Abstract
A toolbox of methods must be available for the remediation of lakes and water bodies suffering from eutrophication. One method suggested is hypolimnetic withdrawal based on a closed-circuit system. Prior to the start of a pilot-scale test at Lake Hönsan, Sweden, a laboratory trial with containers filled with water and bottom sediment from this lake was performed. A peristaltic pump distributed equal bottom water volume to four columns, two filled with glass beads and two with the filter material Polonite, and then back to the surface of the containers. The reactive filter medium (RFM) removed phosphate (PO4-P) efficiently (98.6%), despite the relatively low influent concentration (390 µg L−1). The control column filled with glass beads, removed 2.9% of the PO4-P. The anoxic sediment, containing 2.47 mg P g−1, released PO4-P, which was indicated by the increased concentration in near-bottom water. The redirected water after RFM filtration had high pH (; however, an equalization took place in the water mass to a lower but still increased pH value compared to the control . This article reports the pros and cons of a full-scale system using the proposed method.
1. Introduction
Despite large and costly measures to counteract the effects of lake eutrophication, many of the water bodies in Europe still have poor ecological status. Algal blooms occur regularly or cause problems if the lake is used for, e.g., recreation and as a drinking water supply [1,2]. Among the toolbox of measures to counteract water degradation is in situ treatment of the internal phosphorus (P) load by injection of aluminum chloride or lanthanum-modified bentonite into the sediments [3,4,5]. Nature-based solutions with multiple in-lake techniques can also be applied to suppress eutrophication [6]. A method that addresses the possibility of saving the P resource and applying cycling of P and circular economy is hypolimnetic withdrawal (HW) of water from lakes integrated with reactive media filtration [7,8,9]. Silvonen et al. [10] discussed a solution where withdrawn water would flow through a P-capturing purification unit and subsequently return into the same water body. This method involved, in its original version, withdrawing of the lake’s hypolimnetic water directly through a pipeline and discharging into a downstream water body [8,11]. HW has several risks, such as the impact on the lake´s water budget and impact on downstream waters, including eutrophication, temperature increase, oxygen depletion, and odor development [12]. The applied solutions also call for an integrated approach to eutrophication management [13]. Many Swedish municipalities are working on action plans to improve the water status of lakes, water courses, and coastal bays in accordance with the EU Water Framework Directive [14]. The control of P inputs from catchments can be a successful tool for the recovery of many lakes; however, stored P in the bottom sediment promotes internal loading, which can prevent lakes from being free of algal blooms. Motivated by this fact, and that P is historically buried in lake sediments and occurs in high concentrations in hypolimnion in stratified lakes, we developed an original HW method of pumping P-enriched water using recirculating water through reactive filter media (RFM). The recirculating principle, also called closed circuit [10], implies that the hypolimnetic water is discharged back to the same water body. The advantage is that the lake water volume will be unaffected, while disadvantages can possibly be found in changes of the biogeochemical conditions in the lake. Upon P saturation, after large volumes of water are filtrated, the RFM can be recycled directly to agriculture as fertilizer or soil amendment, e.g., as reported in [15]. This also shows advantages of the method from a sustainability viewpoint. The application of RFM in full-scale systems to trap P as well as the use of P-saturated media in agriculture is proven in research, e.g., [16]. Many granulated filter media have been suggested to be used for P removal in wastewater treatment [17]; however, only a few have achieved high Technology Readiness Level (TRL) and have become products on the market [18]. In stratified lakes with spring and autumn circulation, oxygen deficit can occur in the deeper parts during summer and winter. In this case, historically, P of anthropogenic origin has been stored in the sediments, and P leakage to the water column occurs annually during these two periods, particularly if anoxic conditions prevail [12]. Total P concentrations from 125 to 2500 µg L−1 are observed in hypolimnetic water [12]. It should be possible to reduce these P concentrations to a threshold value below 50 µg L−1 [19] by filtration in high-performance RFM. However, there have been few investigations performed where different P-sorbing materials were tested on total P removal at the comparatively low concentrations present in hypolimnetic water. Hussain et al. [7] used field columns filled with basic oxygen furnace slag to remove P from hypolimnetic water with a concentration of 0.25 to 0.49 mg L−1. Water filtration during two successive summers showed continuous and very high PO4-P removal efficiency, varying from 96.1 to 99.9%. The disadvantages that emerged from the experiment were high pH (11.96) of filter effluent and release of vanadium and aluminum from the virgin filter material. Łożyńska et al. [8,9] used Leca® and crushed limestone, having a grain size of 10–30 mm, in a column experiment fed by hypolimnetic water with a PO4-P concentration of 0.41 to 0.46 mg L−1. The duration of their experiment was less than 8 h. Best performance showed a mixture of Leca and limestone (>50%), and pH of the effluent water was not higher than 7.6.
The objective of this study was to investigate the P removal efficacy of the reactive filter material Polonite® when used in the treatment of hypolimnetic water in a closed-circuit system. The material’s chemical composition is shown in Table 1. Furthermore, pH changes in the recirculating water caused by the alkaline filter material were studied to assess the risk for biogeochemical effects in a full-scale lake application. Polonite was selected among available commercial brands manufactured for use in water and wastewater treatment. Extensive research and tests in field and laboratory environments have been performed with Polonite, mainly involving domestic wastewater treatment [20,21,22]. We present here results of a laboratory pre-study, undertaken prior to a scaling-up, semi-scale field experiment at Lake Hönsan, Sweden, which started in December 2021.

Table 1.
Chemical and physical parameters of the Polonite filter material used in the experiment.
2. Materials and Methods
2.1. Experimental Set-Up
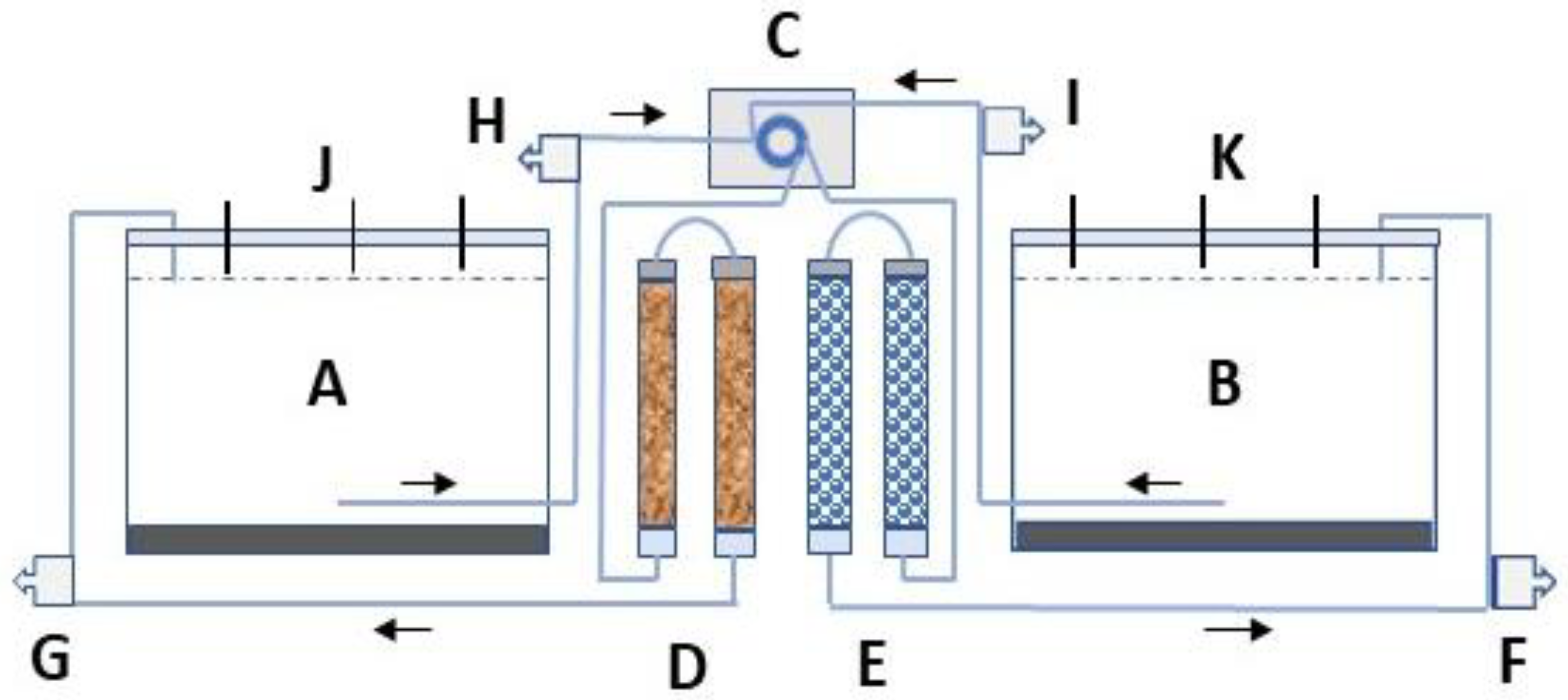
A bench-scale column experiment was constructed, consisting of four transparent pipes having the length and the inner diameter of 270 mm and 20 mm, respectively (Figure 1). Two columns were filled with Polonite® reactive filter medium (particle size 1–4 mm), and two with glass beads (diameter 2–3 mm). The two sizes of beads were mixed to obtain identical pore volume (PV = 70 mL) to the Polonite. The total bed volume (BV) for each material and two series-connected columns was 158 cm3. Two plastic containers were employed and filled with 11 L of sediment and 31 L of near-bottom water from lake Hönsan. Tygon® tubing was used to pump container bottom water via an Ecoline ISM (model VC-MS/CA 8-6. Cole-Parmer GmbH, Wertheim, Germany) multichannel peristaltic pump to the columns and back to the containers’ water surface (Figure 1). The saturated flow rate was 0.67 mL min−1, resulting in a filter hydraulic retention time (HRT) of 40 min for both media. A lid prevented air from circulating into the containers, and nitrogen, N2 (g) was bubbled into the bottom water once per week to keep the dissolved oxygen (DO) below 1 mg L−1, i.e., hypoxic conditions. The Polonite filter material, with a standard particle size of 2−6 mm, was received from the manufacturer (Polonite Nordic AB, Sweden/Poland). The name of the product was for the first time registered in 1999, and its mineralogical background and P sorption characteristics were described by Brogowski and Renman in 2004 [23]. Polonite is produced from opoka, a porous sedimentary calcium-silicate bedrock from the late cretaceous period, by crushing, thermal treatment, and other procedures to obtain desired filter properties. The material was sieved to a particle size that was suitable for the size of the column selected in the experiment. The characterization of the material is shown in Table 1. The glass beads are made of transparent, solid borosilicate glass (Avantor™).
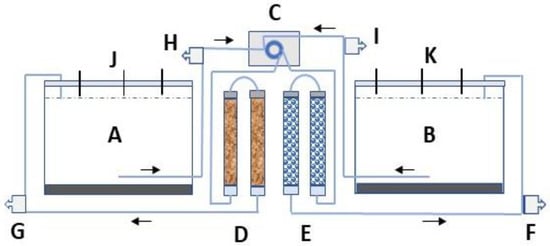
Figure 1.
Schematic presentation (not to scale) of the experimental set-up. Hypolimnetic water was pumped (C) from container (A) to two series-connected columns (D) filled with Polonite and back to the water surface. An identical procedure was performed with container B, but the water was pumped (C) through columns filled with glass beads (E). Openings in the lid (J,K) were used for sampling of water, in situ measurements of physical parameters, and supply of N2. (F–I) indicate ports for sampling column influent water and column effluent water, respectively. The water in the containers was measured randomly at three depths: 5 cm below water surface, 15 cm below, and at 23 cm depth, just above the sediment surface. The 10 cm thick layer of sediment is marked in black.
The containers were placed in shallow plastic barrels with circulating cold (6–8 °C) tap water so that 20 cm of the lower part of the containers was cooled (not shown in Figure 1). Daylight was prevented from entering the containers by covering them with black plastic. No growth of algae was observed in the containers, which usually is seen as growth on the inside. Sampling of water from the containers took place 12 times, with a periodicity of about 3–4 days. Only small water volumes were needed (aggregate samples from three depths, total 25 mL) for PO4-P analysis. Each time water was withdrawn from the containers or from the four sampling ports, it was replaced with the same volume of lake water from Hönsan. The water was stored in a refrigerator at a temperature of 6 °C and was added to the bottom of the containers.
2.2. Collection of Water and Sediment from Lake Hönsan
Water and sediment were collected in the dimictic Lake Hönsan, situated in the city of Hedemora (60°36′12″ N, 15°37′33″ E), Sweden. The samples were taken in February 2020 from the ice-covered lake. The lake (area 0.88 km2) is mainly fed by groundwater at an average inflow rate of 1.8 L s−1. The lake water is characterized by high concentrations of P in the hypolimnion, and algal blooms have occurred during the past years (data from Hedemora Municipality, pers. comm.). There are two deep areas (11 and 12 m) where anoxic/hypoxic conditions occur during the summer and winter stratified periods. Water was pumped from 10.8 m depth and filled into four 25 L containers. Sediment was taken from 11 m depth with means of an Ekman dredge sampler and immediately stored in air-tight containers. The sampling was performed in spring just before the ice melted. The DO concentration, water temperature, electrical conductivity (EC), and pH was at the time of sampling 0.15 mg L−1, 4.1 °C, 350 µS cm−1, and 6.39, respectively. The total-P and PO4-P concentrations of withdrawn bottom water were 620 and 410 µg L−1, respectively. At the start of the experiment, EC, pH, total-P, and PO4-P concentrations in both containers had average values of 374 ± 3 µS cm−1, 6.81 ±0.2, 730 ± 0.5 µg L−1, and 390 ± 0.8 µL−1, respectively. DO was adjusted in the starting volume of container water by bubbling N2, from approximately 1.2 to 0.3 mg L−1.
2.3. Analyses and P Removal Calculation
The water physical conditions (pH, DO, EC, and temperature) were measured in the field and laboratory with a portable Hach HQ40D multimeter (Hach Lange GmbH, Düsseldorf, Germany) with selective probes/sensors for each parameter. The mineralogical composition of Polonite was measured by means of XRD by accredited laboratory ALS Scandinavia AB. The P content in sediment from Lake Hönsan was determined with a portable Thermo Scientific Niton XL3 XRF instrument (Thermo Fisher Scientific Waltham, MA USA). XRF was also used to identify white precipitate at the sediment surface of the container recirculating water through the Polonite filter. The PO4-P concentrations in the water samples were colorimetrically determined using a Seal AA3 Autoanalyzer ((SEAL Analytical GmbH, Norderstedt, Germany).
The PO4-P removal capacity by the column materials, i.e., the retention of PO4-P, was calculated according to the following equation:
where Ce is the average effluent concentration in the containers and Ci is the average influent concentration from the bottom-layer water in the containers.
3. Results and Discussion
This bench-scale experiment lasted only 32 days; however, the results indicate promising possibilities for using hypolimnetic water recirculation combined with filtration in reactive filter media (HWRFM) as a P-trap (Figure 1). The study was limited to investigating if the selected calcium-silicate based filter material could reduce comparatively low P concentrations in lake water to threshold values, which trigger eutrophication. We focused solely on dissolved reactive phosphate (as PO4-P), as this species has a direct impact on algal growth. The removal of particulate bound P is usually very efficient in RFM, and for the experiment we performed in this study, we found that PO4-P constituted 91% of total P in the filter effluent (n = 3). The mechanisms behind P sorption by Polonite have been studied previously, e.g., [24,25,26], and were not investigated in detail in the present paper.
3.1. Phosfate Removal in the Columns and Changes in PO4-P Concentration in the Containers
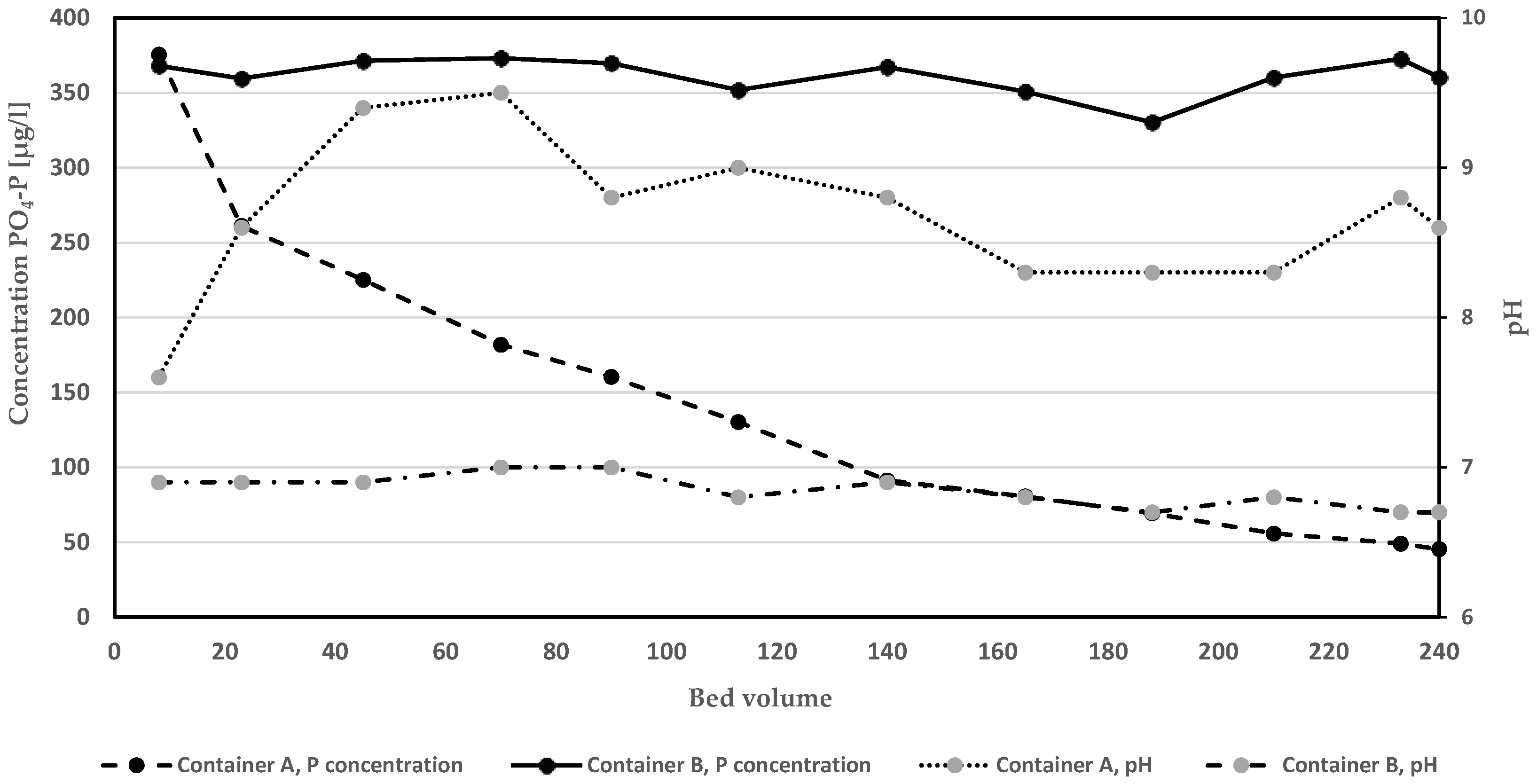
The development in removal efficacy in relation to amount of purified BV is presented in Table 2. The average removal of PO4-P by Polonite and the glass beads was 98.6 and 2.9%, respectively. Polonite removed 100% of PO4-P up to 113 BV and then slowly began to decrease in efficiency, showing 92% removal at 240 BV treated by the filter. The glass bead columns also showed desorption of PO4-P during a short period (BV 22.5−45, −1.5 to −2.2%). The PO4-P concentration in the container (A) fed by water from Polonite columns was successively reduced to a low average value in the surface and middle water layers of 45.3 µg L−1 (Figure 2). The conditions in the container fed by water from glass bead columns (B) did not change much during the pumping, and the corresponding average PO4-P concentration was 361.5 µg L−1 at the end of the experiment. The release of PO4-P from the bottom sediment was almost the same throughout the study period, which was assessed from the concentrations in influent to the glass bead columns (Table 2). The Lake Hönsan sediment was found to have an organic matter content (LOI) of 29 ± 1% and a concentration of 2.468 ± 0.092 mg P g−1 DW, which shows its potential to support eutrophication. The role of sediment P release and seasonality have to be considered when full-scale HWRFM projects are planned, according to studies by Silvonen et al. [10].

Table 2.
Phosphate concentration and removal in hypolimnetic water before (influent) and after (effluent) treatment in Polonite and glass bead columns. Average and standard deviation values are given for the consecutive bed volumes treated. * Calculated as zero removal.
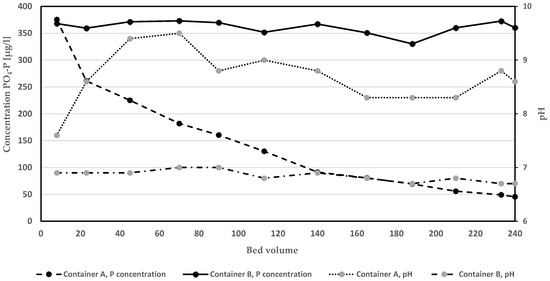
Figure 2.
Development of PO4-P concentration and pH (presented as averages of a measurement in each of three water layers) in containers filled with hypolimnetic water and sediment. Water from container A circulated through filter with Polonite while container B water flowed through columns with glass beads.
The columns filled with glass beads served as a reference to those filled with Polonite. Glass beads are not classified as reactive, as chemical bonds of P to their borosilicate surfaces are not expected. However, they can act as mechanical filters and biofilm carriers, removing particulate matter and forming surfaces for bacteria, which can remove P at least temporarily [27,28].
Many other RFM can be used or developed for the purpose of eradicating algal blooms, predominantly blue-green algae, in water bodies. Reducing P is the best solution, but it is not always obvious which method should be used to obtain the best long-term effect [29]. The experiment presented in this paper used containers that imitated a piece of the hypolimnion in Lake Hönsan. When the experiment ended, the Polonite column effluent had a PO4-P concentration of 20.5 µg L−1, and the average concentration for the total treated volume of water was 3.96 µg L−1. The concentration in surface water of Lake Hönsan (0.5 m depth) is usually below 10 µg L−1 (B. Jonsson, pers. comm.). A certain PO4-P concentration may be a threshold value for an HWRFM system if the reactive filter effluent is redirected to the surface of a water body. The drop in P removal capacity with time is always observed in RFM [20,30], and if the effluent P concentration rises above a set value, a different strategy must be applied. Moreover, nitrogen species occurring in elevated concentrations in hypolimnetic water should not be redirected to the surface water. We therefore suggest pumping hypolimnetic water purified by RFM back to the upper level of the hypolimnion. This solution should work according to the principle demonstrated in our short-term experiment. The filter system in this study was continuously operated. A strategy with intermittent loading or using batch mode [31] could favor the P-sorption capacity of the filter medium and prolong its service life, i.e., the time when it is P-saturated and has to be replaced by virgin material.
3.2. Changes in Physio-Chemical Conditions of the Water during Pumping
The chemical and physical conditions of hypolimnetic water in the container connected to circulation via Polonite columns (marked A in Figure 1) changed during the experiment. The measurements with probes at the bottom were done from the water layer 2 cm above the inlet tube to the filter. It was important to not disturb the sediment surface and cause turbidity in the water. The pH in the bottom layer of the container fed by Polonite-treated water was slightly higher than in the surface, which could have been an effect of N2 addition or the white precipitate of CaCO3 that was observed on the sediment surface (verified by XRF). The release of calcium (Ca2+) from Polonite is documented in previous research [26,31], and the precipitation of CaCO3 or calcium sulphate (CaSO4) is expected to occur in the filter material due to the chemical composition of the hypolimnetic water. These mechanisms can lead to chemical clogging, and in the worst case, hydraulic failure [32]. Dissolved and particulate organic matter can accumulate and form clogging zones in filter material [22]. The release of Ca2+ can lead to precipitation of any new phases, depending on the physio-chemical conditions in the lake water. Precipitates of CaCO3 forming in situ, as observed in the present study, can on the other hand act as capping, immobilizing PO4-P release from the sediment [33]. The effects of humic material on the precipitation of calcium phosphates in the filter should be further studied, as many eutrophic boreal lakes have humic substances [34]. Humic material in soils can in a similar way inhibit the formation of thermodynamically stable calcium phosphates [35]. Humic substances in natural waters often reduce or inhibit calcite (CaCO3) crystal growth [36]. The impact of organic matter in hypolimnetic water on P-retaining mechanisms in RFM should be further investigated and included as a factor for optimal design of an HWRFM system.
The change in pH of the column effluents, i.e., the inflow water to the containers, is shown in Figure 2. After the initial treatment of 7.5 BV, pH was very different between the treated water in Polonite and glass bead control columns, i.e., 12.1 and 6.7, respectively. After the total treatment of 240 BV, pH still was high in the Polonite effluent (pH 10.8) and almost unchanged (pH 6.8) in the glass bead effluent. The alkaline filtrate from Polonite had a great impact on the pH in container A, while no such change was observed in the container B, fed by filtrate from the glass bead column. However, pH approached a maximum of 9.5, despite the fact that 37.9 L of water was pumped in the closed circuit, i.e., 6.9 L more than was filled in the containers. The intermittent bubbling of N2 into the water did not cause the increase in pH, as revealed from the data collected in container B.
The pH seemed to slightly increase in the container water after 210.5 PV treated by the Polonite column (Figure 2). It is known that the P release rate is a function of pH and that PO4-P can be exchanged with OH- at high pH in the sediments [37]. Many studies have reported on processes causing sediment P desorption, e.g., P-solubilizing bacteria, redox, and pH conditions [38,39,40]. Ortuño et al. [41] investigated sediment from a deep wastewater stabilization pond and found that the total-P release increased up to 17.4% in anaerobic conditions and when pH was 9.1. In contrast, a sediment capping with Polonite or Polonite mixed with activated carbon increased both water and sediment pH to 9.1 and 9.0, respectively, while significantly decreasing the PO4-P flux [33].
Thus, the release of alkaline filtrated water to a lake can have additional consequences to merely affecting biological life [42], namely an increased P loading from the sediment to the hypolimnion. We showed in the microcosm experiment presented here, the expected increase in pH (Table 3), which however did not result in short-term mobilization of P. However, the large volume of hypolimnetic water treated in a full-scale system calls for caution and must be carefully monitored in all biogeochemical aspects. The hypolimnetic volume of Lake Hönsan is 18,000 m3 (Unpubl. data from investigations by B. Jonsson and Hedemora Municipality), and this volume could not be treated in RFM in the same short time as our bench-scale experiment. Intermittent pumping (e.g., three one-hour pumping events distributed over a day) should instead be applied to increase the P-sorption capacity of the RFM, as previously mentioned, which could also help to dilute the alkaline filtrate in the lake water. There is no reason to force the pumping, as this can take place over longer periods of time, e.g., one to two years.

Table 3.
The pH changes in container A (Polonite filtration) and B (glass bead filtration) during 32 days pumping of hypolimnetic water. Rounded averages of three measurements in each water layer (surface, middle, bottom) are given as well as inflow pH values in relation to the consecutive bed volumes treated in columns.
The DO concentrations were generally very low in both containers throughout the study period (Table 4). The hypolimnetic water (DO = 0.15 mg L−1) was slowly filled into the containers at the start of the experiment by siphon to avoid oxygen contamination and disturbance of the sediment. However, oxygen was dissolved in the water during the filling and increased in concentration in the surface and middle layers, although still in a hypoxic state. The few temporary pumping events of N2 and openings for sampling probably leaked O2 into the containers and oxygenated the water. The feed of N2 was about 10 cm above the sediment layer, which likely only affected the upper water layers but prevented O2 from oxidizing the sediment. The treated water after columns, i.e., denoted influent in Table 4, had DO values of 4.33 and 5.23 mg L−1 in container A (Polonite) and B (glass beads), respectively, after 7.5 BV. However, more interesting is that the DO concentration was continuously elevated after filtration. Apparently, some processes in the columns took place that caused this increase in DO concentration. Accumulation of organic matter was visible as brownish layers on both glass beads and Polonite. A possible reason for the oxygen increase may have been the activity of photosynthesizing bacteria and microalgae, because light was able to penetrate into the transparent hoses and the columns. On the other hand, the high pH in the Polonite column water does not favor conditions for microorganism growth. The black bottom sediment stayed in an anoxic state, which was supported by the very low concentrations of DO in the near-bottom water.

Table 4.
Development of DO concentrations in container A (Polonite filtration) and B (glass bead filtration) during 32 days pumping of hypolimnetic water. DO was measured in the center of the containers at three depths, and water was collected at the inflow to the containers. Each value represents one measurement, and mean values are given for the water column in relation to the number of BV volume of water.
The cooling of the lower part of the containers helped to keep the temperature close to natural conditions. However, the water temperature was elevated compared to that usually occurring in the lower part of the hypolimnion (4−6 °C). For container A, the temperature gradient was for the bottom, medium, and surface layers, 7 ± 1, 9 ± 2, and 12 ± 1.5 °C, respectively. Container B showed a similar gradient of 7 ± 1.5, 8 ± 1, and 11 ± 2 °C, respectively. The pumped water was heated by ambient room temperature during the flow through hoses and filter material and returned to the containers at a temperature of 18 °C, where it was diluted in the water surface to about 12 °C. The increased pH may have effects in the lake ecosystem and must be considered before an HWRFM system is applied, although pumping distributed over a day can help, as previously discussed. Highly alkaline (pH > 12) filter media effluent can be treated in constructed wetlands (CWs) before it is released to the lake [42]. However, CWs require access to land, which is not always an option in built-up areas. In Lake Kymijärvi in Finland, experiments are underway where the pumped water is treated with various filter materials and then led via a wetland back to the lake [10]. The use of other RFM with lower initial pH values can be considered, such as Sorbulite® [31,43] and Leca® [8]; however, their service life can be much shorter because of their lower P-removal efficiencies. The pumping in our experiment did not affect the DO concentration in the cold bottom water, probably because the redirected water was warmer and slowly mixed with the surface water.
The color of hypolimnetic water was slightly brown, but after the flow through the Polonite, it was completely discolored, which was not the case for the control with glass beads. The strong smell of hydrogen sulfide was also removed by Polonite, but not by the glass beads. As pointed out earlier, humic material causing a brown color of the water, can reduce the life of a filter material consisting of lime. However, when operating a full-scale system, benefits are achieved in terms of clear water recycled to the lake and removal of unpleasant odors (mainly from H2S), which can otherwise be spread into the environment [44]. In fact, the alkaline filter medium Polonite removes CO2 and H2S by forming CaCO3 and CaSO4, respectively, which can contribute to reduced greenhouse gas emissions (G. Renman, unpubl. data).
3.3. Problems Needing Further Study
A filter plant operated during the winter season requires a design such that the reactive media do not freeze solid. A container-based (10- or 20-foot size) mobile system is preferable where the indoor temperature should be 5 °C, i.e., the same temperature as the hypolimnetic water. In summer, the water risks heating up when it passes the filter, but this can be avoided if the container is insulated and possibly equipped with a heat pump. In this proposed manner, combined with installation of a pipe that discharges treated water to the top layer of hypolimnion, no impact on the thermal stratification would be expected [10,11]. The P sorption capacity of most materials is sensitive to low water temperature [45] and HRT [46]. The latter factor can possibly be controlled because the pumping of hypolimnetic water can be distributed over time. The temperature of the water must be regulated so that no disturbances occur in the lake at the same time as when the filter material works best, i.e., when the operating temperature is around 20−25 °C. More research is needed on the optimization of RFM to be used for hypolimnetic water treatment and on P removal structures [47], which can be technically feasible solutions for the lakes to be rephosphorized. However, reactive filtration is nowadays one of the recognized technologies used for recovering nutrients from wastewater [48]. Moreover, if cost-benefit analyses prove that the HWRFM technology is appropriate for water [49] and sediment reuse [50], this increases the possibilities for the method to be implemented on a large scale. Bashar et al. [51], for instance, found that a combined process involving reactive media filtration for wastewater treatment was efficient for P removal, although it did not meet stringent discharge standards. A circular P economy for eutrophicated water bodies should increase the incentives for action by stakeholders; however, barriers have to be overcome, such as the responsibilities along the value chain [52].
4. Conclusions
This simple and short-term microcosm experiment showed that the reactive filter material Polonite was able to remove P from hypolimnetic water to background concentrations. All other types of calcium silicate materials at high TRL should also have the same capacity to remove P from near-bottom water of lakes. It was observed that Polonite removed odor and the brown water color. However, long-term semi-scale field tests should be performed with different materials, including physio-chemical and limnological investigations, to detect any harmful effects of the hypolimnetic withdrawal and use of reactive filter materials in a recirculating system.
Author Contributions
Conceptualization, G.R., A.R.; methodology, A.R.; validation, A.R., G.R.; formal analysis, A.R.; investigation, G.R.; resources, A.R.; data curation, A.R.; writing—original draft preparation, A.R.; writing—review and editing, G.R.; visualization, A.R.; project administration, A.R.; funding acquisition, A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by J. Gustaf Richert Stiftelse, grant number 2020-00651, and Åke and Greta Lissheds Stiftelse, grant number 2021-00169 (to A. Renman).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Böril Jonsson, who contributed with measurement results from Lake Hönsan and the background of its development within the framework of Hedemora Municipality’s monitoring program. Thanks are due to Peter Sennblad, Hedemora Municipality, for sharing information and interest in our project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Noges, P.; Van De Bund, W.; Cardoso, A.C.; Solimini, A.G.; Heiskanen, A.S. Assessment of the ecological status of European surface waters: A work in progress. Hydrobiologia 2009, 633, 197–211. [Google Scholar] [CrossRef]
- Brooks, B.W.; Lazorchak, J.M.; Howard, M.D.; Johnson, M.V.V.; Morton, S.L.; Perkins, D.A.; Reavie, E.D.; Scott, G.I.; Smith, S.A.; Steevens, J.A. Are harmful algal blooms becoming the greatest inland water quality threat to public health and aquatic ecosystems? Environ. Toxicol. Chem. 2016, 35, 6–13. [Google Scholar] [CrossRef]
- Rydin, E.; Welch, E.B. Aluminum dose required to inactivate phosphate in lake sediments. Water Res. 1998, 32, 2969–2976. [Google Scholar] [CrossRef]
- Rydin, E.; Kumblad, L. Capturing past eutrophication in coastal sediments—Towards water-quality goals. Estuar. Coast. Shelf Sci. 2019, 221, 184–188. [Google Scholar] [CrossRef]
- Dithmer, L.; Nielsen, U.G.; Lürling, M.; Spears, B.M.; Yasseri, S.; Lundberg, D.; Moore, A.; Jensen, N.D.; Reitzel, K. Responses in sediment phosphorus and lanthanum concentrations and composition across 10 lakes following applications of lanthanum modified bentonite. Water Res. 2016, 7, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Dondajewska, R.; Kowalczewska-Madura, K.; Gołdyn, R.; Kozak, A.; Messyasz, B.; Cerbin, S. Long-term water quality changes as a result of a sustainable restoration—A case study of dimictic Lake Durowskie. Water 2019, 11, 616. [Google Scholar] [CrossRef]
- Hussain, S.I.; Blowes, D.W.; Ptacek, C.J.; Olding, D. Phosphorus removal from lake water using basic oxygen furnace slag: System performance and characterization of reaction products. Environ. Eng. Sci. 2014, 31, 631–642. [Google Scholar] [CrossRef]
- Łożyńska, J.; Dunalska, J.A.; Bańkowska-Sobczak, A.; Zhang, L.; Mitsch, W.J. Treatment of Hypolimnion Water on Mineral Aggregates as the Second Step of the Hypolimnetic Withdrawal Method Used for Lake Restoration. Minerals 2021, 11, 98. [Google Scholar] [CrossRef]
- Łożyńska, J.; Bańkowska-Sobczak, A.; Popek, Z.; Dunalska, J.A. Selection of P-reactive materials for treatment of hypolimnetic water withdrawn from eutrophic lakes. Ecohydr. Hydrobiol. 2020, 20, 276–288. [Google Scholar] [CrossRef]
- Silvonen, S.; Niemistö, J.; Csibrán, A.; Jilbert, T.; Torma, P.; Krámer, T.; Nurminen, L.; Horppila, J. A biogeochemical approach to evaluate the optimization and effectiveness of hypolimnetic withdrawal. Sci. Total Environ. 2021, 755, 143202. [Google Scholar] [CrossRef]
- Nürnberg, G.K.; Hartley, R.; Davis, E. Hypolimnetic withdrawal in two North American lakes with anoxic phosphorus release from the sediment. Water Res. 1987, 21, 923–928. [Google Scholar] [CrossRef]
- Nürnberg, G.K. Lake responses to long-term hypolimnetic withdrawal treatments. Lake Reserv. Manag. 2007, 23, 388–409. [Google Scholar] [CrossRef]
- Zamparas, M.; Zacharias, I. Restoration of eutrophic freshwater by managing internal nutrient loads. A review. Sci. Total Environ. 2014, 496, 551–562. [Google Scholar] [CrossRef]
- European Union. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 on establishing a framework for community action in the field of water policy. J. Eur. Commun. 2000, L327, 1–72. [Google Scholar]
- Cucarella, V.; Mazurek, R.; Zaleski, T.; Kopeć, M.; Renman, G. Effect of Polonite used for phosphorus removal from wastewater on soil properties and fertility of a mountain meadow. Environ. Pollut. 2009, 157, 2147–2152. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Montaño, V.; Fenton, O.; Moore, B.; Healy, M.G. Evaluation of the fertiliser replacement value of phosphorus-saturated filter media. J. Clean. Prod. 2021, 291, 125943. [Google Scholar] [CrossRef]
- Patyal, V.; Jaspal, D.; Khare, K. Materials for phosphorous remediation: A review. Phosphorus Sulfur Silicon Relat. Elem. 2021, 196, 1025–1037. [Google Scholar] [CrossRef]
- Karczmarczyk, A.; Woja, K.; Bliska, P.; Baryła, A.; Bus, A. The efficiency of filtration materials Polonite® and Leca® supporting phosphorus removal in on site treatment systems with wastewater infiltration. Infrastruktura i Ekologia Terenów Wiejskich 2017, 4, 1401–1413. [Google Scholar] [CrossRef]
- Fastner, J.; Abella, S.; Litt, A.; Morabito, G.; Matthews, D.; Phillips, M.G.; Chorus, I. Combating cyanobacterial proliferation by avoiding or treating inflows with high P load—Experiences from eight case studies. Aquat. Ecol. 2016, 50, 367–383. [Google Scholar] [CrossRef]
- Renman, A.; Renman, G. Long-term phosphate removal by the calcium-silicate material Polonite in wastewater filtration systems. Chemosphere 2010, 79, 659–664. [Google Scholar] [CrossRef]
- Vidal, B.; Hedström, A.; Herrmann, I. Phosphorus reduction in filters for on-site wastewater treatment. J. Water Process Eng. 2018, 22, 210–217. [Google Scholar] [CrossRef]
- Hamisi, R.; Renman, G.; Renman, A.; Wörman, A. Modelling phosphorus sorption kinetics and the longevity of reactive filter materials used for on-site wastewater treatment. Water 2019, 11, 811. [Google Scholar] [CrossRef]
- Brogowski, Z.; Renman, G. Characterization of opoka as a basis for its use in wastewater treatment. Pol. J. Environ. Stud. 2004, 13, 15–20. [Google Scholar]
- Renman, A. On-Site Wastewater Treatment: Polonite and Other Filter Materials for Removal of Metals, Nitrogen and Phosphorus. Ph.D. Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2008. Trita-LWR.PHD, 1043. Available online: http://kth.diva-portal.org/smash/record.jsf?pid=diva2:14097 (accessed on 4 February 2022).
- Eveborn, D.; Gustafsson, J.P.; Hesterberg, D.; Hillier, S. XANES speciation of P in environmental samples: An assessment of filter media for on-site wastewater treatment. Environ. Sci. Technol. 2009, 43, 6515–6521. [Google Scholar] [CrossRef] [PubMed]
- Kolosov, P.V. Chemistry of Phosphorus Removal by Polonite® Media. Ph.D. Thesis, Tennessee Technological University, Cookeville, TN, USA, 2014. [Google Scholar]
- Rubulis, J.; Juhna, T. Evaluating the potential of biofilm control in water supply systems by removal of phosphorus from drinking water. Water Sci. Technol. 2007, 55, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xing, X.H.; Wang, B.Z. Characteristics of phosphorus removal from wastewater by biofilm sequencing batch reactor (SBR). Biochem. Eng. J. 2003, 16, 279–285. [Google Scholar] [CrossRef]
- Schindler, D.W.; Carpenter, S.R.; Chapra, S.C.; Hecky, R.E.; Orihel, D.M. Reducing phosphorus to curb lake eutrophication is a success. Environ. Sci. Technol. 2016, 50, 8923–8929. [Google Scholar] [CrossRef]
- Cucarella, V.; Renman, G. Phosphorus sorption capacity of filter materials used for on-site wastewater treatment determined in batch experiments—A comparative study. J. Environ. Qual. 2009, 38, 381–392. [Google Scholar] [CrossRef]
- Nilsson, C.; Lakshmanan, R.; Renman, G.; Rajarao, G.K. Efficacy of reactive mineral-based sorbents for phosphate, bacteria, nitrogen and TOC removal–column experiment in recirculation batch mode. Water Res. 2013, 47, 5165–5175. [Google Scholar] [CrossRef]
- Courcelles, B.; Modaressi-Farahmand-Razavi, A.; Gouvenot, D.; Esnault-Filet, A. Influence of precipitates on hydraulic performance of permeable reactive barrier filters. Int. J. Geomech. 2011, 11, 142–151. [Google Scholar] [CrossRef]
- Wikström, J.; Bonaglia, S.; Ramö, R.; Renman, G.; Walve, J.; Hedberg, J.; Gunnarsson, J.S. Sediment remediation with new composite sorbent amendments to sequester phosphorus, organic contaminants, and metals. Environ. Sci. Technol. 2021, 55, 11937–11947. [Google Scholar] [CrossRef] [PubMed]
- Tammeorg, O.; Nürnberg, G.; Nõges, P.; Niemistö, J. Sediment phosphorus release in boreal lakes: The role of trophic state and humic substances. Res. Sq. 2021. preprint. [Google Scholar] [CrossRef]
- Alvarez, R.; Evans, L.A.; Milham, P.J.; Wilson, M.A. Effects of humic material on the precipitation of calcium phosphate. Geoderma 2004, 118, 245–260. [Google Scholar] [CrossRef]
- Reddy, M.M.; Hoch, A.R. Calcite crystal growth rate inhibition by aquatic humic substances. In Advances in Crystal Growth Inhibition Technologies; Springer: Boston, MA, USA, 2002; pp. 107–121. [Google Scholar]
- Boström, B. Relations between chemistry, microbial biomass and activity in sediments of a polluted vs. a non-polluted eutrophic lake. Int. Ver. Für Theor. Angew. Limnol. Verh. 1988, 23, 451–459. [Google Scholar]
- Qu, J.H.; Li, H.F.; Chen, N.; Yuan, H.L. Biogeochemical function of phosphorus-solubilising bacteria on cycling of phosphorus at the water-sediment interface under laboratorial simulated conditions. Int. J. Environ. Pollut. 2013, 52, 104–116. [Google Scholar] [CrossRef]
- Qian, Y.; Liang, X.; Chen, Y.; Lou, L.; Cui, X.; Tang, J.; Li, P.; Cao, R. Significance of biological effects on phosphorus transformation processes at the water–sediment interface under different environmental conditions. Ecol. Eng. 2011, 37, 816–825. [Google Scholar] [CrossRef]
- Christophoridis, C.; Fytianos, K. Conditions affecting the release of phosphorus from surface lake sediments. J. Environ. Qual. 2006, 35, 1181–1192. [Google Scholar] [CrossRef]
- Ortuno, J.F.; Saez, J.; Llorens, M.; Soler, A. Phosphorus release from sediments of a deep wastewater stabilization pond. Water Sci. Technol. 2000, 42, 265–272. [Google Scholar] [CrossRef]
- Mayes, W.M.; Batty, L.C.; Younger, P.L.; Jarvis, A.P.; Kõiv, M.; Vohla, C.; Mander, U. Wetland treatment at extremes of pH: A review. Sci. Total Environ. 2009, 407, 3944–3957. [Google Scholar] [CrossRef]
- Renman, G.; Renman, A. Sustainable use of crushed autoclaved aerated concrete (CAAC) as a filter medium in wastewater purification. In Proceedings of the 8th International Conference on Sustainable Management of Waste and Recycled Materials in Construction, Gothenburg, Sweden, 30 May–1 June 2012. ISCOWA and SGI. [Google Scholar]
- Tandyrak, R.; Gołaś, I.; Parszuto, K.; Bowszys, M.; Szymański, D.; Harnisz, M.; Brudniak, A.; Wysocka, I. The effect of lake restoration by the hypolimnetic withdrawal method on the intensity of ambient odour. J. Limnol. 2016, 75, 531–544. [Google Scholar] [CrossRef]
- Fronczyk, J.; Mumford, K.A. The impact of temperature on the removal of inorganic contaminants typical of urban stormwater. Appl. Sci. 2019, 9, 1273. [Google Scholar] [CrossRef]
- Benzing, S.; Couceiro, F.; Barnett, S.; Williams, J.B.; Pearce, P.; Stanford, C. Impact of hydraulic retention time on phosphorus removal from wastewater using reactive media. Water Sci. Technol. 2020, 82, 2920–2928. [Google Scholar] [CrossRef]
- Penn, C.; Chagas, I.; Klimeski, A.; Lyngsie, G. A review of phosphorus removal structures: How to assess and compare their performance. Water 2017, 9, 583. [Google Scholar] [CrossRef]
- Perera, M.K.; Englehardt, J.D.; Dvorak, A.C. Technologies for recovering nutrients from wastewater: A critical review. Environ. Eng. Sci. 2019, 36, 511–529. [Google Scholar] [CrossRef]
- Molinos-Senante, M.; Hernández-Sancho, F.; Sala-Garrido, R. Cost–benefit analysis of water-reuse projects for environmental purposes: A case study for Spanish wastewater treatment plants. J. Environ. Manag. 2011, 92, 3091–3097. [Google Scholar] [CrossRef] [PubMed]
- Braga, B.B.; de Carvalho, T.R.A.; Brosinsky, A.; Foerster, S.; Medeiros, P.H.A. From waste to resource: Cost-benefit analysis of reservoir sediment reuse for soil fertilization in a semiarid catchment. Sci. Total Environ. 2019, 670, 158–169. [Google Scholar] [CrossRef]
- Bashar, R.; Gungor, K.; Karthikeyan, K.G.; Barak, P. Cost effectiveness of phosphorus removal processes in municipal wastewater treatment. Chemosphere 2018, 197, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Barquet, K.; Järnberg, L.; Rosemarin, A.; Macura, B. Identifying barriers and opportunities for a circular phosphorus economy in the Baltic Sea region. Water Res. 2020, 171, 115433. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).