Municipal-to-Industrial Water Reuse via Multi-Stage and Multi-Pass Reverse Osmosis Systems: A Step from Water Scarcity towards Sustainable Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Procedure
2.2. Description of WRRF and Constructed Pilot Plant
2.3. Data Collection and Analysis
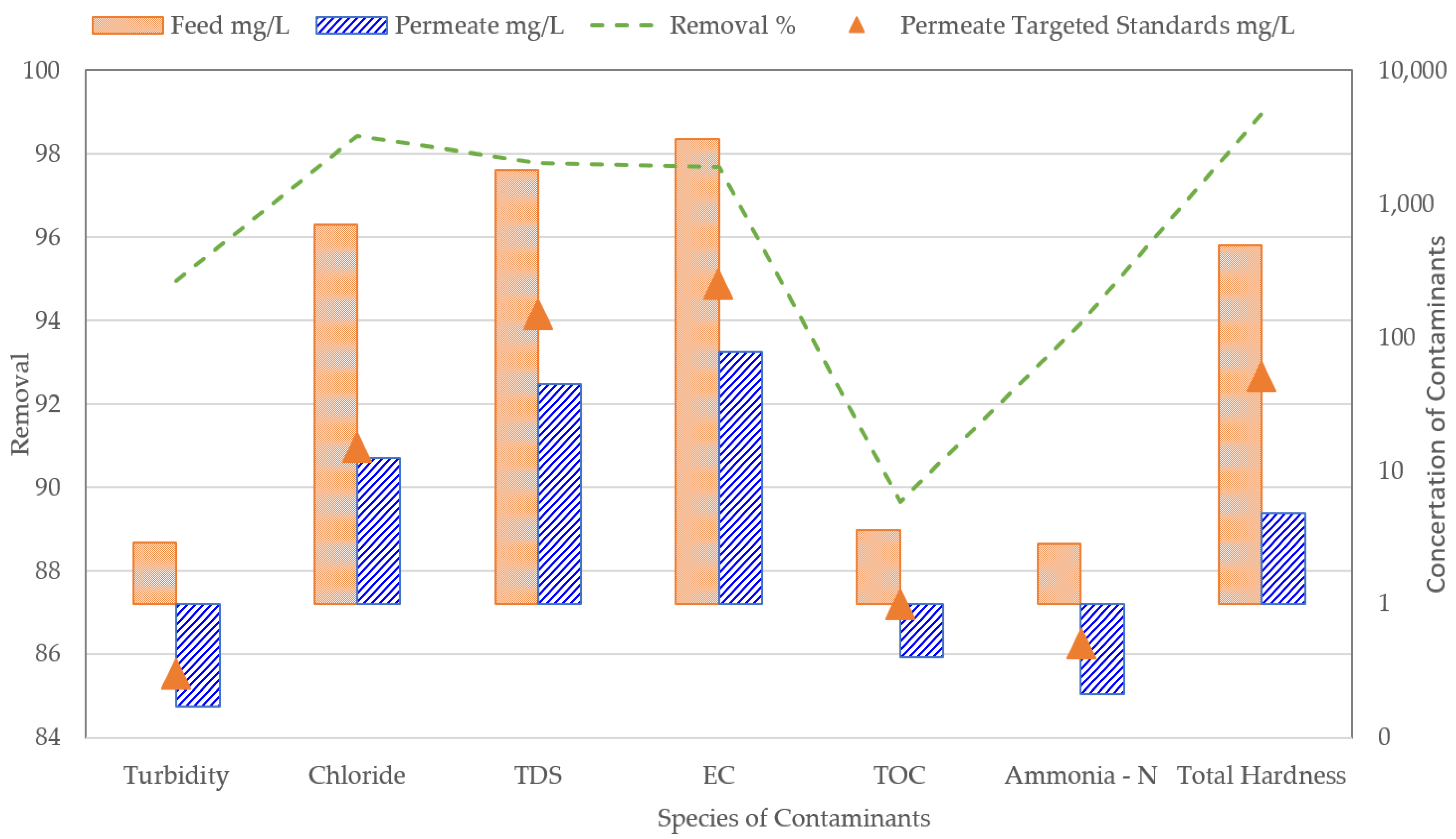
3. Results
3.1. Correlation between Boron and Conductivity in Effluent from Plant A
3.2. Optimization of Plant B
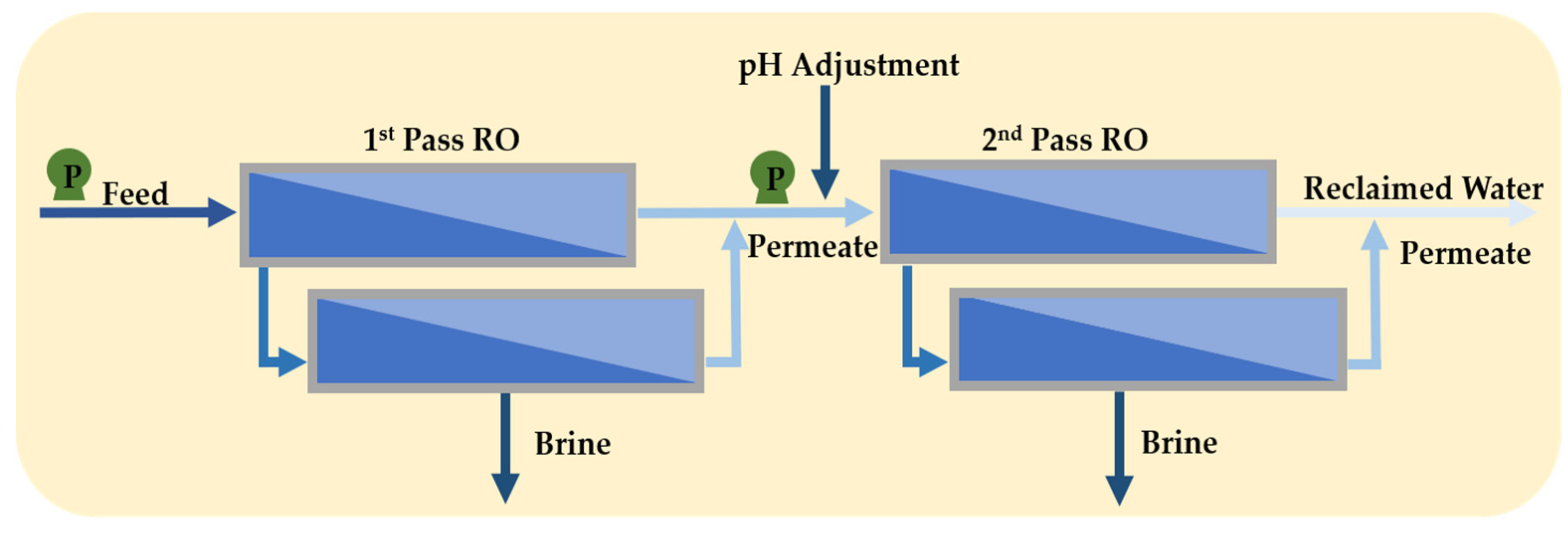
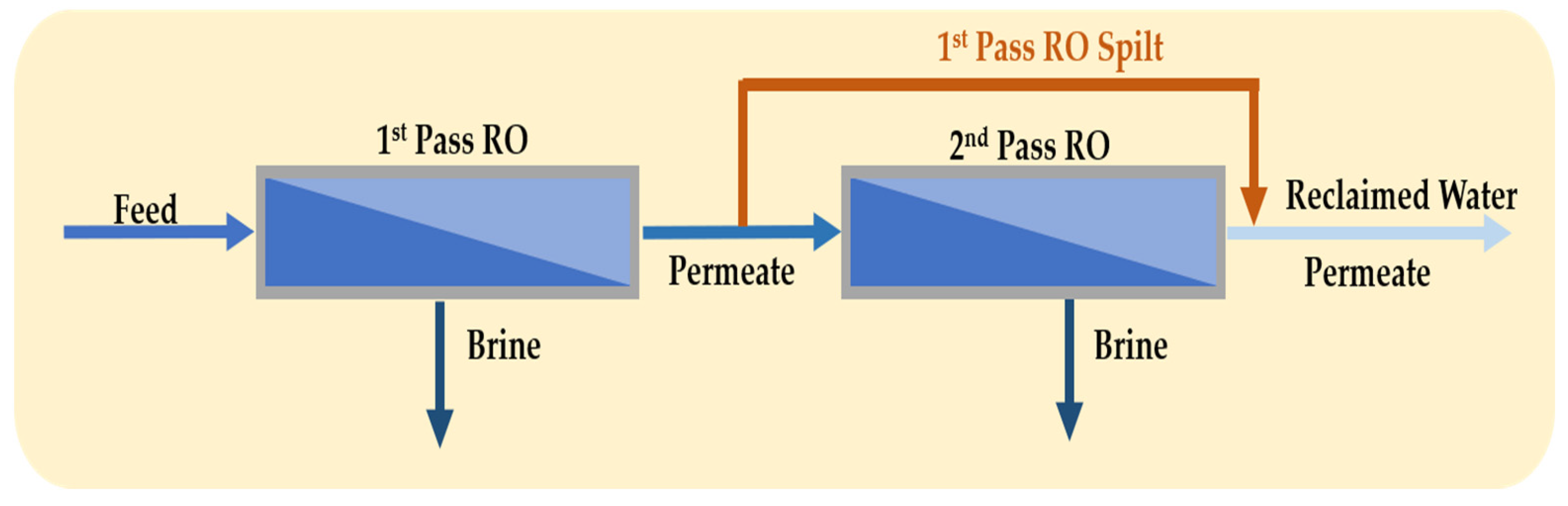
3.3. Full Second-Pass RO and Partial Second-Pass RO
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gómez-Coma, L.; Ortiz-Martínez, V.M.; Fallanza, M.; Ortiz, A.; Ibañez, R.; Ortiz, I. Blue energy for sustainable water reclamation in WWTPs. J. Water Process Eng. 2020, 33, 101020. [Google Scholar] [CrossRef]
- Mancosu, N.; Snyder, R.L.; Kyriakakis, G.; Spano, D. Water Scarcity and Future Challenges for Food Production. Water 2015, 7, 975–992. [Google Scholar] [CrossRef]
- Balfaqih, H.; Al-Nory, M.T.; Nopiah, Z.M.; Saibani, N. Environmental and economic performance assessment of desalination supply chain. Desalination 2017, 406, 2–9. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef]
- Hibbs, M.R.; McGrath, L.K.; Kang, S.; Adout, A.; Altman, S.J.; Elimelech, M.; Cornelius, C.J. Designing a biocidal reverse osmosis membrane coating: Synthesis and biofouling properties. Desalination 2016, 380, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Liu, Y.; He, M.; Su, Y.; Zhao, X.; Elimelechc, M.; Jiang, Z. Antifouling membranes for sustainable water purification: Strategies and mechanisms. Chem. Soc. Rev. 2016, 45, 5888–5924. [Google Scholar] [CrossRef]
- UNESCO World Water Assessment Programme. 2017 UN World Water Development Report: Wastewater, the Untapped Resource; United Nations Educational, Scientific and Cultural Organization: Paris, France, 2017. [Google Scholar]
- Shoushtarian, F.; Negahban-Azar, M. Worldwide Regulations and Guidelines for Agricultural Water Reuse: A Critical Review. Water 2020, 12, 971. [Google Scholar] [CrossRef] [Green Version]
- Oertlé, E.; Hugi, C.; Wintgens, T.; Karavitis, C.A. Poseidon—Decision Support Tool for Water Reuse. Water 2019, 11, 153. [Google Scholar] [CrossRef] [Green Version]
- Cui, B.; Liang, S. Monitoring Opportunistic Pathogens in Domestic Wastewater from a Pilot-Scale Anaerobic Biofilm Reactor to Reuse in Agricultural Irrigation. Water 2019, 11, 1283. [Google Scholar] [CrossRef] [Green Version]
- Dingemans, M.M.L.; Smeets, P.W.M.H.; Medema, G.; Frijns, J.; Raat, K.J.; van Wezel, A.P.; Bartholomeus, R.P. Responsible Water Reuse Needs an Interdisciplinary Approach to Balance Risks and Benefits. Water 2020, 12, 1264. [Google Scholar] [CrossRef]
- Wintgens, T.; Melin, T.; Schäfer, A.; Khan, S.; Muston, M.; Bixio, D.; Thoeye, C. The role of membrane processes in municipal wastewater reclamation and reuse. Desalination 2005, 178, 1–11. [Google Scholar] [CrossRef]
- Garcia-Cuerva, L.; Berglund, E.Z.; Binder, A.R. Public perceptions of water shortages, conservation behaviors, and support for water reuse in the U.S. Resour. Conserv. Recycl. 2016, 113, 106–115. [Google Scholar] [CrossRef] [Green Version]
- TSIA. Review and Outlook of Taiwan’s Semiconductor Industry in the First Quarter of 2021; Taiwan Semiconductor Industry Association Newsletter: Hsinchu, Taiwan, 2021; pp. 32–33. [Google Scholar]
- Huang, H.; Liu, J.; Zhang, P.; Zhang, D.; Gao, F. Investigation on the simultaneous removal of fluoride, ammonia nitrogen and phosphate from semiconductor wastewater using chemical precipitation. Chem. Eng. J. 2017, 307, 696–706. [Google Scholar] [CrossRef]
- Sewage System Office Construction and Planning Agency Ministry of the Interior. Public Sewage Treatment Plant. Available online: https://www.cpami.gov.tw/home.html (accessed on 12 August 2021).
- Levine, A.; Asano, T. Recovering sustainable water from wastewater. Environ. Sci. Technol. 2004, 38, 201A–208A. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keremane, G.B.; McKay, J. Successful wastewater reuse scheme and sustainable development: A case study in Adelaide. Water Environ. J. 2007, 21, 83–91. [Google Scholar] [CrossRef]
- Asano, T.; Levine, A.D. Wastewater reclamation, recycling and reuse: Past, present, and future. Water Sci. Technol. 1996, 33, 1–14. [Google Scholar] [CrossRef]
- Michetti, M.; Raggi, M.; Guerra, E.; Viaggi, D. Interpreting Farmers’ Perceptions of Risks and Benefits Concerning Wastewater Reuse for Irrigation: A Case Study in Emilia-Romagna (Italy). Water 2019, 11, 108. [Google Scholar] [CrossRef] [Green Version]
- Fighir, D.; Teodosiu, C.; Fiore, S. Environmental and Energy Assessment of Municipal Wastewater Treatment Plants in Italy and Romania: A Comparative Study. Water 2019, 11, 1611. [Google Scholar] [CrossRef] [Green Version]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A review on membrane fabrication: Structure, properties and performance relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Singh, R. Production of high-purity water by membrane processes. Desalin. Water Treat. 2009, 3, 99–110. [Google Scholar] [CrossRef] [Green Version]
- Cho, B.-Y.; Kim, H.-W.; Shin, Y.-S. A Study on boron removal for seawater desalination using the combination process of mineral cluster and RO membrane system. Environ. Eng. Res. 2015, 20, 285–289. [Google Scholar] [CrossRef] [Green Version]
- Koseoglu, H.; Kabay, N.; Yüksel, M.; Sarp, S.; Arar, Ö.; Kitis, M. Boron removal from seawater using high rejection SWRO membranes—Impact of pH, feed concentration, pressure, and cross-flow velocity. Desalination 2008, 227, 253–263. [Google Scholar] [CrossRef]
- Mendoza-Grimón, V.; Fernández-Vera, J.R.; Hernández-Moreno, J.M.; Palacios-Díaz, M.d.P. Sustainable Irrigation Using Non-Conventional Resources: What has Happened after 30 Years Regarding Boron Phytotoxicity? Water 2019, 11, 1952. [Google Scholar] [CrossRef] [Green Version]
- Sauer, R.; Gensbittel, D.; Bartz, R.; Rompf, F.; Ziemer-Popp, C.; Wilcox, D.; Malhotra, S. Boron removal experiences at AMD. Ultrapure Water. 2000, 17, 62–68. [Google Scholar]
- Redondo, J.; Busch, M.; De Witte, J.-P. Boron removal from seawater using FILMTECTM high rejection SWRO membranes. Desalination 2003, 156, 229–238. [Google Scholar] [CrossRef]
- Farhat, A.; Ahmad, F.; Hilal, N.; Arafat, H. Boron removal in new generation reverse osmosis (RO) membranes using two-pass RO without pH adjustment. Desalination 2013, 310, 50–59. [Google Scholar] [CrossRef]
- Freger, V.; Shemer, H.; Sagiv, A.; Semiat, R. Chapter 8—Boron Removal Using Membranes. In Boron Separation Processes; Kabay, N., Bryjak, M., Hilal, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 199–217. [Google Scholar] [CrossRef]
- Najid, N.; Kouzbour, S.; Ruiz-García, A.; Fellaou, S.; Gourich, B.; Stiriba, Y. Comparison analysis of different technologies for the removal of boron from seawater: A review. J. Environ. Chem. Eng. 2021, 9, 105133. [Google Scholar] [CrossRef]
- Rybar, S.; Boda, R.; Bartels, C. Split partial second pass design for SWRO plants. Desalin. Water Treat. 2010, 13, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; Zhang, X.; Zhao, X.; Li, F. Removal of high level boron in aqueous solutions using continuous electrodeionization (CEDI). Sep. Purif. Technol. 2018, 192, 297–301. [Google Scholar] [CrossRef]
- Goncharuk, V.V.; Babak, Y.V.; Melńik, L.A.; Trachevskii, V.V. Removal of boron compounds in the course of pressure-driven demineralization. J. Water Chem. Technol. 2011, 33, 307–314. [Google Scholar] [CrossRef]
- Nadav, N. Boron removal from seawater reverse osmosis permeate utilizing selective ion exchange resin. Desalination 1999, 124, 131–135. [Google Scholar] [CrossRef]
- Chillón Arias, M.F.; Valero i Bru, L.; Prats Rico, D.; Varó Galvañ, P. Approximate cost of the elimination of boron in desalinated water by reverse osmosis and ion exchange resins. Desalination 2011, 273, 421–427. [Google Scholar] [CrossRef]
- Taniguchi, M.; Kurihara, M.; Kimura, S. Boron reduction performance of reverse osmosis seawater desalination process. J. Membr. Sci. 2001, 183, 259–267. [Google Scholar] [CrossRef]
- Hyung, H.; Kim, J.-H. A mechanistic study on boron rejection by sea water reverse osmosis membranes. J. Membr. Sci. 2006, 286, 269–278. [Google Scholar] [CrossRef]
- Sagiv, A.; Semiat, R. Analysis of parameters affecting boron permeation through reverse osmosis membranes. J. Membr. Sci. 2004, 243, 79–87. [Google Scholar] [CrossRef]
- Ruiz-García, A.; Nuez, I. Performance evaluation and boron rejection in a SWRO system under variable operating conditions. Comput. Chem. Eng. 2021, 153, 107441. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Y.; Gao, C. Novel high boron removal polyamide reverse osmosis membranes. J. Membr. Sci. 2018, 554, 244–252. [Google Scholar] [CrossRef]
- Ruiz-García, A.; León, F.A.; Ramos-Martín, A. Different boron rejection behavior in two RO membranes installed in the same full-scale SWRO desalination plant. Desalination 2019, 449, 131–138. [Google Scholar] [CrossRef]
- Ruiz-García, A.; Nuez, I.; Carrascosa-Chisvert, M.D.; Santana, J.J. Simulations of BWRO systems under different feedwater characteristics. Analysis of operation windows and optimal operating points. Desalination 2020, 491, 114582. [Google Scholar] [CrossRef]
- Joarder, M.A.M.; Raihan, F.; Alam, J.B.; Hasanuzzaman, S. Regression analysis of ground water quality data of Sunamganj district, Bangladesh. Int. J. Environ. Res. 2008, 2, 291–296. [Google Scholar]
- Li, Y.; Wang, S.; Gu, K.; Zhou, Y.; Gao, C. Progress in the development of boron removal by reverse osmosis membrane technology. Chem. Ind. Eng. Prog. 2020, 39, 596–604. [Google Scholar] [CrossRef]
- Mariñas, B.J. Reverse osmosis technology for wastewater reuse. Water Sci. Technol. 1991, 24, 215–227. [Google Scholar] [CrossRef]
- Kabay, N.; Bryjak, M.; Schlosser, S.; Kitis, M.; Avlonitis, S.; Matejka, Z.; Al-Mutaz, I.; Yuksel, M. Adsorption-membrane filtration (AMF) hybrid process for boron removal from seawater: An overview. Desalination 2008, 223, 38–48. [Google Scholar] [CrossRef]
- Asahi Kasei Corporation Products. Available online: https://www.asahi-kasei.com/services_products/search/ (accessed on 8 May 2021).
- Hydranautics—A Nitto Group Company. Available online: https://membranes.com/product-selection-tools/ (accessed on 8 May 2021).
- American Public Health Association. Standard Method for the Examination of Water and Wastewater, 20th ed.; American Water Works Association & Water Pollution Control Federation: Washington, DC, USA, 1998. [Google Scholar]
- Environmental Protection Administration. Environmental Analysis Laboratory. Available online: https://www.epa.gov.tw/niea/A048BA729D1F7D58 (accessed on 21 May 2021).
- Association, A.P.H. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Water Works Association & Water Environment Federation: Washington, DC, USA, 2017. [Google Scholar]
- Hilal, N.; Kim, G.J.; Somerfield, C. Boron removal from saline water: A comprehensive review. Desalination 2011, 273, 23–35. [Google Scholar] [CrossRef]
- Bouguerra, W.; Mnif, A.; Hamrouni, B.; Dhahbi, M. Boron removal by adsorption onto activated alumina and by reverse osmosis. Desalination 2008, 223, 31–37. [Google Scholar] [CrossRef]
- Stillwell, A.S.; Webber, M.E. Predicting the Specific Energy Consumption of Reverse Osmosis Desalination. Water 2016, 8, 601. [Google Scholar] [CrossRef]
- Khlaif, O.A.; Abdulrazzaq, K.; Mohammed, A.H. Electrical Conductivity as a General Predictor of Multiple Parameters in Tigris River Based on Statistical Regression Model. J. Eng. 2021, 27, 73–82. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Bin Darwish, N.; Hakami, M.W.; Abdullah, A.; Alsadun, A.; Abu Homod, H. Boron Removal by Membrane Distillation: A Comparison Study. Membranes 2020, 10, 263. [Google Scholar] [CrossRef]
- Xu, J.; Gao, X.; Chen, G.; Zou, L.; Gao, C. High performance boron removal from seawater by two-pass SWRO system with different membranes. Water Supply 2010, 10, 327–336. [Google Scholar] [CrossRef]
- Lee, H.; Jin, Y.; Hong, S. Recent transitions in ultrapure water (UPW) technology: Rising role of reverse osmosis (RO). Desalination 2016, 399, 185–197. [Google Scholar] [CrossRef]
- Tu, K.L.; Nghiem, L.D.; Chivas, A.R. Boron removal by reverse osmosis membranes in seawater desalination applications. Sep. Purif. Technol. 2010, 75, 87–101. [Google Scholar] [CrossRef]
- Prats, D.; Chillon-Arias, M.F.; Rodriguez-Pastor, M. Analysis of the influence of pH and pressure on the elimination of boron in reverse osmosis. Desalination 2000, 128, 269–273. [Google Scholar] [CrossRef]
- Hem, J.D. Study and Interpretation the Chemical of Natural of Characteristics Natural Water, 3rd ed.; USGS: Washington, DC, USA, 1985. [Google Scholar]
- Busch, M.; Mickols, W.E. Reducing energy consumption in seawater desalination. Desalination 2004, 165, 299–312. [Google Scholar] [CrossRef]
- Bick, A.; Oron, G. Post-treatment design of seawater reverse osmosis plants: Boron removal technology selection for potable water production and environmental control. Desalination 2005, 178, 233–246. [Google Scholar] [CrossRef]
- Cengeloglu, Y.; Arslan, G.; Tor, A.; Kocak, I.; Dursun, N. Removal of boron from water by using reverse osmosis. Sep. Purif. Technol. 2008, 64, 141–146. [Google Scholar] [CrossRef]
- Kumar, S.; Baskaran, R. Integrated Membrane Operations for Providing Clean Water for Human Needs and Sustainable Industrial Growth. J. Xi’an Univ. Archit. Technol. 2020, XII, 5411. [Google Scholar]
- Urgun-Demirtas, M.; Benda, P.L.; Gillenwater, P.S.; Negri, M.C.; Xiong, H.; Snyder, S.W. Achieving very low mercury levels in refinery wastewater by membrane filtration. J. Hazard. Mater. 2012, 215–216, 98–107. [Google Scholar] [CrossRef]
- Doederer, K.; Farré, M.J.; Pidou, M.; Weinberg, H.S.; Gernjak, W. Rejection of disinfection by-products by RO and NF membranes: Influence of solute properties and operational parameters. J. Membr. Sci. 2014, 467, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Singapore’s National Water Agency. NEWater Technology. Available online: https://www.pub.gov.sg/ (accessed on 17 August 2021).
- Saidan, M.N.; Al-Addous, M.; Al-Weshah, R.A.; Obada, I.; Alkasrawi, M.; Barbana, N. Wastewater Reclamation in Major Jordanian Industries: A Viable Component of a Circular Economy. Water 2020, 12, 1276. [Google Scholar] [CrossRef]
- Saif, Y.; Almansoori, A.; Elkamel, A. Optimal design of split partial second pass reverse osmosis network for desalination applications. AIChE J. 2014, 60, 520–532. [Google Scholar] [CrossRef]
- Du, Y.; Xie, L.; Liu, Y.; Zhang, S.; Xu, Y. Optimization of reverse osmosis networks with split partial second pass design. Desalination 2015, 365, 365–380. [Google Scholar] [CrossRef]
- Bailey, J.R.; Ahmad, S.; Batista, J.R. The Impact of Advanced Treatment Technologies on the Energy Use in Satellite Water Reuse Plants. Water 2020, 12, 366. [Google Scholar] [CrossRef] [Green Version]
- Pindyck, R.S.; Rubinfeld, D.L. Econometric Models and Economic Forecasts, 4th ed.; Irwin/McGraw-Hill: Boston, MA, USA, 1997; p. 634. [Google Scholar]
- Lesjean, B.; Leiknes, T.O. Final MBR-Network Workshop “Salient Outcomes of the European R&D Projects on MBR Technology. 31 March–1 April 2009, Berlin (Germany). Book of Proceedings; Kompetenzzentrum Wasser Berlin gGmbH: Berlin, Germany, 2009; p. 189. [Google Scholar]
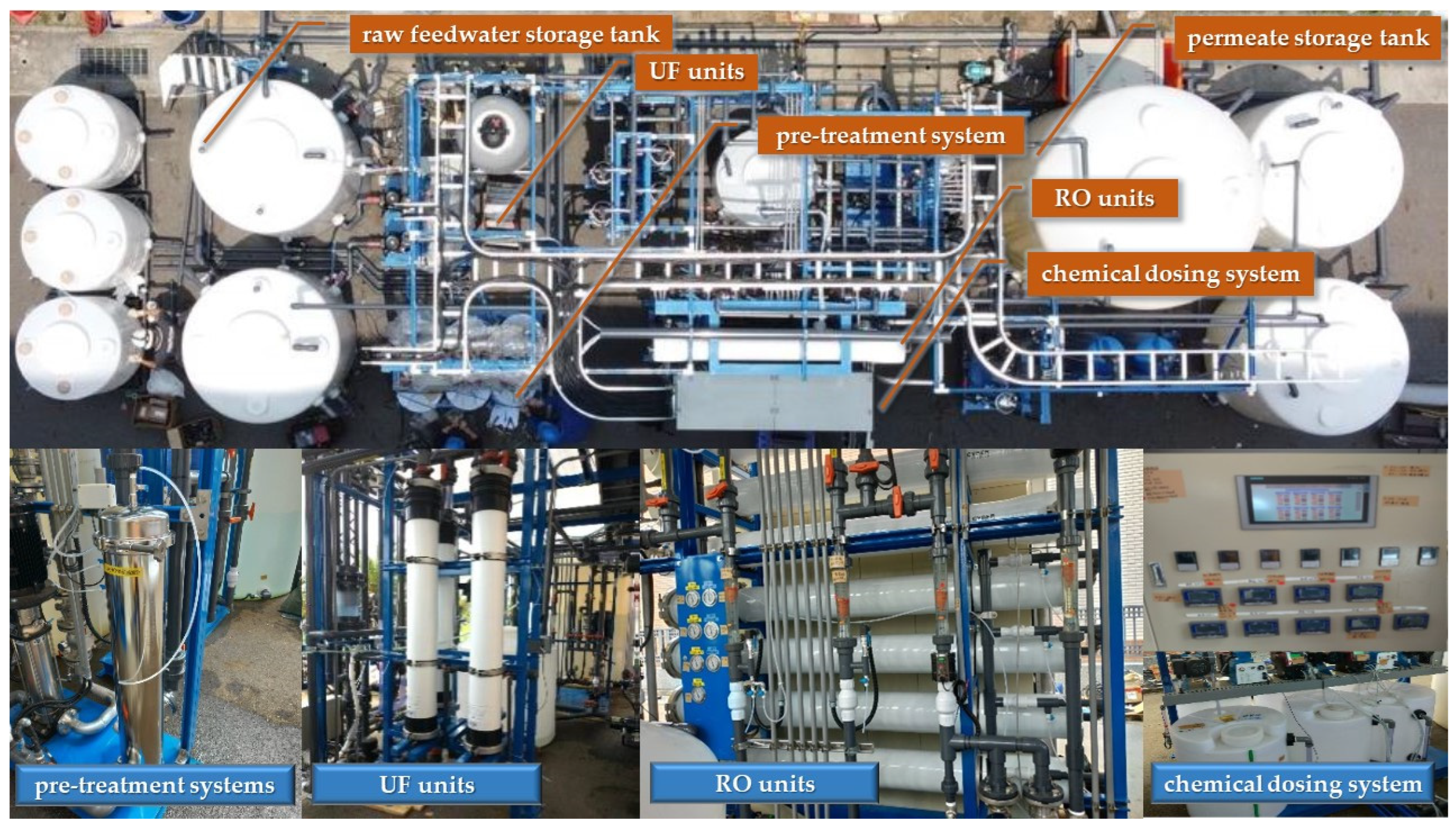



| Parameter | Asahi Kasei, MICROZA UNA-620 A (UF Membrane) | Parameter | Hydranautics Nitto CPA5-LD (RO Membrane) |
|---|---|---|---|
| Maximum Operating Temperature | 40 °C | Maximum Operating Temperature | 45 °C |
| Maximum Inlet Pressure | 45 psi | Maximum Chlorine Concentration | <0.1 ppm |
| pH Range for Cleaning | 0.0–14.0 | Maximum Feedwater Turbidity | 1.0 NTU |
| Membrane Area | 50.0 m2 | Maximum Feedwater SDI (15 min) | 5.0 |
| Module Length | 2418 mm | Maximum Feed Flow | 19.3 m3/h |
| Module Weight Water Filled | 60 kg | Minimum Brine Flow | 2.7 m3/h |
| Module Diameter | 165 mm | Maximum Pressure Drop for Each Element | 15 psi |
| Data | Electrical Conductivity | Boron | Data | Electrical Conductivity | Boron |
|---|---|---|---|---|---|
| μs/cm | mg/L | μs/cm | mg/L | ||
| 20-August-2015 | 8280 | 0.562 | 5-January-2016 | 4010 | 0.374 |
| 8-September-2015 | 7850 | 0.502 | 8-January-2016 | 5050 | 0.405 |
| 24-September-2015 | 7450 | 0.493 | 21-January-2016 | 5340 | 0.374 |
| 8-October-2015 | 8580 | 0.575 | 17-February-2016 | 6290 | 0.475 |
| 29-October-2015 | 3980 | 0.315 | 3-March-2016 | 5670 | 0.433 |
| 11-November-2015 | 4110 | 0.364 | 17-March-2016 | 3100 | 0.273 |
| 27-November-2015 | 3770 | 0.311 | 30-March-2016 | 3000 | 0.296 |
| 3-December-2015 | 3150 | 0.298 | 14-April-2016 | 3900 | 0.398 |
| 16-December-2015 | 4170 | 0.316 | 18-May-2016 | 3550 | 0.265 |
| 23-December-2015 | 4220 | 0.294 | 8-June-2016 | 4600 | 0.376 |
| 29-December-2015 | 4010 | 0.378 | 6-July-2016 | 6680 | 0.457 |
| Model Summary | |||||
|---|---|---|---|---|---|
| Model | R | R2 | Adjusted R2 | Standard Error of the Estimate | |
| 1 | 0.946 1 | 0.895 | 0.889 | 0.030384 | |
| ANOVA 2 | |||||
| Model | Sum of Squares | Df | Mean Square | F | Sig. |
| Regression | 0.157 | 1 | 0.157 | 170.018 | 0.000 1 |
| Residual | 0.018 | 20 | 0.001 | ||
| Total | 0.175 | 21 | |||
| Coefficients 2 | |||||
| Model | Unstandardized Coefficients | Standardized Coefficients | |||
| B | Standard Error | Beta | t | significance probability | |
| (Constant) | 0.138 | 0.020 | 6.841 | 0.000 | |
| Conductivity | 4.959 × 10−5 | 0.000 | 0.946 | 13.039 | 0.000 |
| Date | Feed | 1st Pass RO Permeate | 2nd Pass RO Permeate | 1st Pass RO Removal | 2nd Pass RO Removal | RO System Removal |
|---|---|---|---|---|---|---|
| mg/L | mg/L | mg/L | % | % | % | |
| 15-April-2020 | 0.289 | 0.209 | 0.079 | 27.68 | 62.20 | 72.66 |
| 16-April-2020 | 0.285 | 0.201 | 0.086 | 29.47 | 57.21 | 69.82 |
| 17-April-2020 | 0.241 | 0.187 | 0.091 | 22.41 | 51.34 | 62.24 |
| 18-April-2020 | 0.305 | 0.234 | 0.094 | 23.28 | 59.83 | 69.18 |
| 19-April-2020 | 0.294 | 0.185 | 0.090 | 37.07 | 51.35 | 69.39 |
| 20-April-2020 | 0.313 | 0.202 | 0.094 | 35.46 | 53.47 | 69.97 |
| 21-April-2020 | 0.304 | 0.217 | 0.079 | 28.62 | 63.59 | 74.01 |
| 22-April-2020 | 0.301 | 0.202 | 0.095 | 32.89 | 52.97 | 68.44 |
| 23-April-2020 | 0.335 | 0.207 | 0.051 | 38.21 | 75.36 | 84.78 |
| 24-April-2020 | 0.438 | 0.299 | 0.077 | 31.74 | 74.25 | 82.42 |
| 25-April-2020 | 0.317 | 0.217 | 0.064 | 31.55 | 70.51 | 79.81 |
| 26-April-2020 | 0.286 | 0.209 | 0.056 | 26.92 | 73.21 | 80.42 |
| 27-April-2020 | 0.284 | 0.221 | 0.061 | 22.18 | 72.40 | 78.52 |
| 28-April-2020 | 0.285 | 0.213 | 0.059 | 25.26 | 72.30 | 79.30 |
| 29-April-2020 | 0.396 | 0.255 | 0.077 | 35.61 | 69.80 | 80.56 |
| Date | Feed | 1st Pass RO Permeate | 2nd Pass RO Permeate | 1st Pass RO Removal | 2nd Pass RO Removal | RO System Removal |
|---|---|---|---|---|---|---|
| mg/L | mg/L | mg/L | % | % | % | |
| 30-April-2020 | 0.294 | 0.215 | 0.037 | 26.87 | 82.79 | 87.41 |
| 1-May-2020 | 0.297 | 0.222 | 0.033 | 25.25 | 85.14 | 88.89 |
| 2-May-2020 | 0.286 | 0.216 | 0.025 | 24.48 | 88.43 | 91.26 |
| 3-May-2020 | 0.279 | 0.185 | 0.038 | 33.69 | 79.46 | 86.38 |
| 4-May-2020 | 0.304 | 0.199 | 0.040 | 34.54 | 79.90 | 86.84 |
| 5-May-2020 | 0.393 | 0.254 | 0.051 | 35.37 | 79.92 | 87.02 |
| 6-May-2020 | 0.311 | 0.206 | 0.045 | 33.76 | 78.16 | 85.53 |
| 7-May-2020 | 0.317 | 0.199 | 0.061 | 37.22 | 69.35 | 80.76 |
| 8-May-2020 | 0.304 | 0.225 | 0.049 | 25.99 | 78.22 | 83.88 |
| 9-May-2020 | 0.322 | 0.239 | 0.061 | 25.78 | 74.48 | 81.06 |
| 10-May-2020 | 0.353 | 0.217 | 0.058 | 38.53 | 73.27 | 83.57 |
| 11-May-2020 | 0.397 | 0.259 | 0.065 | 34.76 | 74.90 | 83.63 |
| 12-May-2020 | 0.294 | 0.199 | 0.039 | 32.31 | 80.40 | 86.73 |
| 13-May-2020 | 0.273 | 0.206 | 0.042 | 24.54 | 79.61 | 84.62 |
| 14-May-2020 | 0.296 | 0.198 | 0.050 | 33.11 | 74.75 | 83.11 |
| Electrical Conductivity | Boron 1 | Days of Occurrence | Probability |
|---|---|---|---|
| μs/cm | mg/L | Day | % |
| ≤2000 | ≤0.233 | 11 | 2.48 |
| 2000 < EC ≤ 4000 | 0.233 < EC ≤ 0.333 | 330 | 74.32 |
| 4000 < EC ≤ 6000 | 0.333 < EC ≤ 0.433 | 80 | 18.02 |
| ≥6000 | ≥0.433 | 23 | 5.18 |
| Electrical Conductivity | Boron 1 | SPSP Operation |
|---|---|---|
| μs/cm | mg/L | % |
| ≤1000 | ≤0.188 | 70 Split, 30 2nd Pass RO |
| 1000 < EC ≤ 2000 | 0.188 < EC ≤ 0.237 | 40 Split, 60 2nd Pass RO |
| 2000 < EC ≤ 3000 | 0.237 < EC ≤ 0.287 | 25 Split, 75 2nd Pass RO |
| 3000 < EC ≤ 4000 | 0.287 < EC ≤ 0.336 | 15 Split, 85 2nd Pass RO |
| 4000 < EC ≤ 6000 | 0.336 < EC ≤ 0.436 | 10 Split, 90 2nd Pass RO |
| 6000 < EC ≤ 8000 | 0.436 < EC ≤ 0.535 | 4 Split, 96 2nd Pass RO |
| ≥8000 | ≥0.535 | 0 Split, 100 2nd Pass RO |
| Date | Feed | Boron Concentration in Reclaimed Water 1 | SPSP Operation | Boron Concentration in Reclaimed Water | Boron Removal |
|---|---|---|---|---|---|
| mg/L | mg/L | % | mg/L | % | |
| 20-May-2020 | 1990 | 0.237 | 25 Split, 75 2nd Pass RO | 0.052 | 78.03 |
| 21-May-2020 | 2690 | 0.271 | 25 Split, 75 2nd Pass RO | 0.040 | 85.26 |
| 22-May-2020 | 2560 | 0.265 | 25 Split, 75 2nd Pass RO | 0.044 | 83.39 |
| 23-May-2020 | 3900 | 0.331 | 15 Split, 85 2nd Pass RO | 0.039 | 88.23 |
| 24-May-2020 | 3430 | 0.308 | 15 Split, 85 2nd Pass RO | 0.051 | 83.45 |
| 25-May-2020 | 3580 | 0.316 | 15 Split, 85 2nd Pass RO | 0.058 | 81.62 |
| 26-May-2020 | 4560 | 0.364 | 10 Split, 90 2nd Pass RO | 0.042 | 88.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, S.-S.; Wu, J.-H. Municipal-to-Industrial Water Reuse via Multi-Stage and Multi-Pass Reverse Osmosis Systems: A Step from Water Scarcity towards Sustainable Development. Water 2022, 14, 362. https://doi.org/10.3390/w14030362
Chan S-S, Wu J-H. Municipal-to-Industrial Water Reuse via Multi-Stage and Multi-Pass Reverse Osmosis Systems: A Step from Water Scarcity towards Sustainable Development. Water. 2022; 14(3):362. https://doi.org/10.3390/w14030362
Chicago/Turabian StyleChan, Shih-Shuo, and Jung-Hua Wu. 2022. "Municipal-to-Industrial Water Reuse via Multi-Stage and Multi-Pass Reverse Osmosis Systems: A Step from Water Scarcity towards Sustainable Development" Water 14, no. 3: 362. https://doi.org/10.3390/w14030362





