figure 2.
Schematic of organic materials aerobic degradation integrated and/or external to the bioponic system.
This method makes it possible to use a multitude of organic sources, particularly those generating too high levels of NH4+, e.g., chicken manure, by bringing a certain uniformity and stabilization in the quality of the nutrient solutions. It is based on the biochemical process of aerobic mineralization, and thus requires sufficient and continuous aeration of the solution by air pumps. However, the mineralization of organic materials can also take place in the absence of oxygen. Anaerobic mineralization also called “anaerobic digestion” (AD) can have the advantage of being less energy-demanding since it does not require intensive pumps or motors [136].
3.3. Anaerobic Digestion Method
3.3.1. Principles and Contextualization of Anaerobic Digestion
Anaerobic digestion (AD) consists of the degradation and reduction of organic matter into minerals, other lower-weight organic compounds and gaseous forms of CO2 and methane (CH4) by various microorganisms in the absence of oxygen [136,150,151]. This biogas represents a real economic interest because it can be used as an alternative source of energy to fossil fuels [151]. Besides biogas production, this process also results in a nutrient-rich degraded material called “digestate”, that can be used as an organic fertilizer [150,151]. The quality of the digestate and the efficiency of biogas production are influenced by many factors such as the pH, the C/N ratio, temperature, the nutrient content, and the hydraulic retention time (HRT) in the digester [136,150,151]. Twenty to 95% of the organic matter is degraded during AD [150], depending on the type of feedstock and all the above-mentioned factors. In comparison to untreated residues, digestates are more homogenous and have a higher stability degree, with decreased total and organic carbon, reduced BOD and high mineral content [2,150,151,152,153,154]. They are also less odorous and safer as AD reduces the level of pathogens by inactivating most of them during the process [78,152,155,156]. They also comprise bioactive compounds such as phytohormones, vitamins, nucleotides, monosaccharides, and fulvic acid that can promote plant growth and enhance tolerance to biotic and abiotic stresses [25,150,155]. In soils, digestates appear to be very well-fitted for high N-demanding vegetables with a fast-growing cycle because they rapidly release bioavailable N, mainly in the form of NH4+ [150,157]. They are usually directly spread on cultivated soils, but they can also be pre-treated, e.g., by solid-liquid separation, drying, dilution or filtration [150]. Based on all the benefits of anaerobic digestates as organic fertilizers, more and more researchers are investigating their use in bioponics.
3.3.2. Use of Anaerobic Digestates for Bioponic Cultivation
Liedl et al. (2006) [44] tested the use of the liquid fraction of poultry litter digestate on vegetables grown in bioponics. AD consisted of a 30-day HRT process of broiler litter at a high temperature (56 °C). The yield of lettuce plants was similar to that obtained with a commercial inorganic fertilizer when the digestate was highly diluted, corresponding to a total N concentration of 100 mg/L (Table 2) [44]. Conversely, organic nutrient solutions more concentrated in the digestate (200 and 300 mg/L of total N concentrations) were detrimental to yield. The control hydroponic solution with chemical fertilizers had a total N concentration of 150 mg/L. The importance of the digestate dilution for optimal plant growth was also notably shown by Krishnasamy et al. (2012) [104]: a digestate concentration of 50% resulted in the complete death of bioponic silver beet within 2 weeks, while the same digestate at 20% performed best in terms of foliage yield and plant growth. However, yields were still significantly lower than those obtained with a commercial inorganic solution. The negative effects were associated with too high levels of NH4+ (43.4 mg/L) (Table 2) and low levels of DO in digestate.
In Mupambwa et al.’s (2019) [158] study, a 10% concentrated digestate solution was found suitable for crop use, but phytotoxic when concentrated at 30 or 40%. In Phibunwatthanawong and Riddech’s (2019) [41] study, only a concentration as low as 1% was found suitable. Both studies attributed their phytotoxicity to the high concentrations of NH4+-NH3 and organic compounds. Phibunwatthanawong and Riddech 2019 [41] more specifically investigated the use of digestate on green cos lettuce, also focusing on the AD itself and the impact of different feedstock ratios on digestate quality, i.e., varying the ratios of molasses, distillery slop and sugarcane leaves resulting in six different digestate formulas. After 30 days of AD, all digestate formulas contained plant-growth-promoting bacteria, i.e., N-fixing, phosphate- and K-solubilizing bacteria, and significantly higher levels of indole acetic acid (IAA)—an auxin phytohormone that stimulates root hair development—[159,160,161]. On plants, two digestate formulas diluted at 1% resulted in yields similar to those obtained with chemical fertilizer, showing optimal levels of N (160 mg/L) (Table 2). In Ronga et al.’s (2019) [155] study, the use of a solid fraction of digestate as a growing medium and a liquid digestate-based nutrient solution (6.25%) resulted in yields similar to the yield of a conventional hydroponic system on agri-perlite growing medium and chemical fertilizers. The undiluted liquid digestate was low in total organic carbon (TOC) and P, but rich in K (TOC 3.74%, N 0.34%, K2O 0.95%, P 0%), while the solid fraction was rich in P and TOC (TOC 3.75%, N 0.74%, P2O5 0.60%, K2O 0.76%). A digestate solid fraction as a growing medium can represent a good alternative to conventional substrates.
Regarding crop quality, Liu et al. (2009) [25] showed that the use of diluted digestates for bioponically grown lettuces (1:4 or 1:5 dilution) significantly decreased NO3− concentrations in the shoots in comparison to conventional hydroponics, which is a good-quality sign [119]. Similarly, Wang et al. (2019) [26] observed that when half of the mineral fertilizer was replaced by poultry manure digestate, the lettuce yield was not affected and the nutritional quality was significantly improved: the leaf soluble sugar content increased, and the leaf NO3− content decreased. When the digestate was used as the sole fertilizer, the nutritional quality of the lettuce was also improved, but the shoot weight was only 51% of that obtained with the inorganic fertilizer. This was attributed to the lack of P, Ca, and Mg in the digestate (4.3 mg/L, 8.1 mg/L and 0.7 mg/L, respectively) (Table 2).
This potential nutrient imbalance of the digestate for plant growth has particularly been highlighted in studies focusing on high-nutrient-demanding plants with longer growth cycles, e.g., tomatoes. After testing the liquid fraction of poultry litter digestate on lettuce [44], Liedl et al. (2004)b [162] also studied its use on bioponically grown tomatoes. When the digestate was the sole fertilizer, the plants showed NH3 toxicity and Mg deficiency and produced fewer and smaller tomatoes than under chemical fertilization. The Mg concentration in the organic solution was only 13 mg/L, in comparison to the 50–80 mg Mg/L usually recommended in hydroponics (Table 2), and 100% of N, to which tomato is particularly sensitive, was present in the form of NH4+. The rapid uptake of NH4+ can increase the NH3 concentration within the plant cells and is highly toxic [163]. The increasing demand for carbon skeletons that are required to detoxify this excess NH3 can affect plant growth negatively [120,121,122,123,124,125,163]. Excess NH4+ assimilation also typically generates Mg deficiency, which decreases plant productivity and results in poor fruit quality [106,163,164,165]. As a result, while an N-NH4+:N-NO3− ratio of 25:75 can be recommended for most plants [124,126,127,128,129], a maximum ratio of 10:90 is more suitable for tomatoes [164]. Hence, to counteract NH3 toxicity and improve the N-NH4+:N-NO3− ratio, Liedl et al. (2004)b [162] first heated and air-sparged the solution, which had the effect of removing more than 75% of the total ammonia nitrogen (TAN). Then, calcium nitrate (Ca(NO3)2) was added to adjust the total N to that of the chemical solution. Finally, magnesium sulfate (MgSO4) was added to reach the Mg concentrations of the chemical solution (108 mg/L). With these different adjustments, the digestate nutrient solution resulted in yields similar to those obtained with chemical fertilizer. Mupambwa et al. (2019) [158] made similar observations using the liquid fraction of cow manure digestate on bioponically grown tomatoes. When used as the sole fertilizer, the digestate solution concentrated at 10%—the dilution ratio established to avoid phytotoxicity—did not contain sufficient N and P macro-nutrients for efficient plant growth. Additionally, even when supplemented with chemical fertilizer, the digestate treatments resulted in 275% lower fruit yield on average in comparison to the control treatment, although positive effects on fruit sugar content were observed.
Table 5 summarizes the main studies mentioned above, with the organic materials and anaerobic digestion method used.

Table 5.
Main organic materials and method of anaerobic digestion for the production of nutrient solution used in different types of bioponics.
3.3.3. Drawbacks of the Method
The use of anaerobic digestates to produce nutrient solutions for bioponics leads to rather heterogenous results, caused by potential phytotoxicity of the digestate [25,41,44]. The latter is associated with several aspects.
Firstly, the digestates are often reported to have high levels of BOD, chemical oxygen demand (COD), and residual organic compounds, depending on the efficiency of mineralization [29,78,150]. As already mentioned for the tea method, these aspects can have adverse effects on plant growth because undesired or excessive microbial growth may develop in the bioponic system and asphyxiate plant roots and other beneficial microorganisms [110,131,132,135]. Additionally, residual organic compounds may include plant-toxic compounds [110]. Secondly, digestates usually have a high pH (7–8) [78,150]. This can have adverse effects on nutrient bioavailability and plant growth, as a pH of 5.5–6.5 is usually recommended [1,106]. Furthermore, when the pH in the solution reaches values above 7.5, which was the case for most of the studies mentioned above [25,26,41,104,158], a significant portion of NH4+ in solution is converted into NH3, which is toxic to plants even at low concentrations [166,167,168]. Thirdly, digestates often have an unbalanced N-NH4+/N-NO3− ratio, relative to the usually recommended 25:75 ratio [124,126,127,128,129]. As nitrification cannot take place in anaerobic conditions, NH4+ is the predominant form of mineral N in the digestate and can be found in very large quantities [120,121,122,123,124,125,169]. These too-high levels of BOD, COD, toxic organic compounds and/or NH4+-NH3 explain why digestates are usually strongly diluted, i.e., between 1:4 and 1:100 [25,26,41,44,104,158,162]. However, a compromise has to be found to avoid nutrient deficiencies. Scarcity in Mg, Ca and P has been observed in several studies when using only the liquid fraction of digestate [26,158,162]. Although digestates inherit the mineral characteristics of the original material they are derived from, these low concentrations can also be explained by the very nature of AD: the newly mineralized nutrients P, Mg and Ca tend to precipitate during the AD process in the form of calcium or magnesium phosphate, due to a rise in pH that often occurs during AD [78,150]. This contrasts with the newly mineralized N in the form of NH4+, which mostly remains in solution [150]. Therefore, the liquid fraction of digestates tends to have low concentrations of P, Mg and Ca but be enriched in N minerals, while it is the opposite for the solid fraction [78,150,155].
3.3.4. Discussion on the Method and Potential Lines of Improvement
AD of organic materials can produce organic fertilizer for bioponics, even though several challenges have to be met. If carried out efficiently, this process can mineralize and stabilize all kinds of organic residues [78,150]. Compared to aerobic mineralization, AD is a simpler process with lower energy demand, as it does not require intensive air pumps [136]. It also has the potential to produce biogas and provide an additional resource to the stakeholders, even smallholder farmers, as biogas production can be achieved on a small scale in a low-tech manner.
In crops with a short lifecycle and a relatively low nutrient demand such as lettuce, digestate solution can completely replace chemical fertilizers without facing any adverse effects, even improving the nutritional quality of the produces [25,26]. However, these positive results were obtained only when the digestates were sufficiently diluted, highlighting potential phytotoxicity associated with various aspects: Potential excessive BOD, COD and residual organic compounds; high pH; imbalanced NH4+/NO3− ratio and too-high NH4+-NH3 levels. Furthermore, digestate-based solutions can be depleted in P, Ca and Mg [26,158,162] as liquid digestate tends to be depleted in P, Ca and Mg after liquid-solid separation. Several lines of action can be explored to avoid these deficiencies. Firstly, ways of recovering minerals of the solid fraction should be further explored [78,150], e.g., its use as a growing medium. Secondly, an external supply of nutrients can be made: MgSO4 salts can be added to the nutrient solution to counteract Mg deficiency, giving good results in tomatoes [162], or via foliar application. In the case of P and Ca, phosphoric acid (H3PO4), calcium carbonate or calcium hydroxide during bioponic cultivation could have the double benefit of acting as pH regulators while supplementing the plants in P and Ca minerals. Organic nutrient sources rich in P and Ca could also be used, such as bone meal (Table 1). P can also be added via the foliar application of KH2PO4 [170]. The efficient control of the pH to maintain it below 7.5 during cultivation is a key factor to avoid mineral deficiencies, as digestate often has high initial pH values (8–8.5) that can result in the formation of insoluble calcium phosphate compounds in the nutrient solution [171,172,173]. Finally, the original feedstock for AD should be chosen wisely, according to the plant’s needs. For instance, digestates derived from poultry litter or swine effluent will be more suitable for high-N-demanding crops such as vegetables because they are particularly rich in nitrogen [78] (Table 1). On the other hand, digestates derived from dairy feedstock and plant residues will be more suitable for high K- and P-demanding plants such as leguminous crops or plants at the blooming or reproductive phase (e.g., tomato) because these materials usually have more balanced concentrations of K, P and N (Table 1) [78].
Besides nutrient scarcities, the imbalance of the N-NH4+/N-NO3− ratio and too high NH4+-NH3 concentrations appear to make digestates unsuitable as sole fertilizers of high-nutrient-demanding plants particularly sensitive to NH4+, e.g., tomato. Several strategies could be tested to counteract NH4+ toxicity. Firstly, a balance of the N-NH4+/N-NO3− ratio can be reached by adding N-NO3−, e.g., calcium nitrate, and possibly removing N-NH4+, e.g., by heating and air sparging the solution [162]. Secondly, high N-NH4+ uptake by the plant roots acidifies the nutrient solution, which can be deleterious for optimal nutrient bioavailability [1,106,163,164]. As a consequence, efficient pH management to maintain the pH between 5.5 and 7 is a way of hindering N-NH4+ toxicity. Thirdly, adding silicon (Si) to the bioponic solution could be a promising avenue in bioponics because Si is known to mitigate N-NH4+ toxicity in soils [174,175]. Si supply has already been used in hydroponics as a way of mitigating salt stress on plants via potassium silicate or calcium silicate addition [174,176,177]. The field of application of Si could thus be explored and widened to bioponics to deal with excess N-NH4+ in the nutrient solution and other biotic or abiotic stresses. The high N-NH4+/N-NH3+ ratio found in digestate solutions could also be more acceptable in certain contexts. A ratio of 1:1 can be suitable to reduce the negative impacts of abiotic stress in salinity conditions, resulting in a higher yield and better crop quality [173,178]. Finally, carrying out a second digestion process in aerobic conditions after AD could convert NH4+ into NO3− through nitrification.
3.4. Method Combining Anaerobic Digestion and Microbial Aerobic Mineralization
3.4.1. Principles
Aerobic mineralization could mitigate digestate drawbacks by allowing nitrification, and potentially further mineralizing the remaining organic compounds which would result in a decrease in BOD, DOC, organic acid levels and the N-NH4+/N-NO3− ratio. Moreover, nitrification is a biochemical process that acidifies the medium and can address the issue of the too-high pH of digestates (7.5–8.5) [78]. This could help solubilize other minerals in digestate solutions such as P, Ca or Mg. In Botheju et al.’s (2010) [29] study, the aerobic mineralization of an anaerobic digestate using sequential batch reactors and nitrifying bacteria inoculum not only improved the N-NH4+/N-NO3− ratio of the digestate (more than 75% of N-NH4+ conversion into N-NO3−), but also improved its stability (40% reduced COD and improved aesthetic quality, i.e., an odorless and translucent solution). Additionally, the pH went down from 8 in the original digestate to 5 once nitrified. Heavy metal levels were also reduced, as they remained fixed in the sludge during nitrification through adsorption and sedimentation. Similarly, in Zhang et al.’s (2011) [179] study, the aerobic treatment of pig manure digestate via intermittently aerated sequencing batch reactors showed high nitrification efficiency (71–79%), and COD reduction (89–99%). Parravicini et al. (2008) [180] observed that the aerobic treatment of sewage sludge digestate induced a 16% decrease in the remaining organic content.
3.4.2. Use of Combined Anaerobic Digestion and Aerobic Mineralization Methods for Bioponic Cultivation
With the aim of producing Pak choy (Brassica rapa) in bioponics, Bergstrand et al. (2020) [2] carried out a 2-week nitrification treatment of an anaerobic digestate, using aeration and bio-carriers. During the aerobic treatment, the pH was maintained at 5.5–6.0 by adding un-nitrified digestate (pH 8.0), as nitrification lowered the pH. Following this step, the nitrified digestate was diluted at three different ratios and incorporated into hydroponic systems, according to an EC of 1, 2 or 3 ms/cm. While the product quality was improved in some aspects, i.e., increased vitamin K, beta-carotene and lutein content, the mean fresh weights were 50% lower than under the control conditions, with lower nutrient (N, P, S) content in the leaves. The P, Ca and Mg concentrations in the nutrient solutions were below the typically recommended optimal concentrations, even for the least diluted treatment (8.2 mg/L of P, 99 mg/L of Ca, 8.6 mg/L of Mg in the EC 3 ms/cm treatment, as opposed to the recommended 15–30 mg/L of P, 150–200 mg/L of Ca, 50–80 mg/L of Mg). The nutrient composition of the nitrified digestates also suggests that the nitrification process was not optimal/efficient: relatively high amounts of NH4+ and NO2− remained in the solution when introduced in the hydroponic system, i.e., an N-NO3−:N-NO2−:N-NH4+ ratio of 1.25:1:1 for all three diluted solutions (Table 2). NO2− is well known to be toxic to plants, even at low concentrations [181]. Pelayo Lind et al. (2021) [30] obtained encouraging results on Bok choy (Brassica rapa). They tested the impact of nitrification on a plant-derived anaerobic digestate directly in hydroponic systems and/or prior to its use in the recirculating systems via bioreactors integrated with and/or external to the system. The bioreactors consisted in moving bed biofilm reactors of 100 L barrels with air pumps and biofilm carriers previously inoculated with active sludge from a municipal water treatment plant. When the digestate was sieved and diluted to an appropriate N-NH4+ concentration (200 mg/L), all bioreactors performed nitrification efficiently, and satisfactory yields were obtained. The P, K and Mg concentrations were little impacted by the process (+7.9% P, +4.2% K, −8.7% Mg). The macro and micronutrient concentrations in the final nitrified solution were similar to the ones of the chemical control, except for Ca (−30.9%). The fact that bioreactors were integrated into and/or external to the hydroponic system did not influence plant growth, but pH management did. Yields similar to those obtained with a chemical fertilizer were achievable when the pH in the hydroponic system was automatically controlled during plant growth (i.e., maintained around 6) via the addition of un-nitrified digestate. This method could limit the use of mineral acids in bioponics. In the absence of automation, adding relatively large volumes of digestates every 48 h resulted in wide pH variations (5.0–7.0), which most likely negatively affected plant nutrient uptake. These observations show the importance of pH dynamics and the potential of efficient pH management in bioponics, which have been studied very little so far. Tomato—a more demanding plant particularly sensitive to NH4+—was successfully cultivated by Kechasov et al. (2021) [105] using aerobic mineralization from a pig manure digestate. The biological process was carried out by a nitrification bioreactor integrated with a closed-loop recirculating hydroponic system, similar to an aquaponic system. The organic-based nutrient solution resulted in reduced growth in comparison to conventional hydroponics, but increased fruit size so that the resulting yield was similar under the two conditions. The reduced growth was attributed to lower nutrient content in the organic solution (21.0 ± 15.4 mg/L of N-NO3−, 26.1 ± 16.7 mg/L of P, 30.9 ± 24.6 mg/L of K, 16.8 ± 4.4 mg/L of Ca and 4.8 ± 1.9 mg/L of Mg) (Table 2). However, nitrification was efficient, and no phytotoxicity was observed: N-NH4+ and N-NO2− concentrations remained below 5 mg/L (Table 2).
These studies suggest that an effective nitrification step and efficient pH management of the nutrient solution during plant cultivation can make anaerobic digestates effective as sole fertilizers in bioponics by suppressing or mitigating potential digestate phytotoxicity. Aerobic mineralization can be performed just after AD, but it can also start/continue in the hydroponic systems [30,105], using a biofilter to reinforce the process, as discussed in Section 3.2.3. This would make it possible to add anaerobic digestate during plant cultivation and thus better meet the plant’s needs. Notably, the method developed by Shinohara et al. (2011) [21] can be applied to all kinds of anaerobic digestates, with the creation of bacterial inoculum and controlled addition of digestate in the hydroponic systems to promote nitrification.
Table 6 summarizes the main studies mentioned above, with the organic materials and the combined anaerobic-aerobic degradation method used.

Table 6.
Main organic materials and method of combined anaerobic and aerobic degradation for the production of nutrient solution used in different types of bioponics.
3.4.3. Discussion on the Method
AD followed by aerobic mineralization appears to be a promising way of producing nutrient solutions for bioponics because it combines the benefits of the two methods. When performed efficiently, the first step of AD results in the effective degradation of various organic materials, even plant residues, associated with biogas production. The resulting digestate is a rather homogenized product, free of the majority of pathogens, with high levels of inorganic N in the form of NH4+ [78,150] that can be transformed into NO3− via aerobic mineralization. The aerobic process also results in a lower pH and a decrease in the remaining organic compounds more suitable for plant cultivation [29]. However, this method still faces challenges. The aerobic step and more specifically the nitrification process need to be efficient and fully completed. If not, NO2− produced during the nitrification process can accumulate in the solution [2], and be highly toxic to plants [181]. NO2− accumulation can result from low DO and high levels of NH4+-NH3, as the development of nitrite-oxidizing bacteria can be inhibited and outperformed by ammonium-oxidizing microorganisms [29,145,146,179]. The NH4+ concentration at the initial stage of digestate mineralization should thus be controlled, and oxygenation of the solution should be effective. As discussed in Section 3.2.4, using a microbubble generator to better oxygenate the solution and adding a solid removal compartment during the aerobic stage and/or plant cultivation could improve nitrification efficiency and mineralization, and needs to be further investigated.
Another challenge of this combined method is the complete fertilization of plants by the sole addition of digestate. If the digestate—and more generally the primary organic materials of AD—is limited for P, Ca, K, or other essential minerals; the impact of the method on plant growth will be limited, despite an efficient technique. This brings us back to the choice of the organic materials used for producing nutrient solutions, which should be made wisely (Section 2), and to the ways of compensating for the typical nutrient deficiencies of liquid digestates (Section 3.3.4). The use of the solid fraction of digestates and/or the addition of components during plant cultivation to counteract specific nutrient deficiencies should be further studied. Controlling the pH is also determining the efficacy of the method on plants [2,30,105], and can be optimized using automatic pH control devices. However, this type of equipment, as well as the other devices that this technique could require (an efficient solid removal compartment, an efficient oxygen pump and regular mineral analyses of NO2−, NO3− and NH4+) can be limiting factors for smallholder farmers in remote areas or in developing countries who do not have access to those facilities.
4. General Discussion and Perspectives
The development of methods for producing nutrient solutions based on organic waste is crucial for the deployment of sustainable hydroponics throughout the world. In the last three decades, various methods have been used and tested. This review (1) described the main currently used organic materials, and (2) characterized and categorized the different methods of producing organic nutrient solutions, resulting in four groups based on the main biochemical processes involved: (1) the “tea”-type method, (2) the aerobic microbial degradation method, (3) the anaerobic digestion (AD) method, and (4) the combined anaerobic and aerobic degradation method. While the last three techniques involve in-depth decomposition and mineralization processes of organic materials via relatively long microbial processing, the tea method involves simple dissolving or “maceration” of organic materials over a short time period, solubilization of the minerals already present in the materials, and a low level of mineralization. The resulting tea-based nutrient solution strongly depends on the quality of the primary organic materials and their ability to meet the plant’s needs. This rapid method requires little technical expertise and equipment and could be adapted to short life-cycle crops, and to organic materials rich in nutrients in their mineral form or rapidly mineralizable. Efficient pre-composting of the organic materials before maceration can greatly improve the quality of the solution in relation to the plant’s needs. Conversely, the methods involving in-depth decomposition and mineralization of organic matter can be suitable for more crops, and more types of organic materials can be used. Among them, the AD of materials can lead to a mineral-rich fertilizer made from diverse organic sources, while combining biogas production. However, AD results in high concentrations of ammonium (NH4+), making this method unsuitable for plants particularly sensitive to it (e.g., tomato). The liquid fraction of the digestates also tends to be limiting in P, Ca and Mg, as opposed to the solid fraction. Therefore, the efficient use of biogas digestates in the long life-cycle crops with a high nutrient demand can require adding essential nutrients; the recovery of the solid fraction of the digestate; as well as efficient pH management during plant cultivation. In the meantime, ways of counteracting NH4+ toxicity can also be explored, as well as contexts in which N supply mostly in the form of NH4+ can be positive. Aerobic mineralization following AD, before and/or during cultivation by adding a biofilter compartment to the hydroponic system can also respond to this issue by allowing NH4+ to be converted into nitrate (NO3−) via aerobic nitrification. NO3− can then become the major form of mineral N, preferably taken up by most plants. Together with a solid removal compartment, the use of an oxygenated biofilter in the hydroponic system as a compartment specifically dedicated to mineralization and nitrification can significantly improve the nutrient solution quality and allow adding organic fertilizers during plant cultivation. This form of bioponics, in which the main mineralization process is carried out within the hydroponic system as in aquaponics, could be termed “in-situ bioponics”. It can be opposed to “ex-situ bioponics”, which creates a liquid biofertilizer before plant cultivation, with no compartment dedicated to mineralization within the hydroponic system (e.g., the tea method and AD). Aerobic microbial degradation potentially allows for the cultivation of all kinds of crops, provided that it is led efficiently; otherwise, phytotoxic intermediates may accumulate. This method is complex because it requires technical knowledge and efficient control of the physicochemical parameters in the solution. It is also more energy-intensive because constant oxygenation of the solution is needed.
Each method has its advantages and drawbacks, depending on the context and the crop. Moreover, regardless of the method, the type of initial organic input obviously remains a crucial factor in the quality of the nutrient solution obtained and its effectiveness on plants. Potential nutrient deficiencies or excesses, or the presence of toxic substances in the organic sources remain major challenges for bioponics and its performance, even with efficient and optimized methods. Although some research has been made, more studies are required to expand the knowledge and potential of this technique and make it efficient and accessible to the general public. They should include the study of (1) the dynamics and management of the main physicochemical parameters; (2) the microbiota to better understand the biochemical processes and optimize the method, and explore the potential of plant-growth-promoting microorganisms; (3) the mineral losses occurring during the decomposition and mineralization of the organic materials, and notably the potential pollution generated by the process; (4) the health risks associated with the use of organic materials for bioponics, and ways of mitigating these risks and (5) socio-economic and financial analyses of bioponics to assess its feasibility and viability in different contexts. The research subjects are diverse, and the potential of this technique remains to be explored.
Author Contributions
Writing—original draft preparation and editing, I.S.; Review and editing, I.S. and M.H.J.; Supervision M.H.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was produced with the financial support of the European Union, within the framework of the SWIM project (funding EC ENI/2020/417-484). Its content is the sole responsibility of the University of Liège and does not necessarily reflect the opinions of the European Union.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the writing of the manuscript; or in the decision to publish the study.
References
- Resh, H.M. Hydroponic Food Production: A Definitive Guidebook for the Advanced Home Gardener and the Commercial Hydroponic Grower, 7th ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar] [CrossRef]
- Bergstrand, K.-J.; Asp, H.; Hultberg, M. Utilizing Anaerobic Digestates as Nutrient Solutions in Hydroponic Production Systems. Sustainability 2020, 12, 10076. [Google Scholar] [CrossRef]
- Barman, N.C.; Hasan, M.M.; Islam, M.R.; Banu, N.A. A Review on Present Status and Future Prospective of Hydroponics Technique. Plant Environ. Dev. 2016, 5, 1–7. [Google Scholar]
- Gonnella, M.; Renna, M. The Evolution of Soilless Systems towards Ecological Sustainability in the Perspective of a Circular Economy. Is It Really the Opposite of Organic Agriculture? Agronomy 2021, 11, 950. [Google Scholar] [CrossRef]
- Arancon, N.Q.; Owens, J.D.; Converse, C. The effects of vermicompost tea on the growth and yield of lettuce and tomato in a non-circulating hydroponics system. J. Plant Nutr. 2019, 42, 2447–2458. [Google Scholar] [CrossRef]
- Velazquez-Gonzalez, R.S.; Garcia-Garcia, A.L.; Ventura-Zapata, E.; Barceinas-Sanchez, J.D.O.; Sosa-Savedra, J.C. A Review on Hydroponics and the Technologies Associated for Medium- and Small-Scale Operations. Agriculture 2022, 12, 646. [Google Scholar] [CrossRef]
- Salas, M.; Verdejo, M.; Sanchez, A.; Guzman, M.; Valenzuela, J.; Montero, J. Vertical gardening. Adaptation of hydroponic systems and ornamental species. Acta Hortic. 2012, 937, 1153–1160. [Google Scholar] [CrossRef]
- Basosi, R.; Spinelli, D.; Fierro, A.; Jez, S. Mineral Nitrogen Fertilizers: Environmental Impact of Production and Use; NOVA Science Publishers: New York, NY, USA, 2014; pp. 1–43. [Google Scholar]
- Cordell, D.; Drangert, J.-O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- U.S. Geological Survey. U.S. Mineral Commodity Summaries 2021; U.S. Geological Survey: Reston, VA, USA, 2021. [CrossRef]
- U.S. Geological Survey. USGS: Mineral Commodity Summaries, Phosphate Rock; U.S. Geological Survey: Reston, VA, USA, 2000.
- U.S. Geographical Survey. USGS: Mineral Commodity Summaries, Phosphate Rock; U.S. Geological Survey: Reston, VA, USA, 2011.
- Van Kauwenbergh, S.J. World Phosphate Rock Reserves and Resources; IFDC: Muscle Shoals, AL, USA, 2010. [Google Scholar]
- Kisinyo, P.O.; Opala, P.A. Depletion of phosphate rock reserves and world food crisis: Reality or hoax? Afr. J. Agric. Res. 2020, 16, 1223–1227. [Google Scholar] [CrossRef]
- Edixhoven, J.D.; Gupta, J.; Savenije, H.H.G. Recent revisions of phosphate rock reserves and resources: A critique. Earth Syst. Dyn. 2014, 5, 491–507. [Google Scholar] [CrossRef]
- Van Vuuren, D.; Bouwman, L.; Beusen, A. Phosphorus demand for the 1970–2100 period: A scenario analysis of resource depletion. Glob. Environ. Chang. 2010, 20, 428–439. [Google Scholar] [CrossRef]
- Hebebrand, C.; Laborde, D. High Fertilizer Prices Contribute to Rising Global Food Security Concerns. IFPRI: International Food Policy Research Institute. Available online: https://www.ifpri.org/blog/high-fertilizer-prices-contribute-rising-global-food-security-concerns (accessed on 6 October 2022).
- Chianu, J.N.; Chianu, J.N.; Mairura, F. Mineral Fertilizers in the Farming Systems of Sub-Saharan Africa. A Review. Agron. Sustain. Dev. 2012, 32, 545–566. [Google Scholar] [CrossRef]
- Sunaryo, Y.; Purnomo, D.; Darini, M.T.; Cahyani, V.R. Effects of goat manure liquid fertilizer combined with AB-MIX on foliage vegetables growth in hydroponic. IOP Conf. Ser. Earth Environ. Sci. 2018, 129, 012003. [Google Scholar] [CrossRef]
- Shubha, K.; Mukherjee, A.; Dubey, A.; Koley, T.K. Bioponics—A New Way to Grow Soilless Vegetable Cultivation. Agric. Food 2019, 1. [Google Scholar] [CrossRef]
- Shinohara, M.; Aoyama, C.; Fujiwara, K.; Watanabe, A.; Ohmori, H.; Uehara, Y.; Takano, M. Microbial mineralization of organic nitrogen into nitrate to allow the use of organic fertilizer in hydroponics. Soil Sci. Plant Nutr. 2011, 57, 190–203. [Google Scholar] [CrossRef]
- Giménez, A.; Fernández, J.A.; Pascual, J.A.; Ros, M.; Egea-Gilabert, C. Application of Directly Brewed Compost Extract Improves Yield and Quality in Baby Leaf Lettuce Grown Hydroponically. Agronomy 2020, 10, 370. [Google Scholar] [CrossRef]
- El-Shinawy, M.; Abd-Elmoniem, E.; Abou-Hadid, A. The use of organic manure for lettuce plants grown under NFT conditions. Acta Hortic. 1999, 491, 315–318. [Google Scholar] [CrossRef]
- Mowa, E.; Kalili, M.; Akundabweni, L.; Chimwamurombe, P. Impact of Organic Hydroponic Nutrient Solution on Tomato Fruit Quality. Int. Sci. Technol. J. Namib. 2018, 12, 62–77. [Google Scholar]
- Liu, W.; Du, L.; Yang, Q. Biogas slurry added amino acids decreased nitrate concentrations of lettuce in sand culture. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2009, 59, 260–264. [Google Scholar] [CrossRef]
- Wang, L.; Guo, S.; Wang, Y.; Yi, D.; Wang, J. Poultry biogas slurry can partially substitute for mineral fertilizers in hydroponic lettuce production. Environ. Sci. Pollut. Res. 2019, 26, 659–671. [Google Scholar] [CrossRef]
- Gorenjak, A.H.; Cencic, A. Nitrate in vegetables and their impact on human health. A review. Acta Aliment. 2013, 42, 158–172. [Google Scholar] [CrossRef]
- Jin, E.; Cao, L.; Xiang, S.; Zhou, W.; Ruan, R.; Liu, Y. Feasibility of using pretreated swine wastewater for production of water spinach (Ipomoea aquatic Forsk.) in a hydroponic system. Agric. Water Manag. 2020, 228, 105856. [Google Scholar] [CrossRef]
- Botheju, D.; Svalheim, O.; Bakke, R. Digestate Nitrification for Nutrient Recovery. Open Waste Manag. J. 2010, 3, 1–12. [Google Scholar] [CrossRef]
- Lind, O.P.; Hultberg, M.; Bergstrand, K.-J.; Larsson-Jönsson, H.; Caspersen, S.; Asp, H. Biogas Digestate in Vegetable Hydroponic Production: pH Dynamics and pH Management by Controlled Nitrification. Waste Biomass-Valorization 2021, 12, 123–133. [Google Scholar] [CrossRef]
- Wongkiew, S.; Hu, Z.; Chandran, K.; Lee, J.W.; Khanal, S.K. Nitrogen transformations in aquaponic systems: A review. Aquac. Eng. 2017, 76, 9–19. [Google Scholar] [CrossRef]
- Williams, K.; Nelson, J.S. Challenges of using organic fertilizers in hydroponic production systems. Acta Hortic. 2016, 1112, 365–370. [Google Scholar] [CrossRef]
- Calvet, R.; Chenu, C.; Houot, S. Les Matières Organiques des Sols: Rôles Agronomiques et Environnementaux, 2nd ed.; Editions France Agricole: Paris, France, 2015. [Google Scholar]
- Tikasz, P.; MacPherson, S.; Adamchuk, V.; Lefsrud, M. Aerated chicken, cow, and turkey manure extracts differentially affect lettuce and kale yield in hydroponics. Int. J. Recycl. Org. Waste Agric. 2019, 8, 241–252. [Google Scholar] [CrossRef]
- Atkin, K.; Nichols, M. Organic Hydroponics. Acta Hortic. 2004, 648, 121–127. [Google Scholar] [CrossRef]
- Fang, W.; Chung, H. Bioponics for lettuce production in a plant factory with artificial lighting. Acta Hortic. 2018, 1227, 593–598. [Google Scholar] [CrossRef]
- Zandvakili, O.R.; Barker, A.V.; Hashemi, M.; Etemadi, F.; Autio, W.R. Comparisons of commercial organic and chemical fertilizer solutions on growth and composition of lettuce. J. Plant Nutr. 2019, 42, 990–1000. [Google Scholar] [CrossRef]
- Zhai, Z.; Ehret, D.L.; Forge, T.; Helmer, T.; Lin, W.; Dorais, M.; Papadopoulos, A.P. Organic Fertilizers for Greenhouse Tomatoes: Productivity and Substrate Microbiology. HortScience 2009, 44, 800–809. [Google Scholar] [CrossRef]
- Priadi, D.; Nuro, F. Seedling Production of Pak Choy (Brassica rapa L. var chinensis) using Organic and Inorganic Nutrients. Biosaintifika J. Biol. Biol. Educ. 2017, 9, 217–224. [Google Scholar] [CrossRef][Green Version]
- Kawamura-Aoyama, C.; Fujiwara, K.; Shinohara, M.; Takano, M. Study on the Hydroponic Culture of Lettuce with Microbially Degraded Solid Food Waste as a Nitrate Source. Jpn. Agric. Res. Quart. 2014, 48, 71–76. [Google Scholar] [CrossRef]
- Phibunwatthanawong, T.; Riddech, N. Liquid organic fertilizer production for growing vegetables under hydroponic condition. Int. J. Recycl. Org. Waste Agric. 2019, 8, 369–380. [Google Scholar] [CrossRef]
- Mowa, E. Organic Manure for Vegetable Production under Hydroponic Conditions in Arid Namibia. Int. Sci. Technol. J. Namib. 2015, 5, 3–12. [Google Scholar]
- Wongkiew, S.; Koottatep, T.; Polprasert, C.; Prombutara, P.; Jinsart, W.; Khanal, S.K. Bioponic system for nitrogen and phosphorus recovery from chicken manure: Evaluation of manure loading and microbial communities. Waste Manag. 2021, 125, 67–76. [Google Scholar] [CrossRef]
- Liedl, B.; Cummins, M.; Young, A.; Williams, M.; Chatfield, J. Hydroponic lettuce production using liquid effluent from poultry waste bioremediation as a nutrient source. Acta Hortic. 2004, 659, 721–728. [Google Scholar] [CrossRef]
- Leudtke, B. Use of Compost Tea as a Nutrient Amendment for Plant Growth in a Re-Circulating Hydroponic System; University of Wisconsin System: Madison, WI, USA, 2010. [Google Scholar]
- Latique, S.; Chernane, H.; El Kaoua, M. Seaweed Liquid Fertilizer Effect on Physiological and Biochemical Parameters of Bean Plant (Phaesolus Vulgaris Variety Paulista) under Hydroponic System. Eur. Sci. J. 2013, 9, 174–191. [Google Scholar]
- Yusuf, R.; Laude, S.; Alfiana; Syakur, A.; Ramli. The potential of seaweed used as hydroponic solution on the growth and yields of lettuce (Lactuca sativa L.). IOP Conf. Ser. Earth Environ. Sci. 2021, 653, 012065. [Google Scholar] [CrossRef]
- Steveni, C.M.; Norrington-Davies, J.; Hankins, S.D. Effect of seaweed concentrate on hydroponically grown spring barley. J. Appl. Phycol. 1992, 4, 173–180. [Google Scholar] [CrossRef]
- Saijai, S.; Ando, A.; Inukai, R.; Shinohara, M.; Ogawa, J. Analysis of microbial community and nitrogen transition with enriched nitrifying soil microbes for organic hydroponics. Biosci. Biotechnol. Biochem. 2016, 80, 2247–2254. [Google Scholar] [CrossRef]
- Baweja, P.; Kumar, S.; Kumar, G. Organic Fertilizer from Algae: A Novel Approach Towards Sustainable Agriculture. In Biofertilizers for Sustainable Agriculture and Environment; Springer: Amsterdam, The Netherlands, 2019; pp. 353–370. [Google Scholar] [CrossRef]
- Da Silva, E.F.; Melo, M.F.; Sombra, K.E.S.; Silva, T.S.; De Freitas, D.F.; da Costa, M.E.; Da Silva Santos, E.P.; da Silva, L.F.; Serra, A.P.; de Morais Cavalcante Neitzke, P.R. Organic Nitrogen in Agricultural Systems. In Nitrogen Fixation; IntechOpen: London, UK, 2020; pp. 1–22. [Google Scholar] [CrossRef]
- Shaji, H.; Chandran, V.; Mathew, L. Organic fertilizers as a route to controlled release of nutrients. In Controlled Release Fertilizers for Sustainable Agriculture; Lewu, F.B., Volova, T., Thomas, S., Rakhimol, K.R., Eds.; Academic Press: New York, NY, USA, 2020; pp. 231–245. [Google Scholar] [CrossRef]
- Green, B. Fertilizers in aquaculture. In Feed and Feeding Practices in Aquaculture; Woodhead Publishing: Sawston, Cambridge, UK, 2015; pp. 27–52. [Google Scholar] [CrossRef]
- Eghball, B.; Wienhold, B.J.; Gilley, J.E.; Eigenberg, R.A. Mineralization of Manure Nutrients. J. Soil Water Conserv. 2002, 57, 470–473. [Google Scholar]
- Goss, M.J.; Tubeileh, A.; Goorahoo, D. A Review of the Use of Organic Amendments and the Risk to Human Health. Adv. Agron. 2013, 120, 275–379. [Google Scholar] [CrossRef]
- Viskari, E.-L.; Grobler, G.; Karimäki, K.; Gorbatova, A.; Vilpas, R.; Lehtoranta, S. Nitrogen Recovery with Source Separation of Human Urine—Preliminary Results of Its Fertiliser Potential and Use in Agriculture. Front. Sustain. Food Syst. 2018, 2, 32. [Google Scholar] [CrossRef]
- Yang, L.; Giannis, A.; Chang, V.W.-C.; Liu, B.; Zhang, J.; Wang, J.-Y. Application of hydroponic systems for the treatment of source-separated human urine. Ecol. Eng. 2015, 81, 182–191. [Google Scholar] [CrossRef]
- Barnett, G. Phosphorus forms in animal manure. Bioresour. Technol. 1994, 49, 139–147. [Google Scholar] [CrossRef]
- Sharpley, A.; Moyer, B. Phosphorus Forms in Manure and Compost and Their Release during Simulated Rainfall. J. Environ. Qual. 2000, 29, 1462–1469. [Google Scholar] [CrossRef]
- Yadav, B.K.; Sidhu, A.S. Dynamics of Potassium and Their Bioavailability for Plant Nutrition. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V., Maurya, B., Verma, J., Meena, R., Eds.; Springer: New Delhi, India, 2016; pp. 187–201. [Google Scholar] [CrossRef]
- Mikkelsen, R.; Hartz, T.K. Nitrogen Sources for Organic Crop Production. Better Crops 2008, 92, 16–19. [Google Scholar]
- Chittora, D.; Meena, M.; Barupal, T.; Swapnil, P.; Sharma, K. Cyanobacteria as a source of biofertilizers for sustainable agriculture. Biochem. Biophys. Rep. 2020, 22, 100737. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algal compost–toward sustainable fertilization. Rev. Inorg. Chem. 2013, 33, 161–172. [Google Scholar] [CrossRef]
- Abdullahi, U.A.; Khandaker, M.M.; Alias, N.; Shaari, E.M.; Alam, A.; Badaluddin, N.A.; Mohd, K.S. Seaweed effects on plant growth and environmental remediation: A review. J. Phytol. 2021, 13, 122–129. [Google Scholar] [CrossRef]
- Nabti, E.; Jha, B.; Hartmann, A. Impact of seaweeds on agricultural crop production as biofertilizer. Int. J. Environ. Sci. Technol. 2016, 14, 1119–1134. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Production of seaweed extracts by biological and chemical methods. In Marine Algae Extracts: Processes, Products, and Applications; Wiley: Weinheim, Germany, 2015; Volume 16, pp. 121–144. [Google Scholar] [CrossRef]
- Gale, E.S.; Sullivan, D.M.; Cogger, C.G.; Bary, A.I.; Hemphill, D.D.; Myhre, E.A. Estimating Plant-Available Nitrogen Release from Manures, Composts, and Specialty Products. J. Environ. Qual. 2006, 35, 2321–2332. [Google Scholar] [CrossRef] [PubMed]
- Fine, K.E.; Cole, J.C.; Penn, C.J. Nitrogen Mineralization from Canola Meal or Cottonseed Meal with or without Soapstock. HortScience 2013, 48, 891–896. [Google Scholar] [CrossRef]
- Jamir, L.; Kumar, V.; Kaur, J.; Kumar, S.; Singh, H. Composition, valorization and therapeutical potential of molasses: A critical review. Environ. Technol. Rev. 2021, 10, 131–142. [Google Scholar] [CrossRef]
- Kano, K.; Kitazawa, H.; Suzuki, K.; Widiastuti, A.; Odani, H.; Zhou, S.; Chinta, Y.; Eguchi, Y.; Shinohara, M.; Sato, T. Effects of Organic Fertilizer on Bok Choy Growth and Quality in Hydroponic Cultures. Agronomy 2021, 11, 491. [Google Scholar] [CrossRef]
- Hossain, M.Z.; von Fragstein, P.; Heß, J. Plant Origin Wastes as Soil Conditioner and Organic Fertilizer: A Review. J. Agric. Food Environ. Sci. 2016, 16, 1362–1371. [Google Scholar] [CrossRef]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils, 15th ed.; Pearson: Columbus, OH, USA, 2017. [Google Scholar]
- Moreno-Caselles, J.; Moral, R.; Perez-Murcia, M.D.; Perez-Espinosa, A.; Rufete, B. Nutrient value of animal manures in front of environmental hazards. Commun. Soil Sci. Plant Anal. 2002, 33, 3023–3032. [Google Scholar] [CrossRef]
- Pagliari, P.H.; Laboski, C.A.M. Investigation of the Inorganic and Organic Phosphorus Forms in Animal Manure. J. Environ. Qual. 2012, 41, 901–910. [Google Scholar] [CrossRef]
- Mukai, S.; Oyanagi, W. Decomposition characteristics of indigenous organic fertilisers and introduced quick compost and their short-term nitrogen availability in the semi-arid Ethiopian Rift Valley. Sci. Rep. 2019, 9, 16000. [Google Scholar] [CrossRef]
- Agus, F.; Setyorini, D.; Hartatik, W.; Lee, S.-M.; Sung, J.-K.; Shin, J.-H. Nutrient Balance and Vegetable Crop Production as Affected by Different Sources of Organic Fertilizers. Korean J. Soil Sci. Fertil. 2009, 42, 1–13. [Google Scholar]
- Wortmann, C.S.; Shapiro, C.A. Composting Manure and Other Organic Materials. NebGuide G1315, University of Nebraska Extension. 2012, pp. 1–4. Available online: https://extensionpubs.unl.edu/publication/9000016363380/composting-manure-and-other-organic-materials/ (accessed on 1 December 2022).
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Sengupta, A.; Banerjee, H. Soil-Less Culture in Modern Agriculture. J. Sci. Technol. 2012, 2, 103–108. [Google Scholar]
- Cho, W.-M.; Ravindran, B.; Kim, J.K.; Jeong, K.-H.; Lee, D.J.; Choi, D.-Y. Nutrient status and phytotoxicity analysis of goat manure discharged from farms in South Korea. Environ. Technol. 2016, 38, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Pratt, P.; Castellanos, J. Available Nitrogen from Animal Manures. Calif. Agric. 1981, 35, 24. [Google Scholar]
- Adediran, J.A.; Taiwo, L.B.; Sobulo, R.A. Effect of Organic Wastes and Method of Composting on Compost Maturity, Nutrient Composition of Compost and Yields of Two Vegetable Crops. J. Sustain. Agric. 2003, 22, 95–109. [Google Scholar] [CrossRef]
- Antil, R.S.; Singh, M. Effects of organic manures and fertilizers on organic matter and nutrients status of the soil. Arch. Agron. Soil Sci. 2007, 53, 519–528. [Google Scholar] [CrossRef]
- Hartz, T.K.; Johnstone, P.; Williams, E.; Smith, R. Establishing Lettuce Leaf Nutrient Optimum Ranges through DRIS Analysis. Hortscience 2007, 42, 143–146. [Google Scholar] [CrossRef]
- Basak, H.; Yigit, E. Comparative Effects of Ammonium Nitrate and Blood Meal on Plant Morphology and Leaf Coloring in Green Bean (Phaseolus Vulgaris L.) Seedlings under Salt Stress Condition. Int. J. Agric. Environ. Res. 2018, 4, 708–722. [Google Scholar]
- Genisel, M.; Erdal, S.; Turk, H.; Dumlupınar, R. The availability of bone powder as inorganic element source on growth and development in wheat seedlings. Toxicol. Ind. Health 2012, 28, 458–462. [Google Scholar] [CrossRef]
- Meng, X.; Huang, Q.; Xu, J.; Gao, H.; Yan, J. A review of phosphorus recovery from different thermal treatment products of sewage sludge. Waste Dispos. Sustain. Energy 2019, 1, 99–115. [Google Scholar] [CrossRef]
- Soobhany, N. Insight into the recovery of nutrients from organic solid waste through biochemical conversion processes for fertilizer production: A review. J. Clean. Prod. 2019, 241, 118413. [Google Scholar] [CrossRef]
- Mupambwa, H.A.; Mnkeni, P.N.S. Optimizing the vermicomposting of organic wastes amended with inorganic materials for production of nutrient-rich organic fertilizers: A review. Environ. Sci. Pollut. Res. 2018, 25, 10577–10595. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.-T.; Li, T.-P.; Zhang, Y.; Hu, J.; Bai, Y.-C.; Shan, Y.-H.; Ke, F. Effects of vermicompost amendment as a basal fertilizer on soil properties and cucumber yield and quality under continuous cropping conditions in a greenhouse. J. Soils Sediments 2017, 17, 2718–2730. [Google Scholar] [CrossRef]
- Lazcano, C.; Sampedro, L.; Zas, R.; Domínguez, J. Assessment of Plant Growth Promotion by Vermicompost in Different Progenies of Maritime Pine (Pinus pinaster Ait.). Compos. Sci. Util. 2010, 18, 111–118. [Google Scholar] [CrossRef]
- Suthar, S. Recycling of agro-industrial sludge through vermitechnology. Ecol. Eng. 2010, 36, 1028–1036. [Google Scholar] [CrossRef]
- Tan, J.P.; Jahim, J.M.; Wu, T.Y.; Harun, S.; Mumtaz, T. Use of corn steep liquor as an economical nitrogen source for biosuccinic acid production by Actinobacillus succinogenes. IOP Conf. Ser. Earth Environ. Sci. 2016, 36, 012058. [Google Scholar] [CrossRef]
- Roslan, M.; Sohedein, I.; Ling, P.; Sobri, Z.; Zuan, A.; Cheak, S.; Rahman, N. Sustainable Agronomic Valorization of Unsulfured Molasses and Defatted Soybean Meal as an Optimized Formulation of Bio-Organic Fertilizer Enriched with High Cell Density P-Solubilizing Bacteria. Agronomy 2021, 11, 996. [Google Scholar] [CrossRef]
- Andrews, E.; Kassama, S.; Smith, E.; Brown, P.; Khalsa, S. A Review of Potassium-Rich Crop Residues Used as Organic Matter Amendments in Tree Crop Agroecosystems. Agriculture 2021, 11, 580. [Google Scholar] [CrossRef]
- Damon, P.M.; Bowden, B.; Rose, T.; Rengel, Z. Crop residue contributions to phosphorus pools in agricultural soils: A review. Soil Biol. Biochem. 2014, 74, 127–137. [Google Scholar] [CrossRef]
- Mrad, F. Décomposition de Résidus de Culture et de Matériaux Biosourcés: Impact Sur Les Communautés Microbiennes des Sols Agricoles et Les Fonctions Associées. Ph.D. Thesis, Université de Rouen Normandie & UniLaSalle, Rouen, France, 2018. [Google Scholar]
- USDA; NRCS. Carbon to Nitrogen Ratios in Cropping Systems. 2011. Available online: https://soilhealthnexus.org/resources/soil-properties/soil-chemical-properties/carbon-to-nitrogen-ratio-cn/ (accessed on 7 March 2022).
- Raghunandan, B.L.; Vyas, R.V.; Patel, H.K.; Jhala, Y.K. Perspectives of Seaweed as Organic Fertilizer in Agriculture. In Soil Fertility Management for Sustainable Development; Springer: Singapore, 2019; pp. 267–289. [Google Scholar] [CrossRef]
- Olsson, J.; Toth, G.B.; Albers, E. Biochemical composition of red, green and brown seaweeds on the Swedish west coast. J. Appl. Phycol. 2020, 32, 3305–3317. [Google Scholar] [CrossRef]
- Cole, A.J.; Roberts, D.A.; Garside, A.L.; De Nys, R.; Paul, N.A. Seaweed compost for agricultural crop production. J. Appl. Phycol. 2016, 28, 629–642. [Google Scholar] [CrossRef]
- Charoenpakdee, S. Using Animal Manure to Grow Lettuce (Lactuca Sativa L.) in a Homemade Hydroponics System. Asia-Pac. J. Sci. Technol. 2014, 19, 256–261. [Google Scholar]
- Mowa, E.; Akundabweni, L.; Chimwamurombe, P.; Oku, E.; Mupanbwa, H.A. The influence of organic manure formulated from goat manure on growth and yield of tomato (Lycopersicum esculentum). Afr. J. Agric. Res. 2017, 12, 3061–3067. [Google Scholar] [CrossRef]
- Krishnasamy, K.; Nair, J.; Bäuml, B. Hydroponic system for the treatment of anaerobic liquid. Water Sci. Technol. 2012, 65, 1164–1171. [Google Scholar] [CrossRef]
- Kechasov, D.; Verheul, M.J.; Paponov, M.; Panosyan, A.; Paponov, I.A. Organic Waste-Based Fertilizer in Hydroponics Increases Tomato Fruit Size but Reduces Fruit Quality. Front. Plant Sci. 2021, 12, 680030. [Google Scholar] [CrossRef]
- Jones, J.B., Jr. Complete Guide for Growing Plants Hydroponically, 1st ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Arancon, N.Q.; Pant, A.; Radovich, T.; Hue, N.V.; Potter, J.K.; Converse, C.E. Seed Germination and Seedling Growth of Tomato and Lettuce as Affected by Vermicompost Water Extracts (Teas). HortScience 2012, 47, 1722–1728. [Google Scholar] [CrossRef]
- Shaban, H.; Fazeli-Nasab, B.; Alahyari, H.; Alizadeh, G.; Shahpesandi, S. An Overview of the Benefits of Compost Tea on Plant and Soil Structure. Adv. Biores. 2015, 6, 154–158. [Google Scholar]
- Ingham, E.R. The Compost Tea Brewing Manual, 5th ed.; Soil Foodweb Institute: Corvallis, OR, USA, 2005. [Google Scholar]
- Carballo, T.; Gil, M.; Calvo, L.F.; Moran, A. The Influence of Aeration System, Temperature and Compost Origin on the Phytotoxicity of Compost Tea. Compos. Sci. Util. 2009, 17, 127–139. [Google Scholar] [CrossRef]
- St. Martin, C.C.; Brathwaite, R.A. Compost and compost tea: Principles and prospects as substrates and soil-borne disease management strategies in soil-less vegetable production. Biol. Agric. Hortic. 2012, 28, 1–33. [Google Scholar] [CrossRef]
- Radovich, T.; Pant, A.; Hue, N.; Sugano, J.; Arancon, N. Promoting Plant Growth with Compost Teas. Food Provid. 2011, 7, 1–3. [Google Scholar]
- Scheuerell, S.; Mahaffee, W. Compost Tea: Principles and Prospects for Plant Disease Control. Compos. Sci. Util. 2002, 10, 313–338. [Google Scholar] [CrossRef]
- Hargreaves, J.C.; Adl, M.S.; Warman, P.R. Are compost teas an effective nutrient amendment in the cultivation of strawberries? Soil and plant tissue effects. J. Sci. Food Agric. 2009, 89, 390–397. [Google Scholar] [CrossRef]
- Haghighi, M.; Barzegar, M.R.; da Silva, J.A.T. The effect of municipal solid waste compost, peat, perlite and vermicompost on tomato (Lycopersicum esculentum L.) growth and yield in a hydroponic system. Int. J. Recycl. Org. Waste Agric. 2016, 5, 231–242. [Google Scholar] [CrossRef]
- Haller, H.; Jonsson, A.; Rayo, K.M.; López, A.D. Microbial transport of aerated compost tea organisms in clay loam and sandy loam—A soil column study. Int. Biodeterior. Biodegrad. 2016, 106, 10–15. [Google Scholar] [CrossRef]
- Zaccardelli, M.; Pane, C.; Villecco, D.; Palese, A.M.; Celano, G. Compost tea spraying increases yield performance of pepper (Capsicum annuum L.) grown in greenhouse under organic farming system. Ital. J. Agron. 2018, 13, 229–234. [Google Scholar] [CrossRef]
- Liguori, L.; Pane, C.; Albanese, D.; Celano, G.; Zaccardelli, M.; Di Matteo, M. Compost and Compost Tea Management of Mini Watermelon Cultivations Affects the Chemical, Physical and Sensory Assessment of the Fruits. Agric. Sci. 2015, 6, 117–125. [Google Scholar] [CrossRef]
- Anjana, S.U.; Iqbal, M. Nitrate accumulation in plants, factors affecting the process, and human health implications. A review. Agron. Sustain. Dev. 2007, 27, 45–57. [Google Scholar] [CrossRef]
- Tian, X.; Fang, Y.; Jin, Y.; Yi, Z.; Li, J.; Du, A.; He, K.; Huang, Y.; Zhao, H. Ammonium detoxification mechanism of ammonium-tolerant duckweed (Landoltia punctata) revealed by carbon and nitrogen metabolism under ammonium stress. Environ. Pollut. 2021, 277, 116834. [Google Scholar] [CrossRef] [PubMed]
- Esteban, R.; Ariz, I.; Cruz, C.; Moran, J.F. Review: Mechanisms of ammonium toxicity and the quest for tolerance. Plant Sci. 2016, 248, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Britto, D.T.; Kronzucker, H. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Guo, S.; Bruck, H.; Sattelmacher, B. Effects of supplied nitrogen form on growth and water uptake of French bean (Phaseolus vulgaris L.) plants. Plant Soil 2002, 239, 267–275. [Google Scholar] [CrossRef]
- Ali, A.; Tucker, T.C.; Thompson, T.L.; Salim, M. Effects of Salinity and Mixed Ammonium and Nitrate Nutrition on the Growth and Nitrogen Utilization of Barley. J. Agron. Crop Sci. 2001, 186, 223–228. [Google Scholar] [CrossRef]
- Helali, S.M.; Nebli, H.; Kaddour, R.; Mahmoudi, H.; Lachaâl, M.; Ouerghi, Z. Influence of nitrate—Ammonium ratio on growth and nutrition of Arabidopsis thaliana. Plant Soil 2010, 336, 65–74. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, J.; Dawuda, M.M.; Xie, J.; Yu, J.; Li, J.; Zhang, X.; Tang, C.; Wang, C.; Gan, Y. Appropriate Ammonium-Nitrate Ratio Improves Nutrient Accumulation and Fruit Quality in Pepper (Capsicum annuum L.). Agronomy 2019, 9, 683. [Google Scholar] [CrossRef]
- Hu, L.; Yu, J.; Liao, W.; Zhang, G.; Xie, J.; Lv, J.; Xiao, X.; Yang, B.; Zhou, R.; Bu, R. Moderate ammonium:nitrate alleviates low light intensity stress in mini Chinese cabbage seedling by regulating root architecture and photosynthesis. Sci. Hortic. 2015, 186, 143–153. [Google Scholar] [CrossRef]
- Tabatabaei, S.J.; Fatemi, L.S.; Fallahi, E. Effect of Ammonium: Nitrate Ratio on Yield, Calcium Concentration, and Photosynthesis Rate in Strawberry. J. Plant Nutr. 2007, 29, 1273–1285. [Google Scholar] [CrossRef]
- Liu, G.; Du, Q.; Li, J. Interactive effects of nitrate-ammonium ratios and temperatures on growth, photosynthesis, and nitrogen metabolism of tomato seedlings. Sci. Hortic. 2017, 214, 41–50. [Google Scholar] [CrossRef]
- Garland, J.; Mackowiak, C.; Sager, J. Hydroponic crop production using recycled nutrients from inedible crop residues. SAE Trans. 1993, 102, 1103–1110. [Google Scholar]
- Mackowiak, C.L.; Garland, J.L.; Strayer, R.F.; Finger, B.W.; Wheeler, R.M. Comparison of aerobically-treated and untreated crop residue as a source of recycled nutrients in a recirculating hydroponic system. Adv. Space Res. 1996, 18, 281–287. [Google Scholar] [CrossRef]
- Garland, J.L.; Mackowiak, C.L.; Strayer, R.F.; Finger, B.W. Integration of waste processing and biomass production systems as part of the KSC Breadboard project. Adv. Space Res. 1997, 20, 1821–1826. [Google Scholar] [CrossRef]
- Bohacz, J. Changes in mineral forms of nitrogen and sulfur and enzymatic activities during composting of lignocellulosic waste and chicken feathers. Environ. Sci. Pollut. Res. 2019, 26, 10333–10342. [Google Scholar] [CrossRef]
- Garland, J.L.; Mackowiak, C.L. Utilization of the Water Soluble Fraction of Wheat Straw as a Plant Nutrient Source. NASA Tech. Memo. 1990, 103497, 17–18. [Google Scholar]
- Finger, B.W.; Strayer, R.F. Development of an Intermediate-Scale Aerobic Bioreactor to Regenerate Nutrients from Inedible Crop Residues. SAE Trans. 1994, 103, 1365–1373. [Google Scholar] [CrossRef]
- Delaide, B.; Monsees, H.; Gross, A.; Goddek, S. Aerobic and Anaerobic Treatments for Aquaponic Sludge Reduction and Mineralisation. In Aquaponics Food Production Systems; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 247–266. [Google Scholar] [CrossRef]
- Strayer, R.; Finger, B.; Alazraki, M. Evaluation of an anaerobic digestion system for processing CELSS crop residues for resource recovery. Adv. Space Res. 1997, 20, 2009–2015. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Aoyama, C.; Takano, M.; Shinohara, M. Suppression of Ralstonia solanacearum bacterial wilt disease by an organic hydroponic system. J. Gen. Plant Pathol. 2012, 78, 217–220. [Google Scholar] [CrossRef]
- Chinta, Y.D.; Eguchi, Y.; Widiastuti, A.; Shinohara, M.; Sato, T. Organic hydroponics induces systemic resistance against the air-borne pathogen, Botrytis cinerea (grey mould). J. Plant Interact. 2015, 10, 243–251. [Google Scholar] [CrossRef]
- Mowa, E.; Akundabweni, L.; Chimwamurombe, P.; Oku, E. Formulation of an Organic Hydroponic Nutrient Solution Using Nitrifying Microorganisms. Int. Sci. Technol. J. Namib. 2018, 12, 52–61. [Google Scholar]
- Somerville, C.; Cohen, M.; Pantanella, E.; Stankus, A.; Lovatelli, A. Small-Scale Aquaponic Food Production: Integrated Fish and Plant Farming; FAO Fisheries and Aquaculture Technical Paper; FAO: Rome, Italy, 2014; p. 262. [Google Scholar]
- Rakocy, J.E. Aquaponics—Integrating Fish and Plant Culture. In Aquaculture Production Systems; Wiley-Blackwell: Oxford, UK, 2012; pp. 344–386. [Google Scholar] [CrossRef]
- Vanotti, M.B.; Hunt, P.G. Nitrification treatment of swine wastewater with acclimated nitrifying sludge immobilized in polymer pellets. Trans. Am. Soc. Agric. Eng. 2000, 43, 405–413. [Google Scholar] [CrossRef]
- Cho, K.H.; Kim, J.-O.; Kang, S.; Park, H.; Kim, S.; Kim, Y.M. Achieving enhanced nitrification in communities of nitrifying bacteria in full-scale wastewater treatment plants via optimal temperature and pH. Sep. Purif. Technol. 2014, 132, 697–703. [Google Scholar] [CrossRef]
- Rochmah, W.N.; Mangkoedihardjo, S. Toxicity Effects of Organic Substances on Nitrification Efficiency. IOP Conf. Ser. Earth Environ. Sci. 2020, 506, 012011. [Google Scholar] [CrossRef]
- Prinčič, A.; Mahne, I.; Megušar, F.; Paul, E.A.; Tiedje, J.M. Effects of pH and Oxygen and Ammonium Concentrations on the Community Structure of Nitrifying Bacteria from Wastewater. Appl. Environ. Microbiol. 1998, 64, 3584–3590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, J.; Liu, C.; Zhang, J.; Yang, J. Performance of a fixed-bed biofilm reactor with microbubble aeration in aerobic wastewater treatment. Water Sci. Technol. 2016, 74, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Ikeura, H.; Tsukada, K.; Tamaki, M. Effect of microbubbles in deep flow hydroponic culture on Spinach growth. J. Plant Nutr. 2017, 40, 2358–2364. [Google Scholar] [CrossRef]
- Park, J.-S.; Kurata, K. Application of Microbubbles to Hydroponics Solution Promotes Lettuce Growth. HortTechnology 2009, 19, 212–215. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; de la Fuente, C.; Bernal, M.P. Chemical properties of anaerobic digestates affecting C and N dynamics in amended soils. Agric. Ecosyst. Environ. 2012, 160, 15–22. [Google Scholar] [CrossRef]
- Singh, K.; Lee, K.; Worley, J.; Risse, L.M.; Das, K.C. Anaerobic Digestion of Poultry Litter: A Review. Appl. Eng. Agric. 2010, 26, 677–688. [Google Scholar] [CrossRef]
- Tuszynska, A.; Czerwionka, K.; Obarska-Pempkowiak, H. Phosphorus concentration and availability in raw organic waste and post fermentation products. J. Environ. Manag. 2021, 278 Pt 2, 111468. [Google Scholar] [CrossRef]
- Cioabla, A.E.; Ionel, I.; Dumitrel, G.-A.; Popescu, F. Comparative study on factors affecting anaerobic digestion of agricultural vegetal residues. Biotechnol. Biofuels 2012, 5, 39. [Google Scholar] [CrossRef]
- Ronga, D.; Setti, L.; Salvarani, C.; De Leo, R.; Bedin, E.; Pulvirenti, A.; Milc, J.; Pecchioni, N.; Francia, E. Effects of solid and liquid digestate for hydroponic baby leaf lettuce (Lactuca sativa L.) cultivation. Sci. Hortic. 2019, 244, 172–181. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Mamphweli, S.N.; Meyer, E.L.; Makaka, G.; Simon, M.; Okoh, A.I. An Overview of the Control of Bacterial Pathogens in Cattle Manure. Int. J. Environ. Res. Public Health 2016, 13, 843. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, G.; Zhang, H.; Shen, Y.; Fei, H.; Cheng, W. Biogas slurry use as N fertilizer for two-season Zizania aquatica Turcz. in China. Nutr. Cycl. Agroecosyst. 2017, 107, 303–320. [Google Scholar] [CrossRef]
- Mupambwa, H.A.; Namwoonde, A.S.; Liswaniso, G.M.; Hausiku, M.K.; Ravindran, B. Biogas digestates are not an effective nutrient solution for hydroponic tomato (Lycopersicon esculentum L.) production under a deep water culture system. Heliyon 2019, 5, e02736. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Hussain, S.; Bano, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.A.; Khan, F.; Chen, Y.; Wu, C.; et al. Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: Consequences for changing environment. Environ. Sci. Pollut. Res. 2015, 22, 4907–4921. [Google Scholar] [CrossRef] [PubMed]
- Kurepin, L.V.; Zaman, M.; Pharis, R.P. Phytohormonal basis for the plant growth promoting action of naturally occurring biostimulators. J. Sci. Food Agric. 2014, 94, 1715–1722. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef]
- Liedl, B.; Cummins, M.; Young, A.; Williams, M.; Chatfield, J. Liquid effluent from poultry waste bioremediation as a potential nutrient source for hydroponic tomato production. Acta Hortic. 2004, 659, 647–652. [Google Scholar] [CrossRef]
- Akl, I.A.; Savvas, D.; Papadantonakis, N.; Lydakis-Simantiris, N.; Kefalas, P. Influence of Ammonium to Total Nitrogen Supply Ratio on Growth, Yield and Fruit Quality of Tomato Grown in a Closed Hydroponic System. Eur. J. Hortic. 2003, 68, 204–211. [Google Scholar]
- Siddiqi, M.Y.; Malhotra, B.; Min, X.; Glass, A.D.M. Effects of ammonium and inorganic carbon enrichment on growth and yield of a hydroponic tomato crop. J. Plant Nutr. Soil Sci. 2002, 165, 191–197. [Google Scholar] [CrossRef]
- Chaudhry, A.; Nayab, S.; Hussain, S.; Ali, M.; Pan, Z. Current Understandings on Magnesium Deficiency and Future Outlooks for Sustainable Agriculture. Int. J. Mol. Sci. 2021, 22, 1819. [Google Scholar] [CrossRef]
- Schenk, M.; Wehrmann, J. The influence of ammonia in nutrient solution on growth and metabolism of cucumber plants. Plant Soil 1979, 52, 403–414. [Google Scholar] [CrossRef]
- Krupa, S. Effects of atmospheric ammonia (NH3) on terrestrial vegetation: A review. Environ. Pollut. 2003, 124, 179–221. [Google Scholar] [CrossRef]
- Hageman, R. Ammonium Versus Nitrate Nutrition of Higher Plants. In Nitrogen in Crop Production; American Society of Agronomy, Inc.: Madison, WI, USA, 1984; pp. 67–85. [Google Scholar] [CrossRef]
- Guo, S.; Zhou, Y.; Shen, Q.; Zhang, F. Effect of Ammonium and Nitrate Nutrition on Some Physiological Processes in Higher Plants—Growth, Photosynthesis, Photorespiration, and Water Relations. Plant Biol. 2007, 9, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Rafiullah, R.; Khan, M.J.; Muhammad, D. Foliar Application of Phosphorus to Enhance Phosphorus Utilization and Crop Growth: A Hydroponic Study. Sarhad J. Agric. 2018, 34, 47–53. [Google Scholar] [CrossRef]
- Cerozi, B.; Fitzsimmons, K. Phosphorus dynamics modeling and mass balance in an aquaponics system. Agric. Syst. 2017, 153, 94–100. [Google Scholar] [CrossRef]
- Da Silva Cerozi, B.; Fitzsimmons, K. The effect of pH on phosphorus availability and speciation in an aquaponics nutrient solution. Bioresour. Technol. 2016, 219, 778–781. [Google Scholar] [CrossRef]
- Sambo, P.; Nicoletto, C.; Giro, A.; Pii, Y.; Valentinuzzi, F.; Mimmo, T.; Lugli, P.; Orzes, G.; Mazzetto, F.; Astolfi, S.; et al. Hydroponic Solutions for Soilless Production Systems: Issues and Opportunities in a Smart Agriculture Perspective. Front. Plant Sci. 2019, 10, 923. [Google Scholar] [CrossRef]
- Ma, J.F. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Campos, C.N.S.; da Silva Júnior, G.B.; de Mello Prado, R.; de David, C.H.O.; de Souza Junior, J.P.; Teodoro, P.E. Silicon mitigates ammonium toxicity in plants. Agron. J. 2020, 112, 635–647. [Google Scholar] [CrossRef]
- Ashraf, M.; Rahmatullah; Ahmad, R.; Bhatti, A.; Afzal, M.; Sarwar, A.; Maqsood, M.; Kanwal, S. Amelioration of Salt Stress in Sugarcane (Saccharum officinarum L.) by Supplying Potassium and Silicon in Hydroponics. Pedosphere 2010, 20, 153–162. [Google Scholar] [CrossRef]
- Stamatakis, A.; Papadantonakis, N.; Savvas, D.; Lydakis-Simantiris, N.; Kefalas, P. Effects of silicon and salinity on fruit yield and quality of tomato grown hydroponically. Acta Hortic. 2003, 609, 141–147. [Google Scholar] [CrossRef]
- Bybordi, A.; Tabatabaei, S.J.; Ahmadov, A. Influence of salinity and ammonium: Nitrate ratio on growth, photosynthesis, fatty acid and the activity of antioxidative enzymes in canola. J. Plant Nutr. 2012, 35, 2089–2106. [Google Scholar] [CrossRef]
- Zhang, M.; Lawlor, P.G.; Wu, G.; Lynch, B.; Zhan, X. Partial nitrification and nutrient removal in intermittently aerated sequencing batch reactors treating separated digestate liquid after anaerobic digestion of pig manure. Bioprocess Biosyst. Eng. 2011, 34, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Parravicini, V.; Svardal, K.; Hornek, R.; Kroiss, H. Aeration of anaerobically digested sewage sludge for COD and nitrogen removal: Optimization at large-scale. Water Sci. Technol. 2008, 57, 257–264. [Google Scholar] [CrossRef][Green Version]
- Oke, O.L. Nitrite Toxicity to Plants. Nature 1966, 212, 528. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).