The Diversity of Phytoplankton in a Combined Rice-Shrimp Farming System in the Coastal Area of the Vietnamese Mekong Delta
Abstract
:1. Introduction
2. Materials and Methods
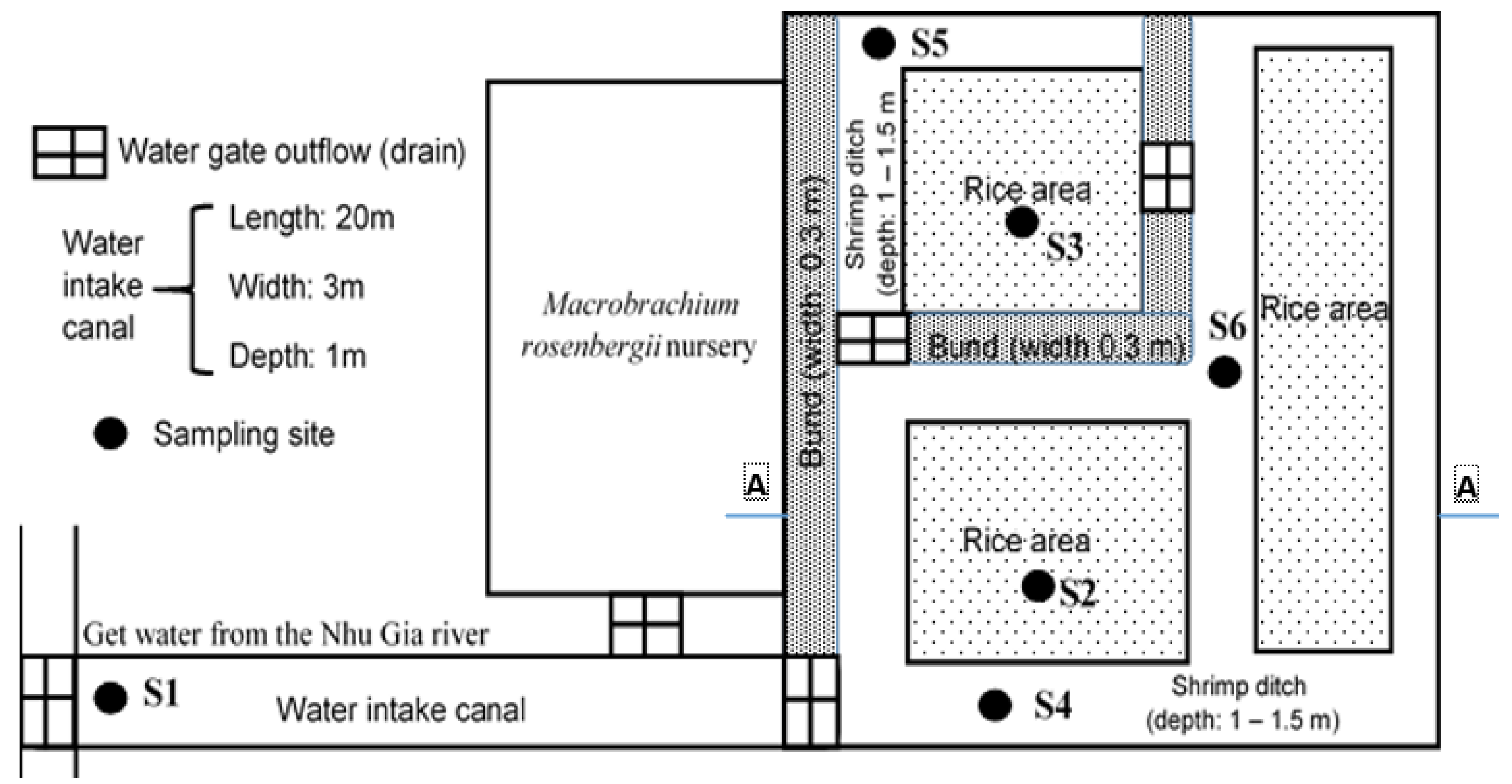
2.1. Description of the Rice-Shrimp System
2.2. Phytoplankton Sampling and Analysis
2.3. Statistical Analyses and Diversity Indices
3. Results
3.1. Composition of Phytoplankton
3.2. Density of Phytoplankton
3.3. Shannon–Wiener Diversity Index (H′)
3.4. Identification of Dominant Phytoplankton Species
4. Discussion
4.1. Composition of Phytoplankton
4.2. Density of Phytoplankton
4.3. Shannon–Wiener Diversity Index (H′)
4.4. Identification of Dominant Phytoplankton Species
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- General Statistics Office of Viet Nam. 2016. Available online: https://www.gso.gov.vn/wp-content/uploads/2019/10/Nien-giam-Thong-ke-2015-1.pdf (accessed on 27 December 2021).
- General Statistics Office of Viet Nam. 2021. Available online: https://www.gso.gov.vn/wp-content/uploads/2021/07/Sach-NGTK-2020Ban-quyen.pdf (accessed on 27 December 2021).
- New, M. Farming Freshwater Prawns: A Manual for the Culture of the Giant River Prawn (Macrobrachium rosenbergii); FAO Fisheries Technical Paper; FAO: Rome, Italy, 2002; Volume 428, 212p. [Google Scholar]
- Tan, L.V.; Thu, T.A.; Long, L.P.; Anh, V.T.V.; Guong, V.T. Some water characteristics of cultivation models on saline soils in Thanh Phu District, Ben Tre Province. J. Agric. Rural. Dev. 2016, 19, 70–78. (In Vietnamese) [Google Scholar]
- DoA. National Action Plan for Shrimp Industry Development in Vietnam until 2025; Vietnam Department of Aquaculture: Ha Noi, Vietnam, 2018. (In Vietnamese) [Google Scholar]
- RIA2-Research Institute for Aquaculture No. 2. Proposal on Giant Freshwater Shrimp Production and Export Development; Ministry of Agriculture and Rural Development: Ho Chi Minh City, Vietnam, 2019. (In Vietnamese) [Google Scholar]
- Hoa, N.M. Characteristics of soil and water in shrimp-rice and shrimp-upland crops systems in saline-affected Acid Sulphate Soils at Hau Giang province in Vietnam. Part I: Characteristics of pond water. Can Tho Univ. J. Sci. 2010, 16, 80–87. (In Vietnamese) [Google Scholar]
- Ha, V.V.; Phuong, T.L.; Linh, H.C.; Tuan, T.H. Assessment of technical and economic efficiency of land-based shrimp production in My Xuyen district, Soc Trang province. Can Tho Univ. J. Sci. 2014, 46, 70–79. (In Vietnamese) [Google Scholar]
- Huy, N.A.; Hiep, N.H. Efficacy of halophillic bacteria, Burkholderia sp. PL9 and Acinetobacter sp. GH1-1, on the growth and yield of rice cultivar LP5 grown on salt affected soil of rice shrimp farming system in My Xuyen district, Soc Trang province. Can Tho Univ. J. Sci. 2019, 55, 24–30. (In Vietnamese) [Google Scholar]
- Li, X.; Yu, H.; Wang, H.; Ma, C. Phytoplankton community structure in relation to environmental factors and ecological assessment of water quality in the upper reaches of the Genhe River in the Greater Hinggan Mountains. Environ. Sci. Pollut. Res. 2019, 26, 17512–17519. [Google Scholar] [CrossRef]
- Tuyen, N.V. Biodiversity in Algae in Vietnam’s Inland Waters-Prospects and Challenges; Agriculture Publishing House: Ha Noi, Vietnam, 2003. (In Vietnamese) [Google Scholar]
- Spence, D.H.N. Factors controlling the distribution of freshwater macrophytes with particular reference to the lochs of Scotland. J. Ecol. 1967, 55, 147–170. [Google Scholar] [CrossRef]
- Seddon, B. Aquatic macrophytes as limnological indicators. Freshw. Biol. 1972, 2, 107–130. [Google Scholar] [CrossRef]
- Leigh, C.; Koster, B.S.; Sang, N.V.; Truc, L.V.; Hiep, L.H.; Xoan, V.B.; Tinh, N.T.N.; An, L.T.; Sammut, J.; Burford, M.A. Rice-shrimp ecosystems in the Mekong Delta: Linking water quality, shrimp and their natural food sources. Sci. Total Environ. 2020, 739, 139931. [Google Scholar] [CrossRef] [PubMed]
- Tien, D.D.; Hanh, V. Freshwater Algae in Vietnam-Classification of Green Algae; Agricultural Publishing House: Ha Noi, Vietnam, 1997; 503p. (In Vietnamese) [Google Scholar]
- Ho, P.H. Algae; Sai Gon Publishing House: Sai Gon City, Vietnam, 1972. (In Vietnamese) [Google Scholar]
- Reynolds, C.S. Ecology of Phytoplankton; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Suthers, I.M.; Rissik, D. Plankton: A Guide to Their Ecology and Monitoring for Water Quality; Commonwealth Scientific and Industrial Research Organisation (CSIRO), CSIRO Publishing: Melbourne, Australia, 2009; 272p. [Google Scholar]
- Soininen, J.; Korhonen, J.J.; Karhu, J.; Vetterli, A. Disentangling the spatial patterns in community composition of prokaryotic andeukaryotic lake plankton. Limnol. Oceanogr. 2011, 56, 508–520. [Google Scholar] [CrossRef]
- Lv, H.; Yang, J.; Liu, L.; Yu, X.; Yu, Z.; Chiang, P. Temperature and nutrients are significant drivers of seasonal shift in phytoplankton community from a drinking water reservoir, subtropical China. Environ. Sci. Pollut. Res. 2014, 21, 5917–5928. [Google Scholar] [CrossRef]
- Nguyen, T.G.; Truong, H.D. Interrelation of phytoplankton and water quality at Bung Binh Thien reservoir, An Giang province, Vietnam. Indones. J. Environ. Manag. Sustain. 2020, 4, 110–115. [Google Scholar]
- Shannon-Wiener. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. Changes in Marine Communities: An Approach to Statistical Analyses and Interpretation; Natural Environment Research Council: Plymouth, UK, 1994. [Google Scholar]
- Feher, I.C.; Zaharie, M.; Oprean, I. Spatial and seasonal variation of organic pollutants in surface water using multivariate statistical techniques. Water Sci. Technol. 2016, 74, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- Giao, N.T. Evaluating the current water quality monitoring system on Hau River, Mekong delta, Vietnam using multivariate statistical technique. Appl. Environ. Res. 2020, 42, 14–25. [Google Scholar]
- Bellinger, E.G.; Sigee, D.C. Freshwater Algae: Identification and Use as Bioindicators; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; 265p. [Google Scholar]
- Ziglio, G.; Siligardi, M.; Flaim, G. Biological Monitoring of Rivers: Applications and Perspectives; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2016; 472p. [Google Scholar]
- Cat, L.V.; Nhung, D.T.H.; Cat, N.N. Aquaculture Water, Water Quality and Solutions to Improve Water Quality; Scientific and Technical Publishing House: Ha Noi, Vietnam, 2006. (In Vietnamese) [Google Scholar]
- Dien, L.D.; Sang, N.V.; Faggotter, S.J.; Chen, C.; Huang, J.; Teasdale, P.R.; Sammut, J.; Burford, M.A. Seasonal nutrient cycling in integrated rice-shrimp ponds. Mar. Pollut. Bull. 2019, 149, 110647. [Google Scholar] [CrossRef]
- Koekemoer, L.; Vuuren, S.J.; Levanets, A. The influence of land use-impacted tributaries on water quality and phytoplankton in the Mooi River, North West Province, South Africa. Bothalia 2021, 51, 1–22. [Google Scholar] [CrossRef]
- Quynh, D.T.T. Survey on the water bio-chemical parameters of shrimp-rice system environment in Phu Thuan commune, Thoai Son district and an Giang province. Sci. Rep. 2009, 36, 34–38. [Google Scholar]
- Nga, B.T. Assess the Situation and Build Models to Improve Household Livelihoods in Areas Affected by Saline Intrusion and Climate Change in Hau Giang Province; Scientific Report; Department of Science and Technology of Hau Giang Province: Hau Giang City, Vietnam, 2019; 131p. (In Vietnamese) [Google Scholar]
- Le, T.H.; Nguyen, T.L.; Bui, T.H. The relationships between environmental factors and phytoplankton diversity indices in some estuarine ecosystems of Vietnam. VNU J. Sci. Nat. Sci. Technol. 2016, 32, 33–38. [Google Scholar]
- Giao, N.T. Evaluating the possible use of phytoplankton and zoobenthos for water quality assessment: A case study at Bung Binh Thien reservoir, An Giang province, Viet Nam. Sci. J. Tra Vinh Univ. 2019, 36, 39–50. [Google Scholar]
- Le, T.T.; Phan, D.D.; Huynh, B.D.K.; Le, V.T.; Nguyen, V.T. Phytoplankton diversity and its relation to the physicochemical parameters in main water bodies of Vinh Long province, Vietnam. J. Vietnam. Environ. 2019, 11, 83–90. [Google Scholar] [CrossRef]
- Reynolds, C.S. Phytoplankton periodicity: The interactions of form, function and environmental variability. Freshw. Biol. 1984, 14, 111–142. [Google Scholar] [CrossRef]
- Hao, N.V. Some Technical Issues of Industrial Tiger Shrimp Farming; Agricultural Publishing: House Ho Chi Minh City, Vietnam, 2002; 210p. (In Vietnamese) [Google Scholar]
- Mai, H.T.B. Fluctuations in Species Composition and Number of Floating Plants in Tiger Shrimp Ponds in Khanh Hoa, Aquaculture. Ph.D. Thesis, Nha Trang University, Nha Trang City, Vietnam, 2015. (In Vietnamese). [Google Scholar]
- Ut, V.N.; Oanh, D.H. Curriculum on Aquatic Plants and Animals; Can Tho University Publishing House: Can Tho City, Vietnam, 2013; 324p. (In Vietnamese) [Google Scholar]
- Thang, N.V. Giant Freshwater Shrimp Farming Techniques; Agricultural Publishing House: Ho Chi Minh City, Vietnam, 1995. (In Vietnamese) [Google Scholar]
- Hai, T.N.; Hien, T.T.T.; Tam, D.H.; Toa, V.T.; Phuong, N.T.; Marcy, M.N. Culture of freshwater prawns (Macrobrachium rosenbergii) in rice-fields using hatchery reared postlarvae in Tam Binh district, Vinh Long province. In Proceedings of the 2001 Annual Workshop of JIRCAS Mekong Delta Project, Can Tho University, Can Tho City, Vietnam, 27–29 November 2001. (In Vietnamese). [Google Scholar]
- Tuan, N.A.; Long, D.N.; Viet, L.Q. Experimental culture of giant freshwater prawn (Macrobrachium Rosenbergii De Man, 1897) at different stocking densities in earthen ponds. Can Tho Univ. J. Sci. 2004, 1, 95–104. (In Vietnamese) [Google Scholar]
- Luu, P.T.; Thai, T.T.; Yen, N.T.M.; Quang, N.X. Phytoplankton community in integrated shrimp-mangrove farming ponds in Ca Mau province. In Proceedings of the The 7th National Science Conference on Ecology and Biological Resources, Hangzhou, China, 19–22 October 2015; pp. 793–900. (In Vietnamese). [Google Scholar]
- Boyd, C.E.; Tucker, C.S. Water Quality and Pond Soil Analyses for Aquaculture; Alabama Agricultural Experiment Station, Auburn University: Auburn, AL, USA, 1992; 188p. [Google Scholar]
- Mai, H.T.B.; Chau, L.H.B.; Khoa, N.D.T. Composition and cell density of microalgae in the “eco-shrimp” ponds at Nam Can and Ngoc Hien distric, Ca Mau province. J. Aquat. Sci. Technol. 2010, 3, 35–40. [Google Scholar]
- Wu, J.T. Phytoplankton as bioindicator for water quality in Taipei. Bot. Bull. Acad. Sinica 1984, 25, 205–214. [Google Scholar]




| Sampling Interval | Site | Bacillariophyta | Cyanophyta | Chlorophyta | Euglenophyta | Pyrrophyta |
|---|---|---|---|---|---|---|
| 1st | S1 | 3 | 10 | 11 | 6 | 0 |
| S2 | 2 | 14 | 10 | 5 | 0 | |
| S3 | 5 | 10 | 9 | 9 | 0 | |
| S4 | 2 | 12 | 9 | 4 | 0 | |
| S5 | 5 | 13 | 9 | 7 | 0 | |
| S6 | 4 | 13 | 10 | 5 | 0 | |
| 2nd | S1 | 4 | 15 | 12 | 13 | 0 |
| S2 | 4 | 12 | 14 | 7 | 2 | |
| S3 | 5 | 15 | 13 | 10 | 0 | |
| S4 | 6 | 14 | 16 | 10 | 0 | |
| S5 | 2 | 13 | 14 | 7 | 0 | |
| S6 | 6 | 15 | 17 | 9 | 1 | |
| 3rd | S1 | 13 | 17 | 21 | 13 | 2 |
| S2 | 3 | 13 | 15 | 9 | 2 | |
| S3 | 10 | 13 | 15 | 9 | 2 | |
| S4 | 5 | 13 | 17 | 9 | 2 | |
| S5 | 9 | 12 | 16 | 7 | 2 | |
| S6 | 6 | 13 | 18 | 7 | 2 | |
| 4th | S1 | 4 | 9 | 13 | 12 | 2 |
| S2 | 3 | 8 | 10 | 8 | 1 | |
| S3 | 2 | 5 | 10 | 9 | 2 | |
| S4 | 4 | 10 | 11 | 9 | 1 | |
| S5 | 3 | 5 | 14 | 10 | 1 | |
| S6 | 4 | 10 | 12 | 11 | 1 | |
| 5th | S1 | 11 | 13 | 11 | 9 | 1 |
| S2 | n/a | n/a | n/a | n/a | n/a | |
| S3 | n/a | n/a | n/a | n/a | n/a | |
| S4 | 8 | 10 | 10 | 9 | 1 | |
| S5 | 5 | 7 | 8 | 7 | 1 | |
| S6 | 8 | 9 | 10 | 11 | 1 | |
| 6th | S1 | 8 | 5 | 9 | 8 | 1 |
| S2 | n/a | n/a | n/a | n/a | n/a | |
| S3 | n/a | n/a | n/a | n/a | n/a | |
| S4 | 6 | 6 | 9 | 12 | 1 | |
| S5 | 5 | 3 | 4 | 3 | 1 | |
| S6 | 7 | 5 | 10 | 17 | 1 |
| Sampling Interval | S1 | S2 | S3 | S4 | S5 | S6 | Water Quality |
|---|---|---|---|---|---|---|---|
| 1st | 2.9 | 2.6 | 2.8 | 2.7 | 2.9 | 2.9 | Medium |
| 2nd | 3.3 | 2.9 | 2.7 | 3.1 | 3.0 | 2.7 | Medium–Good |
| 3rd | 3.2 | 2.7 | 2.6 | 2.9 | 2.8 | 2.7 | Medium–Good |
| 4th | 2.0 | 2.2 | 2.4 | 1.9 | 2.4 | 2.0 | Bad–Medium |
| 5th | 3.3 | – | – | 3.2 | 2.9 | 3.3 | Good |
| 6th | 2.6 | – | – | 2.8 | 2.0 | 2.8 | Medium |
| Phyla and Species | Start of Crop Cycle | Mid-Crop Cycle | End of Crop Cycle | |||
|---|---|---|---|---|---|---|
| Av. Ab | Contrib% | Av. Ab | Contrib% | Av. Ab | Contrib% | |
| Euglenophyta | ||||||
| Euglena nhrenbergii | – | – | – | – | 399 | 5 |
| Phacus lnsmorensis | – | – | – | – | 701 | 7.92 |
| Chlorophyta | ||||||
| Actinastnum hantzschii | 147.5 | 5.64 | 1189 | 10.7 | – | – |
| Closterinm closterioides | – | – | – | – | 565 | 5.99 |
| Closterinm setaceum | – | – | 3685 | 12.9 | – | – |
| Eudorina elegans | 339.5 | 7.53 | – | – | – | – |
| Pandorina morum | 975.5 | 16.33 | – | – | – | – |
| Pediastrnm duplex | – | – | 2060 | 17.1 | – | – |
| Pediastrum simplex | 487.5 | 15.74 | – | – | – | – |
| Scenedesnus acuminatus | – | – | 2016 | 18.9 | 950 | 14.58 |
| Scenedesnus quadricauda | – | – | 2474 | 20.7 | 1233.5 | 20 |
| Staurastrum arcuatum | – | – | – | – | 551 | 6.8 |
| Cyanophyta | ||||||
| Anabaena circinalis | 378 | 14.75 | – | – | – | – |
| Oscillatoria limosa | 245 | 8.21 | – | – | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, N.D.G.; Giao, N.T.; Nguyen, M.N.; Downes, N.K.; Ngan, N.V.C.; Anh, L.H.H.; Trung, N.H. The Diversity of Phytoplankton in a Combined Rice-Shrimp Farming System in the Coastal Area of the Vietnamese Mekong Delta. Water 2022, 14, 487. https://doi.org/10.3390/w14030487
Nam NDG, Giao NT, Nguyen MN, Downes NK, Ngan NVC, Anh LHH, Trung NH. The Diversity of Phytoplankton in a Combined Rice-Shrimp Farming System in the Coastal Area of the Vietnamese Mekong Delta. Water. 2022; 14(3):487. https://doi.org/10.3390/w14030487
Chicago/Turabian StyleNam, Nguyen Dinh Giang, Nguyen Thanh Giao, Minh N. Nguyen, Nigel K. Downes, Nguyen Vo Chau Ngan, Le Hoang Hai Anh, and Nguyen Hieu Trung. 2022. "The Diversity of Phytoplankton in a Combined Rice-Shrimp Farming System in the Coastal Area of the Vietnamese Mekong Delta" Water 14, no. 3: 487. https://doi.org/10.3390/w14030487
APA StyleNam, N. D. G., Giao, N. T., Nguyen, M. N., Downes, N. K., Ngan, N. V. C., Anh, L. H. H., & Trung, N. H. (2022). The Diversity of Phytoplankton in a Combined Rice-Shrimp Farming System in the Coastal Area of the Vietnamese Mekong Delta. Water, 14(3), 487. https://doi.org/10.3390/w14030487








