Assessing Occurrence and Biological Consequences of Contaminants of Emerging Concern on Oceanic Islands
Abstract
:1. Introduction
2. Materials and Methods
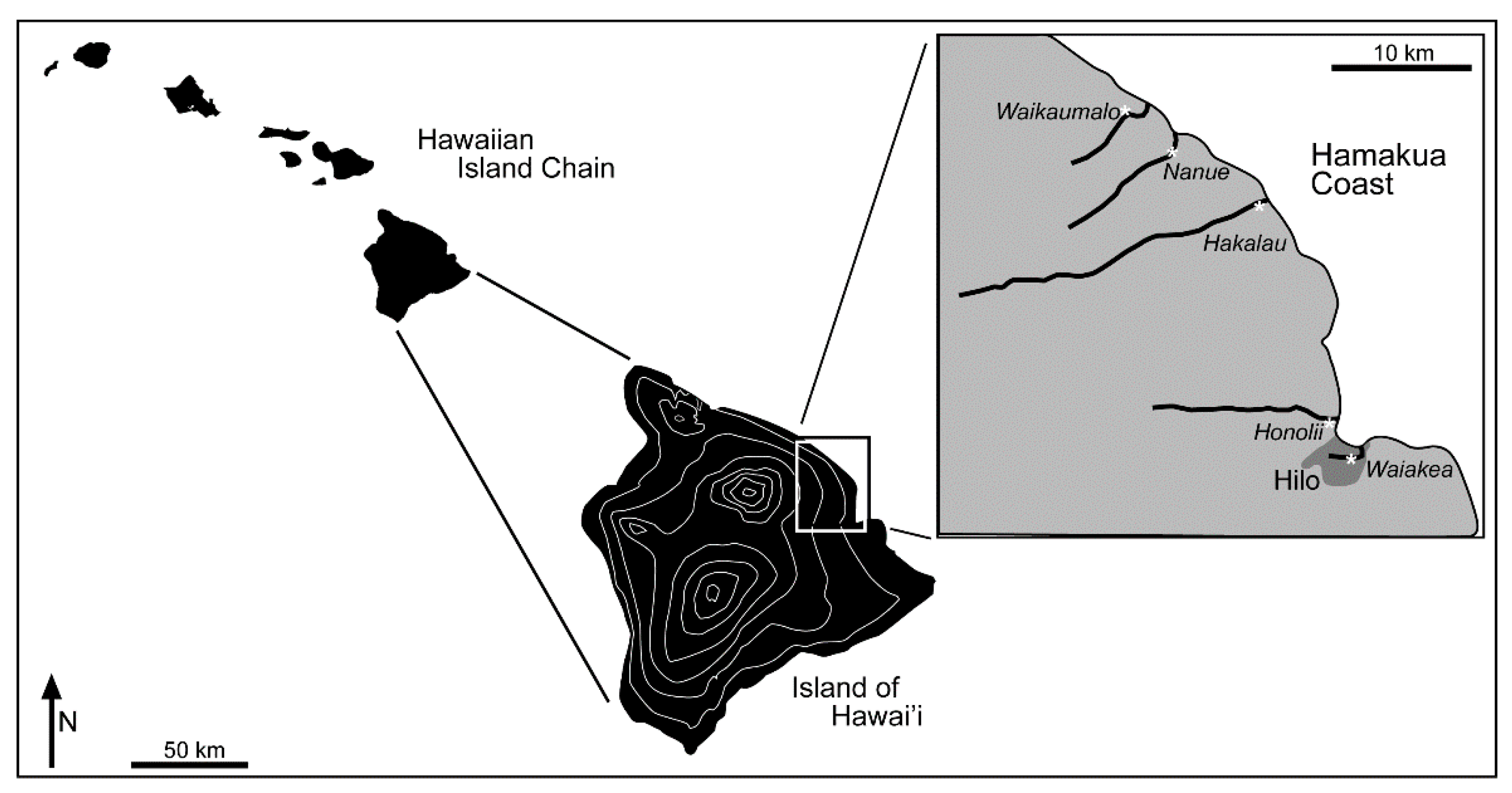
2.1. Sample Collection
2.2. Sample Extraction
2.3. Analytical Methods
2.4. Fish Exposure Experiments
2.5. Predator Avoidance Assay
2.6. Waterfall Climbing Assay
2.7. Exposure-Activity Ratio (EAR)
2.8. Statistical Analysis
3. Results
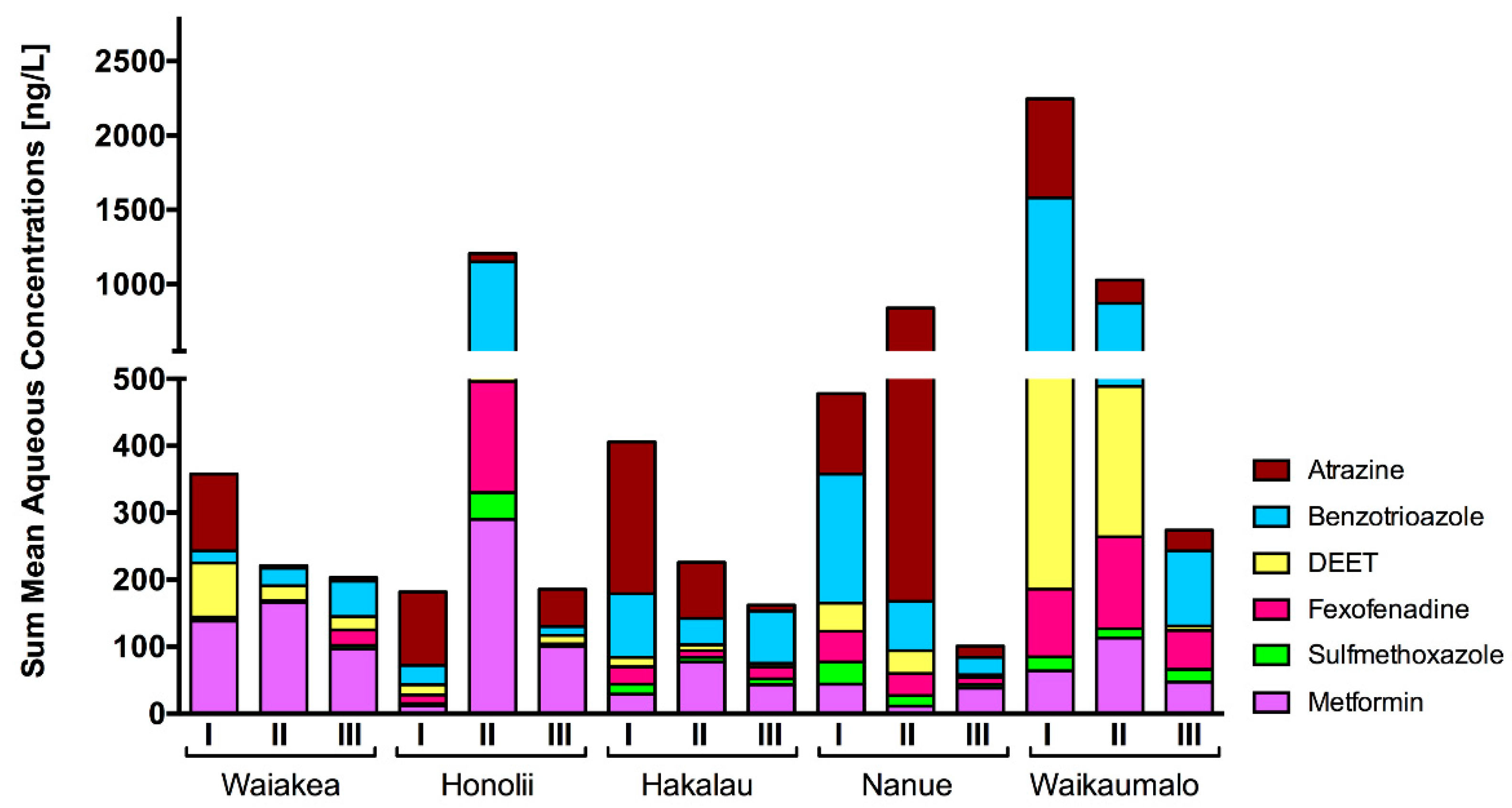
3.1. Chemical Analysis
3.2. Survival and Predator Avoidance Performance
3.3. Climbing Performance
4. Discussion
4.1. Presence of CEC in Hawaiian Streams
4.2. Biological Effects
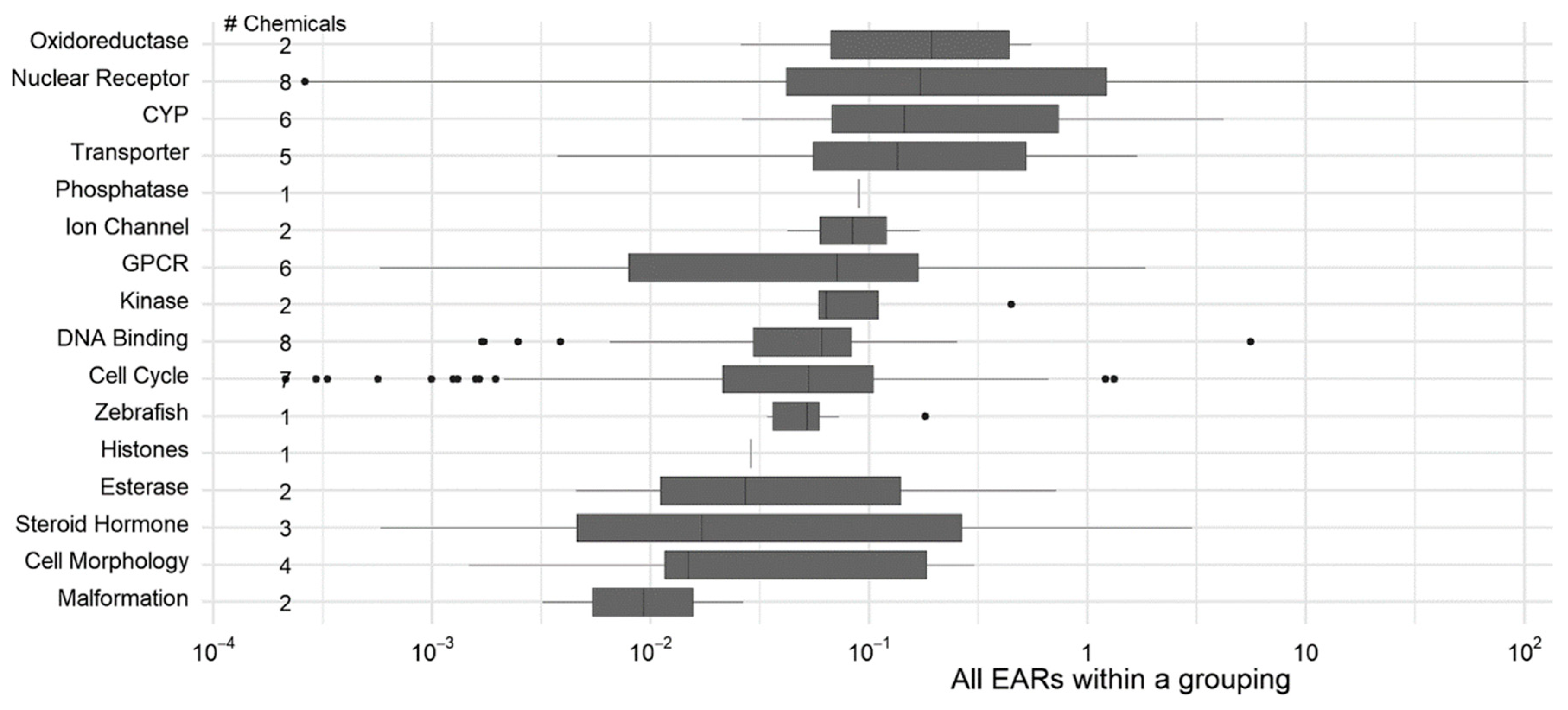
4.3. Mixtures and Exposure–Activity Ratios (EAR)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeMaagd, N. Water Demand in the Residential and Tourism Sectors: Evidence and Implications for Efficient Management. Ph.D. Thesis, University of Hawaii, Manoa, HI, USA, 2020; pp. 55–67. Available online: http://hdl.handle.net/10125/70364 (accessed on 1 December 2021).
- Whittier, R.B.; El-Kadi, A.I. Human Health and Environmental Risk Ranking of On-Site Sewage Disposal Systsems for the Hawaiian Islands of Kauai, Molokai, Maui, and Hawaii; State of Hawaii Department of Health, Safe Drinking Water Branch: Waimalu, HI, USA, 2014.
- Economy, L.M.; Wiegner, T.N.; Strauch, A.M.; Awaya, J.D.; Gerken, T. Rainfall and streamflow effects on estuarine Staphylococcus aureus and fecal indicator bacteria concentrations. J. Environ. Qual. 2019, 48, 1711–1721. [Google Scholar] [CrossRef]
- Abaya, L.M.; Wiegner, T.N.; Beets, J.P.; Colbert, S.L.; Carlson, K.M.; Kramer, K.L. Spatial distribution of sewage pollution on a Hawaiian coral reef. Mar. Pollut. Bull. 2018, 130, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Yoshika, R.M.; Kim, C.J.S.; Tracy, A.M.; Most, R.; Harvell, C.D. Linking sewage pollution and water quality to spatial patterns of Porites lobata growth anomalies in Puako, Hawaii. Mar. Pollut. Bull. 2016, 104, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, Q.A.; Kulikov, S.M.; Graner-O’Neale, L.D.; Metcalf, C.D.; Sultana, T. Contaminants of emerging concern in surface waters in Barbados, West Indies. Environ. Monit. Assess. 2017, 189, 636. [Google Scholar] [CrossRef] [PubMed]
- McDowall, R.M. Hawaiian biogeography and the islands’ freshwater fish fauna. J. Biogeogr. 2003, 30, 703–710. [Google Scholar] [CrossRef]
- Julius, M.L.; Blob, R.W.; Schoenfuss, H.L. The survival of Sicyopterus stimpsoni, an endemic amphidromous Hawaiian gobiid fish, relies on the hydrological cycles of streams: Evidence from changes in algal composition of diet through growth stages. Aquat. Ecol. 2005, 39, 473–484. [Google Scholar] [CrossRef]
- Schoenfuss, H.L.; Blob, R.W. The Importance of Functional Morphology for Fishery Conservation and Management: Applications to Hawaiian Amphidromous Fishes; Bishop Museum Press: Honolulu, HI, USA, 2007; Volume 3, pp. 125–141. [Google Scholar]
- Radtke, R.L.; Kinzie, R.A., III; Folsom, S.D. Age at recruitment of Hawaiian freshwater gobies. Environ. Biol. Fishes 1988, 23, 205–213. [Google Scholar] [CrossRef]
- Schoenfuss, H.L.; Blob, R.W. Kinematics of waterfall climbing in Hawaiian freshwater fishes (Gobiidae): Vertical propulsion at the aquatic-terrestrial interface. J. Zool. 2003, 261, 191–205. [Google Scholar] [CrossRef]
- Rearick, D.C.; Ward, J.; Venturelli, P.; Schoenfuss, H.L. Environmental oestrogens cause predation-induced population decline in a freshwater fish. R. Soc. Open Sci. 2018, 5, 181065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- All, L.N.R.; Monshi, M.; Siddiqua, Z.; Shields, J.; Alame, K.; Wahls, A.; Akemann, C.; Meyer, D.; Crofts, E.J.; Saad, F.; et al. Detection of endocrine disrupting chemicals in Danio rerio and Daphnia pulex: Step-one, behavioral screen. Chemosphere 2021, 271, 129442. [Google Scholar] [CrossRef]
- Blob, R.W.; Kawano, S.M.; Moody, K.N.; Bridges, W.C.; Maie, T.; Ptacek, M.B.; Julius, M.L.; Schoenfuss, H.L. Morphological selection and the evaluation of potential tradeoffs between escape from predators and the climbing of waterfalls in the Hawaiian stream Goby Sicyopterus stimpsoni. Integr. Comp. Biol. 2010, 50, 1185–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, P.M.; Journey, C.A.; Romanok, K.M.; Barber, L.B.; Buxton, H.T.; Foreman, W.T.; Furlong, E.T.; Glassmeyer, S.T.; Hladik, M.L.; Iwanowicz, L.R.; et al. Expanded target-chemical analysis reveals extensive mixed-organic-contaminant exposure in U.S. streams. Environ. Sci. Technol. 2017, 51, 4792–4802. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.M.; Brigham, M.E.; Kiesling, R.L.; Schoenfuss, H.L.; Jorgenson, Z.G. Environmentally Relevant Chemical Mixtures of Concern in U.S. Tributaries to the Great Lakes. Integr. Environ. Assess. Manag. 2018, 14, 509–518. [Google Scholar] [CrossRef]
- Nilsen, E.; Smalling, K.L.; Ahrens, L.; Gros, M.; Miglioranza, K.S.B.; Pico, Y.; Schoenfuss, H.L. Critical Review: Grand challenges in assessing the adverse effects of contaminants of emerging concern on aquatic food webs. Environ. Toxicol. Chem. 2019, 38, 46–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoenfuss, H.L.; Blanchard, T.A.; Kuamo’o, D.G.K. Metamorphosis in the cranium of postlarval Sicyopterus stimpsoni, an endemic Hawaiian stream goby. Micronesica 1997, 30, 93–104. [Google Scholar]
- Keith, P. Biology and Ecology of Amphidromous Gobiidae of the Indo-Pacific and the Caribbean Regions. J. Fish Biol. 2003, 63, 831–847. [Google Scholar] [CrossRef]
- Martin, J.; Buchberger, W.; Santos, J.L.; Alonso, E.; Aparicio, I. High-performance liquid chromatography quadrupole time-of-flight mass spectrometry method for the analysis of antidiabetic drugs in aqueous environmental samples. J. Chromatogr. B 2012, 895, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Diamond, K.M.; Lagarde, R.; Schoenfuss, H.L.; Walker, J.A.; Ponton, D.; Blob, R.W. Relationship of escape performance with predator regime and ontogeny in fishes. J. Linnaean Soc. 2019, 127, 324–336. [Google Scholar] [CrossRef]
- Diamond, K.M.; Schoenfuss, H.L.; Walker, J.A.; Blob, R.W. Flowing water affects fish fast-starts: Escape performance of the Hawaiian stream goby, Sicyopterus stimpsoni. J. Exp. Biol. 2016, 219, 3100–3105. [Google Scholar] [CrossRef] [Green Version]
- Hedrick, T.L. Software techniques for two- and three-dimensional kinematic measurements of biological and biomimetic systems. Bioinspir. Biomim. 2008, 3, 034001. [Google Scholar] [CrossRef] [PubMed]
- Domenici, P.; Blake, R.W. The kinematics and performance of fish fast-start swimming. J. Exp. Biol. 1997, 200, 1165–1178. [Google Scholar] [CrossRef]
- Hale, M.E. Locomotor mechanics during early life history: Effects of size and ontogeny on fast-start performance of salmonid fishes. J. Exp. Biol. 1999, 202, 1465–1479. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.A.; Ghalambor, C.K.; Griset, O.L.; McKenney, D.; Reznick, D.N. Do faster starts increase the probability of evading predators? Funct. Ecol. 2005, 19, 808–815. [Google Scholar] [CrossRef]
- Diamond, K.M.; Lagarde, R.; Griner, J.G.; Ponton, D.; Powder, K.E.; Schoenfuss, H.L.; Walker, J.S.; Blob, R.W. Interactions among multiple selective pressures on the form-function relationship in insular stream fishes. Biol. J. Linn. Soc. 2021, 134, 557–567. [Google Scholar] [CrossRef]
- Ramsey, J.; Ripley, B. Pspline: Penalized Smoothing Splines. 2017. Available online: https://cran.r-project.org/web/packages/pspline/pspline.pdf (accessed on 1 December 2021).
- Blob, R.W.; Rai, R.; Julius, M.L.; Schoenfuss, H.L. Functional diversity in extreme environments: Effects of locomotor style and substrate texture on the waterfall-climbing performance of Hawaiian gobiid fishes. J. Zool. 2006, 268, 315–324. [Google Scholar] [CrossRef]
- Blob, R.W.; Bridges, W.C.; Ptacek, M.B.; Maie, T.; Cediel, R.A.; Bertolas, M.M.; Julius, M.L.; Schoenfuss, H.L. Morphological selection in an extreme flow environment: Body shape and waterfall-climbing success in the Hawaiian stream fish Sicyopterus stimpsoni. Integr. Comp. Biol. 2008, 48, 734–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, R.A.; Friedman, K.P.; Simon, T.W.; Marty, M.S.; Patlewicz, G.; Rowlands, J.C. An exposure: Activity profiling method for interpreting high-throughput screening data for estrogenic activity-proof of concept. Regul. Toxiol. Pharmacol. 2015, 71, 398–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, D.; Mäechler, M.; Bloker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Soft. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Starling, M.C.V.M.; Amorim, C.C.; Leao, M.M.D. Occurrence, control and fate of contaminants of emerging concern in environmental compartments in Brazil. J. Hazard. Mater. 2019, 372, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Tiedeken, E.J.; Tahar, A.; McHugh, B.; Rowan, N.J. Monitoring, sources, receptors, and control measures for three European Union watch list substances of emerging concern in receiving waters—A 20 year systematic review. Sci. Total Environ. 2017, 574, 1140–1163. [Google Scholar] [CrossRef] [PubMed]
- Odendaal, C.; Seaman, M.T.; Kemp, G.; Patterton, H.E.; Patterton, H.-G. An LC-MS/MS based survey of contaminants of emerging concern in drinking water in South Africa. S. Afr. J. Sci. 2015, 111, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Richardson, B.J.; Lam, P.K.S.; Martin, M. Emerging chemicals of concern: Pharmaceuticals and personal care products (PPCPs) in Asia, with particular reference to South China. Mar. Pollut. Bull. 2005, 50, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Meador, J.P.; Bettcher, L.F.; Ellenberger, M.C.; Senn, T.D. Metabolic profiling for juvenile Chinook salmon exposed to contaminants of emerging concern. Sci. Total Environ. 2020, 747, 141097. [Google Scholar] [CrossRef]
- Caldwell, D.J.; D’Aco, V.; Davidson, T.; Kappler, K.; Murray-Smith, R.J.; Owen, S.F.; Robinson, P.F.; Simon-Hettich, B.; Straub, J.O.; Tell, J. Environmental risk assessment of metformin and its transformation product guanylurea: II. occurrence in surface waters of Europe and the United States and derivation of predicated no-effects concentrations. Chemosphere 2019, 216, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Guyader, M.; Warren, L.; Green, E.; Proudian, A.; Kiesling, R.; Schoenfuss, H.L.; Higgins, C.P. Trace Organic Contaminant (TOrC) Mixtures in Minnesota Littoral Zones: Effects of On-Site Wastewater Treatment System (OWTS) Proximity and Biologic Impact. Sci. Total Environ. 2018, 626, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Weeks, J.; Guiney, P.; Nikiforov, A. Assessment of the environmental fate and ecotoxicity of N,N-diethyl-m-toluamide (DEET). Integr. Environ. Assess. Manag. 2012, 8, 120–134. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. National Library of Medicine TOXNET; Toxicological Data Network; U.S. Department of Health and Human Services: Washington, DC, USA, 2017. Available online: https://toxnet.nlm.nih.gov/newtoxnet/index.html (accessed on 21 December 2019).
- Swartz, C.H.; Reddy, S.; Benotti, M.J.; Yin, H.; Barber, L.B.; Brownawell, B.J.; Rudel, R.A. Steroid estrogens, nonylphenol ethoxylate metabolites, and other wastewater contaminants in groundwater affected by a residential septic system on Cape Cod, MA. Environ. Sci. Technol. 2006, 40, 4894–4902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, B.; Price, A.E.; Scott, W.C.; Kristofco, L.A.; Ramirez, A.J.; Chambliss, C.K.; Yelderman, J.C.; Brooks, B.W. Comparison of contaminants of emerging concern removal, discharge, and water quality hazards among centralized and on-site wastewater treatment system effluents receiving common wastewater influent. Sci. Total Environ. 2014, 466, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.-Q.; Liu, Y.-S.; Xiong, Q.; Cai, W.-W.; Ying, G.-G. Occurrence, toxicity and transformation of six typical benzotriazoles in the envrionment: A review. Sci. Total Environ. 2019, 661, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Brasher, A.; Wolff, R.H. Relations between land use and organochlorine pesticides. PCBs, and semi-volatile organic compounds in streambed sediment and fish on the Island of Oahu, Hawaii. Arch. Environ. Contam. Toxicol. 2004, 46, 385–398. [Google Scholar] [CrossRef]
- Brasher, A.M.D. Impacts of human disturbances on biotic communities in Hawaiian streams. BioScience 2003, 53, 1052–1060. [Google Scholar] [CrossRef] [Green Version]
- Brasher, A.; Wolff, R.H.; Luton, C.D. Associations Among Land Use, Habitat Characteristics, and Invertebrate Community Structure in Nine Streams on the Island of Oahu, Hawaii, 1999–2001; U.S. Geological Survey: Sunrise Valley Drive, VA, USA, 2003.
- Hathaway, C.B. Stream Channel Modification in Hawaii, Part C: Tolerance of Native Stream Species to Observed Levels of Environmental Variability. Colombia (MO): US Fish and Wildlife Service, National Stream Alteration Team; US Department of the Interior: Washington, DC, USA, 1978.
- Moody, K.N.; Gagne, R.B.; Heim-Ballew, H.; Alda, F.; Hain, E.F.; Lisi, P.J.; Walter, R.P.; Higashi, G.R.; Hogan, J.D.; McIntyre, P.B.; et al. Invasion hotspots and ecological saturation of streams across the Hawaiian archipelago. Cybium 2017, 41, 127–156. [Google Scholar] [CrossRef]
- Moody, K.N.; Wren, J.L.K.; Kobayashi, D.R.; Blum, M.J.; Ptacek, M.B.; Blob, R.W.; Toonen, R.J.; Schoenfuss, H.L.; Childress, M.J. Evidence of local adaptation in a waterfall-climbing Hawaiian goby derived from coupled biophysical modeling of larval dispersal and post-settlement selection. BMC Evol. Biol. 2019, 19, 88. [Google Scholar] [CrossRef]
- Elizalde-Velázquez, G.A.; Gómez-Oliván, L.M. Occurrence, toxic effects and removal of metformin in the aquatic environments in the world: Recent trends and perspectives. Sci. Total Environ. 2020, 702, 134924. [Google Scholar] [CrossRef] [PubMed]
- Sundlin, A. Ecotoxicological Effects on a Food-Web Exposed to Pharmaceuticals. Master’s Thesis, UmeÅ University, Umeå, Sweden, 2015. [Google Scholar]
- Gatermann, R.; Biselli, S.; Hühnerfuss, H.; Rimkus, G.G.; Hecker, M.; Karbe, L. Synthetic musks in the environment. Part 1: Species-dependent bioaccumulation of polycyclic ad. nitro musk fragrances in freshwater fish and mussels. Arch. Environ. Contam. Toxicol. 2002, 42, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Correa-Reyes, G.; Viana, M.T.; Matquez-Rocha, F.J.; Licea, A.F.; Ponce, E.; Vazques-Duhalt, R. Nonylphenol algal bioaccumulation and its effects through the trophic chain. Chemosphere 2007, 68, 662–670. [Google Scholar] [CrossRef] [PubMed]
- de Albuquerque, F.P.; de Oliveira, J.L.; Moschini-Carlos, V.; Fraceto, L.F. An overview of the potential impacts of atrazine in aquatic environments: Perspectives for tailored solutions based on nanotechnology. Sci. Total Environ. 2020, 700, 134868. [Google Scholar] [CrossRef]
- Jackson, M.C.; Loewen, C.J.G.; Vinebrooke, R.D.; Chimimba, C.T. Net effect of multiple stressors in freshwater ecosystems: A meta-analysis. Glob. Chang. Biol. 2016, 22, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.M.; Minarik, T.A.; Martinovic-Weigelt, D.; Curran, E.A.; Bartell, S.E.; Schoenfuss, H.L. Environmental estrogens in an urban aquatic ecosystem: II. Biological effects. Environ. Int. 2013, 61, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Aluru, N.; Leatherland, J.F.; Vijayan, M.M. Bisphenol A in oocytes leads to growth suppression and altered stress performance in juvenile rainbow trout. PLoS ONE 2010, 5, e10741. [Google Scholar] [CrossRef] [Green Version]
| Chemical | Year | Method | LDL | Log Kow 1 | Ambient | Low | Medium | High | |
|---|---|---|---|---|---|---|---|---|---|
| metformin | 2017 2018 | anti-diabetic | LC–MS | 60 | −2.64 | 85 667 | 34 885 | 1995 2129 | 19,172 14,748 |
| sulfamethoxazole | 2017 2018 | antibiotic | LC–MS | 60 | 0.89 | 6 445 | 121 83 | 253 885 | 4036 5830 |
| fexofenadine | 2017 2018 | antihistamine | LC–MS | 162 | 2.81 | 122 110 | 720 520.5 | 2173 2236 | 18,173 18,638 |
| desvenlafaxine | na | mood-altering | GC–MS | 360 | 2.72 | nm nm | 218 | 583 | 5830 |
| estrone | na | hormone | GC–MS | 4130 | 3.13 | nm | 3 | 30 | 300 |
| DEET | 2017 2018 | repellent | LC–MS | 55 | 2.02 | 609 718 | 1763 388 | 3654 1838 | 16,463 12,859 |
| HHCB 1 | na | fragrance | GC–MS | 321 | 5.90 | nm | 218 | 2180 | 21,800 |
| benzotriazole | 2017 2018 | industrial | GC–MS | 75 | 1.44 | 288 358 | 2215 1350 | 5408 8618 | 68,075 77,479 |
| 4-Nonylphenol | 2017 2018 | surfactant | GC–MS | 17,800 | 5.76 | nm | 400 | 4000 | 40,000 |
| TBEP 2 | 2017 2018 | plasticizer | GC–MS | 2260 | 3.75 | nm | 1500 | 15,000 | 150,000 |
| BPA | 2017 2018 | plasticizer | GC–MS | 2340 | 3.32 | nm | 300 | 3000 | 30,000 |
| atrazine | 2017 2018 | herbicide | LC–MS | 65 | 2.61 | 16 45 | 76 93 | 333 394 | 3103 2801 |
| bromacil | 2017 2018 | herbicide | LC–MS | 150 | 2.11 | 6 10 | 61 28 | 156 171 | 1275 1212 |
| metolachlor | 2017 2018 | herbicide | LC–MS | 145 | 3.13 | 5 8 | 35 30 | 161 165 | 1446 1316 |
| Replicate | Predation Experiment | Control | Low | Medium | High |
|---|---|---|---|---|---|
| 1 | survival (%) | 93.3 | 100 | 100 | 100 |
| no response | 1 | 2 | 0 | 1 | |
| analyzed responses | 12 | 11 | 14 | 12 | |
| latency (ms) | 20 ± 7.3 | 29 ± 14 | 22 ± 9.6 | 24 ± 7.9 | |
| peak acceleration (cm/s2) | 3543 ± 1077 | 2664 ± 1070 | 2650 ± 1136 | 2779 ± 1191 | |
| peak velocity (cm/s) | 95 ± 18 | 86 ± 26 | 80 ± 23 | 89 ± 21 | |
| 2 | survival (%) | 93.3 | 93.3 | 93.3 | 100 |
| no response | 2 | 2 | 1 | 1 | |
| analyzed responses | 9 | 11 | 12 | 14 | |
| latency (ms) | 21 ± 11 | 24 ± 16 | 23 ± 8.3 | 21 ± 9 | |
| peak acceleration (cm/s2) | 3134 ± 1126 | 2886 ± 975 | 3007 ± 797 | 3095 ± 864 | |
| peak velocity (cm/s) | 98 ± 18 | 93 ± 17 | 99 ± 14 | 97 ± 19 | |
| 3 | survival (%) | 93.3 | 80.0 | 86.7 | 60.0 |
| no response | 0 | 2 | 1 | 0 | |
| analyzed responses | 13 | 7 | 10 | 4 | |
| latency (ms) | 43 ± 25 | 38 ± 19 | 31 ± 17 | 22 ± 11 | |
| peak acceleration (cm/s2) | 3057 ± 659 a | 2914 ± 651 a | 3047 ± 902 a | 1374 ± 780 b | |
| peak velocity (cm/s) | 95 ± 15 | 95 ± 11 | 101 ± 19 | 79 ± 29 |
| Climbing Experiment | Control | Low | Medium | High |
|---|---|---|---|---|
| survival (%) | 86.7 | 73.3 | 86.7 | 60.0 |
| non-climber | 3 | 2 | 3 | 2 |
| analyzed responses | 10 | 9 | 10 | 7 |
| time in motion (%) | 62 ± 14 | 52 ± 21 | 56 ± 20 | 51 ± 21 |
| climb velocity (cm/s) | 0.38± 0.06 ab | 0.47± 0.17 a | 0.35± 0.06 b | 0.41± 0.07 ab |
| total velocity (cm/s) | 0.23 ± 0.05 | 0.23 ± 0.09 | 0.19 ± 0.08 | 0.21 ± 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diamond, K.M.; Good, C.J.; Johnny, N.; Sakihara, T.S.; Edmiston, P.L.; Faust, J.A.; Schoenfuss, T.C.; Rubin, A.M.; Blob, R.W.; Schoenfuss, H.L. Assessing Occurrence and Biological Consequences of Contaminants of Emerging Concern on Oceanic Islands. Water 2022, 14, 275. https://doi.org/10.3390/w14030275
Diamond KM, Good CJ, Johnny N, Sakihara TS, Edmiston PL, Faust JA, Schoenfuss TC, Rubin AM, Blob RW, Schoenfuss HL. Assessing Occurrence and Biological Consequences of Contaminants of Emerging Concern on Oceanic Islands. Water. 2022; 14(3):275. https://doi.org/10.3390/w14030275
Chicago/Turabian StyleDiamond, Kelly M., Christopher J. Good, Nina Johnny, Troy S. Sakihara, Paul L. Edmiston, Jennifer A. Faust, Tonya C. Schoenfuss, Alexander M. Rubin, Richard W. Blob, and Heiko L. Schoenfuss. 2022. "Assessing Occurrence and Biological Consequences of Contaminants of Emerging Concern on Oceanic Islands" Water 14, no. 3: 275. https://doi.org/10.3390/w14030275





