Microbial Interactions, Growth, and Health of Aquatic Species in Biofloc Systems
Abstract
:1. The Expansion of Intensive RAS in Aquaculture Production
2. Biofloc Technology and Production Aquaculture
3. Microbial Communities and Biofloc Interactions
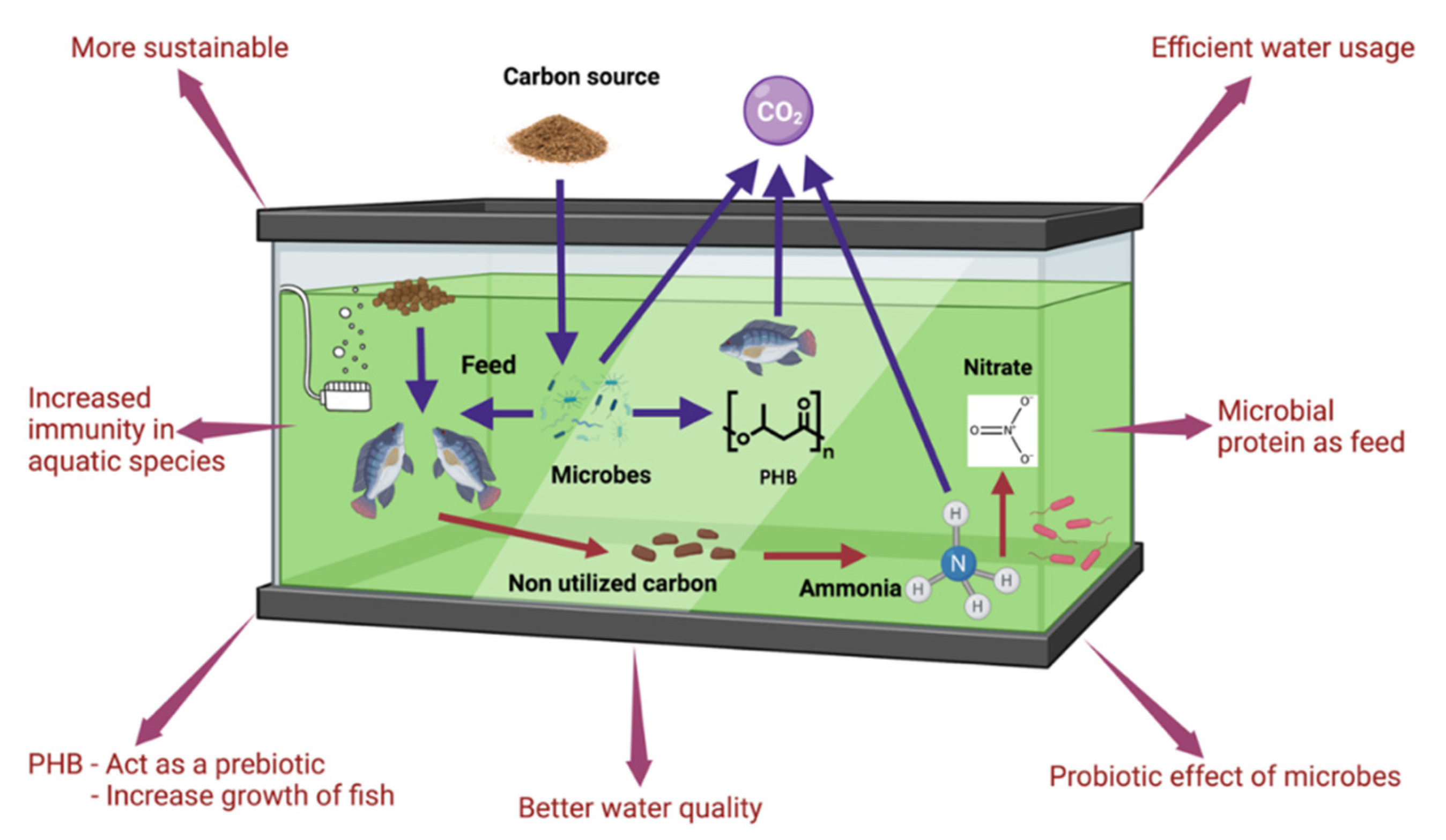
Effect of Carbon Source on the Microbial Community and Structure of Biofloc
4. Probiotic Applications and the Intestinal Microbiota of Aquatic Organisms
5. Microbial Interactions in Biofloc and Implications on Health and Diseases of Cultured Species
6. Future Research and Optimization of Biofloc Systems in Fish and Shrimp Culture
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. In Brief to The State of World Fisheries and Aquaculture 2022; Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar]
- Görs, M.; Schumann, R.; Hepperle, D.; Karsten, U. Quality analysis of commercial Chlorella products used as dietary supplement in human nutrition. J. Appl. Phycol. 2010, 22, 265–276. [Google Scholar] [CrossRef]
- Subasinghe, R.; Soto, D.; Jia, J. Global aquaculture and its role in sustainable development. Rev. Aquac 2009, 1, 2–9. [Google Scholar] [CrossRef]
- Ray, A. Biofloc Technology for Super-Intensive Shrimp Culture. Biofloc Technology—A Practical Guide Book, 2nd ed.; The World Aquaculture Society: Baton Rouge, LA, USA, 2012; pp. 167–188. [Google Scholar]
- Emerenciano, M.; Gaxiola, G.; Cuzon, G. Biofloc technology (BFT): A review for aquaculture application and animal food industry. In Biomass Now-Cultivation and Utilization; Matovic, M.D., Ed.; IntechOpen: London, UK, 2013; pp. 301–328. [Google Scholar]
- Crab, R.; Defoirdt, T.; Bossier, P.; Verstraete, W. Biofloc technology in aquaculture: Beneficial effects and future challenges. Aquaculture 2012, 356, 351–356. [Google Scholar] [CrossRef]
- Rosenthal, H.; Castell, J.D.; Chiba, K.; Forster, J.R.M.; Hilge, V.; Hogendoorn, H.; Mayo, R.D.; Muir, J.F.; Murray, K.F.; Petit, J.; et al. Flow-through and recirculation system. EIFAC 1986, 49. [Google Scholar]
- Summerfelt, S.T.; Sharrer, M.J.; Tsukuda, S.M.; Gearheart, M. Process requirements for achieving full-flow disinfection of recirculating water using ozonation and UV irradiation. Aquac. Eng. 2009, 40, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Attramadal, K.J.; Salvesen, I.; Xue, R.; Øie, G.; Størseth, T.R.; Vadstein, O.; Olsen, Y. Recirculation as a possible microbial control strategy in the production of marine larvae. Aquac. Eng. 2012, 46, 27–39. [Google Scholar] [CrossRef]
- Martins, C.I.M.; Eding, E.H.; Verdegem, M.C.; Heinsbroek, L.T.; Schneider, O.; Blancheton, J.P.; Verreth, J.A. New developments in recirculating aquaculture systems in Europe: A perspective on environmental sustainability. Aquac. Eng. 2010, 43, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Bergheim, A.; Drengstig, A.; Ulgenes, Y.; Fivelstad, S. Production of Atlantic salmon smolts in Europe—Current characteristics and future trends. Aquac. Eng. 2009, 41, 46–52. [Google Scholar] [CrossRef]
- Colson, V.; Sadoul, B.; Valotaire, C.; Prunet, P.; Gaumé, M.; Labbé, L. Welfare assessment of rainbow trout reared in a recirculating aquaculture system: Comparison with a flow-through system. Aquaculture 2015, 436, 151–159. [Google Scholar] [CrossRef]
- Vivanco-Aranda, M.; Gallardo-Escárate, C.J.; del Río-Portilla, M.Á. Low-density culture of red abalone juveniles, Haliotis rufescens Swainson 1822, recirculating aquaculture system and flow-through system. Aquac. Res. 2011, 42, 161–168. [Google Scholar] [CrossRef]
- Ahmad, I.; Babitha Rani, A.M.; Verma, A.K.; Maqsood, M. Biofloc technology: An emerging avenue in aquatic animal healthcare and nutrition. Aquac. Int. 2017, 25, 1215–1226. [Google Scholar] [CrossRef]
- Hargreaves, J.A. Photosynthetic suspended-growth systems in aquaculture. Aquac. Eng. 2006, 34, 344–363. [Google Scholar] [CrossRef]
- Ogello, E.O.; Outa, N.O.; Obiero, K.O.; Kyule, D.N.; Munguti, J.M. The prospects of biofloc technology (BFT) for sustainable aquaculture development. Sci. Afr. 2021, 14, e01053. [Google Scholar] [CrossRef]
- Crab, R.; Avnimelech, Y.; Defoirdt, T.; Bossier, P.; Verstraete, W. Nitrogen removal techniques in aquaculture for a sustainable production. Aquaculture 2007, 270, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Luo, G.; Gao, Q.; Wang, C.; Liu, W.; Sun, D.; Li, L.; Tan, H. Growth, digestive activity, welfare, and partial cost-effectiveness of genetically improved farmed tilapia (Oreochromis niloticus) cultured in a recirculating aquaculture system and an indoor biofloc system. Aquaculture 2014, 422, 1–7. [Google Scholar] [CrossRef]
- Prangnell, D.I.; Castro, L.F.; Ali, A.S.; Browdy, C.L.; Zimba, P.V.; Laramore, S.E.; Samocha, T.M. Some limiting factors in superintensive production of juvenile Pacific white shrimp, Litopenaeus vannamei, in no-water-exchange, biofloc-dominated systems. J. World Aquac. Soc. 2016, 47, 396–413. [Google Scholar] [CrossRef]
- Crab, R.; Kochva, M.; Verstraete, W.; Avnimelech, Y. Bio-flocs technology application in over-wintering of tilapia. Aquac. Eng. 2009, 40, 105–112. [Google Scholar] [CrossRef]
- Jung, J.Y.; Hur, J.W.; Kim, K.; Han, H.S. Evaluation of floc-harvesting technologies in biofloc technology (BFT) system for aquaculture. Bioresour. Technol. 2020, 314, 123719. [Google Scholar] [CrossRef]
- Hargreaves, J.A. Biofloc Production Systems for Aquaculture; Southern Regional Aquaculture Center: Stoneville, MS, USA, 2013; Volume 4503, pp. 1–11. [Google Scholar]
- Jamal, M.T.; Broom, M.; Al-Mur, B.A.; Al Harbi, M.; Ghandourah, M.; Al Otaibi, A.; Haque, M.F. Biofloc technology: Emerging microbial biotechnology for the improvement of aquaculture productivity. Pol. J. Microbiol. 2020, 69, 401–409. [Google Scholar] [CrossRef]
- Tabarrok, M.; Seyfabadi, J.; Salehi Jouzani, G.; Younesi, H. Comparison between recirculating aquaculture and biofloc systems for rearing juvenile common carp (Cyprinus carpio): Growth performance, haemato-immunological indices, water quality and microbial communities. Aquac. Res. 2020, 51, 4881–4892. [Google Scholar] [CrossRef]
- Azim, M.E.; Little, D.C. The biofloc technology (BFT) in indoor tanks: Water quality, biofloc composition, and growth and welfare of Nile tilapia (Oreochromis niloticus). Aquaculture 2008, 283, 29–35. [Google Scholar] [CrossRef]
- Khatoon, H.; Banerjee, S.; Yuan, G.T.G.; Haris, N.; Ikhwanuddin, M.; Ambak, M.A.; Endut, A. Biofloc as a potential natural feed for shrimp postlarvae. Int. Biodeterior. Biodegrad. 2016, 113, 304–309. [Google Scholar] [CrossRef]
- De Schryver, P.; Crab, R.; Defoirdt, T.; Boon, N.; Verstraete, W. The basics of bio-flocs technology: The added value for aquaculture. Aquaculture 2008, 277, 125–137. [Google Scholar] [CrossRef]
- Wilén, B.M.; Balmer, P. The effect of dissolved oxygen concentration on the structure, size and size distribution of activated sludge flocs. Water Res. 1999, 33, 391–400. [Google Scholar] [CrossRef]
- Wilén, B.M.; Keiding, K.; Nielsen, P.H. Anaerobic deflocculation and aerobic reflocculation of activated sludge. Water Res. 2000, 34, 3933–3942. [Google Scholar] [CrossRef]
- Ulloa Walker, D.A.; Morales Suazo, M.C.; Emerenciano, M.G.C. Biofloc technology: Principles focused on potential species and the case study of Chilean river shrimp Cryphiops caementarius. Rev. Aquac. 2020, 12, 1759–1782. [Google Scholar] [CrossRef]
- Poli, M.A.; Legarda, E.C.; de Lorenzo, M.A.; Pinheiro, I.; Martins, M.A.; Seiffert, W.Q.; do Nascimento Vieira, F. Integrated multitrophic aquaculture applied to shrimp rearing in a biofloc system. Aquaculture 2019, 511, 734274. [Google Scholar] [CrossRef]
- Pérez-Fuentes, J.A.; Hernández-Vergara, M.P.; Pérez-Rostro, C.I.; Fogel, I. C: N ratios affect nitrogen removal and production of Nile tilapia Oreochromis niloticus raised in a biofloc system under high density cultivation. Aquaculture 2016, 452, 247–251. [Google Scholar] [CrossRef]
- Choo, H.X.; Caipang, C.M.A. Biofloc technology (BFT) and its application towards improved production in freshwater tilapia culture. Aquac. Aquar. Conserv. Legis. 2015, 8, 362–366. [Google Scholar]
- Jiménez-Ojeda, Y.K.; Collazos-Lasso, L.F.; Arias-Castellanos, J.A. Dynamics and use of nitrogen in Biofloc Technology-BFT. Aquac. Aquar. Conserv. Legis. 2018, 11, 1107–1129. [Google Scholar]
- Emerenciano, M.G.C.; Martínez-Córdova, L.R.; Martínez-Porchas, M.; Miranda-Baeza, A. Biofloc technology (BFT): A tool for water quality management in aquaculture. In Water Quality; Tutu, H., Ed.; InTechOpen: London, UK, 2017; Volume 5, pp. 92–109. [Google Scholar]
- Ebeling, J.M.; Timmons, M.B.; Bisogni, J.J. Engineering analysis of the stoichiometry of photoautotrophic, autotrophic, and heterotrophic removal of ammonia–nitrogen in aquaculture systems. Aquaculture 2006, 257, 346–358. [Google Scholar] [CrossRef] [Green Version]
- Khanjani, M.H.; Sharifinia, M. Biofloc technology as a promising tool to improve aquaculture production. Rev. Aquacult. 2020, 12, 1836–1850. [Google Scholar] [CrossRef]
- Megahed, M.E. The effect of microbial biofloc on water quality, survival and growth of the green tiger shrimp (Penaeus semisulcatus) fed with different crude protein levels. J. Arab. Aquac. Soc. 2010, 5, 119–142. [Google Scholar]
- Mugwanya, M.; Dawood, M.A.; Kimera, F.; Sewilam, H. Biofloc systems for sustainable production of economically important aquatic species: A review. Sustainability 2021, 13, 7255. [Google Scholar] [CrossRef]
- Pinto, P.H.O.; Rocha, J.L.; do Vale Figueiredo, J.P.; Carneiro, R.F.S.; Damian, C.; de Oliveira, L.; Seiffert, W.Q. Culture of marine shrimp (Litopenaeus vannamei) in biofloc technology system using artificially salinized freshwater: Zootechnical performance, economics and nutritional quality. Aquaculture 2020, 520, 734960. [Google Scholar] [CrossRef]
- Emerenciano, M.G.; Arnold, S.; Perrin, T. Sodium metasilicate supplementation in culture water on growth performance, water quality and economics of indoor commercial-scale biofloc-based Litopenaeus vannamei culture. Aquaculture 2022, 560, 738566. [Google Scholar] [CrossRef]
- Betanzo-Torres, E.A.; Piñar-Álvarez, M.d.l.Á.; Sierra-Carmona, C.G.; Santamaria, L.E.G.; Loeza-Mejía, C.-I.; Marín-Muñiz, J.L.; Sandoval Herazo, L.C. Proposal of ecotechnologies for tilapia (Oreochromis niloticus) production in Mexico: Economic, environmental, and social implications. Sustainability 2021, 13, 6853. [Google Scholar] [CrossRef]
- M Hwihy, H.; F Zeina, A.; A El-Damhougy, K. Influence of biofloc technology on economic evaluation of culturing Oreochromis niloticus reared at different stocking densities and feeding rates. EJABF 2021, 25, 737–748. [Google Scholar] [CrossRef]
- Sudirman, A.; Rahardjo, S.; Rukmono, D. Economical analysis of polyculture of catfish and tilapia fish in biofloc system. Int. J. Eng. Sci. 2020, 9, 1–7. [Google Scholar]
- Halim, M.A.; Nahar, S.; Nabi, M.M. Biofloc technology in aquaculture and its potentiality: A review. Int. J. Fish Aquat. 2019, 7, 260–266. [Google Scholar]
- El-Sayed, A.F.M. Use of biofloc technology in shrimp aquaculture: A comprehensive review, with emphasis on the last decade. Rev. Aquacult. 2021, 13, 676–705. [Google Scholar] [CrossRef]
- Faizullah, M.M.; Rajagopalsamy, C.; Ahilan, B.; Daniel, N. Application of biofloc technology (BFT) in the aquaculture system. J. Entomol. Zool. Stud. 2019, 7, 204–212. [Google Scholar]
- Sgnaulin, T.; de Mello, G.L.; Thomas, M.C.; Garcia, J.R.E.; de Oca, G.A.R.M.; Emerenciano, M.G.C. Biofloc technology (BFT): An alternative aquaculture system for piracanjuba Brycon orbignyanus? Aquaculture 2018, 485, 119–123. [Google Scholar] [CrossRef]
- Dauda, A.B. Biofloc technology: A review on the microbial interactions, operational parameters and implications to disease and health management of cultured aquatic animals. Rev. Aquacult. 2020, 12, 1193–1210. [Google Scholar] [CrossRef]
- Abakari, G.; Luo, G.; Kombat, E.O.; Alhassan, E.H. Supplemental carbon sources applied in biofloc technology aquaculture systems: Types, effects and future research. Rev. Aquacult. 2021, 13, 1193–1222. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Mohammadi, A.; Emerenciano, M.G.C. Microorganisms in biofloc aquaculture system. Aquacult. Rep. 2022, 26, 101300. [Google Scholar] [CrossRef]
- Ju, Z.Y.; Forster, I.; Conquest, L.; Dominy, W. Enhanced growth effects on shrimp (Litopenaeus vannamei) from inclusion of whole shrimp floc or floc fractions to a formulated diet. Aquac. Nutr. 2008, 14, 533–543. [Google Scholar] [CrossRef]
- Wei, Y.; Liao, S.A.; Wang, A.L. The effect of different carbon sources on the nutritional composition, microbial community and structure of bioflocs. Aquaculture 2016, 465, 88–93. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, X.; Yin, X.; Lu, H.; Chen, G.; Yu, J.; Ruan, Y. Effect of stock density on the microbial community in biofloc water and Pacific white shrimp (Litopenaeus vannamei) gut microbiota. Appl. Microbiol. Biotechnol. 2019, 103, 4241–4252. [Google Scholar] [CrossRef]
- Martins, P.; Cleary, D.F.; Pires, A.C.; Rodrigues, A.M.; Quintino, V.; Calado, R.; Gomes, N.C. Molecular analysis of bacterial communities and detection of potential pathogens in a recirculating aquaculture system for Scophthalmus maximus and Solea senegalensis. PLoS ONE 2013, 8, e80847. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Huang, J.; Wang, X.H.; Song, X.L.; Yang, C.H.; Zhang, X.G.; Wang, G.C. The application of bioflocs technology in high-intensive, zero exchange farming systems of Marsupenaeus japonicus. Aquaculture 2012, 354, 97–106. [Google Scholar] [CrossRef]
- Wei, G.; Shan, D.; Li, G.; Li, X.; Tian, R.; He, J.; Shao, Z. Prokaryotic communities vary with floc size in a biofloc-technology based aquaculture system. Aquaculture 2020, 529, 735632. [Google Scholar] [CrossRef]
- Xu, W.; Wen, G.; Su, H.; Xu, Y.; Hu, X.; Cao, Y. Effect of input C/N ratio on bacterial community of water biofloc and shrimp gut in a commercial zero-exchange system with intensive production of Penaeus vannamei. Microorganisms 2022, 10, 1060. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Liu, G.; Deng, Y.; Zhu, S.; Ye, Z.; Shao, Y.; Liu, D. Performance and microbial community analysis of combined denitrification and biofloc technology (CDBFT) system treating nitrogen-rich aquaculture wastewater. Bioresour. Technol. 2019, 288, 121582. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, M.; Pandey, P.K.; Aravind, R.; Vennila, A.; Bharti, V.; Purushothaman, C.S. Effect of different biofloc system on water quality, biofloc composition and growth performance in Litopenaeus vannamei (Boone, 1931). Aquac. Res. 2016, 47, 3432–3444. [Google Scholar] [CrossRef]
- Tubin, J.S.B.; Paiano, D.; de Oliveira Hashimoto, G.S.; Furtado, W.E.; Martins, M.L.; Durigon, E.; Emerenciano, M.G.C. Tenebrio molitor meal in diets for Nile tilapia juveniles reared in biofloc system. Aquaculture 2020, 519, 734763. [Google Scholar] [CrossRef]
- Monroy-Dosta, M.D.C.; De Lara-Andrade, R.; Castro-Mejia, J.; Castro-Mejia, G.; Coelho-Emerenciano, M.G. Microbiology community composition and abundance associated to biofloc in tilapia aquaculture. Rev. Biol. Mar. Oceanog. 2013, 48, 511–520. [Google Scholar] [CrossRef]
- Racz, L.; Datta, T.; Goel, R. Effect of organic carbon on ammonia oxidizing bacteria in a mixed culture. Bioresour. Technol. 2010, 101, 6454–6460. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Chen, J.; Gou, J.; Hou, J.; Li, D.; He, X. The effect of different carbon sources on water quality, microbial community and structure of biofloc systems. Aquaculture 2018, 482, 103–110. [Google Scholar] [CrossRef]
- Schrader, K.K.; Green, B.W.; Perschbacher, P.W. Development of phytoplankton communities and common off-flavors in a biofloc technology system used for the culture of channel catfish (Ictalurus punctatus). Aquacul. Eng. 2011, 45, 118–126. [Google Scholar] [CrossRef]
- Avnimelech, Y. Feeding with microbial flocs by tilapia in minimal discharge bio-flocs technology ponds. Aquaculture 2007, 264, 140–147. [Google Scholar] [CrossRef]
- Bossier, P.; Ekasari, J. Biofloc technology application in aquaculture to support sustainable development goals. Microb. Biotechnol. 2017, 10, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Khanjani, M.H.; Sharifinia, M.; Hajirezaee, S. Recent progress towards the application of biofloc technology for tilapia farming. Aquaculture 2022, 552, 738021. [Google Scholar] [CrossRef]
- Dong, S.; Li, Y.; Jiang, F.; Hu, Z.; Zheng, Y. Performance of Platymonas and microbial community analysis under different C/N ratio in biofloc technology aquaculture system. J. Water Process. Eng. 2021, 43, 102257. [Google Scholar] [CrossRef]
- Luo, G.; Chen, X.; Tan, J.; Abakari, G.; Tan, H. Effects of carbohydrate addition strategy and biofloc levels on the establishment of nitrification in biofloc technology aquaculture systems. Aquaculture 2020, 514, 734441. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Su, J.; Tian, Y.; Ning, X.; Hong, H.; Zheng, T. Lysis of a red-tide causing alga, Alexandrium tamarense, caused by bacteria from its phycosphere. Biol. Control. 2010, 52, 123–130. [Google Scholar] [CrossRef]
- Hargreaves, J.A. A simulation model of ammonia dynamics in commercial catfish ponds in the southeastern United States. Aquac. Eng. 1997, 16, 27–43. [Google Scholar] [CrossRef]
- Cardona, E.; Gueguen, Y.; Magré, K.; Lorgeoux, B.; Piquemal, D.; Pierrat, F.; Noguier, F.; Saulnier, D. Bacterial community characterization of water and intestine of the shrimp Litopenaeus stylirostris in a biofloc system. BMC Microbiol. 2016, 1, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ekasari, J.; Azhar, M.H.; Surawidjaja, E.H.; Nuryati, S.; De Schryver, P.; Bossier, P. Immune response and disease resistance of shrimp fed biofloc grown on different carbon sources. Fish Shellfish. Immunol. 2014, 41, 332–339. [Google Scholar] [CrossRef]
- Serra, F.P.; Gaona, C.A.; Furtado, P.S.; Poersch, L.H.; Wasielesky, W. Use of different carbon sources for the biofloc system adopted during the nursery and grow-out culture of Litopenaeus vannamei. Aquac. Int. 2015, 23, 1325–1339. [Google Scholar] [CrossRef]
- García-Ríos, L.; Miranda-Baeza, A.; Coelho-Emerenciano, M.G.; Huerta-Rábago, J.A.; Osuna-Amarillas, P. Biofloc technology (BFT) applied to tilapia fingerlings production using different carbon sources: Emphasis on commercial applications. Aquaculture 2019, 502, 26–31. [Google Scholar] [CrossRef]
- Deng, Y.; Borewicz, K.; van Loo, J.; Olabarrieta, M.Z.; Kokou, F.; Sipkema, D.; Verdegem, M.C. In-situ biofloc affects the core prokaryotes community composition in gut and enhances growth of Nile tilapia (Oreochromis niloticus). Microb. Ecol. 2021, 84, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Fuentes, J.A.; Pérez-Rostro, C.I.; Hernández-Vergara, M.P.; del Carmen Monroy-Dosta, M. Variation of the bacterial composition of biofloc and the intestine of Nile tilapia Oreochromis niloticus, cultivated using biofloc technology, supplied different feed rations. Aquac. Res. 2018, 49, 3658–3668. [Google Scholar] [CrossRef]
- Huang, L.; Guo, H.; Chen, C.; Huang, X.; Chen, W.; Bao, F.; Zhang, D. The bacteria from large-sized bioflocs are more associated with the shrimp gut microbiota in culture system. Aquaculture 2020, 523, 735159. [Google Scholar] [CrossRef]
- Ferreira, M.G.; Melo, F.P.; Lima, J.P.; Andrade, H.A.; Severi, W.; Correia, E.S. Bioremediation and biocontrol of commercial probiotic in marine shrimp culture with biofloc. Lat. Am. J. Aquat. Res. 2017, 45, 167–176. [Google Scholar] [CrossRef]
- Kuebutornye, F.K.; Abarike, E.D.; Lu, Y. A review on the application of Bacillus as probiotics in aquaculture. Fish Shellfish. Immunol. 2019, 87, 820–828. [Google Scholar] [CrossRef]
- Kathia, C.M.; del Carmen, M.D.M.; Aida, H.P.; Jorge, C.M.; Félix, A.G.J.; Amadeo, B.M.J. Effect of two probiotics on bacterial community composition from biofloc system and their impact on survival and growth of tilapia (Oreochromis niloticus). Int. J. Fish. Aquat. Stud. 2018, 6, 525–533. [Google Scholar]
- Daniel, N.; Nageswari, P. Exogenous probiotics on biofloc based aquaculture: A review. Curr. Agric. Res. J. 2017, 5, 88. [Google Scholar] [CrossRef]
- Kathia, C.M.; del Carmen, M.D.M.; Aida, H.P.; Jorge, C.M.; Daniel, B.C. Probiotics used in biofloc system for fish and crustacean culture. Rev. Aquac. 2017, 23, 28. [Google Scholar]
- Bostock, J.; McAndrew, B.; Richards, R.; Jauncey, K.; Telfer, T.; Lorenzen, K.; Corner, R. Aquaculture: Global status and trends. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2897–2912. [Google Scholar] [CrossRef] [Green Version]
- Meyer, F.P. Aquaculture disease and health management. J. Anim. Sci. 1991, 69, 4201–4208. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.C.; Rise, M.L.; Christian, S.L. A comparison of the innate and adaptive immune systems in cartilaginous fish, ray-finned fish, and lobe-finned fish. Front. Immunol. 2019, 10, 2292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minaz, M.; Kubilay, A. Operating parameters affecting biofloc technology: Carbon source, carbon/nitrogen ratio, feeding regime, stocking density, salinity, aeration, and microbial community manipulation. Aquacult. Int. 2021, 29, 1121–1140. [Google Scholar] [CrossRef]
- Rollo, A.; Sulpizio, R.; Nardi, M.; Silvi, S.; Orpianesi, C.; Caggiano, M.; Carnevali, O. Live microbial feed supplement in aquaculture for improvement of stress tolerance. Fish Physiol. Biochem. 2006, 32, 167–177. [Google Scholar] [CrossRef]
- Ekasari, J.; Rivandi, D.R.; Firdausi, A.P.; Surawidjaja, E.H.; Zairin Jr., M.; Bossier, P.; De Schryver, P. Biofloc technology positively affects Nile tilapia (Oreochromis niloticus) larvae performance. Aquaculture 2015, 441, 72–77. [Google Scholar] [CrossRef]
- Emerenciano, M.; Ballester, E.L.; Cavalli, R.O.; Wasielesky, W. Biofloc technology application as a food source in a limited water exchange nursery system for pink shrimp Farfantepenaeus brasiliensis (Latreille, 1817). Aquac. Res. 2012, 43, 447–457. [Google Scholar] [CrossRef]
- Defoirdt, T.; Boon, N.; Bossier, P.; Verstraete, W. Disruption of bacterial quorum sensing: An unexplored strategy to fight infections in aquaculture. Aquaculture 2004, 240, 69–88. [Google Scholar] [CrossRef]
- Tepaamorndech, S.; Nookaew, I.; Higdon, S.M.; Santiyanont, P.; Phromson, M.; Chantarasakha, K.; Mhuantong, W.; Plengvidhya, V.; Visessanguan, W. Metagenomics in bioflocs and their effects on gut microbiome and immune responses in Pacific white shrimp. Fish Shellfish. Immunol. 2020, 106, 733–774. [Google Scholar] [CrossRef]
- Gustilatov, M.; Ekasari, J.; Pande, G.S.J. Protective effects of the biofloc system in Pacific white shrimp (Penaeus vannamei) culture against pathogenic Vibrio parahaemolyticus infection. Fish Shellfish. Immunol. 2022, 124, 66–73. [Google Scholar] [CrossRef]
- Jang, I.K.; Pang, Z.; Yu, J.; Kim, S.K.; Seo, H.C.; Cho, Y.R. Selectively enhanced expression of prophenoloxidase activating enzyme 1 (PPAE1) at a bacteria clearance site in the white shrimp, Litopenaeus vannamei. BMC Immunol. 2010, 12, 1–11. [Google Scholar] [CrossRef] [Green Version]
- de Jesus Becerra-Dorame, M.; Martinez-Cordova, L.R.; Martínez-Porchas, M.; Hernández-López, J.; López-Elías, J.A.; Mendoza-Cano, F. Effect of using autotrophic and heterotrophic microbial-based-systems for the pre-grown of Litopenaeus vannamei, on the production performance and selected haemolymph parameters. Aquac. Res. 2014, 45, 944–948. [Google Scholar] [CrossRef]
- Betanzo-Torres, E.A.; Piñar-Álvarez, M.D.L.Á.; Sandoval-Herazo, L.C.; Molina-Navarro, A.; Rodríguez-Montoro, I.; González-Moreno, R.H. Factors that limit the adoption of biofloc technology in aquaculture production in Mexico. Water 2020, 12, 2775. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padeniya, U.; Davis, D.A.; Wells, D.E.; Bruce, T.J. Microbial Interactions, Growth, and Health of Aquatic Species in Biofloc Systems. Water 2022, 14, 4019. https://doi.org/10.3390/w14244019
Padeniya U, Davis DA, Wells DE, Bruce TJ. Microbial Interactions, Growth, and Health of Aquatic Species in Biofloc Systems. Water. 2022; 14(24):4019. https://doi.org/10.3390/w14244019
Chicago/Turabian StylePadeniya, Uthpala, Donald Allen Davis, Daniel E. Wells, and Timothy J. Bruce. 2022. "Microbial Interactions, Growth, and Health of Aquatic Species in Biofloc Systems" Water 14, no. 24: 4019. https://doi.org/10.3390/w14244019
APA StylePadeniya, U., Davis, D. A., Wells, D. E., & Bruce, T. J. (2022). Microbial Interactions, Growth, and Health of Aquatic Species in Biofloc Systems. Water, 14(24), 4019. https://doi.org/10.3390/w14244019






