Using Sawdust Derived Biochar as a Novel 3D Particle Electrode for Micropollutants Degradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Biochar
2.3. Physicochemical Characterization Techniques
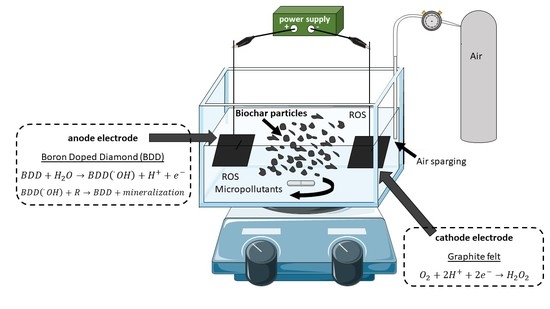
2.4. Experimental Set-Up and Procedure
2.5. Analytical Methods
3. Results
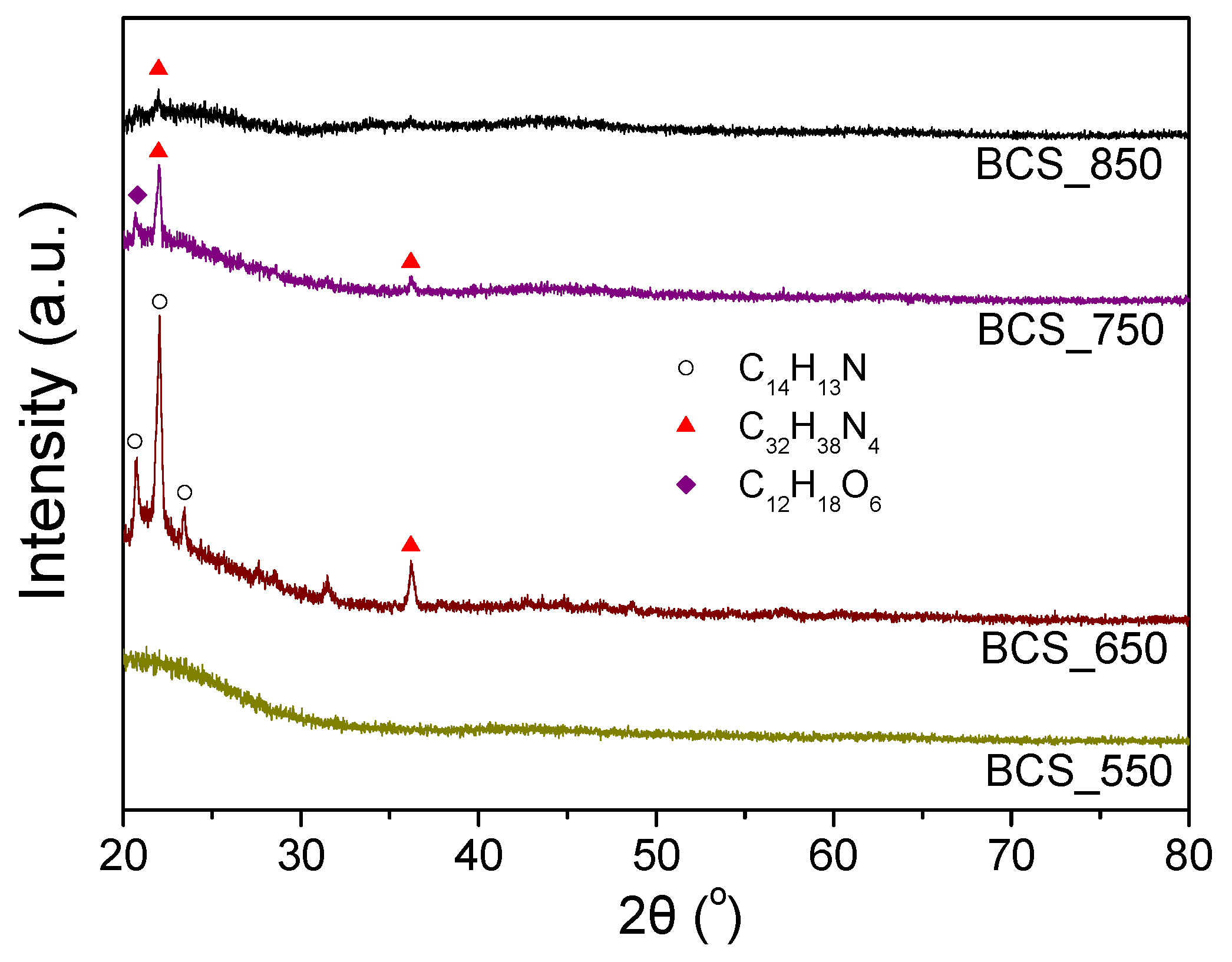
Physicochemical Characterization
4. Discussion
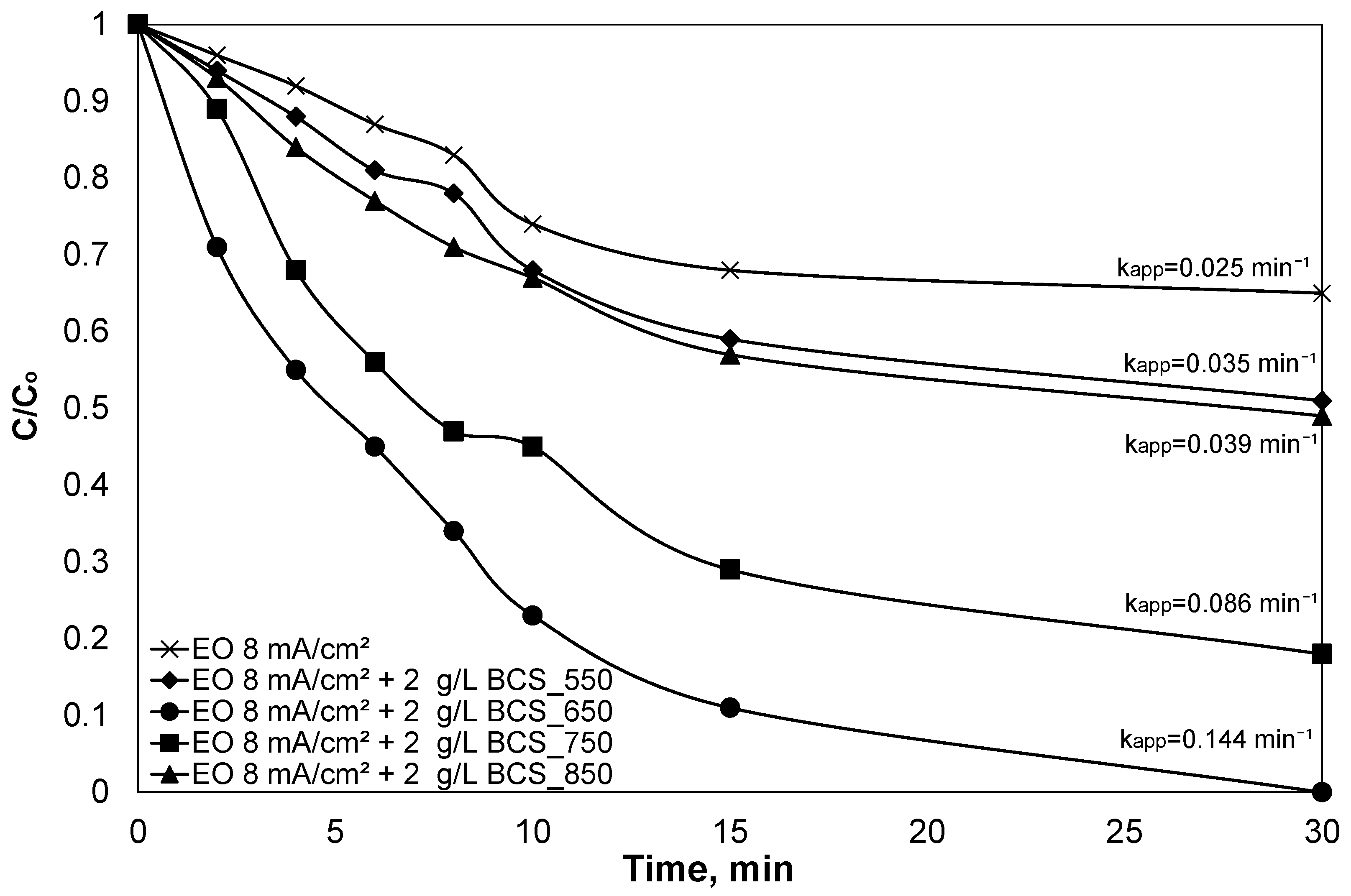
4.1. Effect of Particle Electrode
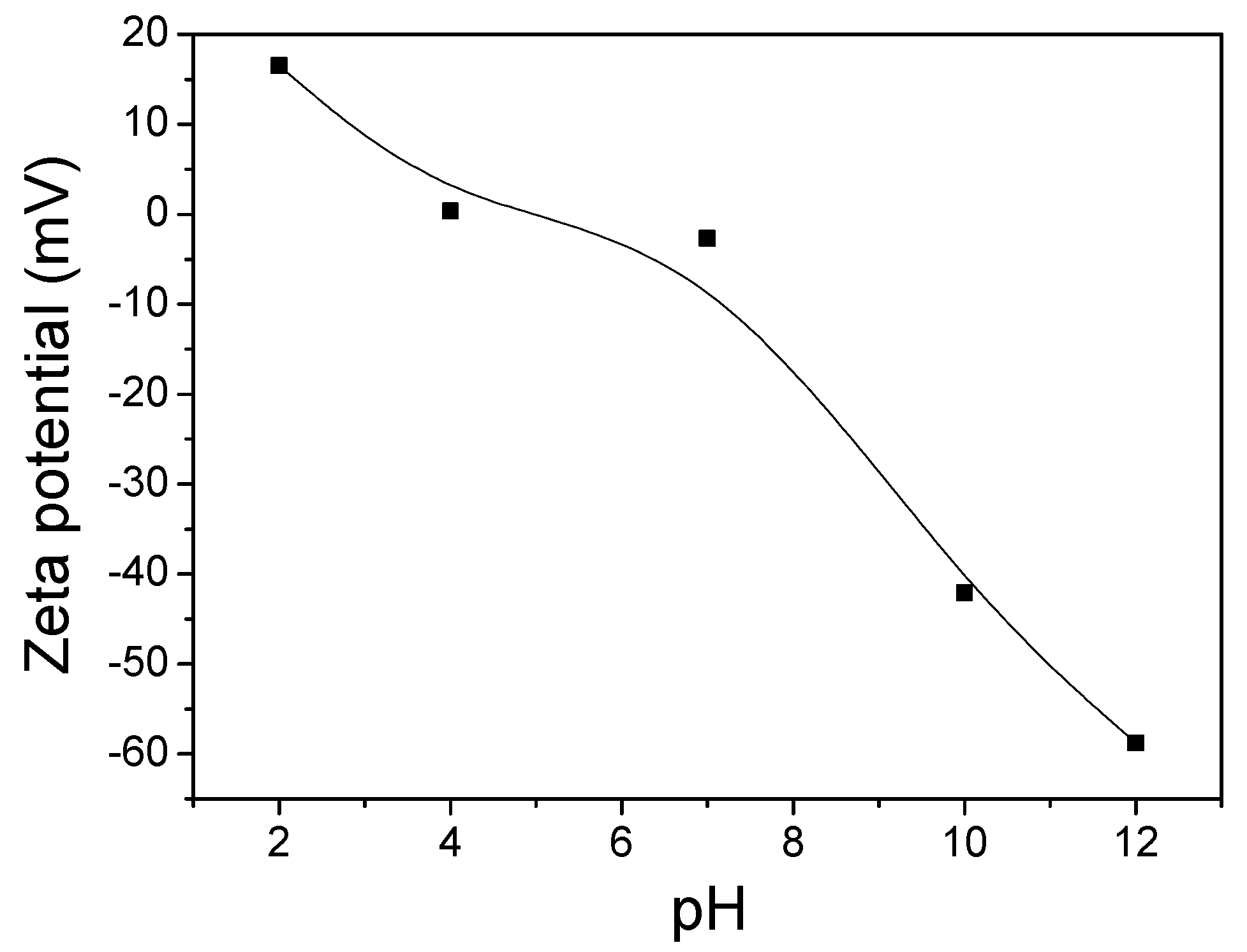
4.2. Effect of pH
4.3. Effect of Water Matrices
4.4. Biochar Reuse
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Olasupo, A.; Suah, F.B.M. Recent advances in the removal of pharmaceuticals and endocrine-disrupting compounds in the aquatic system: A case of polymer inclusion membranes. J. Hazard. Mater. 2021, 406, 124317. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.L.S.; Bassin, J.P.; Peixoto, R.S. Water contamination by endocrine disruptors: Impacts, microbiological aspects and trends for environmental protection. Environ. Pollut. 2018, 235, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Gonsioroski, A.; Mourikes, V.E.; Flaws, J.A. Endocrine Disruptors in Water and Their Effects on the Reproductive System. Int. J. Mol. Sci. 2020, 21, 1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannakis, S.; Lin, K.-Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- García-Espinoza, J.D.; Nacheva, P.M. Degradation of pharmaceutical compounds in water by oxygenated electrochemical oxidation: Parametric optimization, kinetic studies and toxicity assessment. Sci. Total Environ. 2019, 691, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Herraiz-Carboné, M.; Cotillas, S.; Lacasa, E.; Moratalla, Á.; Cañizares, P.; Rodrigo, M.A.; Sáez, C. Improving the biodegradability of hospital urines polluted with chloramphenicol by the application of electrochemical oxidation. Sci. Total Environ. 2020, 725, 138430. [Google Scholar] [CrossRef]
- Brillas, E.; Martínez-Huitle, C.A. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl. Catal. B Environ. 2015, 166–167, 603–643. [Google Scholar] [CrossRef]
- Cotillas, S.; Llanos, J.; Cañizares, P.; Clematis, D.; Cerisola, G.; Rodrigo, M.A.; Panizza, M. Removal of Procion Red MX-5B dye from wastewater by conductive-diamond electrochemical oxidation. Electrochim. Acta 2018, 263, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ganiyu, S.O.; Oturan, N.; Raffy, S.; Cretin, M.; Causserand, C.; Oturan, M.A. Efficiency of plasma elaborated sub-stoichiometric titanium oxide (Ti4O7) ceramic electrode for advanced electrochemical degradation of paracetamol in different electrolyte media. Sep. Purif. Technol. 2019, 208, 142–152. [Google Scholar] [CrossRef] [Green Version]
- Särkkä, H.; Bhatnagar, A.; Sillanpää, M. Recent developments of electrooxidation in water treatment—A review. J. Electroanal. Chem. 2015, 754, 46–56. [Google Scholar] [CrossRef]
- Radjenovic, J.; Sedlak, D.L. Challenges and Opportunities for Electrochemical Processes as Next-Generation Technologies for the Treatment of Contaminated Water. Environ. Sci. Technol. 2015, 49, 11292–11302. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Sun, Y.; Shah, K.J.; Zheng, H.; Ma, B. Electrochemical degradation of oxytetracycline by Ti-Sn-Sb/γ-Al2O3 three-dimensional electrodes. J. Environ. Manag. 2019, 241, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Bampos, G.; Petala, A.; Frontistis, Z. Recent Trends in Pharmaceuticals Removal from Water Using Electrochemical Oxidation Processes. Environments 2021, 8, 85. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, H.; Croué, J.-P. Production of Sulfate Radical from Peroxymonosulfate Induced by a Magnetically Separable CuFe2O4 Spinel in Water: Efficiency, Stability, and Mechanism. Environ. Sci. Technol. 2013, 47, 2784–2791. [Google Scholar] [CrossRef]
- Pan, G.; Jing, X.; Ding, X.; Shen, Y.; Xu, S.; Miao, W. Synergistic effects of photocatalytic and electrocatalytic oxidation based on a three-dimensional electrode reactor toward degradation of dyes in wastewater. J. Alloys Compd. 2019, 809, 151749. [Google Scholar] [CrossRef]
- Shen, B.; Wen, X.; Huang, X. Enhanced removal performance of estriol by a three-dimensional electrode reactor. Chem. Eng. J. 2017, 327, 597–607. [Google Scholar] [CrossRef]
- Ji, J.; Li, X.; Xu, J.; Yang, X.; Meng, H.; Yan, Z. Zn-Fe-rich granular sludge carbon (GSC) for enhanced electrocatalytic removal of bisphenol A (BPA) and Rhodamine B (RhB) in a continuous-flow three-dimensional electrode reactor (3DER). Electrochim. Acta 2018, 284, 587–596. [Google Scholar] [CrossRef]
- Zhan, J.; Li, Z.; Yu, G.; Pan, X.; Wang, J.; Zhu, W.; Han, X.; Wang, Y. Enhanced treatment of pharmaceutical wastewater by combining three-dimensional electrochemical process with ozonation to in situ regenerate granular activated carbon particle electrodes. Sep. Purif. Technol. 2019, 208, 12–18. [Google Scholar] [CrossRef]
- Nezamaddin, M.; Hamidreza, P.; Khodadadi, S.M.; Yaghoub, H.; Iman, P.; Saeed, P.; Noureddin, N. Electrochemical Degradation of Reactive Black 5 Using Three-Dimensional Electrochemical System Based on Multiwalled Carbon Nanotubes. J. Environ. Eng. 2019, 145, 4019021. [Google Scholar] [CrossRef]
- Hassan, M.F.; Sabri, M.A.; Fazal, H.; Hafeez, A.; Shezad, N.; Hussain, M. Recent trends in activated carbon fibers production from various precursors and applications—A comparative review. J. Anal. Appl. Pyrolysis 2020, 145, 104715. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, C.; Yang, Z.; Qin, Q.; Zhang, Z.; Wang, X.; Shen, J. Preparation of Carbon Aerogel Electrode for Electrosorption of Copper Ions in Aqueous Solution. Materials 2019, 12, 1864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.; Zhang, S.; Liu, L.; Xu, J. Graphite particle electrodes that enhance the detoxification of municipal solid waste incineration fly ashes in a three-dimensional electrokinetic platform and its mechanisms. Environ. Pollut. 2018, 243, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Wang, Y.; Li, J.; Shen, H.; Zhang, C.; Qu, T. Reduction of Chemical Oxygen Demand from Refinery Wastewater by Three-Dimensional Electrode-Electro-Fenton Process. Bull. Chem. Soc. Jpn. 2015, 89, 50–57. [Google Scholar] [CrossRef]
- Li, J.; Yan, J.; Yao, G.; Zhang, Y.; Li, X.; Lai, B. Improving the degradation of atrazine in the three-dimensional (3D) electrochemical process using CuFe2O4 as both particle electrode and catalyst for persulfate activation. Chem. Eng. J. 2019, 361, 1317–1332. [Google Scholar] [CrossRef]
- Chaudhari, N.K.; Jin, H.; Kim, B.; Lee, K. Nanostructured materials on 3D nickel foam as electrocatalysts for water splitting. Nanoscale 2017, 9, 12231–12247. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Li, J.; Zhao, C.; He, X.; Yang, G. Fabrication of slag particle three-dimensional electrode system for methylene blue degradation: Characterization, performance and mechanism study. Chemosphere 2018, 213, 377–383. [Google Scholar] [CrossRef]
- Sun, Y.; Li, P.; Zheng, H.; Zhao, C.; Xiao, X.; Xu, Y.; Sun, W.; Wu, H.; Ren, M. Electrochemical treatment of chloramphenicol using Ti-Sn/γ-Al2O3 particle electrodes with a three-dimensional reactor. Chem. Eng. J. 2017, 308, 1233–1242. [Google Scholar] [CrossRef]
- Sun, W.; Sun, Y.; Shah, K.J.; Chiang, P.-C.; Zheng, H. Electrocatalytic oxidation of tetracycline by Bi-Sn-Sb/γ-Al2O3 three-dimensional particle electrode. J. Hazard. Mater. 2019, 370, 24–32. [Google Scholar] [CrossRef]
- Habib, A.; Bhatti, H.N.; Iqbal, M. Metallurgical Processing Strategies for Metals Recovery from Industrial Slags. Z. Phys. Chem. 2020, 234, 201–231. [Google Scholar] [CrossRef]
- Hong, L.; Yang, Q.; Liying, Z.; Yingyan, C.; Bing, W. Investigation of a novel pyrolusite particle electrode effects in the chlorine-containing wastewater. Water Sci. Technol. 2018, 78, 1427–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Brown, R. Biochar Production Technology. In Biochar for Environmental Management: Science and Technology; Routledge: Abingdon, UK, 2012; ISBN 9781849770552. [Google Scholar]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation; Routledge: Abingdon, UK, 2021; ISBN 9780367779184-0367779188. [Google Scholar]
- Chen, B.; Chen, Z.; Lv, S. A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour. Technol. 2011, 102, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Gao, J.; Liu, C.; Dionysiou, D.D.; Wang, Y.; Zhou, D. Key Role of Persistent Free Radicals in Hydrogen Peroxide Activation by Biochar: Implications to Organic Contaminant Degradation. Environ. Sci. Technol. 2014, 48, 1902–1910. [Google Scholar] [CrossRef] [PubMed]
- Kemmou, L.; Frontistis, Z.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D. Degradation of antibiotic sulfamethoxazole by biochar-activated persulfate: Factors affecting the activation and degradation processes. Catal. Today 2018, 313, 128–133. [Google Scholar] [CrossRef]
- Ioannidi, A.; Arvaniti, O.S.; Nika, M.C.; Aalizadeh, R.; Thomaidis, N.S.; Mantzavinos, D.; Frontistis, Z. Removal of drug losartan in environmental aquatic matrices by heat-activated persulfate: Kinetics, transformation products and synergistic effects. Chemosphere 2022, 287, 131952. [Google Scholar] [CrossRef]
- Arvaniti, O.S.; Petala, A.; Zalaora, A.A.; Mantzavinos, D.; Frontistis, Z. Solar light-induced photocatalytic degradation of methylparaben by g-C3N4 in different water matrices. J. Chem. Technol. Biotechnol. 2020, 95, 2811–2821. [Google Scholar] [CrossRef]
- Petala, A.; Tsikritzis, D.; Kollia, M.; Ladas, S.; Kennou, S.; DI, K. Synthesis and characterization of N-doped TiO2 photocatalysts with tunable response to solar radiation. Appl. Surf. Sci. 2014, 305, 281–291. [Google Scholar] [CrossRef]
- Dimitriadou, S.; Frontistis, Z.; Petala, A.; Bampos, G.; Mantzavinos, D. Carbocatalytic activation of persulfate for the removal of drug diclofenac from aqueous matrices. Catal. Today 2020, 355, 937–944. [Google Scholar] [CrossRef]
- Kouskouki, A.; Chatzisymeon, E.; Mantzavinos, D.; Frontistis, Z. Electrochemical Degradation of Piroxicam on a Boron-Doped Diamond Anode: Investigation of Operating Parameters and Ultrasound Synergy. ChemElectroChem 2019, 6, 841–847. [Google Scholar] [CrossRef]
- Yuan, J.-H.; Xu, R.-K. The amelioration effects of low temperature biochar generated from nine crop residues on an acidic Ultisol. Soil Use Manag. 2011, 27, 110–115. [Google Scholar] [CrossRef]
- Batista, E.M.C.C.; Shultz, J.; Matos, T.T.S.; Fornari, M.R.; Ferreira, T.M.; Szpoganicz, B.; de Freitas, R.A.; Mangrich, A.S. Effect of surface and porosity of biochar on water holding capacity aiming indirectly at preservation of the Amazon biome. Sci. Rep. 2018, 8, 10677. [Google Scholar] [CrossRef] [PubMed]
- Fahmi, A.H.; Samsuri, A.W.; Jol, H.; Singh, D. Physical modification of biochar to expose the inner pores and their functional groups to enhance lead adsorption. RSC Adv. 2018, 8, 38270–38280. [Google Scholar] [CrossRef] [Green Version]
- Qian, L.; Chen, B. Interactions of Aluminum with Biochars and Oxidized Biochars: Implications for the Biochar Aging Process. J. Agric. Food Chem. 2014, 62, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Zimmerman, A.R.; Harris, W. Surface chemistry variations among a series of laboratory-produced biochars. Geoderma 2011, 163, 247–255. [Google Scholar] [CrossRef]
- Samsuri, A.W.; Sadegh-Zadeh, F.; Seh-Bardan, B.J. Characterization of biochars produced from oil palm and rice husks and their adsorption capacities for heavy metals. Int. J. Environ. Sci. Technol. 2014, 11, 967–976. [Google Scholar] [CrossRef]
- Liu, Q.; Bai, X.; Su, X.; Huang, B.; Wang, B.; Zhang, X.; Ruan, X.; Cao, W.; Xu, Y.; Qian, G. The promotion effect of biochar on electrochemical degradation of nitrobenzene. J. Clean. Prod. 2020, 244, 118890. [Google Scholar] [CrossRef]
- Frontistis, Z.; Mantzavinos, D.; Meriç, S. Degradation of antibiotic ampicillin on boron-doped diamond anode using the combined electrochemical oxidation—Sodium persulfate process. J. Environ. Manag. 2018, 223, 878–887. [Google Scholar] [CrossRef]
- Stathoulopoulos, A.; Mantzavinos, D.; Frontistis, Z. Coupling Persulfate-Based AOPs: A Novel Approach for Piroxicam Degradation in Aqueous Matrices. Water 2020, 12, 1530. [Google Scholar] [CrossRef]
- Li, H.; Yang, H.; Cheng, J.; Hu, C.; Yang, Z.; Wu, C. Three-dimensional particle electrode system treatment of organic wastewater: A general review based on patents. J. Clean. Prod. 2021, 308, 127324. [Google Scholar] [CrossRef]
- Correia-sá, L.; Soares, C.; Freitas, O.M.; Moreira, M.M.; Nouws, H.P.A.; Correia, M.; Paíga, P.; Rodrigues, A.J.; Oliveira, C.M.; Figueiredo, S.A.; et al. A three-dimensional electrochemical process for the removal of carbamazepine. Appl. Sci. 2021, 11, 6432. [Google Scholar] [CrossRef]
- Hai, H.; Xing, X.; Li, S.; Xia, S.; Xia, J. Electrochemical oxidation of sulfamethoxazole in BDD anode system: Degradation kinetics, mechanisms and toxicity evaluation. Sci. Total Environ. 2020, 738, 139909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zheng, X.; Chen, B.; Ma, J.; Niu, X.; Zhang, D.; Lin, Z.; Fu, M.; Zhou, S. Enhanced adsorption of sulfamethoxazole from aqueous solution by Fe-impregnated graphited biochar. J. Clean. Prod. 2020, 256, 120662. [Google Scholar] [CrossRef]







| Notation | Carbon Type | Thermal Treatment (°C) | SSA (m2 g−1) |
|---|---|---|---|
| BCS_850 | Biochar derived from sawdust | 850 | 2.3 ± 0.1 |
| BCS_750 | Biochar derived from sawdust | 750 | 3.05 ± 0.7 |
| BCS_650 | Biochar derived from sawdust | 650 | 2.3 ± 0.3 |
| BCS_550 | Biochar derived from sawdust | 550 | 1.1 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petala, A.; Bampos, G.; Frontistis, Z. Using Sawdust Derived Biochar as a Novel 3D Particle Electrode for Micropollutants Degradation. Water 2022, 14, 357. https://doi.org/10.3390/w14030357
Petala A, Bampos G, Frontistis Z. Using Sawdust Derived Biochar as a Novel 3D Particle Electrode for Micropollutants Degradation. Water. 2022; 14(3):357. https://doi.org/10.3390/w14030357
Chicago/Turabian StylePetala, Athanasia, Georgios Bampos, and Zacharias Frontistis. 2022. "Using Sawdust Derived Biochar as a Novel 3D Particle Electrode for Micropollutants Degradation" Water 14, no. 3: 357. https://doi.org/10.3390/w14030357