Taxonomic and Functional Responses of Species-Poor Riverine Fish Assemblages to the Interplay of Human-Induced Stressors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Design and Fish Sampling
2.3. Beta Diversity Analyses
2.4. Estimation of Functional Diversity Indices
2.5. The Effects of Stressors on Functional Diversity
3. Results
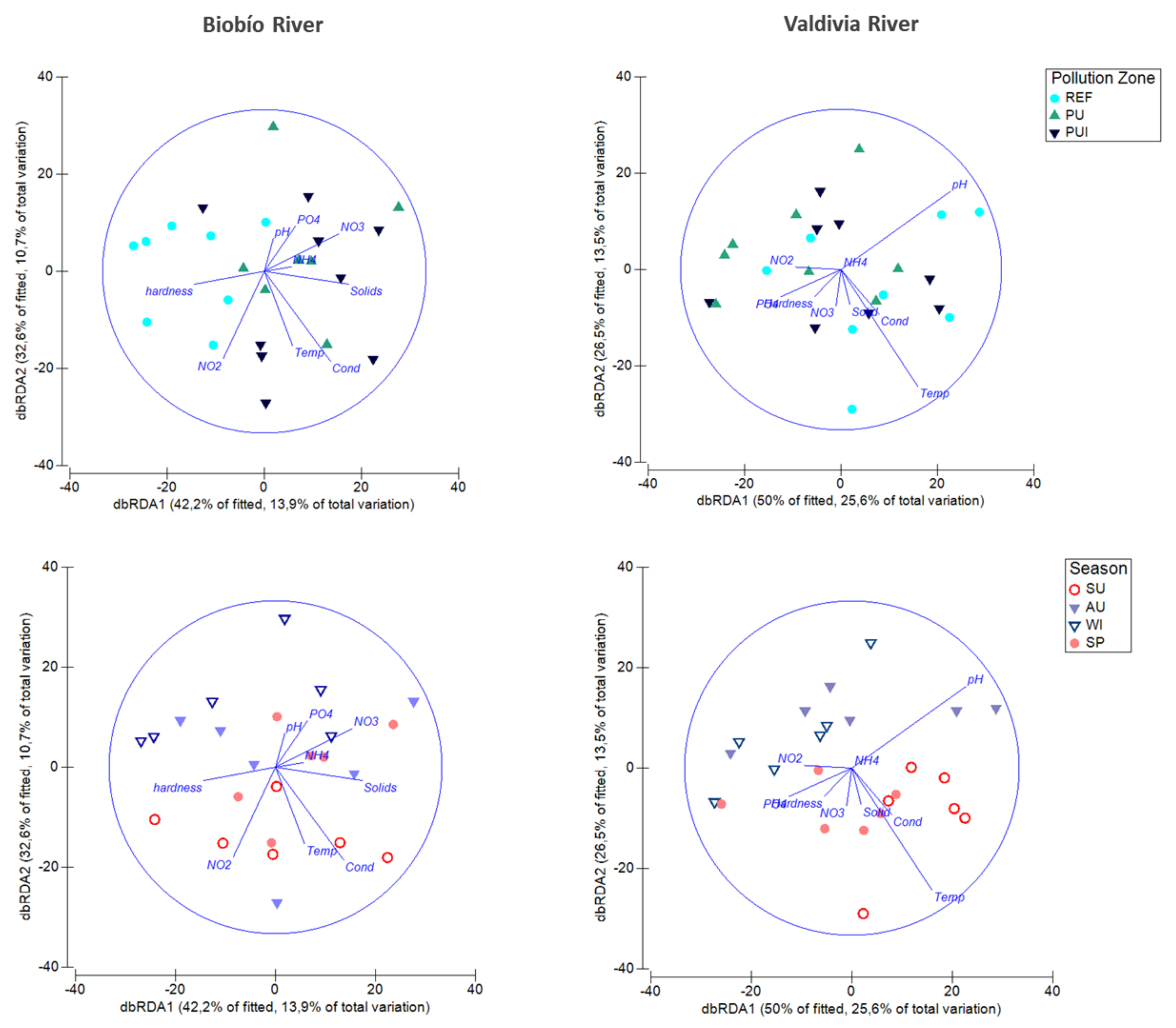
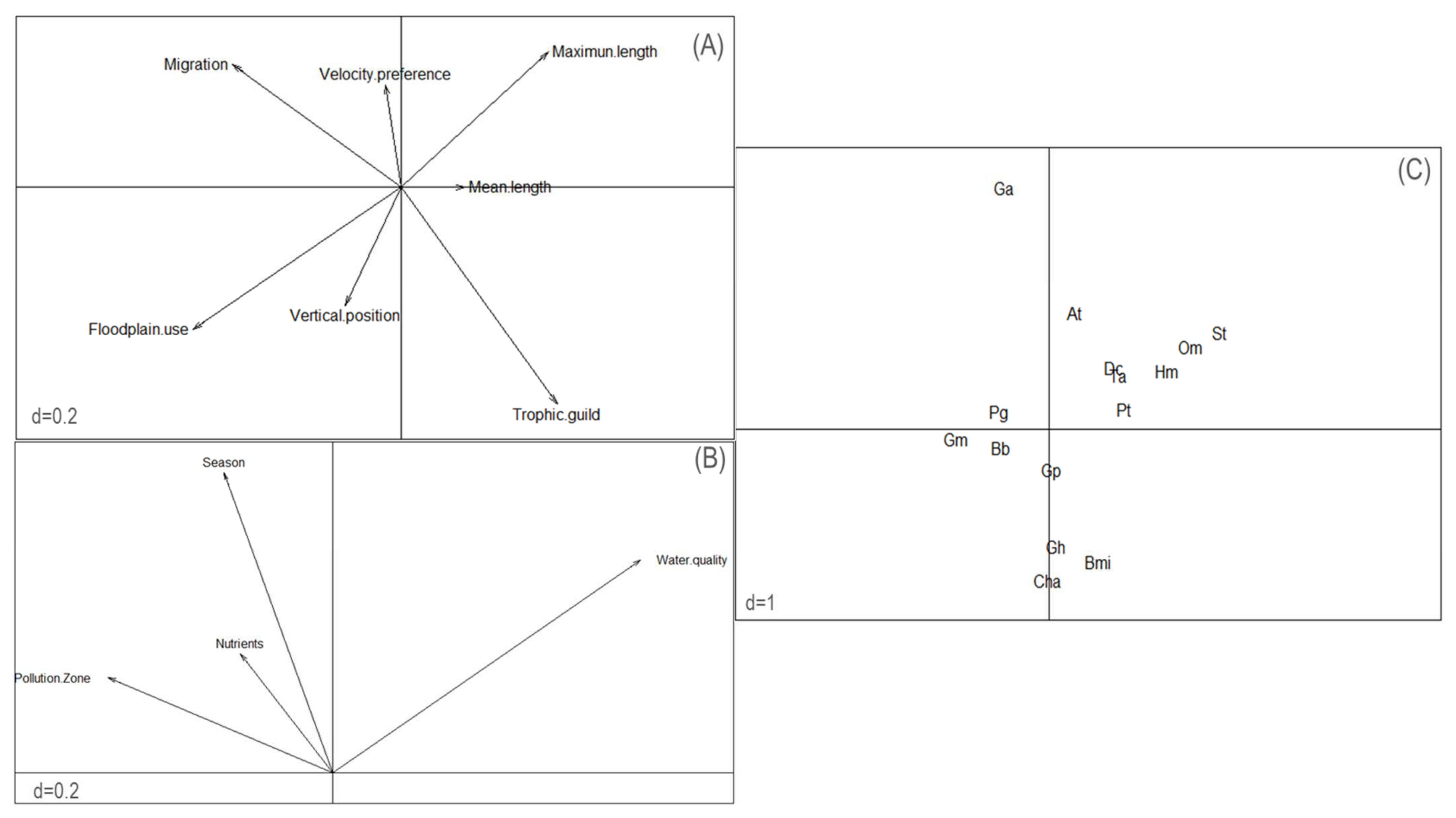
3.1. Taxonomic Assemblage Structure and Environmental Effects
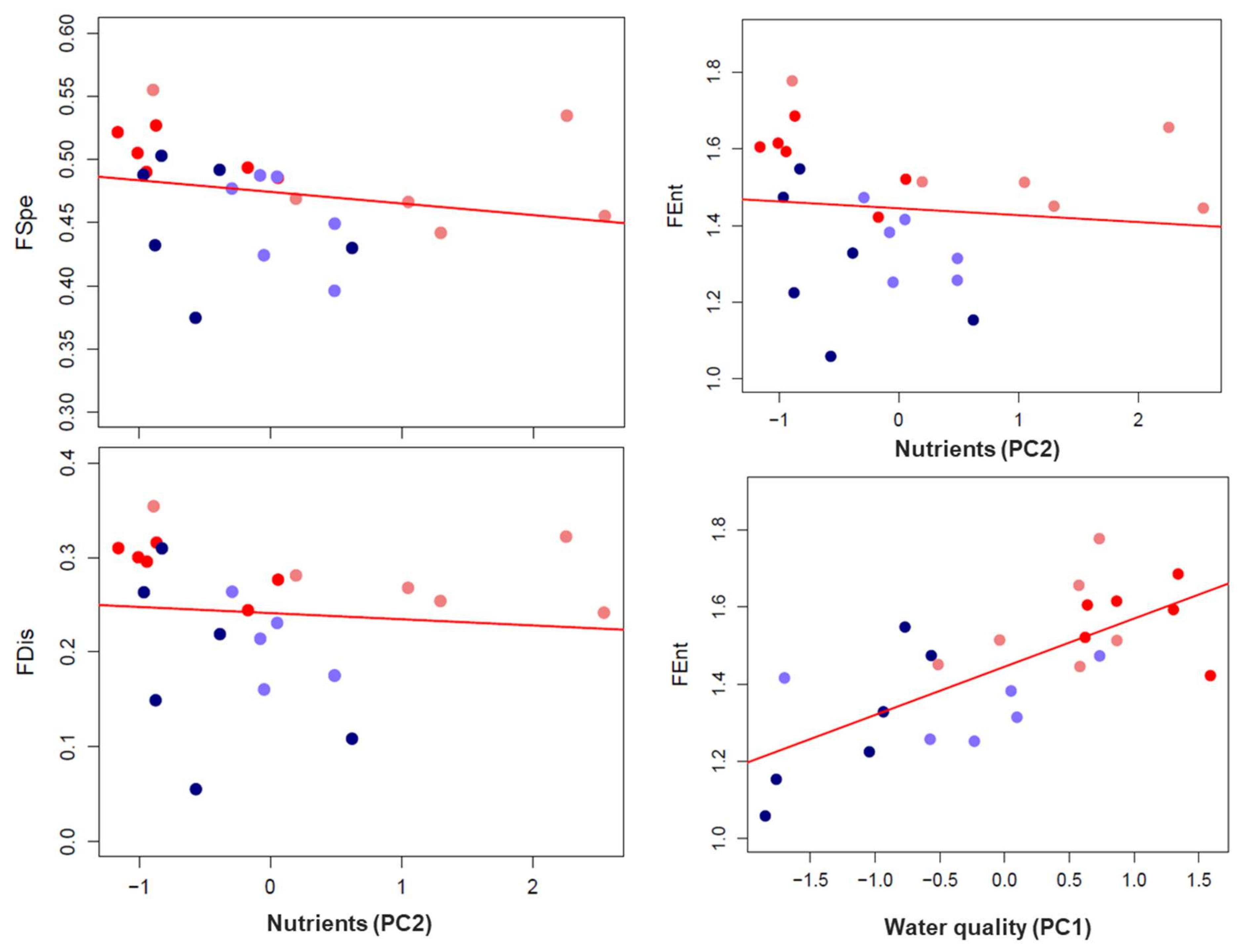
3.2. The Effects of Stressors on Functional Diversity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Code | Common Name | Family | Origin | Basin | |
|---|---|---|---|---|---|---|
| Biobio River | Valdivia River | |||||
| Australoheros facetus | Af | chanchito | Cichlidae | non-native | x | |
| Aplochiton taeniatus | At | peladilla | Galaxiidae | native | x | |
| Bullockia maldonadoi | Bma | bagrecito | Tricomycteridae | native | x | |
| Basilychthys microlepidotus | Bmi | pejerrey chileno | Atherinopsinae | native | x | x |
| Brachylaxias bullocki | Bb | puye rojo | Galaxiidae | native | x | |
| Cyprinus carpio | Cc | carpa | Ciprinidae | non-native | x | |
| Cheirodon kiliani | Cha | pocha | Characidae | native | x | |
| Cheirodon galusdae | Chg | pocha | Characidae | native | x | |
| Diplomystes camposensis | Dc | tollo | Diplomystidae | native | x | |
| Geotria australis | Ga | lamprea | Geotriidae | native | x | x |
| Gambusia holbrooki | Gh | pez mosquito | Poeciliidae | non-native | x | x |
| Galaxias maculatus | Gm | puye | Galaxiidae | native | x | x |
| Galaxias platei | Gp | puye grande | Galaxiidae | native | x | |
| Hatcheria macrei | Hm | bagre grande | Tricomycteridae | native | x | |
| Onchorrynchus mykiss | Om | trucha arcoiris | Salmonidae | non-native | x | x |
| Percilia irwini | Pi | carmelita de Concepción | Perciilidae | native | x | |
| Percilia gillissii | Pg | Carmelita | Perciilidae | native | x | |
| Percicthys melanops | Pm | perca negra | Percichthyidae | native | x | |
| Percicthys trucha | Pt | perca | Percichthyidae | native | x | x |
| Salmo trutta | St | trucha café | Ssalmonidae | non-native | x | x |
| Trichomycterus areolatus | Ta | bagre chico | Tricomycteridae | native | x | x |
References
- Strayer, D.L.; Dudgeon, D. Freshwater biodiversity conservation: Recent progress and future challenges. J. N. Am. Benthol. Soc. 2010, 29, 344–358. [Google Scholar] [CrossRef] [Green Version]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Reidy Liermann, C.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Araya-Osses, D.; Casanueva, A.; Román-Figueroa, C.; Uribe, J.M.; Paneque, M. Climate change projections of temperature and precipitation in Chile based on statistical downscaling. Clim. Dyn. 2020, 54, 4309–4330. [Google Scholar] [CrossRef]
- Bozkurt, D.; Rojas, M.; Boisier, J.P.; Valdivieso, J. Projected hydroclimate changes over Andean basins in central Chile from downscaled CMIP5 models under the low and high emission scenarios. Clim. Chang. 2018, 150, 131–147. [Google Scholar] [CrossRef]
- Habit, E.; Górski, K.; Alò, D.; Ascencio, E.; Astorga, A.; Colin, N.; Contador, T.; de los Ríos, P.; Delgado, V.; Dorador, C.; et al. Biodiversidad de Ecosistemas de Agua Dulce. Mesa Biodiversidad-Comité Científico COP25; Ministerio de Ciencia, Tecnología, Conocimiento e Innovación: Santiago, Chile, 2019; p. 64. [Google Scholar]
- Pastén, P.; Vega, A.; Guerra, P.; Pizarro, J.; Lizama, K. Water Quality in Chile: Progress, Challenges and Perspectives. In Water Quality in the Americas; IANAS: Ciudad de México, Mexico, 2019; p. 160. [Google Scholar]
- Schinegger, R.; Pucher, M.; Aschauer, C.; Schmutz, S. Configuration of multiple human stressors and their impacts on fish assemblages in Alpine river basins of Austria. Sci. Total Environ. 2018, 616, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Colin, N.; Villéger, S.; Wilkes, M.; de Sostoa, A.; Maceda-Veiga, A. Functional diversity measures revealed impacts of non-native species and habitat degradation on species-poor freshwater fish assemblages. Sci. Total Environ. 2018, 625, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pérez, A.; Oliva-Paterna, F.J.; Colin, N.; Torralva, M.; Górski, K. Functional response of fish assemblage to multiple stressors in a highly regulated Mediterranean river system. Sci. Total Environ. 2020, 730, 138989. [Google Scholar] [CrossRef] [PubMed]
- Birk, S.; Chapman, D.; Carvalho, L.; Spears, B.M.; Andersen, H.E.; Argillier, C.; Auer, S.; Baattrup-Pedersen, A.; Banin, L.; Beklioğlu, M.; et al. Impacts of multiple stressors on freshwater biota across spatial scales and ecosystems. Nat. Ecol. Evol. 2020, 4, 1060–1068. [Google Scholar] [CrossRef]
- Ormerod, S.J.; Dobson, M.; Hildrew, A.G.; Townsend, C. Multiple stressors in freshwater ecosystems. Freshw. Biol. 2010, 55 (Suppl. S1), 1–4. [Google Scholar] [CrossRef]
- Schinegger, R.; Trautwein, C.; Melcher, A.; Schmutz, S. Multiple human pressures and their spatial patterns in european running waters. Water Environ. J. 2012, 26, 261–273. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Cánovas, C.; Millán, A.; Velasco, J.; Vaughan, I.P.; Ormerod, S.J. Contrasting effects of natural and anthropogenic stressors on beta diversity in river organisms. Glob. Ecol. Biogeogr. 2013, 22, 796–805. [Google Scholar] [CrossRef]
- Colin, N.; Maceda-Veiga, A.; Monroy, M.; Ortega-Ribera, M.; Llorente, M.; de Sostoa, A. Trends in biomarkers, biotic indices, and fish population size revealed contrasting long-term effects of recycled water on the ecological status of a Mediterranean river. Ecotoxicol. Environ. Saf. 2017, 145, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Amiard-Triquet, C.; Cossu-Leguille, C.; Mouneyrac, C. Biomarkers of defense, tolerance, and ecological consequences. In Ecological Biomarkers: Indicators of Ecotoxicological Effects; CRC Press: London, UK, 2013; pp. 45–74. [Google Scholar]
- Vila, I.; Fuentes, L.; Contreras, M. Peces límnicos de Chile. Bol. Mus. Nac. Hist. Nat. 1999, 48, 61–75. [Google Scholar]
- Dyer, B.S. Revisión sistemática de los Pejerreyes de Chile (Teleostei, Atheriniforme). Estud. Oceanol 2000, 200019, 99–127. [Google Scholar]
- Habit, E.; Dyer, B.; Vila, I. Estado de conocimiento de los peces dulceacuícolas de Chile. Gayana (Concepc) 2006, 70, 100–113. [Google Scholar] [CrossRef] [Green Version]
- Habit, E.; Piedra, P.; Ruzzante, D.E.; Walde, S.J.; Belk, M.C.; Cussac, V.E.; González, J.; Colin, N. Changes in the distribution of native fishes in response to introduced species and other anthropogenic effects. Glob. Ecol. Biogeogr. 2010, 19, 697–710. [Google Scholar] [CrossRef]
- MMA. Ministerio del Medio Ambiente. 2021. Available online: http://especies.mma.gob.cl/CNMWeb/Web/WebCiudadana/Default.aspx (accessed on 16 January 2021).
- Castro, S.A.; Rojas, P.; Vila, I.; Habit, E.; Pizarro-Konczak, J.; Abades, S.; Jaksic, F.M. Partitioning β-diversity reveals that invasions and extinctions promote the biotic homogenization of Chilean freshwater fish fauna. PLoS ONE 2020, 15, e0238767. [Google Scholar] [CrossRef]
- Díaz, G.; Górski, K.; Heino, J.; Arriagada, P.; Link, O.; Habit, E. The longest fragment drives fish beta diversity in fragmented river networks: Implications for river management and conservation. Sci. Total Environ. 2021, 766, 144323. [Google Scholar] [CrossRef]
- Vellend, M. Do commonly used indices of β-diversity measure species turnover? J. Veg. Sci. 2001, 12, 545–552. [Google Scholar] [CrossRef]
- Socolar, J.B.; Gilroy, J.J.; Kunin, W.E.; Edwards, D.P. How should beta-diversity inform biodiversity conservation? Trends Ecol. Evol. 2016, 31, 67–80. [Google Scholar] [CrossRef] [Green Version]
- Mouillot, D.; Graham, N.A.; Villéger, S.; Mason, N.W.; Bellwood, D.R. A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 2013, 28, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Rojas, P.; Castro, S.A.; Vila, I.; Jaksic, F.M. Exotic species elicit decoupled responses in functional diversity components of freshwater fish assemblages in Chile. Ecol. Indic. 2021, 133, 108364. [Google Scholar] [CrossRef]
- Teichert, N.; Lepage, M.; Sagouis, A.; Borja, A.; Chust, G.; Ferreira, M.T.; Argillier, C. Functional redundancy and sensitivity of fish assemblages in European rivers, lakes and estuarine ecosystems. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, A.; Manning, P.; Cadotte, M.W.; Cowles, J.; Isbell, F.; Jousset, A.L.; Wagg, C. Lost in trait space: Species-poor communities are inflexible in properties that drive ecosystem functioning. Adv. Ecol. Res. 2019, 61, 91–131. [Google Scholar] [CrossRef]
- Villéger, S.; Mason, N.W.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef] [Green Version]
- Colin, N.; Piedra, P.; Habit, E. Variaciones espaciales y temporales de las comunidades ribereñas de peces en un sistema fluvial no intervenido: Río San Pedro, cuenca del río Valdivia (Chile). Gayana (Concepc) 2012, 76, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Díaz, G.; Arriagada, P.; Górski, K.; Link, O.; Karelovic, B.; Gonzalez, J.; Habit, E. Fragmentation of Chilean Andean rivers: Expected effects of hydropower development. Rev. Chil. Hist. Nat. 2019, 92, 1–13. [Google Scholar] [CrossRef]
- Elgueta, A.; Górski, K.; Thoms, M.; Fierro, P.; Toledo, B.; Manosalva, A.; Habit, E. Interplay of geomorphology and hydrology drives macroinvertebrate assemblage responses to hydropeaking. Sci. Total Environ. 2021, 768, 144262. [Google Scholar] [CrossRef]
- Chiang, G.; Munkittrick, K.R.; McMaster, M.E.; Barra, R.; Servos, M. Marco conceptual de Monitoreo Regional de Efectos Acumulativos: Brechas y Desafíos para la Cuenca del rio Biobío en el Centro-Sur de Chile. Gayana (Concepc) 2014, 78, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Alonso, Á.; Figueroa, R.; Castro-Díez, P. Pollution assessment of the Biobío River (Chile): Prioritization of substances of concern under an ecotoxicological approach. Environ. Manag. 2017, 59, 856–869. [Google Scholar] [CrossRef]
- Orrego, R.; Hewitt, L.M.; McMaster, M.; Chiang, G.; Quiroz, M.; Munkittrick, K.; Gavilán, F.; Barra, R. Assessing wild fish exposure to ligands for sex steroid receptors from pulp and paper mill effluents in the Biobio River Basin, Central Chile. Ecotoxicol. Environ. Saf. 2019, 171, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, K.; Einax, J.W. Analytical and chemometric characterization of the Cruces River in South Chile. Environ. Sci. Pollut. Res. 2010, 17, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Marín, V.H.; Delgado, L.E.; Vila, I.; Tironi, A.; Barrera, V.; Ibáñez, C. Regime shifts of Cruces River wetland ecosystem: Current conditions, future uncertainties. Lat. Am. J. Aquat. Res. 2014, 42, 160–171. [Google Scholar] [CrossRef]
- Barra, R.O.; Chiang, G.; Saavedra, M.F.; Orrego, R.; Servos, M.R.; Hewitt, L.M.; McMaster, M.; Bahamonde, P.; Tucca, F.; Munkittrick, K.R. Endocrine Disruptor Impacts on Fish From Chile: The Influence of Wastewaters. Front. Endocrinol. 2021, 12, 208. [Google Scholar] [CrossRef] [PubMed]
- Maire, E.; Grenouillet, G.; Brosse, S.; Villéger, S. How many dimensions are needed to accurately assess functional diversity? A pragmatic approach for assessing the quality of functional spaces. Glob. Ecol. Biogeogr. 2015, 24, 728–740. [Google Scholar] [CrossRef]
- Elgueta, A.; Thoms, M.C.; Górski, K.; Díaz, G.; Habit, E. Functional process zones and their fish communities in temperate Andean river networks. River Res. Appl. 2019, 35, 1702–1711. [Google Scholar] [CrossRef]
- Quiroz-Jara, M.; Casini, S.; Fossi, M.C.; Orrego, R.; Gavilán, J.F.; Barra, R. Integrated Physiological Biomarkers Responses in Wild Fish Exposed to the Anthropogenic Gradient in the Biobío River, South-Central Chile. Environ. Manag. 2021, 67, 1145–1157. [Google Scholar] [CrossRef]
- Pauchard, A.; Aguayo, M.; Peña, E.; Urrutia, R. Multiple effects of urbanization on the biodiversity of developing countries: The case of a fast-growing metropolitan area (Concepción, Chile). Biol. Conserv. 2006, 127, 272–281. [Google Scholar] [CrossRef]
- Niemeyer, H.; Cereceda, P. Hidrografía. Ediciones Geografía de Chile; Instituto Geográfico Militar: Santiago, Chile, 1984. [Google Scholar]
- Mulsow, S.; Grandjean, M. Incompatibility of sulphate compounds and soluble bicarbonate salts in the Rio Cruces waters: An answer to the disappearance of Egeria densa and black-necked swans in a RAMSAR sanctuary. Ethics Sci. Environ. Politics 2006, 2006, 5–11. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Baselga, A.; Orme, C.D.L. betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- Baselga, A. Separating the two components of abundance-based dissimilarity: Balanced changes in abundance vs. abundance gradients. Methods Ecol. Evol. 2013, 4, 552–557. [Google Scholar] [CrossRef]
- McArdle, B.H.; Anderson, M.J. Anderson. Fitting multivariate models to community data: A comment on distance-based redundancy analysis. Ecology 2001, 82, 290–297. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Valdovinos, C.; Habit, E.; Jara, A.; Piedra, P.; González, J.; Salvo, J. Dinámica espacio-temporal de 13 especies de peces nativos en un ecotono lacustre-fluvial de la Cuenca del Río Valdivia (Chile). Gayana (Concepc) 2012, 76, 45–58. [Google Scholar]
- Vila, I.; Habit, E. Current situation of the fish fauna in the Mediterranean region of Andean river systems in Chile. FiSHMED Fishes Mediterr. Environ. 2015, 2, 19. [Google Scholar] [CrossRef] [Green Version]
- Bellwood, D.R.; Hughes, T.P.; Hoey, A.S. Sleeping functional group drives coral-reef recovery. Curr. Biol. 2006, 16, 2434–2439. [Google Scholar] [CrossRef] [Green Version]
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef]
- Ricotta, C.; Szeidl, L. Diversity partitioning of Rao’s quadratic entropy. Theor. Popul. Biol. 2009, 76, 299–302. [Google Scholar] [CrossRef]
- Queen, J.P.; Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists; Cambridge University Press: Cambridge, UK, 2002; p. 527. [Google Scholar]
- Dray, S.; Choler, P.; Dolédec, S.; Peres-Neto, P.R.; Thuiller, W.; Pavoine, S.; ter Braak, C.J. Combining the fourth-corner and the RLQ methods for assessing trait responses to environmental variation. Ecology 2014, 95, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Habit, E.; Belk, M.C.; Cary Tuckfield, R.; Parra, O. Response of the fish community to human-induced changes in the Biobío River in Chile. Freshw. Biol. 2006, 51, 1–11. [Google Scholar] [CrossRef]
- Valenzuela-Aguayo, F.; McCracken, G.R.; Manosalva, A.; Habit, E.; Ruzzante, D.E. Human-induced habitat fragmentation effects on connectivity, diversity, and population persistence of an endemic fish, Percilia irwini, in the Biobío River basin (Chile). Evol. Appl. 2020, 13, 794–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vivancos, A.; Górski, K.; Manosalva, A.; Toledo, B.; Reid, M.; Habit, E. Hydrological connectivity drives longitudinal movement of endangered endemic Chilean darter Percilia irwini (Eigenmann, 1927). J. Fish Biol. 2021, 98, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Habit, E.; Belk, M.C.; Parra, O. Response of the riverine fish community to the construction and operation of a diversion hydropower plant in central Chile. Aquat. Conserv. Mar. Freshw. Ecosyst. 2007, 17, 37–49. [Google Scholar] [CrossRef]
- García, A.; Jorde, K.; Habit, E.; Caamaño, D.; Parra, O. Downstream environmental effects of dam operations: Changes in habitat quality for native fish species. River Res. Appl. 2011, 27, 312–327. [Google Scholar] [CrossRef]
- Górski, K.; Winter, H.V.; De Leeuw, J.J.; Minin, A.E.; Nagelkerke, L.A.J. Fish spawning in a large temperate floodplain: The role of flooding and temperature. Freshw. Biol. 2010, 55, 1509–1519. [Google Scholar] [CrossRef]
- Górski, K.; Collier, K.J.; Hamilton, D.P.; Hicks, B.J. Effects of flow on lateral interactions of fish and shrimps with off-channel habitats in a large river-floodplain system. Hydrobiologia 2014, 729, 161–174. [Google Scholar] [CrossRef]
- Fierro, P.; Valdovinos, C.; Arismendi, I.; Díaz, G.; Jara-Flores, A.; Habit, E.; Vargas-Chacoff, L. Examining the influence of human stressors on benthic algae, macroinvertebrate, and fish assemblages in Mediterranean streams of Chile. Sci. Total Environ. 2019, 686, 26–37. [Google Scholar] [CrossRef]
- Maceda-Veiga, A.; Mac Nally, R.; de Sostoa, A. Environmental correlates of food-chain length, mean trophic level and trophic level variance in invaded riverine fish assemblages. Sci. Total Environ. 2018, 644, 420–429. [Google Scholar] [CrossRef]
- Oyanedel, A.; Habit, E.; Belk, M.C.; Solis-Lufí, K.; Colin, N.; Gonzalez, J.; Jara, A.; Muñoz-Ramírez, C. Movement patterns and home range in Diplomystes camposensis (Siluriformes: Diplomystidae), an endemic and threatened species from Chile. Neotrop. Ichthyol. 2018, 16, e170134. [Google Scholar] [CrossRef]
- Muñoz-Ramírez, C.P.; Unmack, P.J.; Habit, E.; Johnson, J.B.; Cussac, V.E.; Victoriano, P. Phylogeography of the ancient catfish family Diplomystidae: Biogeographic, systematic, and conservation implications. Mol. Phylogenet. Evol. 2014, 73, 146–160. [Google Scholar] [CrossRef]
- Victoriano, P.F.; Vera, I.; Olmos, V.; Dib, M.; Insunza, B.; Muñoz-Ramírez, C.; Montoya, R.; Jara, A.; Habit, E. Patrones idiosincráticos de diversidad genética de peces nativos del Río San Pedro (Cuenca del Río Valdivia), un sistema de la región glaciada del sur de Chile. Gayana (Concep) 2012, 76, 01–09. [Google Scholar] [CrossRef] [Green Version]
- Habit, E. Aspectos de la biología y hábitat de un pez endémico de Chile en peligro de extinción (Diplomystes nahuelbutaensis Arratia, 1987). Interciencia 2005, 30, 8–11. [Google Scholar]
- Habit, E.; Jara, A.; Colin, N.; Oyanedel, A.; Victoriano, P.; Gonzalez, J.; Solis-Lufí, K. Threatened fishes of the world: Diplomystes camposensis Arratia, 1987 (Diplomystidae). Environ. Biol. Fishes 2009, 84, 393–394. [Google Scholar] [CrossRef]
- Habit, E.; García, A.; Díaz, G.; Arriagada, P.; Link, O.; Parra, O.; Thoms, M. River science and management issues in Chile: Hydropower development and native fish communities. River Res. Appl. 2019, 35, 489–499. [Google Scholar] [CrossRef]
- Shrestha, J.; Niklaus, P.A.; Pasquale, N.; Huber, B.; Barnard, R.L.; Frossard, E.; Schleppi, P.; Tockner, K.; Luster, J. Flood pulses control soil nitrogen cycling in a dynamic river floodplain. Geoderma 2014, 228, 14–24. [Google Scholar] [CrossRef]
- Smith, V.H.; Tilman, G.D.; Nekola, J.C. Eutrophication: Impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ. Pollut. 1999, 100, 179–196. [Google Scholar] [CrossRef]
- Camargo, J.A.; Alonso, Á. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Environ. Int. 2006, 32, 831–849. [Google Scholar] [CrossRef] [PubMed]
- Hord, N.G.; Beaver, L.; Axton, E.; Truong, L.; St. Marie, L.; Tanguay, R.; Logan, C.; Choi, J.; Spagnoli, S.; Stevens, J.F. Nitrate and nitrite exposure affect cognitive behavior and oxygen consumption during exercise in zebrafish. FASEB J. 2017, 31 (Suppl. S1), lb277. [Google Scholar] [CrossRef]
- Pizarro, J.; Vergara, P.M.; Rodriguez, J.A.; Sanhueza, P.A.; Castro, S.A. Nutrients dynamics in the main river basins of the centre-southern region of Chile. J. Hazard. Mater. 2010, 175, 608–613. [Google Scholar] [CrossRef]
- Lagos, N.A.; Paolini, P.; Jaramillo, E.; Lovengreen, C.; Duarte, C.; Contreras, H. Environmental processes, water quality degradation, and decline of waterbird populations in the Rio Cruces wetland, Chile. Wetlands 2008, 28, 938–950. [Google Scholar] [CrossRef] [Green Version]




| Trait Type | Function | Trait | Categories | Categorical Value |
|---|---|---|---|---|
| Ordinal | Trophic interaction | Trophic guilds | Detritivore | 1 |
| Omnivore-invertivore | 2 | |||
| Omnivore-piscivore | 3 | |||
| Omnivore | 4 | |||
| Ordinal | Habitat use | Vertical position | Benthic | 1 |
| Benthopelagic | 2 | |||
| Pelagic | 3 | |||
| Velocity preference | Reophilic | 1 | ||
| Limnophilic | 2 | |||
| Eurytopic | 3 | |||
| Floodplain use | Frequently | 1 | ||
| Scarcely | 2 | |||
| Ordinal | Life history | Migration | Facultative amphidromous | 1 |
| Anadromous | 2 | |||
| Facultative catadromous | 3 | |||
| Non-migratory | 4 | |||
| Continuous | Morphology | Mean length | ||
| Maximum length |
| Biobío River | Valdivia River | |||
|---|---|---|---|---|
| PC1 (39%) | PC2 (33%) | PC1 (38%) | PC2 (35%) | |
| pH | 0.6076 | −0.2326 | 0.8375 | 0.0959 |
| Temperature | 0.7225 | 0.4152 | 0.8939 | 0.1486 |
| Conductivity | 0.7607 | -0.1050 | 0.6449 | −0.2490 |
| Hardness | 0.6277 | 0.0108 | 0.0454 | −0.6015 |
| Nitrite | 0.0970 | 0.9186 | 0.2425 | 0.7647 |
| Nitrate | −0.0948 | −0.1542 | −0.0858 | 0.7947 |
| Ammonium | −0.3662 | 0.6901 | −0.0176 | −0.0382 |
| Phosphate | 0.2669 | 0.4748 | 0.1685 | 0.4762 |
| FSpe | FOri | FDis | FEnt | |||||
|---|---|---|---|---|---|---|---|---|
| Chi sq | p | Chi sq | p | Chi sq | p | Chi sq | p | |
| Biobío | ||||||||
| Physical water quality (PC1) | 4.9166 | 0.0266 * | 0.4903 | 0.4838 | 2.0049 | 0.15679 | 1.9758 | 0.1598 |
| Zone | 6.4841 | 0.03908 * | 7.7944 | 0.0203 * | ||||
| Valdivia | ||||||||
| Nutrient pollution (PC2) | 5.280 | 0.02157 * | 1.2546 | 0.2627 | 3.1996 | 0.07366 | 4.5285 | 0.008486 ** |
| Season | 30.343 | <0.001 *** | 23.2988 | <0.001 *** | 2.9122 | 0.40537 | 11.6996 | 0.033334 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colin, N.; Habit, E.; Manosalva, A.; Maceda-Veiga, A.; Górski, K. Taxonomic and Functional Responses of Species-Poor Riverine Fish Assemblages to the Interplay of Human-Induced Stressors. Water 2022, 14, 355. https://doi.org/10.3390/w14030355
Colin N, Habit E, Manosalva A, Maceda-Veiga A, Górski K. Taxonomic and Functional Responses of Species-Poor Riverine Fish Assemblages to the Interplay of Human-Induced Stressors. Water. 2022; 14(3):355. https://doi.org/10.3390/w14030355
Chicago/Turabian StyleColin, Nicole, Evelyn Habit, Aliro Manosalva, Alberto Maceda-Veiga, and Konrad Górski. 2022. "Taxonomic and Functional Responses of Species-Poor Riverine Fish Assemblages to the Interplay of Human-Induced Stressors" Water 14, no. 3: 355. https://doi.org/10.3390/w14030355







