Abstract
The active development of water purification functional materials based on multicomponent spinel ferrites makes it necessary to search for new efficient methods of obtaining initial nanostructured powders. In this study, a two-stage method for the synthesis of perspective pollutant absorption agents based on NixZn1−xFe2O4 (x = 0, 0.3, 0.7, 1.0) spinel ferrites are proposed and implemented. The approach is based on the synthesis of the initial powder using the solution combustion method and its subsequent thermal treatment in the air. It was found that synthesized samples are single-phase Ni-Zn ferrites with an average crystallite size of 41.4 to 35.7 nm and a degree of crystallinity of ~95–96%. The analysis of antimicrobial activity against four diverse test-cultures: Escherichia coli ATCC 11229 (non-spore-forming gram-negative), Bacillus cereus ATCC 10702 (spore-forming gram-positive), Staphylococcus citreus NCTC 9379 (non-spore-forming gram-positive), and Candida tropicalis ATCC 750 (yeast) showed that almost all of the synthesized powders exhibit an advanced ability to inhibit the growth of the microorganisms mentioned above. The compositions obtained can be a perspective basis for both natural and wastewater purificators with magnetic separation ability and can find biotechnological and biomedical applications as promising antimicrobial materials.
1. Introduction
Ferrites of various compositions appear to be an essential class of magnetic functional materials that are known foremost by means of their wide usage in the production of various electronic components [1,2,3,4,5]. Since the discovery of ferrites in the middle of the last century, an intensive study of the possibility to improve their functional properties by modifying the structural, morphological, and microstructural parameters has been constantly conducted. Currently, a large number of works have been published, in which the influence of doping cations of transition metals on the magnetic and electrical properties of both initial ferrite powders and final ceramic materials has been thoroughly studied [6,7,8,9]. Among the whole variety of ferrites, stand out. Like multi-component lithium ferrites, Ni-Zn ferrites are the basis for obtaining microwave ceramic products for civil and military applications [10,11,12]. Furthermore, it has been discovered that nanostructured ferrite powders are capable of acting as efficient catalysts [13,14] and photocatalysts (due to their crystalline structure, able to absorb visible light and conduct oxidation processes) [15,16,17] and antimicrobial materials [18,19]; they are also used for the purification of natural water sources along with wastewater from various pollutants (utilizing photocatalysis and absorption) [20,21]. In recent years, they have been used in the water purification field and seem to be promising future materials for this particular application as adsorbents of metal ions (e.g., Ag, Hg, Cd, Co, Cr, Cu, Ni, Mn, Mo, Pb, Sn, and Zn), dyes, pesticides and insecticides, pharmaceuticals (separately or in a combination with other materials, e.g., chitosan), and other refractory organic pollutants [22,23,24,25,26,27]. Cobalt-, zinc-, and copper-doped ferrite powders are of main scientific interest due to their remarkable physical properties (e.g., high magnetic crystalline anisotropy, coercivity, magnetization), high photocatalytic activity under visible light, outstanding chemical stability, and biocompatibility [15,28,29]. Coupled with this, when using ferrites as water purificators, they can be effortlessly selectively recovered from suspension using an external magnet. From the point of biomedical application, ferrites of copper, nickel, zinc, and cobalt, which have shown particular perspective in this field (as core and coating materials, magnetic nanocarriers, antimicrobial materials, adsorbents, etc.) [30,31], are especially actively studied.
Thus far, the main method for the industrial production of ferrites remains the classical solid-phase technology, which is the most applicable to produce large volumes of ferrite powder [32]. Although constant attempts to improve the functional properties (such as varying the temperature regimes of ferritization and agglomerating [33,34] and the selection of optimal conditions for mechanochemical processing [35,36] of the resulting powdery and ceramic products) were made using this technology, but it is still difficult to obtain powders with a controlled particle size in the nanometer range. Therefore, a several studies have been aimed at developing new methods for obtaining nanostructured ferrites [37]. Among the currently known methods, the methods of hydrothermal treatment [38,39], sonochemical synthesis [40,41], sol-gel synthesis [42,43], the method of solution combustion synthesis (SCS) stand out [44,45]. All methods of “wet chemistry” listed above make it possible to obtain controlled nanostructures in a wide range of functional parameters applicable for a plethora of applications, including the biotechnological field [46]. Particularly, it should be mentioned that plenty of solution combustion methods have wide and promising prospects for industrial scale-up and allow varying multiple synthesis parameters, which simplify the process of obtaining nanopowders with controlled morphology and structure [47,48]. The authors of the current study have developed a two-stage method of thermal treatment of X-ray amorphous combustion products based on the synthesis of a completely X-ray amorphous powder under conditions of glycine-nitrate combustion with a significant lack of organic fuel (glycine) and its subsequent heat treatment at temperatures of 500–800 °C. This specific method has been successfully applied to the synthesis of several systems of rare earth orthoferrites [49,50] and multicomponent spinel-type ferrites [51,52]. In addition, the solution combustion method is low-cost, offers a high performance, and is affordable even for small-scale production. This leads to the fact that water purification methods based on ferrite powders obtained by the SCS-method result in a significant reduction of investment and preparation expenses and, in addition, are environment-friendly.
In the presented work, this technique was successfully applied for the synthesis of multicomponent Ni-Zn ferrites of the composition NixZn1−xFe2O4 (x = 0, 0.3, 0.7, 1.0), perspective water pollutant adsorbents, and antimicrobial materials. The current paper provides a detailed description of both structural and morphological, as well as magnetic and antimicrobial properties of the synthesized compositions. The selection of the thermal treatment mode was based on the previous research of the authors [49,52] and literature data [53,54].
2. Materials and Methods
2.1. Materials
In the current study, nickel nitrate 6-aqueous Ni(NO3)2 × 6H2O (chemically pure 99%—Neva-reactive, Saint Petersburg, Russia), zinc nitrate 6-aqueous Zn(NO3)2 × 6H2O (chemically pure 99%)—Neva-reactive, Saint Petersburg, Russia), iron nitrate 9-aqueous Fe(NO3)3 × 9H2O (chemically pure 99%—Neva-reactive, Saint Petersburg, Russia), glycine C2H5NO2 (chemically pure 99%—Neva-reactive, Saint Petersburg, Russia), and nitric acid HNO3 (chemically pure 99%—Neva-reactive, Saint Petersburg, Russia) were used to synthesize the initial pre-ceramic powder.
2.2. Synthesis of Initial Powder
The initial ferrite nanopowder of NixZn1−xFe2O4 (x = 0, 0.3, 0.7, 1.0) composition was synthesized using two stages: During the first stage of the synthesis, the initial X-ray amorphous powder was obtained under the conditions of solution combustion with a significant lack of organic fuel, while during the second stage, the synthesized products were thermally treated in the air at a temperature of 650 °C for 5 h [49]. The calculation of the main mass portions was carried out following the reaction of the formation of the final products:
where φ = 1—corresponds to the stoichiometric ratio of glycine (in our case, φ = 0.2 was chosen—a significant lack).
The reagents used for the synthesis were dissolved in 100 mL of bidistilled water under constant mechanical stirring (stirring speed was 200 rpm) and heating (heating up to 30–35 °C). After the complete dissolution, 2 mL of nitric acid were added to the obtained reaction solution to prevent the precipitation of metal complex compounds. Then, the solution was heated on an electric stove (HS-191—Supra, Tokyo, Japan) until the water was almost completely removed and the autoignition point, where the brown solid reaction product was formed and reached. The obtained powders were mechanically ground in a mortar, thermally treated at a temperature of 650 °C for 5 h in an air atmosphere, and then analyzed.
2.3. Physicochemical Characterization
X-ray phase and X-ray structural analysis of the synthesized powders were performed using a SmartLab 3 diffractometer (Rigaku Corp., Tokyo, Japan) via SmartLab Studio II software package. The survey was carried out under the CuKα emission (0.15405 nm), and the diffraction peaks were correlated using the ICDD PDF-2 powder database. The average crystallite size and the crystallite size distribution were calculated in compliance with the Scherrer formula and methods of fundamental parameters, respectively. Infrared spectra were obtained by FTIR spectroscopy using an IRTracer-100 spectrometer (Shimadzu, Tokyo, Japan) in the wavenumber range from 3300 to 300 cm−1 using KBr tablets. The low-temperature nitrogen adsorption-desorption isotherm measurements were held by the means of a high-performance adsorption analyzer ASAP 2020 (Micrometrics Instrument Corp., Atlanta, GA, USA). According to the data obtained, values of specific surface area and the porosity of synthesized powders were determined by Brunauer-Emmet-Teller (BET) and Barret-Joyner-Halenda (BJN) methods. All samples were preliminarily heat-treated under vacuum at 300 °C for 4 h. The morphology and elemental analysis of the obtained powders were determined by scanning electron microscopy and energy-dispersive microscopy using a Vega 3 SBH electron microscope (Tescan, Brno, Czech Republic) with INCA 200 detector (Tescan, Oxford, UK). The remanence, saturation magnetization, and coercive force were determined using a 7410 vibrating sample magnetometer (Lake Shore Cryotronics, Westville, OH, USA) at room temperature.
2.4. Antimicrobial Activity Test
2.4.1. Preparation of Bacterial and Yeast Cell Culture Media
The antimicrobial activity of the obtained samples was determined against four test-cultures: Escherichia coli ATCC 11229 (non-spore-forming gram-negative), Bacillus cereus ATCC 10702 (spore-forming gram-positive), Staphylococcus citreus NCTC 9379 (non-spore-forming gram-positive), Candida tropicalis ATCC 750 (yeast).
The inoculum was prepared from a daily culture of microorganisms in isotonic solution (0.85% NaCl) according to standard turbidity of 0.5 McFarland. The concentration of the bacterial suspension, the density of which corresponds to the McFarland 0.5 turbidity standard, is 1.5 × 108 CFU/mL, of the yeast suspension—1.5 × 106 CFU/mL.
Meat Enzyme Hydrolysate (agarized)—36 g of “MEH-agar” (9358-058-39484474-2009—NICF, Saint Petersburg, Russia) and 20 g of “microbiological agar-agar” (imp, 17206-96—LenReactiv, Saint Petersburg, Russia) were added to a 1 L tap water and heated to get a homogeneous liquid (until the full agar dissolving). Afterward, the obtained liquid was filtered through a cotton-gauze filter, poured into 750-mL Erlenmeyer flasks, and sterilized by autoclaving at 121 °C for 30 min. Then, the medium was cooled to a temperature of 48 ± 2 °C and transferred into sterile Petri dishes forming a layer of 4–5 mm. After solidification of the medium, following the antiseptic rules, the dishes were dried at a temperature of 37 ± 1 °C for 40–60 min. In this form, MEH-agar can be used for 7 days if stored at a temperature of 2–8 °C.
Sabouraud agar—60 g of “Sabouraud agar” (9385-024-39484474-2012—NICF, Saint Petersburg, Russia) and 20 g of “microbiological agar-agar” (imp, 17206-96—LenReactiv, Saint Petersburg, Russia) were added to 1 L of tap water and heated to get a homogeneous liquid (until the full agar dissolving). Afterward, the obtained liquid was filtered through a cotton-gauze filter, poured into 750-mL Erlenmeyer flasks, and sterilized by autoclaving at 121 °C for 30 min. Then, the medium was cooled to a temperature of 48 ± 2 °C and transferred into sterile Petri dishes forming a layer of 4–5 mm. After solidification of the medium, following the antiseptic rules, the dishes were dried at a temperature of 37 ± 1 °C for 40–60 min. In this form, Sabouraud-agar can be used for 7 days if stored at a temperature of 2–8 °C.
LB broth—10 g of tryptone (P/N 1612—Pronadisa, Conda, Getafe, Spain), 5 g of yeast extract (9385-007-39484474-2003—NICF, Saint-Petersburg, Russia), and 10 g of sodium chloride (ACS—LenReactiv, Saint Petersburg, Russia) were added to 1 L of tap water and heated to get a homogeneous liquid. Afterward, the obtained liquid was filtered through a cotton-gauze filter, poured into 250-mL flasks, and sterilized by autoclaving at 121 °C for 30 min.
Sabouraud broth—60 g of “Sabouraud agar” (9385-024-39484474-2012—NICF, Saint Petersburg, Russia) were added to 1 L of tap water and heated to get a homogeneous liquid. Afterward, the obtained liquid was filtered through a cotton-gauze filter, poured into 250-mL flasks, and sterilized by autoclaving at 121 °C for 30 min.
2.4.2. Inhibition Zone Assay
Initially, agarized nutrient media, Meat Enzyme Hydrolysate, and Sabouraud agar, were poured into Petri dishes. The inoculum (bacteria—1.5 × 108 CFU/mL, yeast—1.5 × 106 CFU/mL) was then applied to the surface of the nutrient media in an amount of 100 μL/dish and spread with a spatula. Then, four wells were made in each plate with a sterile drill in the thickness of the agar. Solutions of the studied ferrites in dimethyl sulfoxide (DMSO) with a concentration of 20 mg/mL were preliminarily prepared using an ultrasonic bath (UZV-9.5 TTC (RMD)—Sapphire LLC, Saint Petersburg, Russia) until complete dissolution.
The solutions were added to the wells, 50 μL per each. DMSO was used as a control. Samples with B. cereus, S. citreus, and C. tropicalis were incubated at 28 °C, with E. coli—at 37 °C, all for 24 h. The results were evaluated by the presence and size of the zone of inhibition of the growth of microorganisms.
2.4.3. Growth Curve Assay
The magnetic NiZnFe nanocomposites were irradiated with ultraviolet light for 30 min and added to LB (for E. coli, B. cereus, and S. citreus) or Sabouraud (for C. tropicalis) broths to prepare a suspension (5 mL) with the concentration of 20 mg/mL. E. coli, B. cereus or S. citreus of 1.5 × 108 CFU/mL, or C. tropicalis of 1.5 × 106 CFU/mL (100 µL) were added into the suspension and incubated for 24 h at 28 °C or 37 °C, depending on the culture of the microorganism: B. cereus, S. citreus, and C. tropicalis or E. coli, respectively. Two hundred microliters of bacterial and yeast liquids were taken at 1 h, 6 h, 12 h, and 24 h, separately, to test the OD600 value. LB broth was used as a blank control, LB broth and inoculum as a negative control, LB broth, inoculum, and 100 ng/mg penicillin (for bacteria) or 2 µg/mL of fluconazole (for yeast) as a positive control.
2.4.4. MIC and MBC Assay
Also, the minimum inhibitory concentration of the studied ferrites was determined by the serial dilution method using the same 4 test cultures: Escherichia coli (non-spore-forming gram-negative), Bacillus cereus (spore-forming gram-positive), Staphylococcus citreus (non-spore-forming gram-positive), Candida tropicalis (yeast). LB and Sabouraud broths were used for bacterial cultures and yeast, respectively. Stock solutions of the studied ferrites in dimethyl sulfoxide with a concentration of 400 mg/mL were preliminarily prepared using an ultrasonic bath (UZV-9.5 TTC (RMD)—Sapphire LLC, Saint Petersburg, Russia) until complete dissolution.
The inoculum was prepared from a daily culture of microorganisms in isotonic solution (0.85% NaCl) according to standard turbidity of 0.5 McFarland. The concentration of the bacterial suspension, the density of which corresponds to the McFarland 0.5 turbidity standard, is 1.5 × 108 CFU/mL, of the yeast suspension—1.5 × 106 CFU/mL.
The following series of ferrite dilutions were prepared: 100, 50, 25, 12.5, 6.25, and 3.125 mg/mL. Five control wells were made. In the first well, the culture medium and inoculum were introduced (negative control-1); in the second, the culture medium with DMSO and inoculum (negative control-2); in the third, the culture medium and DMSO (positive control-1); in the fourth, the culture medium, DMSO, and 100 ng/mg penicillin (for bacteria) or 2 µg/mL of fluconazole (for yeast) (positive control-2), in the fifth, LB broth (blank control). The plates were then incubated for 24 h at temperatures of 28 °C or 37 °C, depending on the culture of the microorganism: B. cereus, S. citreus, and C. tropicalis or E. coli, respectively. The results were assessed by the presence of microbial growth. Additionally, the OD600 value was tested exactly in the 96-well plates using a Multiscan FC microplate photometer (Thermo Fisher Scientific, Inc., Helsinki, Finland) through SkanIt RE 6.0.2; the minimum inhibitory concentration (MIC) was determined by the lowest concentration of ferrite solution that suppresses the visible growth of microorganisms.
Lastly, 100 μL of culture fluid was taken from each well, where no visible bacterial/yeast growth was present, and the OD600 value was similar to the blank group. All taken culture fluids were applied to the surface of the nutrient media in Petri dishes and spread with a spatula. Samples with B. cereus, S. citreus, and C. tropicalis were incubated at 28 °C and with E. coli at 37 °C, all for 24 h. The minimal bactericidal concentration (MBC) was the lowest concentration, which grew no test cultures.
3. Results
3.1. Powder X-ray Diffraction and Elemental Characterization
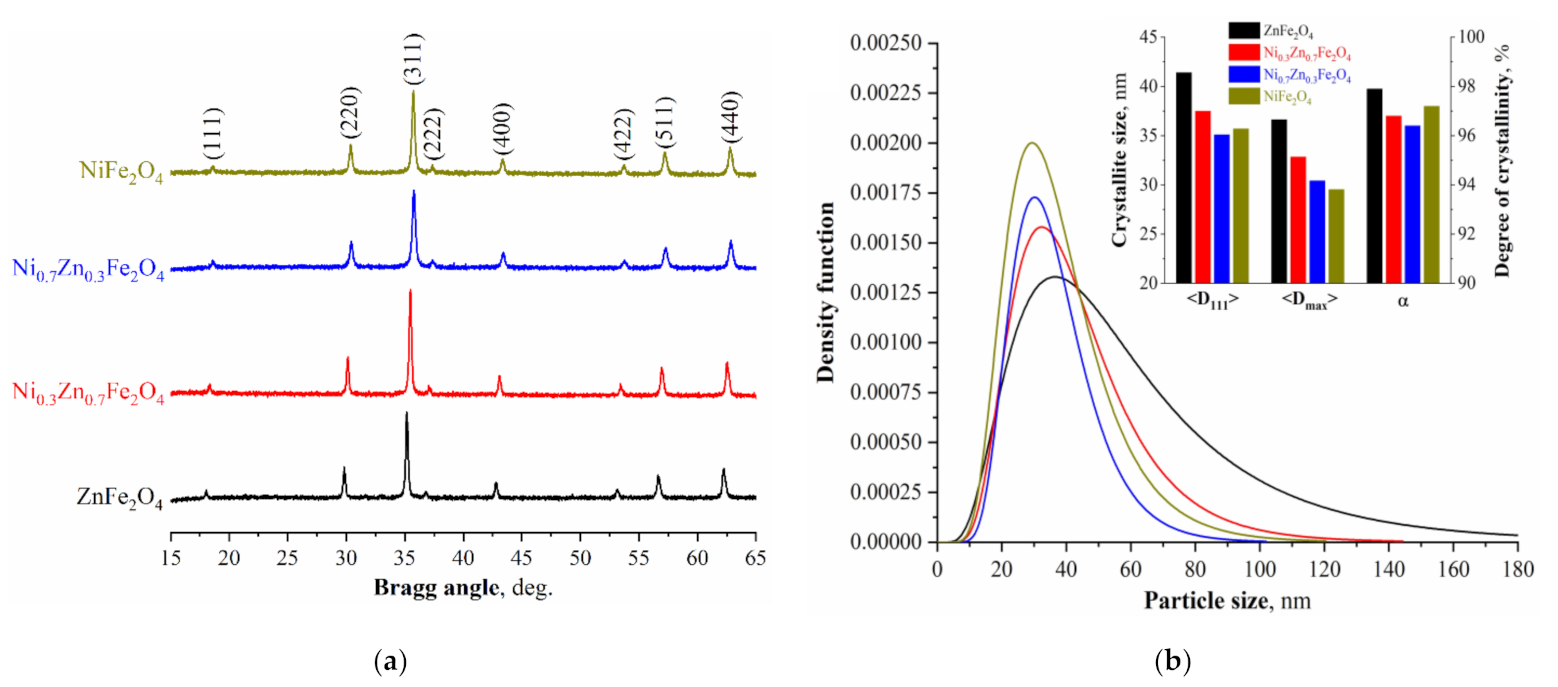
Results of powder X-ray diffractometry of synthesized samples NixZn1-xFe2O4 (x = 0, 0.3, 0.7, 1.0) are shown in the Figure 1. The data obtained confirm that all four ferrite samples contain only one phase with a cubic structure (JCPDS # 080234). The peak shift is explained by a structural parameter change caused by the doping nickel cations’ introduction. The values of the crystalline lattice parameters, average crystallite size, and X-ray density are represented in Table 1.
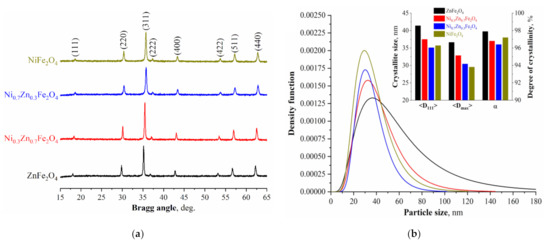
Figure 1.
(a) X-ray diffraction patterns of the Ni-Zn ferrite powders; (b) crystallite size distribution of the obtained NixZn1-xFe2O4 (x = 0, 0.3, 0.7, 1.0) ferrites.

Table 1.
Structural parameters of NixZn1-xFe2O4 (x = 0, 0.3, 0.7, 1.0) nanopowders.
The obtained unit cell parameters values are in good agreement with the results of previous studies [53,55], in which systems of similar compositions were synthesized. The change in the unit cell parameters is caused by the replacement of octahedral and tetrahedral positions in it by the Ni2+ cations and their smaller (0.69 Å) radius in comparison with Zn2+ cations (0.75 Å). It should be noted that the largest average crystallite size calculated using the Scherrer formula is observed in the ZnFe2O4 sample (41.4 nm), and with an increase in Ni2+ content, it decreases to 35.1 nm. The maximum crystallite size obtained from the crystallite size distribution curves (Figure 1b) is also in good agreement with the average size calculated by the Scherrer formula. Nonetheless, it is noteworthy that some studies [55,56] reported on the opposite situation when the average crystallite size increased upon the addition of cations Ni2+, which authors of [55] explained by the derivation of the α-Fe2O3 impurity phase. For powders synthesized in this study, the presence of impurity phases was not observed, which allows us to conclude that the change in the crystallite size is most likely associated with a change in the combustion temperature of the flame during the synthesis of the initial amorphous precursor, which, as is known, depends on the composition of the reaction solution [57].
In addition, the elemental composition of the obtained ferrites was examined by the energy-dispersive analysis method. The data acquired confirmed that all synthesized powders correspond to the calculated composition in terms of the main elements within the error of the determination method.
3.2. FT-IR Spectroscopy
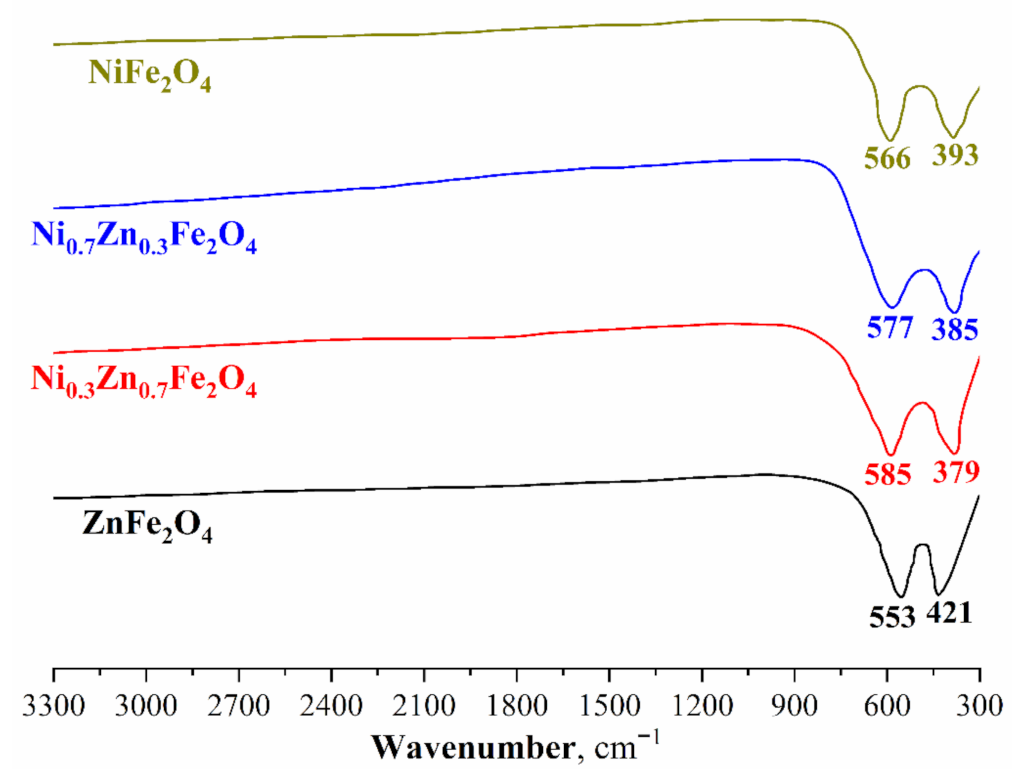
Ni-Zn ferrites’ IR-spectra of various compositions are shown in Figure 2. In all the samples examined, only two main absorption bands are observed in the range from 379 I kto 553 cm−1. The lack of absorption bands related to zinc, nickel, and iron crystalline hydrates should also be noted. Consequently, this indirectly confirms that all the starting materials have either reacted completely or were removed from the powders during heat treatment. Moreover, absorption bands varying from 379 to 553 cm−1 are characteristic of this class of compounds and additionally confirm the successful formation of nickel-zinc ferrites [56,58].
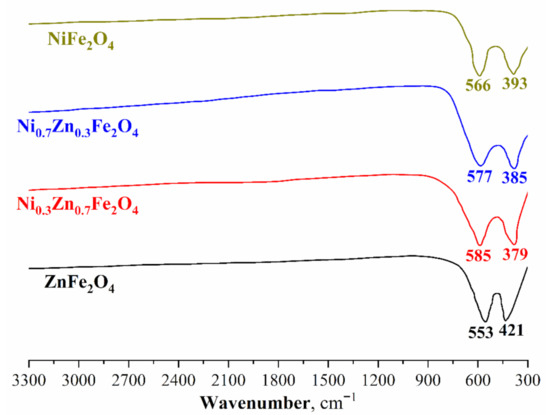
Figure 2.
FT-IR spectra of the synthesized Ni-Zn ferrite nanopowders.
Absorption bands in the range of 553–585 cm−1 refer to the oxygen stretching vibrations in M-O-Fe (M-Ni, Zn) and Fe-O-Fe systems, while absorption bands comprised between 379 and 421 cm−1 characterize the O-Fe-O bending vibrations. The absorption bands shift with an increase in the Ni content in the crystal lattice—this is also associated with the structural changes. Therefore, the results of FTIR spectroscopy confirm the data of powder X-ray diffractometry and indicate the successful synthesis of single-phase nickel-zinc ferrites.
3.3. Low-Temperature N2 Sorption-Desorption Analysis
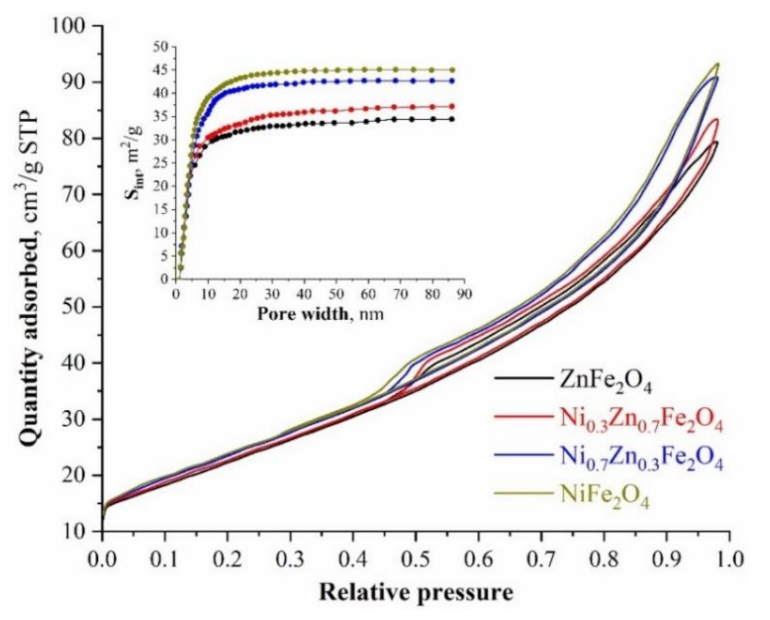
Liquid nitrogen low-temperature sorption-desorption was used to assess the surface characteristics of the synthesized samples. Figure 3 shows the obtained isotherms and differential dependences of the surface area on the pore diameter. In concordance with the data obtained, the largest specific surface area (~93.76 m2/g) is observed for pure nickel ferrite, while the smallest (~79.89 m2/g) is observed for ZnFe2O4. The more nickel cations are present in the synthesized ferrites’ structure, the more the specific surface area increases, which is most likely associated with a decrease in the average crystallite size. In conjunction with the published works [59,60], the specific surface area is one of the most important parameters in terms of both antimicrobial activity and the possible use of ferrites for wastewater treatment. It follows that values within ~80–90 m2/g are sufficient for the efficient use of the synthesized powders for this purpose. On top of this, the presence of a large mesopore number (insert in Figure 3) suggests a possible high sorption activity of the synthesized compositions [61].
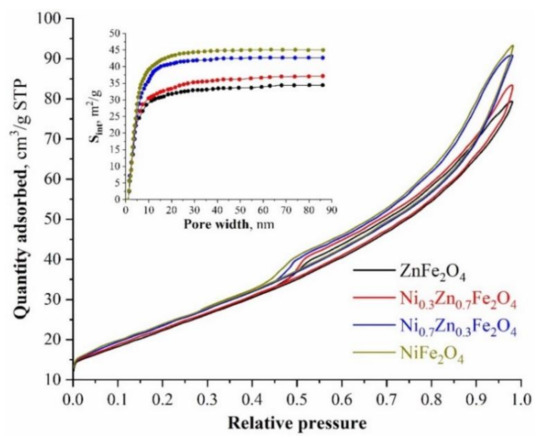
Figure 3.
Low-temperature nitrogen adsorption-sorption isotherms of the nanostructured nickel-zinc ferrites.
3.4. Morphology Analysis
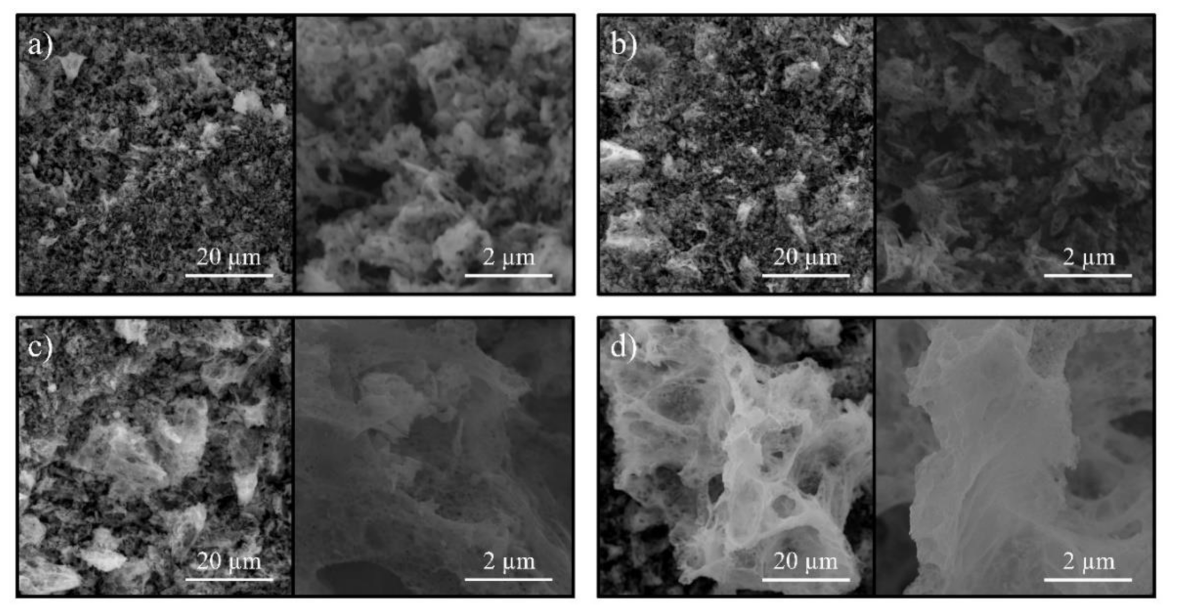
The SEM morphology of the obtained nanopowders of nickel-zinc ferrites is shown in Figure 4. The obtained micrographs demonstrate a typical morphology for the solution combustion products. Notwithstanding, it is to be noted that changes in a ZnFe2O4—NiFe2O4 row affect not only the structural but also the morphological features of the synthesized samples. Thereupon, in the zinc ferrite micrographs, the micron agglomerates are of an average size of about 2–5 microns, while in the case of nickel ferrite, the average size of these agglomerates increases to 25–30 microns.
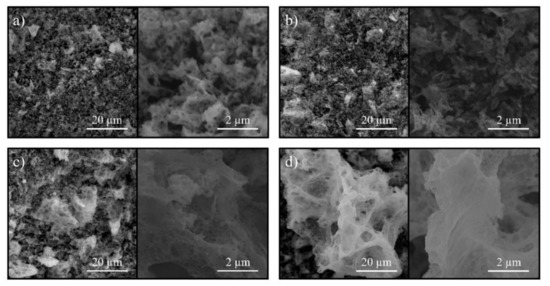
Figure 4.
SEM images of the synthesized nanopowders (a) ZnFe2O4; (b) Ni0.3Zn0.7Fe2O4; (c) Ni0.7Zn0.3Fe2O4; (d) NiFe2O4.
This variance is most likely associated with a change in the combustion temperature depending on the composition of the reaction medium and was considered in detail in previous works [12,44]. The outstanding is the spongy structure of the obtained ferrites, which plays an important role in the process of water sources purifying and can increase the sorption activity [62]. It should be noted that the morphology of all synthesized samples corresponds to typical products of solution combustion obtained using glycine as an organic fuel [12,44,58].
3.5. Magnetic Properties
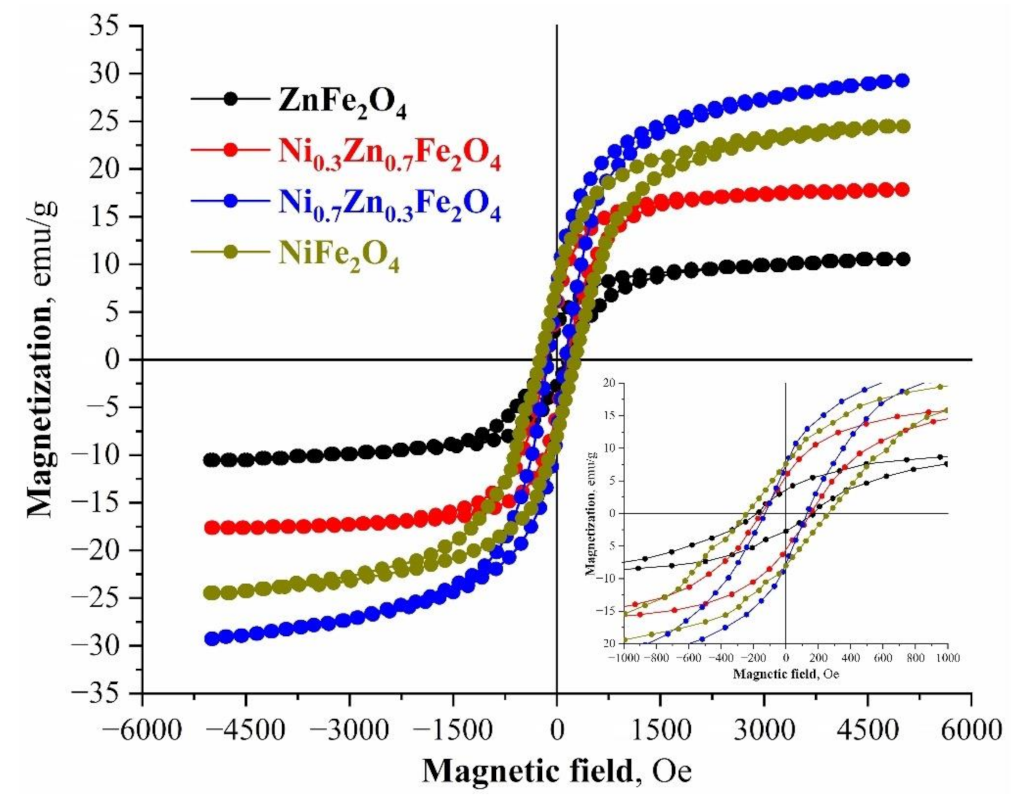
The magnetic nature of the synthesized compositions represents a typical ferrimagnetic behavior model. From the point of magnetic characteristics and the process of magnetic separation of ferrite nanoparticles from aqueous solutions, the most promising samples are NiFe2O4 и Ni0.7Zn0.3Fe2O4, which characterize the most prominent parameters of remanent magnetization and saturation magnetization (Mr = 7.62 and 7.31 emu/g, Ms = 29.34 and 24.57 emu/g). Conversely, from the point of the coercive force and, as a consequence, the ability of ferrite to react to an external magnetic field and to be magnetized, the most propitious are NiFe2O4 and ZnFe2O4 (Hc = 230.7 and 180.4 Oe) (Figure 5). The fact that both in the average crystallite sizes and the values of specific surfaces, the magnetic parameters (except for the coercive force) change linearly with an increase of the amount of the Ni2+ cations in the spinel structure, is of particular note. This is also related to the structural changes in the synthesized compositions. It is known that the magnetic properties of ferrites are greatly influenced by the average particle size, which significantly changes within the synthesized series [63,64].
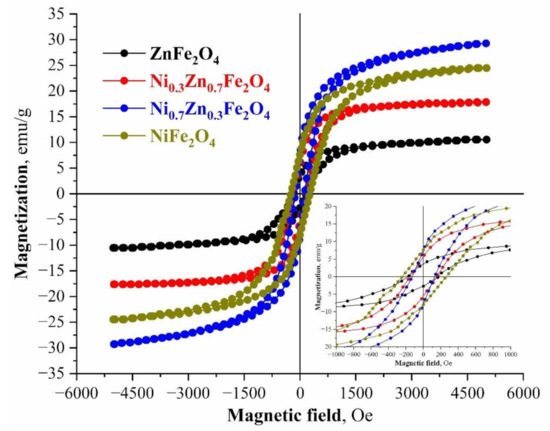
Figure 5.
M-H hysteresis loops of the synthesized nickel-zinc ferrites samples at room temperature.
3.6. Inhibition Zone Assay
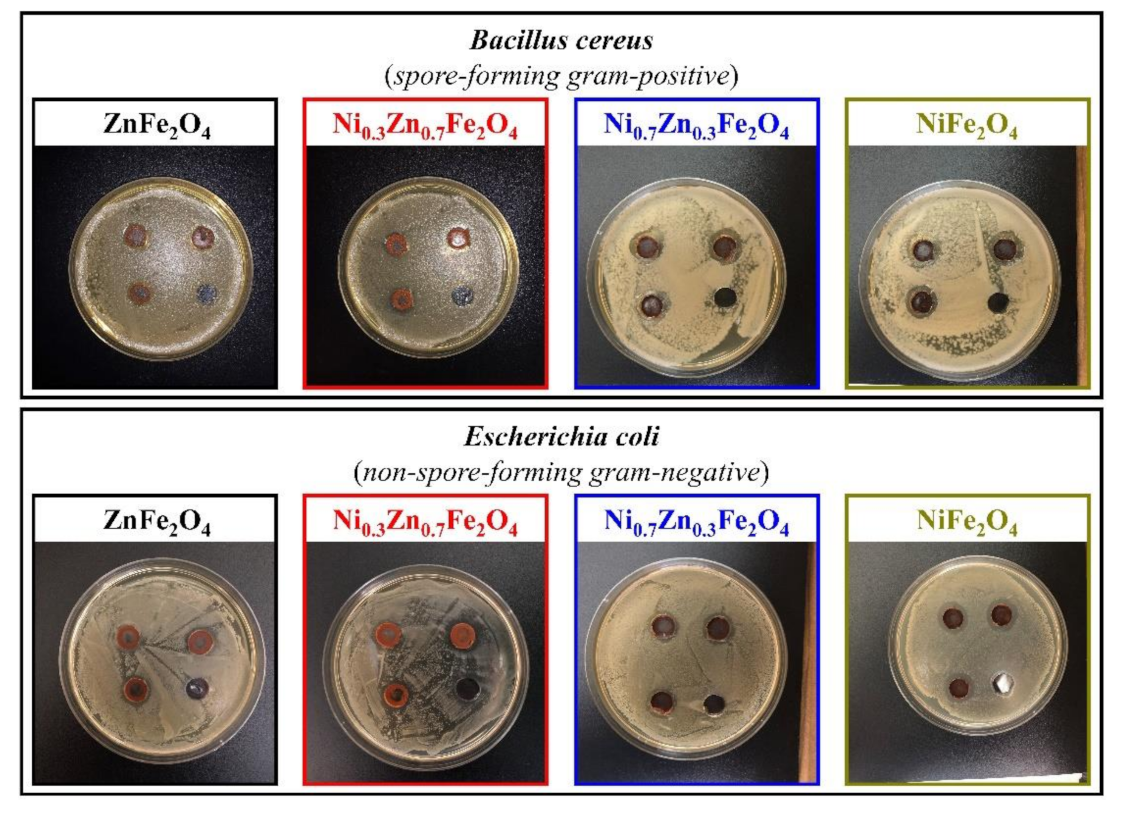
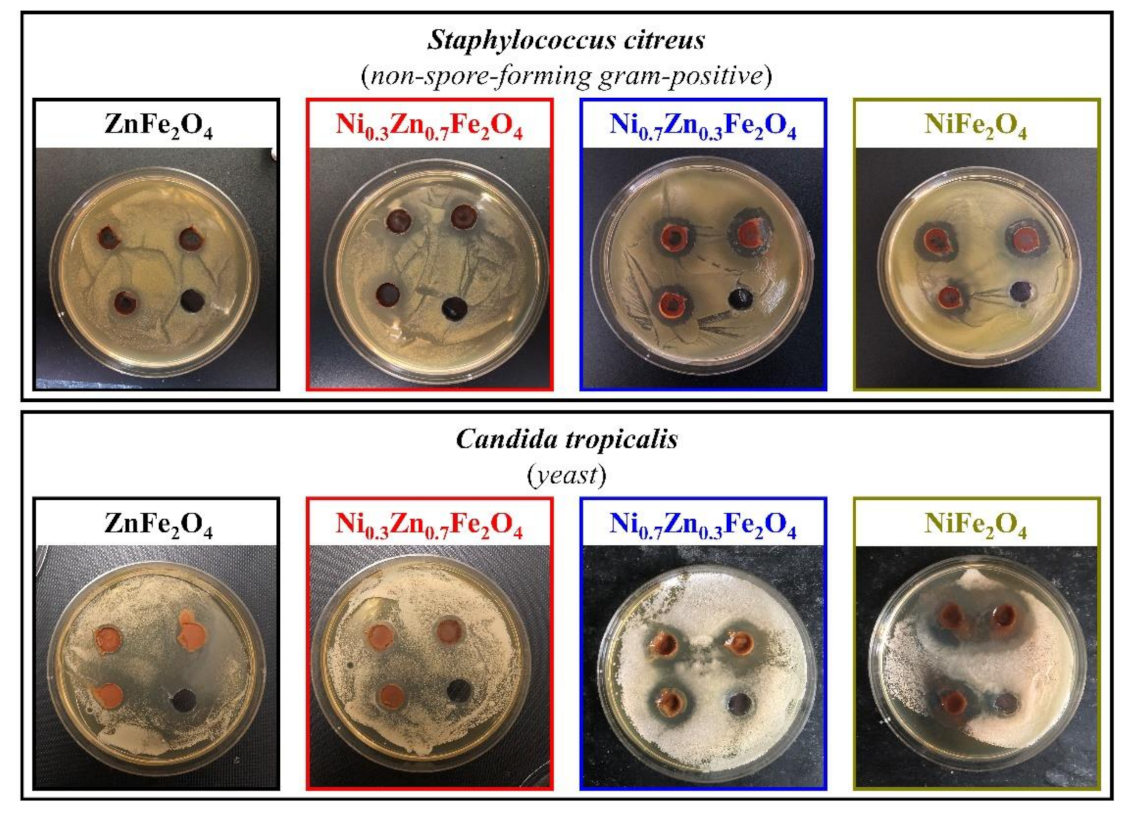
The ability to inhibit the growth of microorganisms of the synthesized ferrites was determined against four test cultures, Escherichia coli (non-spore-forming gram-negative), Bacillus cereus (spore-forming gram-positive), Staphylococcus citreus (non-spore-forming gram-positive), and Candida tropicalis (yeast), using the agar diffusion method. Figure 6 and Figure 7 depict the results of a study of four obtained ferrites by the method of wells, according to which all formulations showed moderate inhibition of the growth of test cultures.
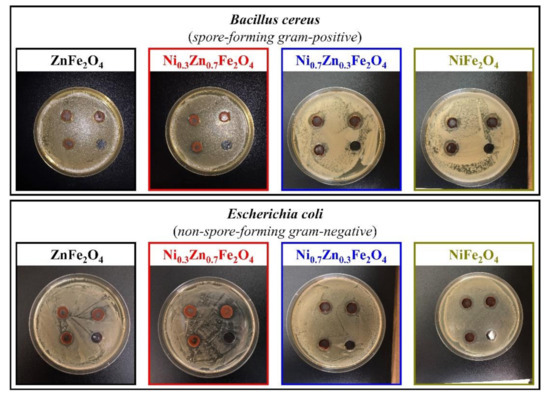
Figure 6.
Inhibition zone assay of the obtained NixZn1-xFe2O4 (x = 0, 0.3, 0.7, 1.0) ferrites toward B. cereus and E. coli.
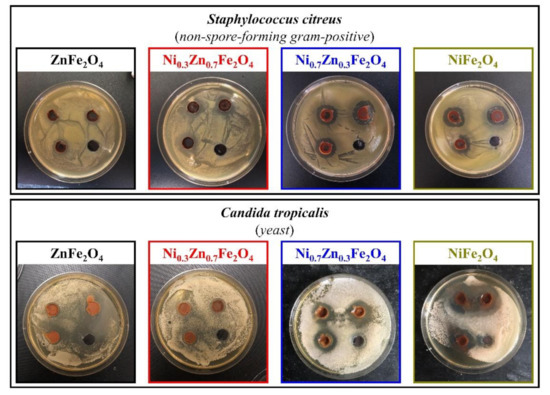
Figure 7.
Inhibition zone assay of the obtained NixZn1-xFe2O4 (x = 0, 0.3, 0.7, 1.0) ferrites toward S. citreus and C. tropicalis.
In the case of B. cereus, Ni0.3Zn0.7Fe2O4 (sample 2) and Ni0.7Zn0.3Fe2O4 (sample 3) showed approximately the same zones of inhibition, amounting to 15 ± 1 mm and 16 ± 1 mm, respectively; ZnFe2O4 (sample 1) showed the least bactericidal activity (zone of inhibition—13 mm), unlike NiFe2O4 (sample 4), which displayed the highest antibacterial activity toward B. cereus, producing an inhibition zone of 18 ± 1 mm (here and further control—10 mm).
Although all the studied ferrites showed the least significant differences to E. coli, as sample 1 has an inhibition zone of 14 ± 1 mm, samples 2 and 3 have an inhibition zone of 15 ± 1 mm, and sample 4 has an inhibition zone of17 ± 1 mm; however, they still demonstrate the noticeable antibacterial activity.
Furthermore, regarding S. citreus, the bactericidal activity of the synthesized ferrites is somewhat stronger than in the cases of the previously described cultures. The zones of inhibition of samples 1 and 2 turned out to be nearly equal, 21 ± 1 mm and 20 ± 1 mm, respectively, coupled with composition 3, which showed an inhibition zone of 23 ± 2 mm. On top of that, the inhibition zone of sample 4 was the largest and amounted to 26 ± 2 mm. The diameter of the zone of inhibition of all synthesized samples and their comparison with the literature data are presented in Table 2.

Table 2.
Elemental composition of synthesized ferrite samples.
The most noticeable differences in the inhibition of the growth of microorganisms in the studied ferrite samples were found against C. tropicalis: samples 1 and 2 showed approximately the same zones of inhibition (1st—18 ± 1 mm, 2nd—17 ± 1 mm), by contrast, in the third sample the zone of inhibition was up to 24 ± 2 mm, and in the fourth, it was 27 ± 2 mm.
It is known that even though an increase in the mass fraction of zinc increases the growth of the zone of inhibition to various test cultures, zinc ferrite itself does not have significant antibacterial activity, which is associated with the presence of a large amount of Fe3+ cations in its composition [65,66]. This is confirmed by the results obtained in this work, in which ZnFe2O4 demonstrated the worst antibacterial activity concerning all four selected test cultures (Table 3). In turn, nickel ferrite, due to the smallest particle size (~30 nm) and as a consequence of the highest specific surface area among all synthesized samples (~100 m2/g), has the most pronounced antibacterial properties. The importance of these parameters is associated with the peculiarities of the mechanism of interaction of ferrite particles with the cell of the studied microorganisms [67].

Table 3.
Comparison of inhibition zone of ferrites toward different test cultures.
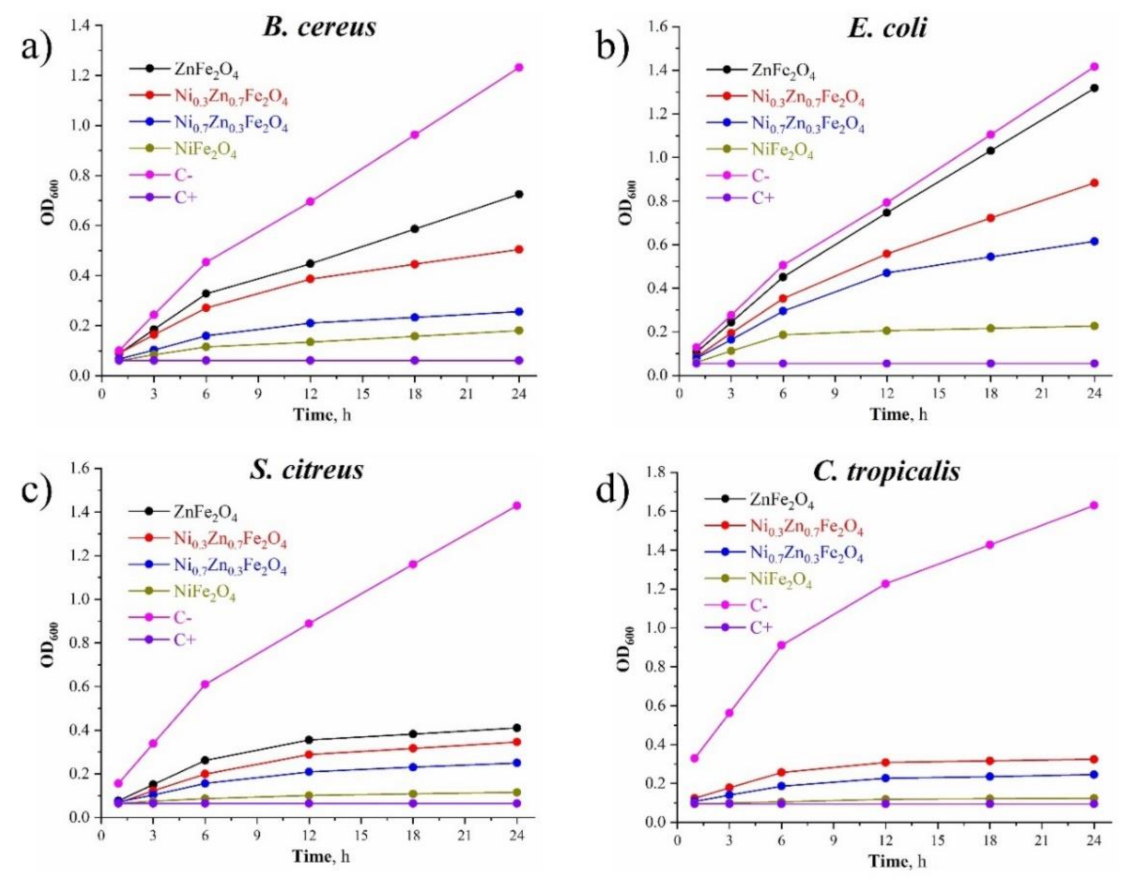
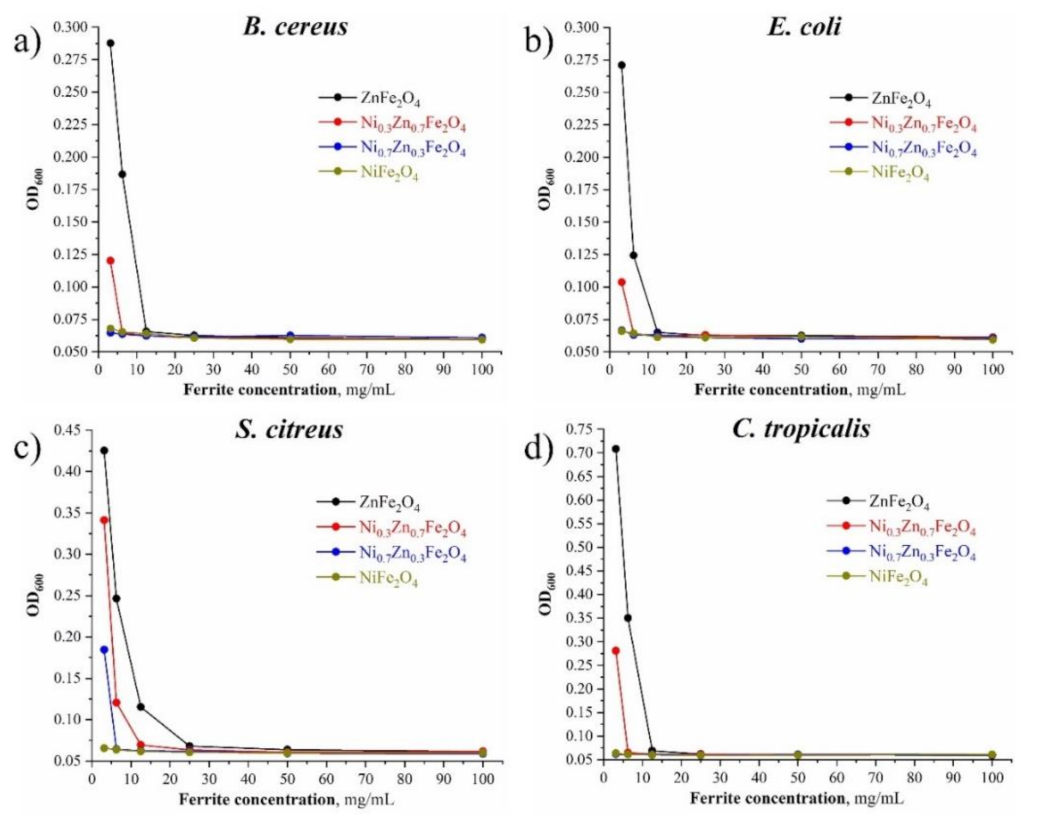
3.7. Growth Curve Assay
The effects of the materials on the growth curve of the selected test cultures are shown in Figure 8. It follows that the antimicrobial activity of the synthesized composites increased with the increase of Ni content: NiFe2O4 demonstrated the closest effect toward the positive control group when being tested on all the chosen cultures (both bacteria and yeast). Also, the antimicrobial performance of Ni0.7Zn0.3Fe2O4 was better than that of Ni0.3Zn0.7Fe2O4, and the antimicrobial performance of Ni0.3Zn0.7Fe2O4 was better than that of ZnFe2O4 due to the reduction of the nickel content in these composites, respectively, toward all the chosen test-cultures.
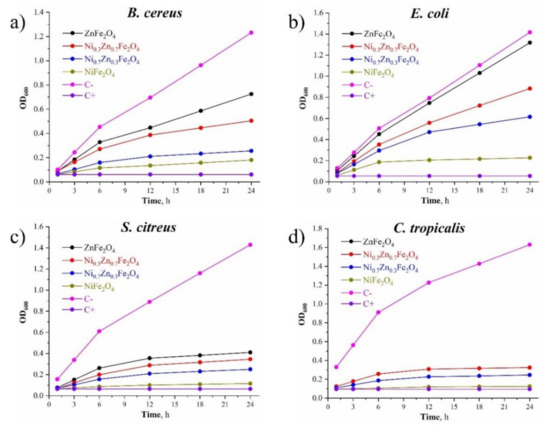
Figure 8.
(a) The effect of the obtained NixZn1-xFe2O4 (x = 0, 0.3, 0.7, 1.0) materials on the B. cereus growth curve; (b) the effect of the obtained NixZn1-xFe2O4 (x = 0, 0.3, 0.7, 1.0) materials on the E. coli growth curve; (c) the effect of the obtained NixZn1-xFe2O4 (x = 0, 0.3, 0.7, 1.0) materials on the S. citreus growth curve; (d) the effect of the obtained NixZn1-xFe2O4 (x = 0, 0.3, 0.7, 1.0) materials on the C. tropicalis growth curve.
3.8. MIC and MBC Assay
In determining the sensitivity of microorganisms to the obtained ferrites, 4 same test cultures were also used.
From the data obtained regarding B. cereus, E. coli, and C. tropicalis, the highest minimum inhibitory concentration (MIC) is shown by sample 2 (25 mg/mL), whereas using the first sample it was determined as 6.25 mg/mL and for samples 3 and 4, it was 3.125 mg/mL (Figure 9).
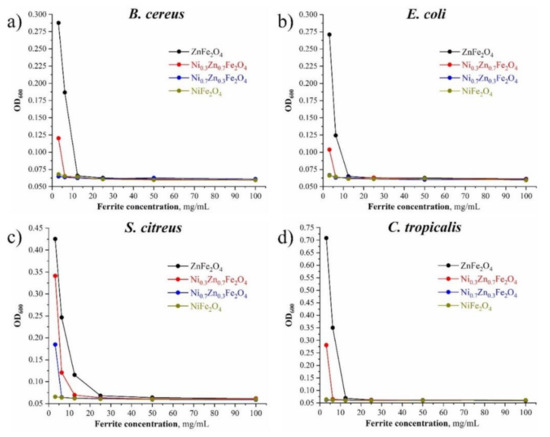
Figure 9.
(a) MIC image of the obtained NixZn1-xFe2O4 (x = 0, 0.3, 0.7, 1.0) composites for B. cereus; (b) MIC image of the obtained NixZn1-xFe2O4 (x = 0, 0.3, 0.7, 1.0) composites for E. coli; (c) MIC image of the obtained NixZn1-xFe2O4 (x = 0, 0.3, 0.7, 1.0) composites for S. citreus; (d) MIC image of the obtained NixZn1-xFe2O4 (x = 0, 0.3, 0.7, 1.0) composites for C. tropicalis.
As for S. citreus, it was determined that the sensitivity of this culture to the studied ferrites is slightly lower than that of the rest of the test cultures used, so the MIC of sample 2 came out to 25 mg/mL for samples 1, 3, and 4, 12.5, 6.25, and 3.125 mg/mL, respectively (Table 4).

Table 4.
The MBC of NixZn1−xFe2O4 (x = 0, 0.3, 0.7, 1.0) nanopowders.
The synthesized Ni-ferrites have successfully demonstrated the ability to inhibit the growth of various microorganisms. The increase of Ni content contributed to the enhancement of antimicrobial activity. If comparing with Li-ferrites studied previously in [68], it should be noted that the studied samples showed a significantly higher result of antimicrobial activity against S. citreus. It was also found that Ni-ferrites inhibit the growth of yeast, all samples successfully suppressed the growth of C. tropicalis culture.
4. Conclusions
Thus, in this work, we have shown the possibility of obtaining nanopowders of Ni-Zn ferrites with the composition NixZn1−xFe2O4 (x = 0, 0.3, 0.7, 1.0) by the method of two-stage synthesis using solution combustion, which display a significant antimicrobial activity against E. coli, B. cereus, S. citreus, and C. tropicalis. It was found that the largest diameter of the inhibition zone is exhibited by simple nickel ferrite powders with a specific surface area of ~90 m2/g. The most optimal from the point of possible biotechnological application and purification of natural water sources are ferrites of NiFe2O4 and Ni0.7Zn0.3Fe2O4 compositions. All synthesized samples exhibit typical ferrimagnetic properties and, therefore, can be used for magnetic separation.
Author Contributions
Conceptualization: K.D.M.; methodology: K.D.M. and V.I.P.; validation: I.B.P. and V.N.N.; formal analysis: A.D.B. and K.D.M.; investigation: A.D.B., D.D.S., I.D.B. and V.E.B.; resources: G.G.N. and V.N.N.; data curation: A.D.B., I.D.B., and I.B.P.; writing—original draft: K.D.M. and A.D.B. with contribution from G.G.N. and V.I.P.; writing—review and editing: G.G.N., D.D.S., and V.E.B.; visualization: I.B.P. with contribution from K.D.M. All authors have read and agreed to the published version of the manuscript.
Funding
The current research was funded by RFBR, project number 20-03-00976.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The study was partially performed on the equipment of the Engineering Center of Saint Petersburg State Institute of Technology.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ranga, R.; Kumar, A.; Kumari, P.; Singh, P.; Madaan, V.; Kumar, K. Ferrite application as an electrochemical sensor: A review. Mater. Charact. 2021, 178, 111269. [Google Scholar] [CrossRef]
- Abbas, N.; Rubab, N.; Sadiq, N.; Manzoor, S.; Khan, M.I.; Garcia, J.F.; Aragao, I.B.; Tariq, M.; Akhtar, Z.; Yasmin, G. Aluminum-doped cobalt ferrite as an efficient photocatalyst for the abatement of methylene blue. Water 2021, 12, 2285. [Google Scholar] [CrossRef]
- Thakur, P.; Taneja, S.; Chahar, D.; Ravelo, D.; Thakur, B. Recent advances on synthesis, characterization and high frequency applications of Ni-Zn ferrite nanoparticles. J. Magn. Magn. Mater. 2021, 530, 167925. [Google Scholar] [CrossRef]
- Kumar, E.R.; Jayaprakash, R.; Seehra, M.S.; Prakash, T.; Kumar, S. Effect of α-Fe2O3 phase on structural, magnetic and dielectric properties of Mn-Zn ferrite nanoparticles. J. Phys. Chem. Solids 2013, 74, 943–949. [Google Scholar] [CrossRef]
- Kumar, E.R.; Reddy, P.S.P.; Devi, G.S.; Sathiyaraj, S. Structural, dielectric and gas sensing behavior of Mn substituted spinel MFe2O4 (M = Zn, Cu, Ni, and Co) ferrite nanoparticles. J. Magn. Magn. Mater. 2016, 398, 281–288. [Google Scholar] [CrossRef]
- Ramadevi, P.; Kousi, F.; Sangeetha, A.; Gibson, M.S.M.; Shanmugavadivu, Ra. Structural and electrochemical investigation on pure and nickel doped cobalt ferrite nanoparticles for supercapacitor application. Mater. Today Proc. 2020, 33, 2238–2243. [Google Scholar] [CrossRef]
- Martinson, K.D.; Kozyritskaya, S.S.; Panteleev, I.B.; Popkov, V.I. Low coercivity ceramics based on LiZnMn ferrite synthesized via glycine-nitrate combustion. Nanosyst. Phys. Chem. Math. 2019, 10, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; He, Z.; Wang, Y.; Lu, M. Electrochemical/Peroxymonosulfate/NrGO-MnFe2O4 for advanced treatment of landfill leachate nanofiltration concentrate. Water 2021, 13, 413. [Google Scholar] [CrossRef]
- Deepty, M.; Srinivas, Ch.; Kumar, E.R.; Mohan, N.K.; Prajapat, C.L.; Rao, T.V.C.; Meena, S.S.; Verma, A.K.; Sastry, D.L. XRD, EDX, FTIR and ESR spectroscopic studies of co-precipitated Mn-substituted Zn-ferrite nanoparticles. Ceram. Int. 2019, 45, 8037–8044. [Google Scholar] [CrossRef]
- Sorescu, M.; Diamandescu, L.; Peelamedu, R.; Roy, R.; Yadoji, P. Structural and magnetic properties of NiZn ferrites prepared by microwave sintering. J. Magn. Magn. Mater. 2004, 279, 195–201. [Google Scholar] [CrossRef]
- Yoo, J.-E.; Kang, Y.-M. Electromagnetic wave absorbing properties of Ni-Zn ferrite powder-epoxy composites in GHz range. J. Magn. Magn. Mater. 2020, 513, 167075. [Google Scholar] [CrossRef]
- Martinson, K.D.; Ivanov, A.A.; Panteleev, I.B.; Popkov, V.I. Effect of sintering temperature on the synthesis of LiZnMnFe microwave ceramics with controllable electro/magnetic properties. Ceram. Int. 2021, 47, 30071–30081. [Google Scholar] [CrossRef]
- Borade, R.M.; Kale, S.B.; Tekale, S.U.; Jadhav, K.M.; Pawar, R.P. Cobalt ferrite magnetic nanoparticles as highly efficient catalyst for the mechanochemical synthesis of 2-aey; benzimidazoles. Catal. Commun. 2021, 159, 106349. [Google Scholar] [CrossRef]
- El-Hafiz, D.R.A.; Sakr, A.A.-E.; Ebiad, M.A. Methane Bi-reforming for direct ethanol production over smart Cu/Mn-ferrite catalyst. Renew. Energy 2021, 167, 236–247. [Google Scholar] [CrossRef]
- Sonu; Sharma, S.; Dutta, V.; Raizada, P.; Hosseini-Bandegharaei, A.; Thakur, V.; Nguyen, V.-H.; VanLe, Q.; Singh, P. An overview of heterojunctioned ZnFe2O4 photocatalyst for enhanced oxidative water purification. J. Environ. Chem. Eng. 2021, 9, 105812. [Google Scholar] [CrossRef]
- Park, C.M.; Kim, Y.M.; Kim, K.-H.; Wang, D.; Su, C.; Yoon, Y. Potential utility of graphene-based nano spinel ferrites as adsorbent and photocatalyst for removing organic/inorganic contaminants from aqueous solutions: A mini-review. Chemosphere. 2019, 221, 392–402. [Google Scholar] [CrossRef]
- Singh, S.; Singhal, S. Transition metal-doped cobalt ferrite nanoparticles: Efficient photocatalyst for photodegradation of textile dye. Mater. Today: Proc. 2019, 14, 453–460. [Google Scholar] [CrossRef]
- Bhosale, S.V.; Ekamble, P.S.; Bhoraskar, S.V.; Mathe, V.L. Effect of surface properties of NiFe2O4 nanoparticles synthesized by dc thermal plasma route on antimicrobial activity. Appl. Surf. Sci. 2018, 441, 724–733. [Google Scholar] [CrossRef]
- Ashour, A.H.; El-Batal, A.I.; Abdel Maksoud, M.I.A.; El-Sayyad, G.S.; Labib, Sh.; Abdeltwab, E.; El-Okr, M.M. Antimicrobial activity of metal-substituted cobalt ferrite nanoparticles synthesized by sol-gel technique. Particuology 2018, 40, 141–151. [Google Scholar] [CrossRef]
- Taguba, M.A.M.; Ong, D.C.; Ensano, B.M.B.; Kan, C.-C.; Grisdanurak, N.; Yee, J.-J.; de Luna, M.D.G. Nonlinear isotherm and kinetic modeling of Cu(II) and Pb(II) uptake from water by MnFe2O4/Chitosan nanoadsorbents. Water 2021, 13, 1662. [Google Scholar] [CrossRef]
- Mylarappa, M.; Lakshmi, V.V.; Mahesh, K.R.V.; Nagaswarupa, H.P.; Raghavendra, N. Recovery of Mn-Zn ferrite from waste batteries and development of rGO/Mn-Zn ferrite nanocomposite for water purification. Mater. Today Proc. 2019, 9, 256–265. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Yun, Y.-S. Spinel ferrite magnetic adsorbents: Alternative future materials for water purification? Coord. Chem. Rev. 2016, 315, 90–111. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Lyu, L.; Hu, C. Surface oxygen vacancy inducing peroxymonosulfate activation through electron donation of pollutants over cobalt-zinc ferrite for water purification. Appl. Catal. B Environ. 2020, 270, 118874. [Google Scholar] [CrossRef]
- Masunga, N.; Mmelesi, O.K.; Kefeni, K.K.; Mamba, B.B. Recent advances in copper ferrite nanoparticles and nanocomposites synthesis, magnetic properties and application in water treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103179. [Google Scholar] [CrossRef]
- Sharma, L.; Kakkar, R. Magnetically retrievable one-pot fabrication of mesoporous magnesium ferrite (MgFe2O4) for the remediation of chlorpyrifos and real pesticide wastewater. J. Environ. Chem. Eng. 2018, 6, 6891–6903. [Google Scholar] [CrossRef]
- Dobaradaran, S.; Nodehi, R.N.; Yaghmaeian, K.; Jaafari, J.; Niari, M.H.; Bharti, A.K.; Agarwal, S.; Gupta, V.K.; Azari, A.; Shariatifar, N. Catalytic decomposition of 2-chlorophenol using ultrasonic-assisted Fe3O4-TiO2@MWCNT systems: Influence factors, pathway and mechanism study. J. Colloid Interface Sci. 2018, 512, 172–189. [Google Scholar] [CrossRef] [Green Version]
- Simeonidis, K.; Mourdikoudis, S.; Kaprara, E.; Mitrakas, M.; Polavarapu, L. Inorganic engineered nanoparticles in drinking water treatment: A critical review. Environ. Sci. Water Res. Technol. 2016, 2, 43–70. [Google Scholar] [CrossRef] [Green Version]
- Bohara, R.A.; Throat, N.D.; Mulla, N.A.; Pawar, S.H. Surface-modified cobalt ferrite nanoparticles for rapid capture, detection, and removal of pathogens: A potential material for water purification. Appl. Biochem. Biotechnol. 2017, 182, 598–608. [Google Scholar] [CrossRef]
- Springer, V.; Barreiros, L.; Avena, M.; Segundo, M.A. Nickel ferrite nanoparticles for removal of polar pharmaceuticals from water samples with multi-purpose features. Adsorption 2018, 24, 431–441. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, Y.; Li, G.; Su, M.; Chen, X.; Zhang, H.; Zhang, Y.; Jiao, W.; He, Y.; Yi, J.; et al. Engineering ferrite nanoparticles with enhanced magnetic response for advanced biomedical applications. Mater. Today Adv. 2020, 8, 100119. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.A.M.; Nkambule, T.T.; Mamba, B.B. Spinel ferrite nanoparticles and nanocomposites for biomedical applications and their toxicity. Mater. Sci. Eng. C 2020, 107, 110314. [Google Scholar] [CrossRef] [PubMed]
- Thakur, P.; Chahar, D.; Taneja, S.; Bhalla, N.; Thakur, A. A review on MnZn ferrites: Synthesis, characterization and applications. Ceram. Int. 2020, 46, 15740–15763. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.S.; Amaral, F.; Graca, M.P.F.; Costa, L.C. Comparison of lithium ferrite powders prepared by sol-gel and solid-state reaction methods. Mater. Sci. Eng. B. 2020, 255, 114529. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Liu, B.; Wang, J.; Han, G.; Zhang, Y. Characterization and property of magnetic ferrite ceramics with interesting multilayer structure prepared by solid-state reaction. Ceram. Int. 2021, 47, 10927–10939. [Google Scholar] [CrossRef]
- Harris, V.G.; Sepelak, V. Mechanochemically processed zinc ferrite nanoparticles: Evolution of structure and impact of induced cation inversion. J. Magn. Magn. Mater. 2018, 465, 603–610. [Google Scholar] [CrossRef]
- Luo, J. Nanosize Ce3+ added MgCuZn ferrite powders prepared by mechanochemical process: Synthesis, characterization and magnetic properties. Mater. Lett. 2013, 98, 174–177. [Google Scholar] [CrossRef]
- Qin, H.; He, Y.; Xu, P.; Huang, D.; Wang, Z.; Wang, H.; Wang, Z.; Zhao, Y.; Tian, Q.; Wang, C. Spinel ferrites (MFe2O4): Synthesis, improvement and catalytic application in environment and energy field. Adv. Colloid Interface Sci. 2021, 294, 102486. [Google Scholar] [CrossRef]
- Tsay, C.-Y.; Chiu, Y.-C.; Tseng, Y.-K. Investigation on structure, magnetic, and FMR properties for hydrothermally-synthesized magnesium-zinc ferrite nanoparticles. Phys. B Condens. Matter. 2019, 570, 29–34. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, L.; Zhao, B.; Du, Y.; Zhou, X. Growth mechanism of octahedral-like nickel ferrite crystals prepared by modified hydrothermal method and morphology dependent magnetic performance. Ceram. Int. 2018, 44, 9809–9815. [Google Scholar] [CrossRef]
- Das, J.; Moholkar, V.S.; Chakma, S. Structural, magnetic and optical properties of sonochemically synthesized Zr-ferrite nanoparticles. Powder Technol. 2018, 328, 1–6. [Google Scholar] [CrossRef]
- Yadav, R.S.; Kuritka, I.; Vilcakova, J.; Jamatia, T.; Machovsky, M.; Skoda, D.; Urbanek, P.; Masar, M.; Urbanek, M.; Kalina, L.; et al. Impact of sonochemical synthesis condition on the structural and physical properties of MnFe2O4 spinel ferrite nanoparticles. Ultrason. Sonochemistry 2020, 61, 104839. [Google Scholar] [CrossRef]
- Berezhnaya, M.V.; Mittova, I.Ya.; Perov, N.S.; Al’myasheva, O.V.; Nguyen, A.T.; Mittova, V.O.; Bessalova, V.V.; Viryutina, E.L. Production of zinc-doped yttrium ferrite nanopowders by the sol-gel method. Russ. J. Inorg. Chem. 2018, 63, 742–746. [Google Scholar] [CrossRef]
- De-Leon-Prado, L.E.; Cortes-Hernandez, D.A.; Almanza-Robles, J.M.; Escobedo-Bocardo, J.C.; Sanchez, J.; Reyes-Rdz, P.Y.; Jasso-Teran, R.A.; Hurtado-Lopez, G.F. Synthesis and characterization of nanosized MgxMn1-xFe2O4 ferrites by both sol-gel and thermal decomposition methods. J. Magn. Magn. Mater. 2017, 427, 230–234. [Google Scholar] [CrossRef]
- Martinson, K.D.; Panteleev, I.B.; Shevchik, A.P.; Popkov, V.I. Effect of Red/Ox ratio on the structure and magnetic behavior of Li0.5Fe2.5O4 nanocrystals synthesized by solution combustion approach. Lett. Mater. 2019, 9, 475–479. [Google Scholar] [CrossRef] [Green Version]
- Popkov, V.I.; Almjasheva, O.V.; Nevedomskiy, V.N.; Panchuk, V.V.; Semenov, V.G.; Gusarov, V.V. Effect of spatial constraints on the phase evolution of YFeO3-based nanopowders under heat treatment of glycine-nitrate combustion products. Ceram. Int. 2018, 44, 20906–20912. [Google Scholar] [CrossRef]
- Amiri, M.; Salavati-Niasari, M.; Akbari, A. Magnetic nanocarriers: Evolution of spinel ferrites for medical applications. Adv. Colloid Interface Sci. 2019, 265, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Martinson, K.D.; Cherepkova, I.A.; Panteleev, I.B.; Popkov, V.I. Single-step solution-combustion synthesis of magnetically soft NiFe2O4 nanopowders with controllable parameters. Int. J. Self-Propagating High-Temp. Synth. 2019, 28, 266–270. [Google Scholar] [CrossRef]
- Aruna, S.T.; Mukasyan, A.S. Combustion synthesis and nanomaterials. Curr. Opin. Solid State Mater. Sci. 2008, 12, 44–50. [Google Scholar] [CrossRef]
- Popkov, V.I.; Martinson, K.D.; Kondrashkova, I.S.; Enikeeva, M.O.; Nevedomskiy, V.N.; Panchuk, V.V.; Semenov, V.G.; Volkov, M.P.; Pleshakov, I.V. SCS-assisted production of EuFeO3 core-shell nanoparticles: Formation process, structural features and magnetic behavior. J. Alloy. Compd. 2021, 859, 157812. [Google Scholar] [CrossRef]
- Tikhanova, S.M.; Lebedev, L.A.; Martinson, K.D.; Chebanenko, M.I.; Buryanenko, I.V.; Semenov, V.G.; Nevedomskiy, V.N.; Popkov, V.I. The synthesis of novel heterojunction h-YbFeO3/o-YbFeO3 photocatalyst with enhanced Fenton-like activity under visible light. New J. Chem. 2021, 45, 1541–1550. [Google Scholar] [CrossRef]
- Martinson, K.D.; Ivanov, A.A.; Panteleev, I.B.; Popkov, V.I. Pre-ceramic nanostructured LiZnMn-ferrite powders: Synthesis, structure, and electromagnetic properties. Glas. Ceram. 2020, 77, 215–220. [Google Scholar] [CrossRef]
- Martinson, K.D.; Sakhno, D.D.; Belyak, V.E.; Kondrashkova, I.S. Ni0.4Zn0.6Fe2O4 nanopowders by solution-combustion synthesis: Influence of Red/Ox ratio on their morphology, structure and magnetic properties. Int. J. Self-Propagating High-Temp. Synth. 2020, 29, 202–207. [Google Scholar] [CrossRef]
- Kazin, A.P.; Rumyantseva, M.N.; Prusakov, V.E.; Suzdalev, I.P.; Gaskov, A.M. Cation distribution in nanocrystalline NixZn1-xFe2O4 spinel ferrites. Inorg. Mater. 2012, 48, 525–530. [Google Scholar] [CrossRef]
- Ghosh, B.; Sardar, M.; Banerjee, S. Effect of antisite formation on magnetic properties of nickel-zinc ferrite particles. J. Appl. Phys. 2013, 114, 183903. [Google Scholar] [CrossRef]
- El-Sayed, A.M. Influence of zinc content on some properties of Ni-Zn ferrites. Ceram. Int. 2002, 28, 363–367. [Google Scholar] [CrossRef]
- Srinivas, Ch.; Tirupanyam, B.V.; Meena, S.S.; Yusuf, S.M.; Babu, Ch.S.; Ramakrishna, K.S.; Potukuchi, D.M.; Sastry, D.L. Structural and magnetic characterization of co-precipitated NixZn1-xFe2O4 ferrite nanoparticles. J. Magn. Magn. Mater. 2016, 407, 135–141. [Google Scholar] [CrossRef]
- Varma, A.; Mukasyan, A.S.; Rogachev, A.S.; Manukyan, K.V. Solution combustion synthesis of nanoscale materials. Chem. Rev. 2016, 116, 14493–14586. [Google Scholar] [CrossRef]
- Martinson, K.D.; Cherepkova, I.A.; Sokolov, V.V. Formation of cobalt ferrite nanoparticles during the burning of glycine-nitrate and their magnetic properties. Glas. Phys. Chem. 2018, 44, 21–25. [Google Scholar] [CrossRef]
- Zawadzka, K.; Kadziota, K.; Felczak, A.; Wronska, N.; Piwonska, I.; Kisielewska, A.; Lisowska, K. Surface area or diameter—which factor really determines the antibacterial activity of silver nanoparticles grown on TiO2 coatings? New J. Chem. 2014, 38, 3275–3281. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, O. Influence of particle size on the antibacterial activity of zinc oxide. Int. J. Inorg. Mater. 2001, 3, 643–646. [Google Scholar] [CrossRef]
- Bedel, N.S.; Tezcan, M.; Ceylan, O.; Gurdag, G.; Cicek, H. Effects of pore morphology and size on antimicrobial activity of chitosan/poly(ethylene glycol) diacrylate macromer semi-IPN hydrogels. J. Appl. Polym. Sci. 2015, 132, 42707. [Google Scholar] [CrossRef]
- Allgayer, R.; Yousefi, N.; Tufenkji, N. Graphene oxide sponge as adsorbent for organic contaminants: Comparison with granular activated carbon and influence of water chemistry. Environ. Sci. Nano. 2020, 7, 2669–2680. [Google Scholar] [CrossRef]
- Massoudi, J.; Smari, M.; Khirouni, K.; Dhahri, E.; Bessais, L. Impact of particle size on the structural and magnetic properties of superparamagnetic Li-ferrite nanoparticles. J. Magn. Magn. Mater. 2021, 528, 167806. [Google Scholar] [CrossRef]
- Peddis, D.; Cannas, C.; Musini, A.; Ardu, A.; Orru, F.; Fiorani, D.; Laureti, S.; Rinaldi, D.; Muscas, G.; Concas, G.; et al. Beyond the effect of particle size: Influence of CoFe2O4 nanoparticle arrangements on magnetic properties. Chem. Mater. 2013, 25, 2005–2013. [Google Scholar] [CrossRef]
- Ishaq, K.; Saka, A.A.; Kamardeen, A.O.; Ahmed, A.; Alhassan, M.I.; Abdullahi, H. Characterization and antibacterial activity of nickel doped a-alumina nanoparticles. Eng. Sci. Technol. Int. J. 2017, 20, 563–569. [Google Scholar] [CrossRef]
- Maksoud, M.I.A.A.; El-Sayyad, G.S.; Abokhadra, A.; Soliman, L.I.; El-Bahnasawy, H.H.; Ashour, A.H. Influence of Mg2+ substitution on structural, optical, magnetic, and antimicrobial properties of Mn-Zn ferrite nanoparticles. J. Mater. Sci. Mater. Electron. 2020, 31, 2598–2616. [Google Scholar] [CrossRef]
- Gheidari, D.; Mehrdad, M.; Maleki, S.; Samanesadat, H. Synthesis and potent antimicrobial activity of CoFe2O4 nanoparticles under visible light. Heliyon 2020, 6, 05058. [Google Scholar] [CrossRef]
- Martinson, K.D.; Beliaeva, A.D.; Nianikova, G.G.; Panteleev, I.B. SCS-assisted preparation of magnetically soft Li and LiZnMn ferrite nanopowders with enhanced bioavailability and bioactivity suitable for use in food technology. IOP Conf. Ser. Earth Environ. Sci. 2021, 723, 032107. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).