Assessment of Spatio-Temporal Variability of Faecal Pollution along Coastal Waters during and after Rainfall Events
Abstract
:1. Introduction
2. Materials and Methods
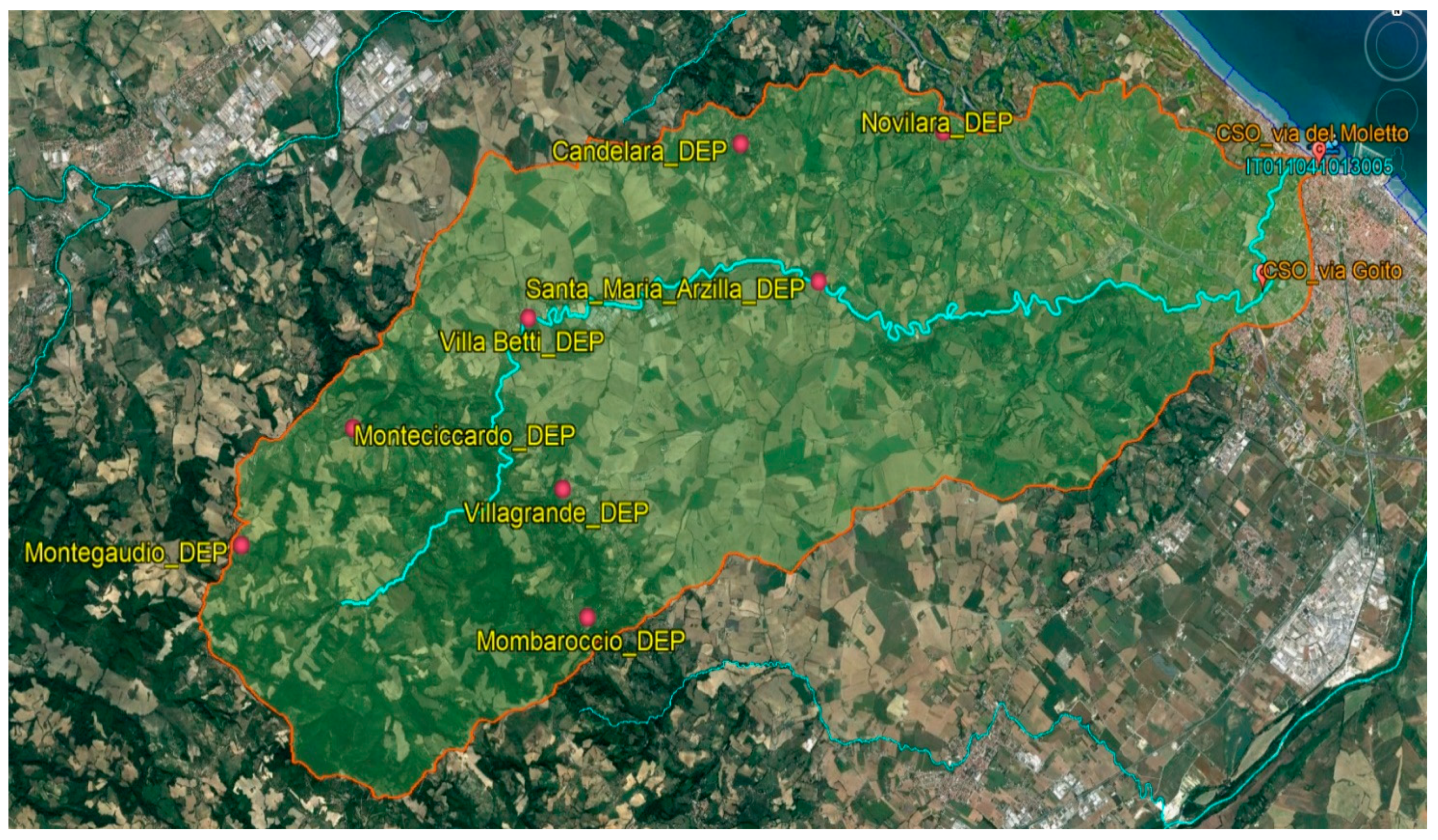
2.1. Study Area and Sampling Strategy
2.2. Environmental Variable Analysis
2.3. Microbiological Analysis
2.4. Statistical Analysis
3. Results
Spatial and Temporal Variability of the Environmental Parameters and Faecal Bacteria Distribution in the Arzilla River and Bathing Water
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McLellan, S.L.; Eren, A.M. Discovering new indicators of fecal pollution. Trends Microbiol. 2014, 22, 697–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newton, R.J.; Bootsma, M.J.; Morrison, H.G.; Sogin, M.L.; McLellan, S.L. A Microbial Signature Approach to Identify Fecal Pollution in the Waters Off an Urbanized Coast of Lake Michigan. Microb. Ecol. 2013, 65, 1011–1023. [Google Scholar] [CrossRef]
- Oliver, D.M.; Hanley, N.D.; van Niekerk, M.; Kay, D.; Heathwaite, A.L.; Rabinovici, S.J.M.; Kinzelman, J.L.; Fleming, L.E.; Porter, J.; Shaikh, S.; et al. Molecular tools for bathing water assessment in Europe: Balancing social science research with a rapidly developing environmental science evidence-base. Ambio 2016, 45, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Luna, G.M.; Manini, E.; Turk, V.; Tinta, T.; D’Errico, G.; Baldrighi, E.; Baljak, V.; Buda, D.; Cabrini, M.; Campanelli, A.; et al. Status of faecal pollution in ports: A basin-wide investigation in the Adriatic Sea. Mar. Pollut. Bull. 2019, 147, 219–228. [Google Scholar] [CrossRef]
- Barco, J.; Papiri, S.; Stenstrom, M.K. First flush in a combined sewer system. Chemosphere 2008, 71, 827–833. [Google Scholar] [CrossRef]
- Al Aukidy, M.; Verlicchi, P. Contributions of combined sewer overflows and treated effluents to the bacterial load released into a coastal area. Sci. Total Environ. 2017, 607–608, 483–496. [Google Scholar] [CrossRef]
- He, Y.; He, Y.; Sen, B.; Li, H.; Li, J.; Zhang, Y.; Zhang, J.; Jiang, S.C.; Wang, G. Storm runoff differentially influences the nutrient concentrations and microbial contamination at two distinct beaches in northern China. Sci. Total Environ. 2019, 663, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Mongruel, R.; Vanhoutte-Brunier, A.; Fiandrino, A.; Valette, F.; Ballé-Béganton, J.; Pérez Agúndez, J.A.; Gallai, N.; Derolez, V.; Roussel, S.; Lample, M.; et al. Why, How, and how far should microbiological contamination in a coastal zone be mitigated? An application of the systems approach to the Thau lagoon (France). J. Environ. Manag. 2013, 118, 55–71. [Google Scholar] [CrossRef] [Green Version]
- Grilli, F.; Accoroni, S.; Acri, F.; Aubry, F.B.; Bergami, C.; Cabrini, M.; Campanelli, A.; Giani, M.; Guicciardi, S.; Marini, M.; et al. Seasonal and Interannual Trends of Oceanographic Parameters over 40 Years in the Northern Adriatic Sea in Relation to Nutrient Loadings Using the EMODnet Chemistry Data Portal. Water 2020, 12, 2280. [Google Scholar] [CrossRef]
- Chu, Y.; Tournoud, M.G.; Salles, C.; Got, P.; Perrin, J.L.; Rodier, C.; Caro, A.; Troussellier, M. Spatial and temporal dynamics of bacterial contamination in South France coastal rivers: Focus on in-stream processes during low flows and floods. Hydrol. Process 2014, 28, 3300–3313. [Google Scholar] [CrossRef]
- Basili, M.; Campanelli, A.; Frapiccini, E.; Luna, G.M.; Quero, G.M. Occurrence and distribution of microbial pollutants in coastal areas of the Adriatic Sea influenced by river discharge. Environ. Pollut. 2021, 285, 117672. [Google Scholar] [CrossRef]
- Jalliffier-Verne, I.; Heniche, M.; Madoux-Humery, A.S.; Galarneau, M.; Servais, P.; Prévost, M.; Dorner, S. Cumulative effects of fecal contamination from combined sewer overflows: Management for source water protection. J. Environ. Manag. 2016, 174, 62–70. [Google Scholar] [CrossRef]
- Ferrarin, C.; Penna, P.; Penna, A.; Spada, V.; Ricci, F.; Bilić, J.; Krzelj, M.; Ordulj, M.; Šikoronja, M.; Ðuračić, I.; et al. Modelling the quality of bathing waters in the adriatic sea. Water 2021, 13, 1525. [Google Scholar] [CrossRef]
- Line, D.E.; White, N.M.; Kirby-Smith, W.W.; Potts, J.D. Fecal coliform export from four coastal North Carolina areas. J. Am. Water Resour. Assoc. 2008, 44, 606–617. [Google Scholar] [CrossRef]
- Schlitzer, R. Ocean Data View 2020. Available online: https://odv.awi.de (accessed on 2 February 2022).
- Field, K.G.; Samadpour, M. Fecal source tracking, the indicator paradigm, and managing water quality. Water Res. 2007, 41, 3517–3538. [Google Scholar] [CrossRef]
- Amorim, E.; Ramos, S.; Bordalo, A.A. Relevance of temporal and spatial variability for monitoring the microbiological water quality in an urban bathing area. Ocean Coast. Manag. 2014, 91, 41–49. [Google Scholar] [CrossRef]
- Zenelaj, R.; Hila, F. Impact of Urban Wastewater Discharges on the Microbiological Pollution of Rivers Debouching into the Adriatic Sea; ESE Publications: Tirana, Albania, 2007. [Google Scholar]
- Ostoich, M.; Aimo, E.; Fassina, D.; Barbaro, J.; Vazzoler, M.; Soccorso, C.; Rossi, C. Biologic impact on the coastal belt of the province of Venice (Italy, Northern Adriatic Sea): Preliminary analysis for the characterization of the bathing water profile. Environ. Sci. Pollut. Res. 2011, 18, 247–259. [Google Scholar] [CrossRef]
- Liberatore, L.; Murmura, F.; Scarano, A. Bathing water profile in the coastal belt of the province of Pescara (Italy, Central Adriatic Sea). Mar. Pollut. Bull. 2015, 95, 100–106. [Google Scholar] [CrossRef]
- Organizzazione delle Nazioni Unite. Trasformare il Nostro Mondo: L’Agenda 2030 per lo Sviluppo Sostenibile (Agenda 2030); Organizzazione delle Nazioni Unite: New York, NY, USA, 2015. [Google Scholar]
- Spagnoli, F.; Dell’Anno, A.; de Marco, A.; Dinelli, E.; Fabiano, M.; Gadaleta, M.V.; Ianni, C.; Loiacono, F.; Manini, E.; Marini, M.; et al. Biogeochemistry, grain size and mineralogy of the central and southern Adriatic sea sediments: A review. Chem. Ecol. 2010, 26, 19–44. [Google Scholar] [CrossRef] [Green Version]
- Spagnoli, F.; De Marco, R.; Dinelli, E.; Frapiccini, E.; Frontalini, F.; Giordano, P. Sources and metal pollution of sediments from a coastal area of the central western adriatic sea (Southern Marche region, Italy). Appl. Sci. 2021, 11, 1118. [Google Scholar] [CrossRef]
- Reynolds, L.J.; Martin, N.A.; Sala-Comorera, L.; Callanan, K.; Doyle, P.; O’Leary, C.; Buggy, P.; Nolan, T.M.; O’Hare, G.M.P.; O’Sullivan, J.J.; et al. Identifying Sources of Faecal Contamination in a Small Urban Stream Catchment: A Multiparametric Approach. Front. Microbiol. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Mallin, M.A.; McIver, M.R. Pollutant impacts to Cape Hatteras National Seashore from urban runoff and septic leachate. Mar. Pollut. Bull. 2012, 64, 1356–1366. [Google Scholar] [CrossRef]
- Napier, A.D.; Depledge, M.; Knipper, M.; Lovell, R.; Ponarin, E.; Sanabria, E.; Thomas, F. Culture Matters: Using a Cultural Contexts of Health Approach to Enhance Policy-Making; WHO: Copenhagen, Denmark, 2017. [Google Scholar]
- Penna, P.; Baldrighi, E.; Betti, M.; Bolognini, L.; Campanelli, A.; Capellacci, S.; Casabianca, S.; Ferrarin, C.; Giuliani, G.; Grilli, F.; et al. Water quality integrated system: A strategic approach to improve bathing water management. J. Environ. Manag. 2021, 295, 113099. [Google Scholar] [CrossRef]
- Locatelli, L.; Russo, B.; Acero Oliete, A.; Carlos Sánchez Catalán, J.; Martínez-Gomariz, E.; Martínez, M. Modeling of E. coli distribution for hazard assessment of bathing waters affected by combined sewer overflows. Nat. Hazards Earth Syst. Sci. 2020, 20, 1219–1232. [Google Scholar] [CrossRef]
- Strickland, J.D.H.; Parsons, T.R. A Practical HANDBOOK of Seawater Analysis; Bulletin of Fisheries Research Board of Canada 167: Ottawa, ON, Canada, 1972. [Google Scholar]
- Alpha Analytical Quality. Total Suspended Solids Dried at 103–105 °C Total Volatile Suspended Solids Dried at 500 °C. In Standard Methods for the Examination of Water and Wastewater; APHA-AWWA-WPCF; Alpha Analytical, Inc.: Holmes, PA, USA, 2013; pp. 1–9. [Google Scholar]
- Clarke, D.A.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E: Plymouth, MA, USA, 2006. [Google Scholar]
- Marche, D.C. 1991/676/CE-D.M. n. 9 del 25/02/2016 A. del P. d’azione preliminare zone vulnerabili da nitrati di origine agricola della R. In Delibera della Giunta Regionale 1282_2019; Regione Marche Giunta Regionale: Ancona, Italy, 2019; pp. 1–64. [Google Scholar]
- EU Directive 2008/98/EC of the European Parliament and of the Council on Waste and Repealing Certain Directives; Official Journal of the European Union: Luxembourg, 2008; pp. 3–30.
- DIRECTIVE 2000/60/EC of the European Parliament and of the Council Establishing a Framework for Community Action in the Field of Water Policy; Official Journal of the European Communities: Aberdeen, UK, 2000; pp. 1–72.
- DM 18354 27-11-2009: Disposizioni per L’attuazione dei Regolamenti (CE) n. 834/2007, n. 889/2008, n. 1235/2008 e Successive Modifiche Riguardanti la Produzione Biologica e L’etichettatura dei Prodotti Biologici; Il Ministro delle Politiche Agricole Alimentari e Forestali: Rome, Italy, 2009; pp. 1–21.
- Ostoich, M.; Ghezzo, M.; Umgiesser, G.; Zambon, M.; Tomiato, L.; Ingegneri, F.; Mezzadri, G. Modelling as decision support for the localisation of submarine urban wastewater outfall: Venice lagoon (Italy) as a case study. Environ. Sci. Pollut. Res. 2018, 25, 34306–34318. [Google Scholar] [CrossRef]
- Hong, H.; Qiu, J.; Liang, Y. Environmental factors influencing the distribution of total and fecal coliform bacteria in six water storage reservoirs in the Pearl River Delta Region, China. J. Environ. Sci. 2010, 22, 663–668. [Google Scholar] [CrossRef]
- Sidhu, J.P.S.; Skelly, E.; Hodgers, L.; Ahmed, W.; Li, Y.; Toze, S. Prevalence of Enterococcus species and their virulence genes in fresh water prior to and after storm events. Environ. Sci. Technol. 2014, 48, 2979–2988. [Google Scholar] [CrossRef]
- Lipp, E.K.; Kurz, R.; Vincent, R.; Rodriguez-Palacios, C.; Farrah, S.R.; Rose, J.B. The effects of seasonal variability and weather on microbial fecal pollution and enteric pathogens in a subtropical estuary. Estuaries 2001, 24, 266–276. [Google Scholar] [CrossRef]
- Pongmala, K.; Autixier, L.; Madoux-Humery, A.S.; Fuamba, M.; Galarneau, M.; Sauvé, S.; Prévost, M.; Dorner, S. Modelling total suspended solids, E. coli and carbamazepine, a tracer of wastewater contamination from combined sewer overflows. J. Hydrol. 2015, 531, 830–839. [Google Scholar] [CrossRef]
- Boldrin, A.; Carniel, S.; Giani, M.; Marini, M.; Bernardi Aubry, F.; Campanelli, A.; Grilli, F.; Russo, A. Effects of bora wind on physical and biogeochemical properties of stratified waters in the northern Adriatic. J. Geophys. Res. 2009, 114, 1–19. [Google Scholar] [CrossRef]
- Ishii, S.; Ksoll, W.B.; Hicks, R.E.; Sadowsky, M.J. Presence and growth of naturalized Escherichia coli in temperate soils from lake superior watersheds. Appl. Environ. Microbiol. 2006, 72, 612–621. [Google Scholar] [CrossRef] [Green Version]
- Wilkes, G.; Edge, T.A.; Gannon, V.P.J.; Jokinen, C.; Lyautey, E.; Neumann, N.F.; Ruecker, N.; Scott, A.; Sunohara, M.; Topp, E.; et al. Associations among pathogenic bacteria, parasites, and environmental and land use factors in multiple mixed-use watersheds. Water Res. 2011, 45, 5807–5825. [Google Scholar] [CrossRef]
- Illuminati, S.; Annibaldi, A.; Truzzi, C.; Tercier-Waeber, M.-L.; Nöel, S.; Braungardt, C.B.; Achterberg, E.P.; Howell, K.A.; Turner, D.; Marini, M.; et al. In-situ trace metal (Cd, Pb, Cu) speciation along the Po River plume (Northern Adriatic Sea) using submersible systems. Mar. Chem. 2019, 212, 47–63. [Google Scholar] [CrossRef]
- Viaroli, P.; Soana, E.; Pecora, S.; Laini, A.; Naldi, M.; Fano, E.A.; Nizzoli, D. Space and time variations of watershed N and P budgets and their relationships with reactive N and P loadings in a heavily impacted river basin (Po river, Northern Italy). Sci. Total Environ. 2018, 639, 1574–1587. [Google Scholar] [CrossRef]
- Samavati, Z.; Borhani, T.N.; Samavati, A.; Ismail, A.F.; Rahman, M.A.; Othman, M.H.D.; Soleymani, M. Optical fiber sensor based on magneto-plasmonic features of Ag-Co nanostructure for ppm ammonium detection in aqueous solutions. Opt. Fiber Technol. 2021, 67, 102730. [Google Scholar] [CrossRef]





| 2019–2020 | N-NH4+ | N-NO3− | Rain | Enterococci | E. coli | |
|---|---|---|---|---|---|---|
| N-NH4+ | 1 | |||||
| N-NO3− | 0.159 | 1 | ||||
| rain | −0.059 | 0.209 | 1 | |||
| Enterococci | 0.346 | 0.225 | 0.422 | 1 | ||
| E. coli | 0.244 | 0.197 | 0.490 | 0.850 | 1 | |
| 2019 | N-NH4 + | N-NO3− | rain | Enterococci | E. coli | |
| N-NH4+ | 1 | |||||
| N-NO3− | −0.382 | 1 | ||||
| rain | 0.461 | 0.070 | 1 | |||
| Enterococci | 0.947 | −0.348 | 0.543 | 1 | ||
| E. coli | 0.968 | −0.340 | 0.476 | 0.926 | 1 | |
| 2020 | N-NH4+ | N-NO3− | rain | Enterococci | E. coli | |
| N-NH4+ | 1 | |||||
| N-NO3− | 0.158 | 1 | ||||
| rain | −0.270 | 0.159 | 1 | |||
| Enterococci | −0.011 | 0.446 | 0.498 | 1 | ||
| E. coli | −0.172 | 0.252 | 0.492 | 0.852 | 1 |
| Variable | SS | F | p | Prop% | Cum.Prop% | ||
|---|---|---|---|---|---|---|---|
| (a) Arzilla River | 2019-2020 | N-NH4+ | 5.74 × 108 | 4.703 | 0.032 | 7.6 | 7.6 |
| Chl-a | 5.27 × 108 | 4.587 | 0.023 | 7.0 | 14.6 | ||
| TSM | 4.59 × 108 | 4.452 | 0.039 | 6.1 | 20.7 | ||
| 2019 | N-NH4+ | 2.27 × 109 | 240.990 | 0.001 | 92.0 | 91.9 | |
| Chl-a | 3.87 × 107 | 4.872 | 0.006 | 1.6 | 93.6 | ||
| 2020 | T | 1.13 × 109 | 10.920 | 0.001 | 24.3 | 24.3 | |
| S | 1.19 × 109 | 16.920 | 0.001 | 25.7 | 50.0 | ||
| TN | 5.68 × 108 | 10.365 | 0.003 | 12.2 | 62.2 | ||
| (b) Seawater | 2019–2020 | N-NH4+ | 1.12 × 108 | 113.310 | 0.001 | 69.8 | 69.8 |
| pH | 3.86 × 106 | 4.174 | 0.040 | 2.4 | 72.2 | ||
| 2019 | S | 5.06 × 107 | 65.969 | 0.001 | 75.0 | 75.0 | |
| N-NH4+ | 4.86 × 106 | 8.493 | 0.008 | 7.2 | 82.2 | ||
| pH | 1.94 × 106 | 4.459 | 0.043 | 2.9 | 85.1 | ||
| 2020 | N-NH4+ | 6.46 × 107 | 60.956 | 0.001 | 70.9 | 70.9 | |
| Redox | 3.87 × 106 | 4.099 | 0.031 | 4.2 | 75.2 | ||
| S | 2.95 × 106 | 5.333 | 0.030 | 3.2 | 78.4 | ||
| T | 2.27 × 106 | 6.979 | 0.012 | 2.5 | 80.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manini, E.; Baldrighi, E.; Ricci, F.; Grilli, F.; Giovannelli, D.; Intoccia, M.; Casabianca, S.; Capellacci, S.; Marinchel, N.; Penna, P.; et al. Assessment of Spatio-Temporal Variability of Faecal Pollution along Coastal Waters during and after Rainfall Events. Water 2022, 14, 502. https://doi.org/10.3390/w14030502
Manini E, Baldrighi E, Ricci F, Grilli F, Giovannelli D, Intoccia M, Casabianca S, Capellacci S, Marinchel N, Penna P, et al. Assessment of Spatio-Temporal Variability of Faecal Pollution along Coastal Waters during and after Rainfall Events. Water. 2022; 14(3):502. https://doi.org/10.3390/w14030502
Chicago/Turabian StyleManini, Elena, Elisa Baldrighi, Fabio Ricci, Federica Grilli, Donato Giovannelli, Michele Intoccia, Silvia Casabianca, Samuela Capellacci, Nadia Marinchel, Pierluigi Penna, and et al. 2022. "Assessment of Spatio-Temporal Variability of Faecal Pollution along Coastal Waters during and after Rainfall Events" Water 14, no. 3: 502. https://doi.org/10.3390/w14030502









