Benthic Macroinvertebrate Diversity as Affected by the Construction of Inland Waterways along Montane Stretches of Two Rivers in China
Abstract
:1. Introduction
2. Materials and Methods
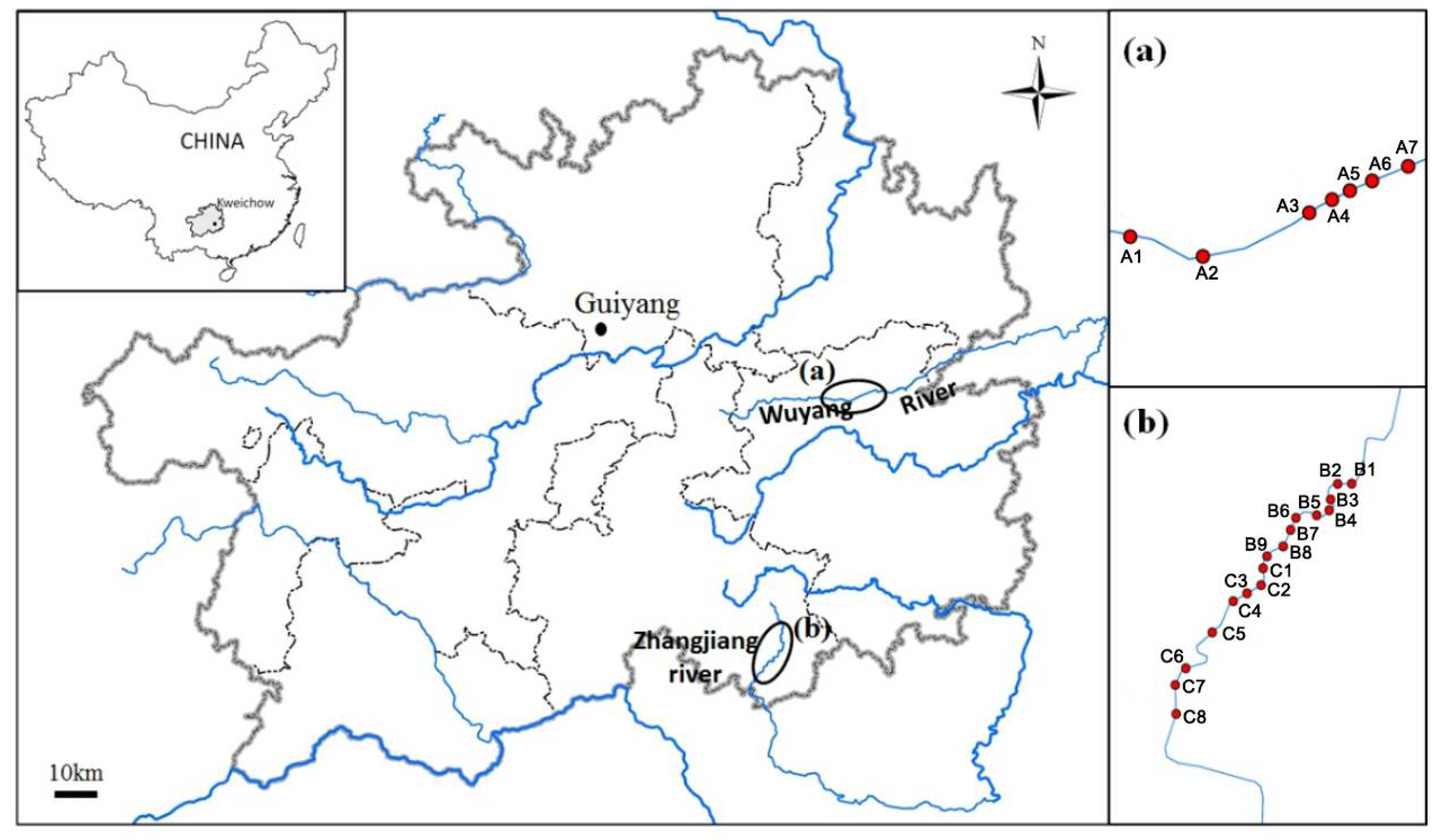
2.1. Study Area
2.2. Benthic Macroinvertebrate Sampling
2.3. Analyses of Environmental Factors
2.4. Statistical Analyses
3. Results
3.1. Analysis of Environmental Variables
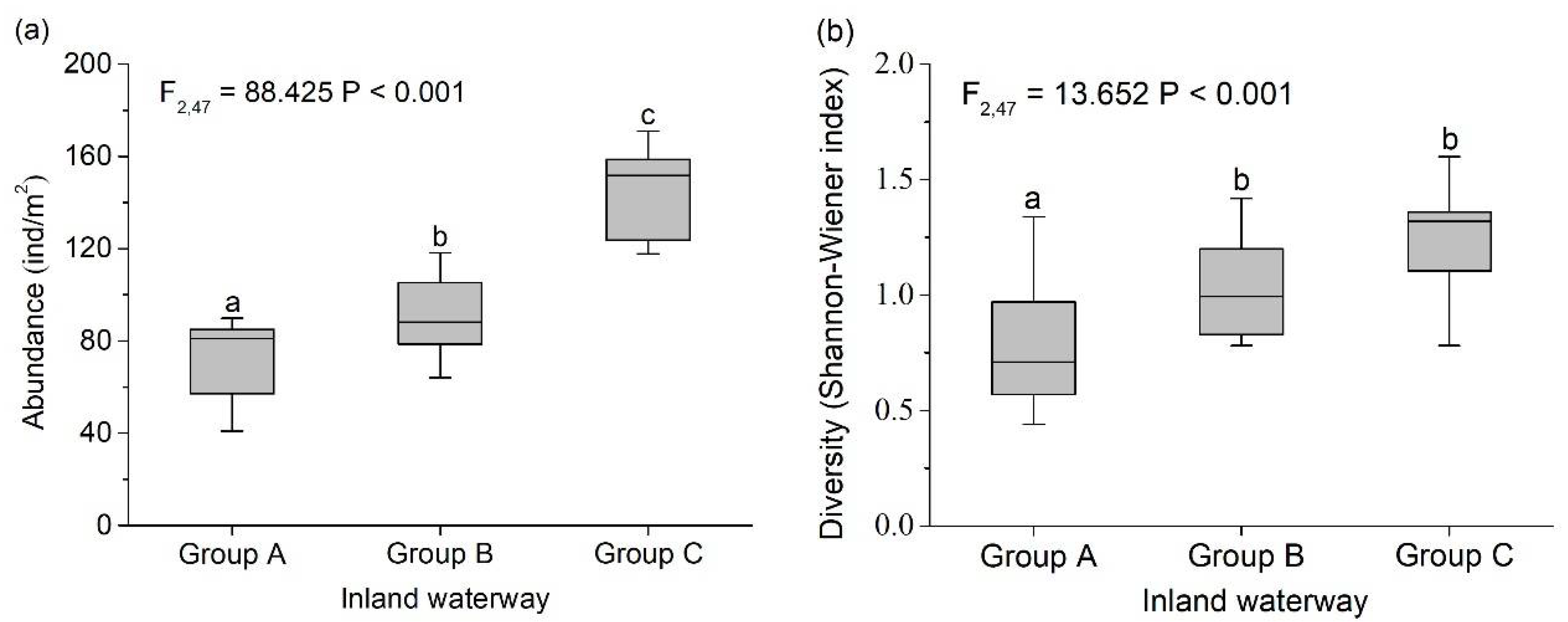
3.2. Characteristics of the Macroinvertebrate Community
3.3. Influence of Environmental Variables on Benthic Macroinvertebrate Communities
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dębska, K.; Rutkowska, B.; Szulc, W. Influence of the catchment area use on the water quality in the Utrata River. Environmental Monitoring and Assessment. Environ. Monit. Assess. 2022, 194, 165. [Google Scholar] [CrossRef] [PubMed]
- Arbaciauskas, K.; Semenchenk, V.; Grabowski, M. Assessment of biological contamination of benthic macroinvertebrate communities in European inland waterways. Aquatic Invasions 2018, 3, 211–230. [Google Scholar] [CrossRef]
- Paarlberg, A.J.; Guerrero, M.; Huthoff, F. Optimizing Dredge-and-Dump Activities for River Navigability Using a Hydro-Morphodynamic Model. Water 2015, 7, 3943–3962. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Zhang, Z.X.; Yang, H.J.; Bian, H.F.; Jiang, H.B.; Sheng, L.X.; He, C.G. Effects of instream restoration measures on the physical habitats and benthic macroinvertebrates in an agricultural headwater stream. Ecol. Eng. 2018, 122, 252–262. [Google Scholar] [CrossRef]
- Fraser, M.A.; Baldwin, D.S.; Rees, G.N.; Silvester, E.J.; Whitworth, K.L. Rehabilitation options for inland waterways impacted by sulfidic sediments-Field trials in a south-eastern Australian wetland. J. Environ. Manag. 2012, 102, 71–78. [Google Scholar] [CrossRef] [PubMed]
- La Guardia, M.J.; Hale, R.C.; Newman, B. Brominated Flame-Retardants in Sub-Saharan Africa: Burdens in Inland and Coastal Sediments in the eThekwini Metropolitan Municipality, South Africa. Environ. Sci. Technol. 2013, 47, 9643–9650. [Google Scholar] [CrossRef]
- Lorenz, S.; Pusch, M.T. Filtration activity of invasive mussel species under wave disturbance conditions. Biol. Invasions 2013, 15, 2681–2690. [Google Scholar] [CrossRef]
- Conder, J.M.; Fuchsman, P.C.; Grover, M.M.; Magar, V.S.; Henning, M.H. Critical review of mercury sediment quality values for the protection of benthic invertebrates. Environ. Toxicol. Chem. 2014, 34, 6–21. [Google Scholar] [CrossRef] [Green Version]
- Hanafiah, M.M.; Leuven, R.S.E.W.; Sommerwerk, N.; Tockner, K.; Huijbregts, M.A.J. Including the introduction of exotic species in life cycle impact assessment: The case of inland shipping. Environ. Sci. Technol. 2013, 47, 13924–13940. [Google Scholar] [CrossRef]
- Whitfield, A.K.; Becker, A. Impacts of recreational motorboats on fishes: A review. Mar. Pollut. Bull. 2014, 83, 24–31. [Google Scholar] [CrossRef]
- Weber, A.; Wolter, C. Habitat rehabilitation for juvenile fish in urban waterways: A case study from Berlin, Germany. J. Appl. Ichthyol. 2016, 33, 136–143. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Xu, Y.P.; Han, L.F.; Yu, Z.H.; Yang, M.N.; Pan, G.B. Assessment of river health based on an improved entropy-based fuzzy matter-element model in the Taihu Plain, China. Ecol. Indic. 2015, 57, 85–95. [Google Scholar] [CrossRef]
- Adkins, J.K.; Barton, C.D.; Grubbs, S.; Stringer, J.W.; Kolka, R.K. Assessment of Streamside Management Zones for Conserving Benthic Macroinvertebrate Communities Following Timber Harvest in Eastern Kentucky Headwater Catchments. Water 2016, 8, 261. [Google Scholar] [CrossRef] [Green Version]
- Gabel, F.; Garcia, X.F.; Schnauder, I.; Pusch, M.T. Effects of ship-induced waves on littoral benthic Invertebrates. Freshw. Biol. 2012, 57, 2425–2435. [Google Scholar] [CrossRef]
- Weber, A.; Zhang, J.; Nardin, A.; Sukhodolova, T.; Wolter, C. Modelling the Influence of Aquatic Vegetation on the Hydrodynamics of an Alternative Bank Protection Measure in a Navigable Waterway. River Res. Appl. 2016, 32, 2071–2080. [Google Scholar] [CrossRef]
- Feiler, U.; Höss, S.; Ahlf, W.; Gilberg, D.; Hammers-Wirtz, M.; Hollert, H.; Heininger, P. Sediment contact tests as a tool for the assessment of sediment quality in German waters. Environ. Toxicol. 2012, 32, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Tricarico, E.; Junqueira, A.O.R.; Dudgeon, D. Alien species in aquatic environments: A selective comparison of coastal and inland waters in tropical and temperate latitudes. Aquat. Conserv.-Mar. Freshw. Ecosyst. 2016, 26, 872–891. [Google Scholar] [CrossRef] [Green Version]
- Sukhodolova, T.; Weber, A.; Zhang, J.X.; Wolter, C. Effects of macrophyte development on the oxygen metabolism of an urban river rehabilitation structure. Sci. Total Environ. 2017, 574, 1125–1130. [Google Scholar] [CrossRef]
- Verbrugge, L.N.H.; Schipper, A.M.; Huijbregts, M.A.J.; Velde, G.V.; Leuven, R.S.E.W. Sensitivity of native and non-native mollusc species to changing river water temperature and salinity. Biol. Invasions 2012, 14, 1187–1199. [Google Scholar] [CrossRef] [Green Version]
- Aufdenkampe, A.K.; Mayorga, E.; Raymond, P.A.; Melack, J.M.; Doney, S.C.; Alin, S.R.; Aalto, R.E.; Yoo, K. Riverine coupling of biogeochemical cycles between land, oceans, and atmosphere. Front. Ecol. Environ. 2011, 9, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.L.; Jiang, X.M.; Li, Z.F.; Wang, J.; Cooper, K.M.; Xie, Z.C. Responses of macroinvertebrates and local environment to short-term commercial sand dredging practices in a flood-plain lake. Sci. Total Environ. 2018, 631–632, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Dilts, T.E.; Weisberg, P.J.; Leitner, P.; Matocq, M.D.; Inman, R.D.; Nussear, K.E.; Esque, T.C. Multiscale connectivity and graph theory highlight critical areas for conservation under climate change. Ecol. Appl. 2016, 26, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Jaumann, L.; Lerche, N.; Renatus, F.; Buchs, A.K.; Gade, R.; Geldermann, J.; Sauter, M. Selection of the Best Inland Waterway Structure: A Multicriteria Decision Analysis Approach. Water Resour. Manag. 2015, 29, 2733–2749. [Google Scholar] [CrossRef]
- Leitner, P.; Borgwardt, F.; Birk, S.; Graf, W. Multiple stressor effects on benthic macroinvertebrates in very large European rivers –A typology-based evaluation of faunal responses as a basis for future bioassessment. Sci. Total Environ. 2021, 756, 143472. [Google Scholar] [CrossRef]
- Gergs, R.; Koester, M.; Schulz, R.S. Potential alteration of cross-ecosystem resource subsidies by an invasive aquatic macroinvertebrate: Implications for the terrestrial food web. Freshw. Biol. 2014, 59, 2645–2655. [Google Scholar] [CrossRef]
- Rico, A.; Van den Brink, P.J.; Graf, W.; Focks, A. Relative influence of chemical and non-chemical stressors on invertebrate communities: A case study in the Danube River. Sci. Total Environ. 2016, 571, 1370–1382. [Google Scholar] [CrossRef]
- Hawkins, C.P.; Yuan, L.L. Multitaxon distribution models reveal severe alteration in the regional biodiversity of freshwater invertebrates. Freshw. Sci. 2016, 35, 1365–1376. [Google Scholar] [CrossRef] [Green Version]
- Lintott, P.R.; Bunnefeld, N.; Park, K.J. Opportunities for improving the foraging potential of urban waterways for bats. Biol. Conserv. 2015, 191, 224–233. [Google Scholar] [CrossRef]
- Harrison, S.S.C.; Pretty, J.L.; Shepherd, D.; Hildrew, A.G.; Smith, C.; Hey, R.D. The effect of instream rehabilitation structures on macroinvertebrates in lowland rivers. J. Appl. Ecol. 2004, 41, 1140–1154. [Google Scholar] [CrossRef]
- Weber, A.; Lautenbach, S.; Wolter, C. Improvement of aquatic vegetation in urban waterways using protected artificial shallows. Ecol. Eng. 2012, 42, 160–167. [Google Scholar] [CrossRef]
- Liedermann, M.; Tritthart, M.; Gmeiner, P.; Hinterleitner, M.; Schludermann, E.; Keckeis, H.; Habersack, H. Typification of vessel-induced waves and their interaction with different bank types, including management implications for river restoration projects. Hydrobiologia 2014, 729, 17–31. [Google Scholar] [CrossRef]
- Kupilas, B.; McKie, B.G.; Januschke, K.; Friberg, N.; Hering, D. Stable isotope analysis indicates positive effects of river restoration on aquatic-terrestrial linkages. Ecol. Indic. 2020, 113, 106242. [Google Scholar] [CrossRef]
- Habersack, H.; Hein, T.; Stanica, A.; Liska, L.; Mair, R.; Jager, E.; Hauer, C.; Bradley, C. Challenges of river basin management: Current status of, and prospects for, the River Danube from a river engineering perspective. Sci. Total Environ. 2016, 543, 828–845. [Google Scholar] [CrossRef] [Green Version]
- Cron, N.; Quick, I.; Zumbroich, T. Assessing and predicting the hydromorphological and ecological quality of federal waterways in Germany: Development of a methodological framework. Hydrobiologia 2018, 814, 75–87. [Google Scholar] [CrossRef]
- Navarro-Llacer, C.; Baeza, D.; de las Heras, J. Assessment of regulated rivers with indices based on macroinvertebrates, fish and riparian forest in the southeast of Spain. Ecol. Indic. 2010, 10, 935–942. [Google Scholar] [CrossRef]
- Vries, J.; Kraak, M.H.S.; Verdonschot, R.C.M.; Verdonschot, P.F.M. Quantifying cumulative stress acting on macroinvertebrate assemblages in lowland streams. Sci. Total Environ. 2019, 694, 133630. [Google Scholar] [CrossRef]
- Paganelli, D.; Kamburska, L.; Zaupa, S.; Garzoli, L.; Boggero, A. Impacts Analysis of Alien Macroinvertebrate Species in the Hydrographic System of a Subalpine Lake on the Italian–Swiss Border. Water 2021, 13, 3146. [Google Scholar] [CrossRef]
- Theodoropoulos, C.; Stamou, A.; Vardakas, L.; Papadaki, C.; Dimitriou, E.; Skoulikidis, N.; Kalogianni, E. River restoration is prone to failure unless pre-optimized within a mechanistic ecological framework | Insights from a model-based case study. Water Res. 2020, 173, 115550. [Google Scholar] [CrossRef]
- Kosnicki, E.; Sefick, S.A.; Paller, M.H.; Jerrell, M.S.; Prusha, B.A.; Sterrett, S.C. A Stream Multimetric Macroinvertebrate Index (MMI) for the Sand Hills Ecoregion of the Southeastern Plains, USA. Environ. Manag. 2016, 58, 741–751. [Google Scholar] [CrossRef]
- Feld, C.K.; Bello, F.; Doledec, S. Biodiversity of traits and species both show weak responses to hydromorphological alteration in lowland river macroinvertebrates. Freshw. Biol. 2014, 59, 233–248. [Google Scholar] [CrossRef]
- Mantyka-Pringle, C.S.; Martin, T.G.; Moffatt, D.B.; Linke, S.; Rhodes, J.R. Understanding and Predicting the Combined Effects of Climate Change and Land-Use Change on Freshwater Macroinvertebrates and Fish. J. Appl. Ecol. 2014, 51, 572–581. [Google Scholar] [CrossRef]
- Legendre, P.; Caceres, M. Beta diversity as the variance of community data: Dissimilarity coefficients and partitioning. Ecol. Lett. 2013, 16, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Soininen, J. A quantitative analysis of species sorting across organisms and ecosystems. Ecology 2014, 95, 3284–3292. [Google Scholar] [CrossRef]
- Pinto, U.; Maheshwari, B.L. River health assessment in peri-urban landscapes: An application of multivariate analysis to identify the key variables. Water Res. 2011, 45, 3915–3924. [Google Scholar] [CrossRef]
- ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide, Software for Canonical Community Ordination; Version 4.5; Microcomputer Power: Ithaca, NY, USA, 2002. [Google Scholar]
- Halabowski, D.; Lewin, I. Triggers for the Impoverishment of the Macroinvertebrate Communities in the Human-Impacted Rivers of Two Central European Ecoregions. Water Air Soil Pollut. 2021, 232, 55. [Google Scholar] [CrossRef]
- Martel, N.; Rodriguez, M.A.; Bérubé, P. Multi-scale analysis of responses of stream benthic macroinvertebrate to forestry activities and environmental context. Freshw. Biol. 2007, 52, 85–97. [Google Scholar] [CrossRef]
- Stewart, B.A. An assessment of the impacts of timber plantations on water quality and biodiversity values of Marbellup Brook, Western Australia. Environ. Monit. Assess. 2011, 173, 941–953. [Google Scholar] [CrossRef]
- Bunn, S.E.; Abal, E.G.; Smith, M.J.; Choy, S.C.; Fellows, C.S.; Harch, B.D. Integration of science and monitoring of river ecosystem health to guide investments in catchment protection and rehabilitation. Freshw. Biol. 2010, 55, 223–240. [Google Scholar] [CrossRef] [Green Version]
- Norris, R.H.; Linke, S.; Prosser, I.; Young, W.J.; Liston, P.; Bauer, N. Very-broad-scale assessment of human impacts on river condition. Freshw. Biol. 2007, 52, 959–976. [Google Scholar] [CrossRef]
- Langston, W.J.; O’Hara, S.; Pope, N.D.; Davey, M.; Shortidge, E.; Imamura, M. Bioaccumulation surveillance in Milford Haven Waterway. Environ. Monit. Assess. 2012, 184, 289–311. [Google Scholar] [CrossRef] [Green Version]
- Launois, L.; Veslot, J.; Irz, P. Development of a fish-based index (FBI) of biotic integrity for French lakes using the hindcasting approach. Ecol. Indic. 2011, 11, 1572–1583. [Google Scholar] [CrossRef]
- Holmes, R.W.; Anderson, B.S.; Phillips, B.M.; Hunt, J.W.; Crane, D.B.; Mekebri, A.; Connor, V. Statewide Investigation of the Role of Pyrethroid Pesticides in Sediment Toxicity in California’s Urban Waterways. Environ. Sci. Technol. 2008, 42, 7003–7009. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, W.; Nakano, Y.; Nakai, S.; Okuda, T.; Imai, T.; Okada, M. Macrobenthic succession and characteristics of a man-made intertidal sandflat constructed in the diversion channel of the Ohta River Estuary. Mar. Pollut. Bull. 2014, 82, 101–108. [Google Scholar] [CrossRef]
- Sang, L.Z.; Wall, A.; Mao, Z.; Yan, X.P.; Wang, J. A novel method for restoring the trajectory of the inland waterway ship by using AIS data. Ocean Eng. 2015, 110, 183–194. [Google Scholar] [CrossRef] [Green Version]
- Saxena, G.; Marzinelli, E.M.; Naing, N.N.; He, Z.L.; Liang, Y.T.; Tom, L. Metagenomics Reveals the Influence of Land Use and Rain on the Benthic Microbial Communities in a Tropical Urban Waterway. Msystems 2018, 3, e00136-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hein, T.; Schwarz, U.; Habersack, H.; Nichersu, I.; Preiner, S.; Willby, N.; Weigelhofer, G. Current status and restoration options for floodplains along the Danube River. Sci. Total Environ. 2016, 543, 778–790. [Google Scholar] [CrossRef]



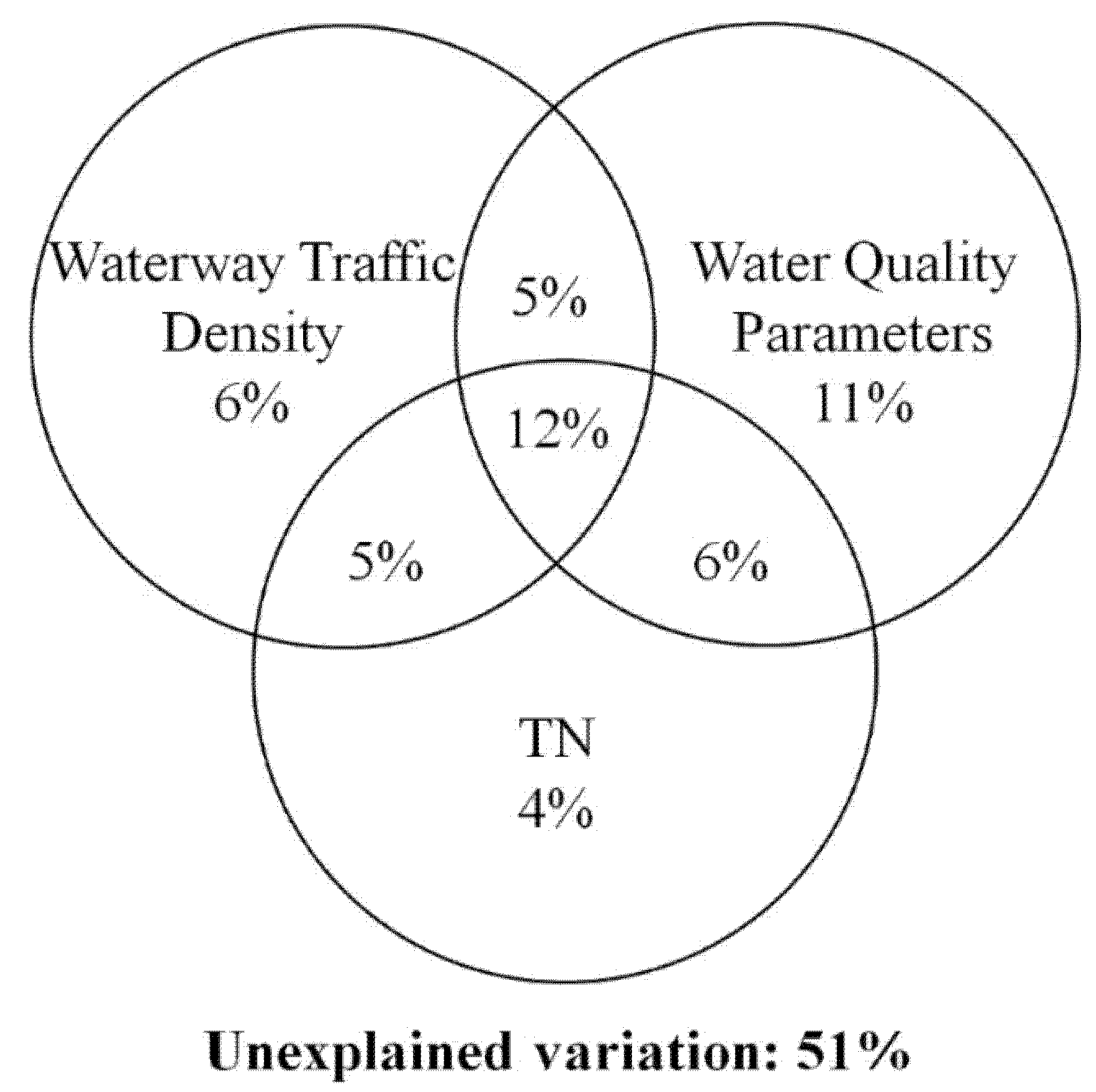
| Environmental Variables | Total Eigenvalues | % Variance | Cumulative % |
|---|---|---|---|
| Total nitrogen (TN) | 4.355 | 48.391 | 48.391 |
| Nitrate nitrogen (NO3-N) | 1.528 | 16.973 | 65.364 |
| Total phosphorus (TP) | 1.006 | 11.112 | 76.476 |
| Chemical oxygen demand (COD) | 0.811 | 9.025 | 85.502 |
| Suspended solids (SS) | 0.509 | 5.652 | 91.154 |
| Dissolved oxygen (DO) | 0.462 | 5.135 | 96.289 |
| Water velocity (VEL) | 0.181 | 2.015 | 98.304 |
| Temperature (TEM) | 0.105 | 1.163 | 99.467 |
| pH | 0.048 | 0.501 | 99.968 |
| Ammonium nitrogen (NH4–N) | 0.003 | 0.032 | 100.00 |
| Benthic Macroinvertebrate Taxa | Abbreviation | Group A | Group B | Group C | |
|---|---|---|---|---|---|
| Oligochaeta | Limnodrilus hoffmeisteri | Lim | 6.6 ± 0.7 | 6.4 ± 0.2 | 11.96 ± 0.1 |
| Branchiura sowerbyi | Bra sow | 10.2 ± 2.2 | 11.2 ± 1.1 | 16.1 ±1.3 | |
| Hirudinea | Glossiphonia sp. | Glo | 4 ± 0.5 | 2.76 ± 0.5 | |
| Gastropoda | Lithoglyphopsis sp. | Lith | 12.88 ± 0.5 | ||
| Sinotaia aeruginosa | Sin | 14.26 ± 0.9 | |||
| Bithynia sp. | Bith | 9.66 ± 0.8 | |||
| Radix swinhoei | Rad | 10.8 ± 0.5 | 20.56 ± 0.9 | ||
| Stenothyra glabra | Sten | 12.6 ± 1.8 | 12.4 ± 0.4 | 6.44 ± 0.1 | |
| Oncomelania sp. | Onc | 10.58 ± 0.1 | |||
| Gyraulus convexiusculus | Gyr | 2.76 ± 0.1 | |||
| Physa acuta | Phy | 5.6 ± 0.2 | |||
| Bivalvia | Corbicula fluminea | Cor | 7.8 ± 1.0 | 8.8 ± 0.6 | 8.28 ± 0.2 |
| Crustacea | Caridina sp. | Car | 5.4 ± 0.5 | 16.8 ± 1.8 | 5.98 ± 0.4 |
| Isecta | Baetis sp. | Bae | 7.2 ± 0.5 | ||
| Caenis sp. | Cae | 1.2 ± 0.1 | 1.2 ± 0.2 | 1.84 ± 0.2 | |
| Hydropsychidae sp. | Hyd | 1.6 ± 0.7 | |||
| Gomphidae sp. | Gom | 7.8 ± 0.7 | 2.76 ± 0.1 | ||
| Macromia sp. | Mac | 6.0 ± 1.4 | 3.68 ± 0.1 | ||
| Corixidae sp. | Cor | 1.84 ± 0.1 | |||
| Elmidae sp. | Elm | 1.84 ± 0.1 | |||
| Psephenidae sp. | Pse | 1.2 ± 0.2 | |||
| Parapoynx sp. | Par | 1.2 ± 0.4 | |||
| Bezzia sp. | Bez | 2.76 ± 0.1 | |||
| Tipulidae sp. | Tip | 1.2 ± 0.9 | |||
| Tabanidae sp. | Tab | 1.84 ± 0.4 | |||
| Chironomus sp. | Chi | 8.4 ± 0.4 | 4.8 ± 0.6 | 7.36 ± 0.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, P.; Wang, X.; Lan, Y.; Cui, B.; Bai, J.; Xie, T. Benthic Macroinvertebrate Diversity as Affected by the Construction of Inland Waterways along Montane Stretches of Two Rivers in China. Water 2022, 14, 1080. https://doi.org/10.3390/w14071080
Dou P, Wang X, Lan Y, Cui B, Bai J, Xie T. Benthic Macroinvertebrate Diversity as Affected by the Construction of Inland Waterways along Montane Stretches of Two Rivers in China. Water. 2022; 14(7):1080. https://doi.org/10.3390/w14071080
Chicago/Turabian StyleDou, Peng, Xuan Wang, Yan Lan, Baoshan Cui, Junhong Bai, and Tian Xie. 2022. "Benthic Macroinvertebrate Diversity as Affected by the Construction of Inland Waterways along Montane Stretches of Two Rivers in China" Water 14, no. 7: 1080. https://doi.org/10.3390/w14071080
APA StyleDou, P., Wang, X., Lan, Y., Cui, B., Bai, J., & Xie, T. (2022). Benthic Macroinvertebrate Diversity as Affected by the Construction of Inland Waterways along Montane Stretches of Two Rivers in China. Water, 14(7), 1080. https://doi.org/10.3390/w14071080









