Appraisal of Surface Water Quality of Nile River Using Water Quality Indices, Spectral Signature and Multivariate Modeling
Abstract
:1. Introduction
2. Materials and Methods
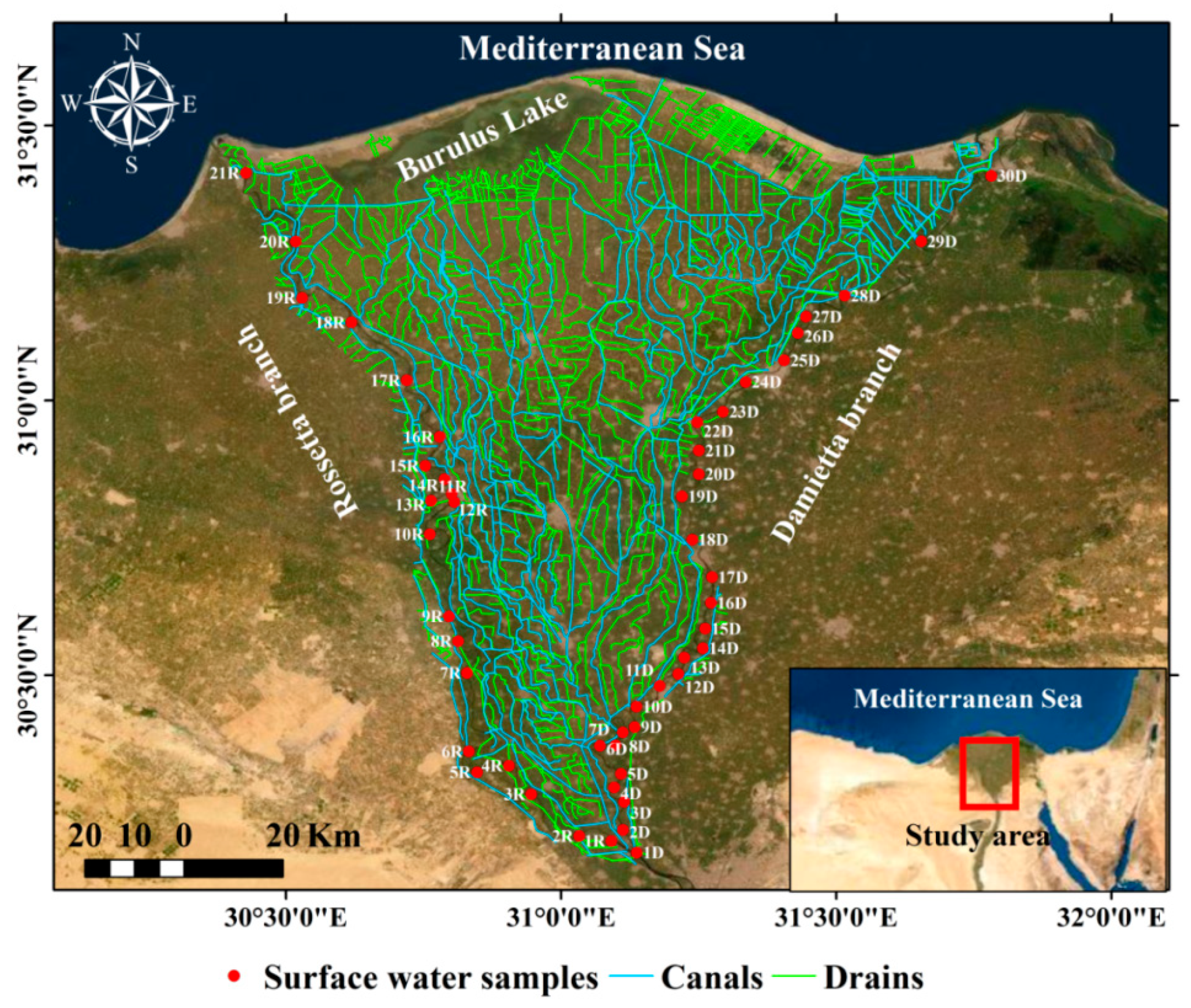
2.1. Study Area
2.2. Sampling and Analyses
2.3. Indexing Approach
2.3.1. Drinking Water Quality Index (DWQI)
2.3.2. Pollution Indices (PIs)
Metal Index (MI)
Pollution Index (PI)
2.4. Proximate Hyperspectral Measurements
2.5. Selected Spectral Reflectance Indices (SRIs) in This Study
2.6. Partial Least Squares Regression (PLSR)
2.7. Data Analysis
3. Results and Discussion
3.1. Physical and Chemical Parameters
3.2. Geochemical Facies and Controlling Mechanisms
3.3. Water Quality Indices (WQIs)
3.3.1. Drinking Water Quality Index (DWQI)
3.3.2. Pollution Indices (PIs)
3.4. Multivariate Statistical Analysis for Physicochemical Parameters
3.4.1. Cluster Analysis (CA)
3.4.2. Principal Component Analysis (PCA)
3.5. Performance of Different SRIs in the Assessment of Water Quality Indicators
3.6. Prediction of Different WQIs Using PLSR Models
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Higler, L.W.G. Fresh Surface Water: Biology and Biodiversity of River Systems. In Encyclopedia of Life Support Systems (EOLSS); ALTERRA: Wageningen, The Netherlands, 2012. [Google Scholar]
- Loucks, D.P.; van Beek, E. Water quality modeling and prediction. In Water Resource Systems Planning and Management: An Introduction to Methods, Models, and Applications; Springer International Publishing: Cham, Switzerland, 2017; pp. 417–467. [Google Scholar]
- Gupta, S.; Gupta, S.K. A critical review on water quality index tool: Genesis, evolution and future directions. Ecol. Inform. 2021, 63, 101299. [Google Scholar] [CrossRef]
- Devi, P.; Singh, P.; Kansal, S.K. Inorganic Pollutants in Water; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Kareem, S.L.; Mohammed, A.A. Removal of tetracycline from wastewater using circulating fluidized bed. Iraqi J. Chem. Pet. Eng. 2020, 21, 29–37. [Google Scholar] [CrossRef]
- Kareemb, S.L.; Jaberc, W.S.; Al-Malikia, L.A.; Al-husseinyb, R.A.; Al-Mamooria, S.K.; Alansarid, N. Water quality assessment and phosphorus effect using water quality indices: Euphrates River-Iraq as a case study. Groundw. Sustain. Dev. 2021, 14, 100630. [Google Scholar] [CrossRef]
- Elhaddad, E.; Al-Zyoud, S. The quality assessment of pollution of Rosetta branch, Nile River, Egypt. Arab. J. Geosci. 2017, 10, 97. [Google Scholar] [CrossRef]
- Duda, R.; Klebert, I.; Zdechlik, R. Groundwater pollution risk assessment based on vulnerability to pollution and potential impact of land use forms. Pol. J. Environ. Stud. 2020, 29, 87–99. [Google Scholar] [CrossRef]
- Khazheeva, Z.I.; Plyusnin, A.M.; Smirnova, O.K.; Peryazeva, E.G.; Zhambalova, D.I.; Doroshkevich, S.G.; Dabaeva, V.V. Mining activities and the chemical composition of R. Modonkul, Transbaikalia. Water 2020, 12, 979. [Google Scholar] [CrossRef] [Green Version]
- Tomaszewska, B.; Bundschuh, J.; Pająk, L.; Dendys, M.; Quezada, V.D.; Bodzek, M.; Armienta, M.A.; Muñoz, M.O.; Kasztelewicz, A. Use of low-enthalpy and waste geothermal energy sources to solve arsenic problems in freshwater production in selected regions of Latin America using a process membrane distillation—Research into model solutions. Sci. Total Environ. 2020, 714, 136853. [Google Scholar] [CrossRef]
- Elsayed, S.; Hussein, H.; Moghanm, F.S.; Khedher, K.M.; Eid, E.M.; Gad, M. Application of irrigation water quality indices and multivariate statistical techniques for surface water quality assessments in the Northern Nile Delta, Egypt. Water 2020, 12, 3300. [Google Scholar] [CrossRef]
- El Bouraie, M.M.; El Barbary, A.A.; Yehia, M.M.; Motawea, E.A. Heavy metal concentrations in surface river water and bed sediments at Nile Delta in Egypt. Suoseura 2010, 61, 1–12. [Google Scholar]
- Abdel-Satar, A.M.; Ali, M.H.; Goher, M.E. Indices of water quality and metal pollution of Nile River, Egypt. Egypt. J. Aquat. Res. 2017, 43, 21–29. [Google Scholar] [CrossRef]
- El Sayed, S.M.; Hegab, M.H.; Mola, H.R.A.; Ahmed, N.M.; Goher, M.E. An integrated water quality assessment of Damietta and Rosetta branches (Nile River, Egypt) using chemical and biological indices. Environ. Monit. Assess. 2020, 192, 228. [Google Scholar] [CrossRef] [PubMed]
- Taher, M.E.; Ghoneium, A.M.; Hopcroft, R.R.; ElTohamy, W.S. Temporal and spatial variations of surface water quality in the Nile River of Damietta Region, Egypt. Environ. Monit. Assess. 2021, 193, 128. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.; Abou El-Safa, M.M.; Farouk, M.; Hussein, H.; Alnemari, A.M.; Elsayed, S.; Khalifa, M.M.; Moghanm, F.S.; Eid, E.M.; Saleh, A.H. Integration of Water Quality Indices and Multivariate Modeling for Assessing Surface Water Quality in Qaroun Lake, Egypt. Water 2021, 13, 2258. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, B.X.; Wang, P.; Chen, J.; Yang, L.; Xiao, K. Hydrogeochemical evolution and heavy metal contamination in groundwater of a reclaimed land on Zhoushan Island. Water 2018, 10, 316. [Google Scholar] [CrossRef] [Green Version]
- Gad, M.; El-Hattab, M. Integration of water pollution indices and DRASTIC model for assessment of groundwater quality 640 in El Fayoum Depression, Western Desert, Egypt. J. Afr. Earth Sci. 2019, 158, 103554. [Google Scholar] [CrossRef]
- Gad, M.; El Osta, M. Geochemical controlling mechanisms and quality of the groundwater resources in El Fayoum Depression, Egypt. Arab. J. Geosci. 2020, 13, 861. [Google Scholar] [CrossRef]
- Semiromi, F.B.; Hassani, A.; Torabian, A.; Karbassi, A.; Lotfi, F.H. Water quality index development using fuzzy logic: A case study of the Karoon River of Iran. Afr. J. Biotechnol. 2011, 10, 10125–10133. [Google Scholar]
- Vinod, J.; Satish, D.; Sapana, G. Assessment of water quality index of industrial area surface water samples. Int. J. ChemTech Res. 2013, 5, 278–283. [Google Scholar]
- Guo, Q.; Wang, Y. Impact of geothermal wastewater drainage on arsenic species in environmental media: A case study at the Yangbajing geothermal field, Tibet, China. Procedia Earth Planet. Sci. 2013, 7, 317–320. [Google Scholar] [CrossRef] [Green Version]
- Gitau, M.W.; Chen, J.; Ma, Z. Water quality indices as tools for decision making and management. Water Resour. Manag. 2016, 30, 2591–2610. [Google Scholar] [CrossRef]
- Mukate, S.; Wagh, V.; Panaskar, D.; Jacobs, J.A.; Sawant, A. Development of new integrated water quality index (IWQI) model to evaluate the drinking suitability of water. Ecol. Indic. 2019, 101, 348–354. [Google Scholar] [CrossRef]
- Wątor, K.; Zdechlik, R. Application of water quality indices to the assessment of the effect of geothermal water discharge on river water quality—Case study from the Podhale region (Southern Poland). Ecol. Indic. 2021, 121, 107098. [Google Scholar] [CrossRef]
- Bhargava, D.; Saxena, B.; Dewakar, A. A Study of Geopollutants in the Godavary River Basin in India, Asian Environment; IOS Press: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Dwivedi, S.; Tiwari, I.; Bhargava, D. Water Quality of the River Ganga at Varanasi. J. Inst. Eng. India Part E Environ. Eng. Div. 1997, 78, 1–4. [Google Scholar]
- Noori, R.; Berndtsson, R.; Hosseinzadeh, M.; Adamowski, J.F.; Abyaneh, M.R. A critical review on the application of the national sanitation foundation water quality index. Environ. Pollut. 2019, 244, 575–587. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, R.H.; Lee, J.; Cheong, T.J.; Yum, B.W.; Chang, H.W. Multivariate statistical analysis to identify the major factors governing groundwater quality in the coastal area of Kimje, South Korea. Hydrol. Process. Int. J. 2005, 19, 1261–1276. [Google Scholar] [CrossRef]
- Noori, R.; Sabahi, M.S.; Karbassi, A.R.; Baghvand, A.; Zadeh, H.T. Multivariate statistical analysis of surface water quality based on correlations and variations in the data set. Desalination 2010, 260, 129–136. [Google Scholar] [CrossRef]
- Hamid, A.; Bhat, S.A.; Bhat, S.U.; Jehangir, A. Environmetric techniques in water quality assessment and monitoring: A case study. Environ. Earth Sci. 2016, 75, 321. [Google Scholar] [CrossRef]
- Jung, K.Y.; Lee, K.L.; Im, T.H.; Lee, I.J.; Kim, S.; Han, K.Y.; Ahn, J.M. Evaluation of water quality for the Nakdong River watershed using multivariate analysis. Environ. Technol. Innov. 2016, 5, 67–82. [Google Scholar] [CrossRef]
- Barzegar, R.; Moghaddam, A.A.; Tziritis, E.; Adamowski, J.; Nassar, J.B.; Noori, M.; Kazemian, N. Exploring the hydrogeochemical evolution of cold and thermal waters in the Sarein-Nir area, Iran using stable isotopes (δ18O and δD), geothermometry and multivariate statistical approaches. Geothermics 2020, 85, 101815. [Google Scholar] [CrossRef]
- El Osta, M.; Masoud, M.; Alqarawy, A.; Elsayed, S.; Gad, M. Groundwater Suitability for Drinking and Irrigation Using Water Quality Indices and Multivariate Modeling in Makkah Al-Mukarramah Province, Saudi Arabia. Water 2022, 14, 483. [Google Scholar] [CrossRef]
- Tariq, R.S.; Shah, M.H.; Shaheen, N.; Khalique, A.; Manzoor, S.; Jaffar, M. Multivariate analysis of selected metals in tannery effluents and related soil. J. Hazard. Mater. 2005, A122, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.R.; Shaheen, N.; Khalique, A.; Sha, M.H. Distribution, correlation, and source apportionment of selected metals in tannery effluents, related soils, and groundwater—A case studies from Multan, Pakistan. Environ. Monit. Assess. 2010, 166, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, S.; Karmegam, U.; Prasanna, M.V.; Sasidhar, P. A study on evaluation of probable sources of heavy metal pollution in groundwater of Kalpakkam region, South India. Environmentalist 2012, 32, 371–382. [Google Scholar] [CrossRef]
- Prasanna, M.V.; Praveena, S.M.; Chidambaram, S.; Nagarajan, R.; Elayaraja, A. Evaluation of water quality pollution for heavy metal contamination monitoring: A case study from Curtin Lake. Environ. Earth Sci. 2012, 67, 1987–2001. [Google Scholar] [CrossRef]
- Sharma, G.; Lata, R.; Thakur, N.; Bajala, V.; Kuniyal, J.C.; Kumar, K. Application of multivariate statistical analysis and water quality index for quality characterization of Parbati River, Northwestern Himalaya, India. Discov. Water 2021, 1, 5. [Google Scholar] [CrossRef]
- Franco-Uria, A.; Lopez-Mateo, C.; Roca, E.; Eernandez-Marcos, M.L. Source identification of heavy metals in pastureland by multivariate analysis in NW Spain. J. Hazard. Mater. 2009, 165, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Kwaya, M.Y.; Hamidu, H.; Kachalla, M.; Abdullahi, I.M. Preliminary Ground and Surface Water Resources Trace Elements Concentration, Toxicity and Statistical Evaluation in Part of Yobe State, North Eastern Nigeria. Geosciences 2017, 7, 117–128. [Google Scholar]
- De Bartolomeo, A.; Poletti, L.; Sanchini, G.; Sebastiani, B.; Morozzi, G. Relationship among parameters of lake polluted sediments evaluated by multivariate statistical analysis. Chemosphere 2004, 55, 1323–1329. [Google Scholar] [CrossRef]
- Kalamaras, N.; Michalopoulou, H.; Byun, H.R. Detection of drought events in Greece using daily precipitation. Hydrol. Res. 2010, 41, 126–133. [Google Scholar] [CrossRef]
- Wang, M.; Markert, B.; Chen, W.; Peng, C.; Ouyang, Z. Identification of heavy metal pollutants using multivariate analysis and effects of land uses on their accumulation in urban soils in Beijing, China. Environ. Monit. Assess. 2012, 184, 5889–5897. [Google Scholar] [CrossRef]
- Ahmad, T.; Gupta, G.; Sharma, A.; Kaur, B.; Alsahli, A.A.; Ahmad, P. Multivariate Statistical Approach to Study Spatiotemporal Variations in Water Quality of aHimalayan Urban Fresh Water Lake. Water 2020, 12, 2365. [Google Scholar] [CrossRef]
- Gitelson, A.; Garbuzov, G.; Szilagyi, F.; Mittenzwey, K.H.; Karnieli, A.; Kaiser, A. Quantitative remote-sensing methods for real-time monitoring of inland waters quality. Int. J. Remote Sens. 1993, 14, 1269–1295. [Google Scholar] [CrossRef]
- Xing, Z.; Chen, J.; Zhao, X.; Li, Y.; Li, X.; Zhang, Z.; Lao, C.; Wang, H. Quantitative estimation of wastewater quality parameters by hyperspectral band screening using GC, VIP and SPA. PeerJ 2019, 7, e8255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, L.; Huang, C.; Zhong, Y.; Wang, Z.; Hu, X.; Lin, L. Inland waters suspended solids concentration retrieval based on PSO-LSSVM for UAV-borne hyperspectral remote sensing imagery. Remote Sens. 2019, 11, 1455. [Google Scholar] [CrossRef] [Green Version]
- Gholizadeh, M.H.; Melesse, A.M.; Reddi, L.A. A Comprehensive review on water quality parameters estimation using remote sensing techniques. Sensors 2016, 16, 1298. [Google Scholar] [CrossRef] [Green Version]
- Song, K.; Li, L.; Li, S.; Tedesco, L.; Hall, B.; Li, L. Hyperspectral remote sensing of total phosphorus (TP) in three central Indiana water supply reservoirs. Water Air Soil Pollut. 2012, 223, 1481–1502. [Google Scholar] [CrossRef]
- Giardino, C.; Bresciani, M.; Cazzaniga, I.; Schenk, K.; Rieger, P.; Braga, F.; Matta, E.; Brando, V.E. Evaluation of multi-resolution satellite sensors for assessing water quality and bottom depth of Lake Garda. Sensors 2014, 14, 24116–24131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khadr, M.; Gad, M.; El-Hendawy, S.; Al-Suhaibani, N.; Dewir, Y.H.; Tahir, M.U.; Mubushar, M.; Elsayed, S. The integration of multivariate statistical approaches, hyperspectral reflectance, and data-driven modeling for assessing the quality and suitability of groundwater for Irrigation. Water 2021, 13, 35. [Google Scholar] [CrossRef]
- Brando, V.E.; Braga, F.; Zaggia, L.; Giardino, C.; Bresciani, M.; Matta, E.; Bellafiore, D.; Ferrarin, C.; Maicu, F.; Benetazzo, A.; et al. High-resolution satellite turbidity and sea surface temperature observations of river plume interactions during a significant flood event. Ocean Sci. 2015, 11, 909–920. [Google Scholar] [CrossRef] [Green Version]
- Shareef, M.A.; Khenchaf, A.; Toumi, A. Integration of passive and active microwave remote sensing to estimate water quality parameters. In Proceedings of the Radar Conference, Philadelphia, PA, USA, 2–6 May 2016; pp. 1–4. [Google Scholar]
- Xiao, R.; Wang, G.; Zhang, Q.; Zhang, Z. Multi-scale analysis of relationship between landscape pattern and urban river water quality in different seasons. Sci. Rep. 2016, 6, 25250. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Gao, B.; Hao, H.; Zhou, H.; Lu, J. Nitrogen and phosphorus in sediments in china: A national-scale assessment and review. Sci. Total Environ. 2017, 576, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Elhag, M.; Gitas, I.; Othman, A.; Bahrawi, J.; Gikas, P. Assessment of water quality parameters using temporal remote sensing spectral reflectance in arid environments, Saudi Arabia. Water 2019, 11, 556. [Google Scholar] [CrossRef] [Green Version]
- Elsayed, S.; Ibrahim, H.; Hussein, H.; Elsherbiny, O.; Elmetwalli, A.H.; Moghanm, F.S.; Ghoneim, A.M.; Danish, S.; Datta, R.; Gad, M. Assessment of water quality in Lake Qaroun using ground-based remote sensing data and artificial neural networks. Water 2021, 13, 3094. [Google Scholar] [CrossRef]
- Garriga, M.; Romero-Bravo, S.; Estrada, F.; Escobar, A.; Matus, I.A.; del Pozo, A.; Astudillo, C.A.; Lobos, G.A. Assessing wheat traits by spectral reflectance: Do we really need to focus on predicted trait-values or directly identify the elite genotypes group? Front. Plant Sci. 2017, 8, 280. [Google Scholar] [CrossRef] [Green Version]
- Gad, M.; El-Hendawy, S.; Al-Suhaibani, N.; Tahir, M.U.; Mubushar, M.; Elsayed, S. Combining hydrogeochemical characterization and a hyperspectral reflectance tool for assessing quality and suitability of two groundwater resources for irrigation in Egypt. Water 2020, 12, 2169. [Google Scholar] [CrossRef]
- Ezzet, S.M.; Mahdy, H.M.; Abo-State, M.A.; El Shakour, E.H.A.; El-Bahnasawy, M.A. Water quality assessment of Nile River at Rosetta branch: Impact of drains discharge. Middle East J. Sci. Res. 2012, 12, 413–423. [Google Scholar]
- Eltohamy, W.S.; Abdel-Baki, S.N.; Abdel-Aziz, N.E.; Khidr, A.A. Evaluation of spatial and temporal variations of surface water quality in the Nile River Damietta branch. Ecol. Chem. Eng. S 2018, 25, 569–580. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Ayandiran, T.A.; Fawole, O.O.; Dahunsi, S.O. Water quality assessment of bitumen polluted Oluwa River, South-Western Nigeria. Water Resour. Ind. 2018, 19, 13–24. [Google Scholar] [CrossRef]
- Kachroud, M.; Trolard, F.; Kefi, M.; Jebari, S.; Bourrie, G. Water quality indices: Challenges and application limits in the literature. Water 2019, 11, 361. [Google Scholar] [CrossRef] [Green Version]
- Lone, S.A.; Bhat, S.U.; Hamid, A.; Bhat, F.A.; Kumar, A. Quality assessment of springs for drinking water in the Himalaya of South Kashmir, India. Environ. Sci. Pollut. Control Ser. 2021, 28, 2279–2300. [Google Scholar] [CrossRef]
- Rawat, H.; Singh, R.; Namtak, S.; Deep, A.; Mamgain, S.; Sharma, A.; Kumar, R. Water quality assessment of Garhwal Himalayan Lake Tarakund based on the application of WQI and mitigation measures for its conservation and management. Int. J. Energy Water Resour. 2021, 5, 73–84. [Google Scholar] [CrossRef]
- Brown, R.M.; McCleiland, N.J.; Deininger, R.A.; O’Connor, M.F. A Water Quality Index—Crossing the Psychological Barrier. Proc. Int. Conf. Water Poll. Res. 1972, 6, 787–797. [Google Scholar]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; Incorporating the 1st Addendum; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Tamasi, G.; Cini, R. Heavy metals in drinking waters from Mount Amiata (Tuscany, Italy) possible risks from arsenic for public health in the province of Siena. Sci. Total Environ. 2004, 327, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Ojekunle, O.Z.; Ojekunle, O.V.; Adeyemi, A.A.; Taiwo, A.G.; Sangowusi, O.R.; Taiwo, A.M.; Adekitan, A.A. Evaluation of surface water quality indices and ecological risk assessment for heavy metals in scrap yard neighbourhood. SpringerPlus 2016, 5, 2–16. [Google Scholar] [CrossRef] [Green Version]
- Caerio, S.; Costa, M.H.; Ramos, T.B.; Fernandes, F.; Silveira, N.; Coimbra, A.; Painho, M. Assessing heavy metal contamination in Sado Estuary sediment: An index analysis approach. Ecol. Indic. 2005, 5, 155–169. [Google Scholar] [CrossRef]
- Goher, M.E.; Mahdy, E.M.; Abdo, M.H.; El Dars, F.M.; Korium, M.A.; Elsherif, A.S. Water quality status and pollution indices of Wadi El-Rayan lakes, El-Fayoum, Egypt. Sustain. Water Resour. Manag. 2019, 5, 387–400. [Google Scholar]
- Chipman, J.W.; Olmanson, L.G.; Gitelson, A.A. Remote Sensing Methods for Lake Management: A Guide for Resource Managers and Decision-Makers; North American Lake Management Society: Madison, WI, USA, 2009. [Google Scholar]
- Somvanshi, S.; Kunwar, P.; Singh, N.; Shukla, S.; Pathak, V. Integrated remote sensing and GIS approach for water quality analysis of gomti river, Uttar Pradesh. Int. J. Environ. Sci. 2012, 3, 62–74. [Google Scholar]
- Bhatti, A.M.; Nasu, S.; Takagi, M.; Nojiri, Y. Assessing the potential of remotely sensed data for water quality monitoring of coastal and inland waters. Res. Bull. Kochi Univ. Technol. 2008, 5, 201–207. [Google Scholar]
- Wang, Z.; Kawamura, K.; Sakuno, Y.; Fan, X.; Gong, Z.; Lim, J. Retrieval of chlorophyll-a and total suspended solids using iterative stepwise elimination partial least squares (ISE-PLS) regression based on field hyperspectral measurements in irrigation ponds in Higashihiroshima, Japan. Remote Sens. 2017, 9, 264. [Google Scholar] [CrossRef] [Green Version]
- Shafique, N.A.; Autrey, B.C.; Fulk, F.; Cormier, S.M. Hyperspectral narrow wavebands selection for optimizing water quality monitoring on the Great Miami River, Ohio. J. Spat. Hydrol. 2001, 1, 1–22. [Google Scholar]
- Elsayed, S.; Gad, M.; Farouk, M.; Saleh, A.H.; Hussein, H.; Elmetwalli, A.H.; Elsherbiny, O.; Moghanm, F.S.; Moustapha, M.E.; Taher, M.A.; et al. Using Optimized Two and Three-Band Spectral Indices and Multivariate Models to Assess Some Water Quality Indicators of Qaroun Lake in Egypt. Sustainability 2021, 13, 10408. [Google Scholar] [CrossRef]
- Vinciková, H.; Hanuš, J.; Pechar, L. Spectral reflectance is a reliable water-quality estimator for small, highly turbid wetlands. Wetl. Ecol. Manag. 2015, 23, 933–946. [Google Scholar] [CrossRef]
- Menken, K.D.; Brezonik, P.L.; Bauer, M.E. Influence of chlorophyll and colored dissolved organic matter (CDOM) on Lake Reflectance Spectra: Implications for Measuring Lake Properties by Remote Sensing. Lake Reserv. Manag. 2006, 22, 179–190. [Google Scholar] [CrossRef] [Green Version]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Piper, A.M. A graphic procedure in the geochemical interpretation of water-analyses. EOS Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Chadha, D.K. A proposed new diagram for geochemical classification of natural waters and interpretation of chemical 617 data. Hydrogeol. J. 1999, 7, 431–439. [Google Scholar] [CrossRef]
- Giménez-Forcada, E.; San Román, F.J.S. An excel macro to plot the HFE-Diagram to identify seawater intrusion phases. Groundwater 2015, 53, 819–824. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A.; Kumar, R.; Bhardwaj, R.; Thukral, A.K.; Rodrigo-Comino, J. Assessment of heavy-metal pollution in three different Indian water bodies by combination of multivariate analysis and water pollution indices. Hum. Ecol. Risk Assess. 2018, 26, 1–16. [Google Scholar] [CrossRef]
- Ahipathy, M.V.; Puttaiah, E.T. Ecological characteristics of Vrishabhavathy River in Bangalore (India). Environ. Geol. 2006, 49, 1217–1222. [Google Scholar] [CrossRef]
- Charles, E.R. Investigating Water Problems: A Water Analysis Manual; Publishing by LaMotte Chemical Products Company: Chestertown, MD, USA, 1970. [Google Scholar]
- WRC, Water Resources Commission. Ghana Raw Water Criteria and Guidelines. Vol. 1. Domestic Water; CSIR-Water Research Institute: Accra, Ghana, 2003.
- Gad, M.; Elsayed, S.; Moghanm, F.S.; Almarshadi, M.H.; Alshammari, A.S.; Khedher, K.M.; Eid, E.M.; Hussein, H. Combining Water Quality Indices and Multivariate Modeling to Assess Surface Water Quality in the Northern Nile Delta, Egypt. Water 2020, 12, 2142. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, X.; Chen, Y.; Cai, Y.; Deng, J. Assessing river water quality using water quality index in Lake Taihu Basin, China. Sci. Total Environ. 2018, 612, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, G.; Liu, H.; Lam, P.K. Multivariate statistical evaluation of dissolved trace elements and a water quality assessment in the middle reaches of Huaihe River, Anhui, China. Sci. Total Environ. 2017, 583, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Bao, F.; Wang, S.; Cui, F. Water quality assessment of the Huaihe River segment of Bengbu (China) using multivariate statistical techniques. Water Res. 2016, 43, 166–176. [Google Scholar] [CrossRef]
- Duan, W.; He, B.; Takara, K.; Luo, P.; Nover, D.; Sahu, N.; Yamashiki, Y. Spatiotemporal evaluation of water quality incidents in Japan between 1996 and 2007. Chemosphere 2013, 93, 946–953. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Li, D.; Shi, K.; Zhang, Y. Temporal and spatial dynamics of phytoplankton primary production in Lake Taihu derived from MODIS data. Remote Sens. 2017, 9, 195. [Google Scholar] [CrossRef]
- Saleh, A.H.; Elsayed, S.; Gad, M.; Elmetwalli, A.H.; Elsherbiny, O.; Hussein, H.; Moghanm, F.S.; Qazaq, A.S.; Eid, E.M.; El-Kholy, A.S.; et al. Utilization of pollution indices, hyperspectral reflectance indices, and data-driven multivariate modelling to assess the bottom sediment quality of Lake Qaroun, Egypt. Water 2022, 14, 890. [Google Scholar] [CrossRef]
- Zhang, Y.; Giardino, C.; Li, L. Water optics and water colour remote sensing. Remote Sens. 2017, 9, 818. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, F.; Ding, J. Evaluation of water quality based on a machine learning algorithm and water quality index for the Ebinur Lake Watershed, China. Sci. Rep. 2017, 7, 12858. [Google Scholar] [CrossRef] [Green Version]
- Seyhan, E.; Dekker, A. Application of remote sensing techniques for water quality monitoring. Hydrol. Biol. Bull. 1986, 20, 41–50. [Google Scholar] [CrossRef]
- El-Din, M.S.; Gaber, A.; Koch, M.; Ahmed, R.S.; Bahgat, I. Remote sensing application for water quality assessment in lake timsah, Suez Canal, Egypt. J. Remote Sens. Technol. 2013, 1, 61–74. [Google Scholar] [CrossRef]
- Wu, J.L.; Ho, C.R.; Huang, C.C.; Srivastav, A.L.; Tzeng, J.H.; Lin, Y.T. Hyperspectral sensing for turbid water quality monitoring in freshwater rivers: Empirical relationship between reflectance and turbidity and total solids. Sensors 2014, 14, 22670–22688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerch, R.N.; Baffaut, C.; Kitchen, N.R.; Sadler, E.J. Long-term agroecosystem research in the Central Mississippi River Basin: Dissolved nitrogen and phosphorus transport in a high-runoff-potential watershed. J. Environ. Qual. 2015, 44, 44–57. [Google Scholar] [CrossRef] [PubMed]









| Physicochemical Parameters | Measured Sample | WHO (2017) Si | Unit Weight wi | Sub Index Qi | Qi × Wi |
|---|---|---|---|---|---|
| pH | 8.0 | 8.5 | 0.00105 | 66.0000 | 0.0692 |
| Turb. | 3.22 | 5 | 0.00178 | 64.4840 | 0.1149 |
| TDS | 261 | 500 | 0.00002 | 52.2000 | 0.0009 |
| EC | 408 | 1500 | 0.00001 | 27.1875 | 0.0002 |
| TH | 107.56 | 500 | 0.00002 | 21.5120 | 0.0004 |
| K+ | 10.61 | 12 | 0.00074 | 88.4365 | 0.0656 |
| Na+ | 29.39 | 200 | 0.00004 | 14.6941 | 0.0007 |
| Mg2− | 11.60 | 50 | 0.00018 | 23.2000 | 0.0041 |
| Ca2+ | 24.00 | 75 | 0.00012 | 32.0000 | 0.0038 |
| Cl− | 53.90 | 250 | 0.00004 | 21.5600 | 0.0008 |
| SO42− | 11.00 | 250 | 0.00004 | 4.4000 | 0.0002 |
| HCO32− | 115.20 | 120 | 0.00007 | 96.0000 | 0.0071 |
| CO3− | 0.00 | 350 | 0.00003 | 0.0000 | 0.0000 |
| NO3− | 4.08 | 50 | 0.00018 | 8.1600 | 0.0015 |
| Al | 0.2616 | 0.1 | 0.08907 | 261.6000 | 23.3012 |
| Ba | 0.0439 | 0.3 | 0.02969 | 14.6333 | 0.4345 |
| Cr | 0.0056 | 0.05 | 0.17814 | 11.2000 | 1.9952 |
| Cu | 0.0066 | 2 | 0.00445 | 0.3300 | 0.0015 |
| Fe | 0.3596 | 0.3 | 0.02969 | 119.8667 | 3.5589 |
| Mn | 0.0547 | 0.1 | 0.08907 | 54.7000 | 4.8722 |
| Mo | 0.0003 | 0.07 | 0.12725 | 0.4286 | 0.0545 |
| Ni | 0.0096 | 0.02 | 0.44536 | 48.0000 | 21.3772 |
| Zn | 0.0159 | 3 | 0.00297 | 0.5300 | 0.0016 |
| ∑ (wi) = 1 |
| Class | PI Value | Effect |
|---|---|---|
| 1 | <1 | No effect |
| 2 | 1–2 | Slightly affected |
| 3 | 2–3 | Moderately affected |
| 4 | 3–5 | Strongly affected |
| 5 | >5 | Seriously affected |
| SRIs | Formula | References |
|---|---|---|
| Published SRIs | ||
| Ratio between blue and red | Blue/Red | [74] |
| Ratio between green and red | Green/Red | [75] |
| Ratio between NIR and red | NIR/Red | [76] |
| Normalized difference index (NDI704,698) | (R704 − R698)/(R704 + R698) | [77] |
| Ratio spectral index | ||
| RSI717,630 | R717/R630 | [78] |
| RSI620,608 | R620/R608 | [79] |
| RSI670,470 | R670/R470 | [79] |
| RSI806,670 | R806/R670 | [80] |
| RSI850,550 | R850/R550 | [80] |
| RSI700,670 | R705/R675 | [81] |
| Newly SRIs | ||
| RSI584,628 | R584/R628 | This work |
| RSI530,680 | R530/R680 | |
| RSI640,590 | R640/R590 | |
| RSI760,560 | R760/R560 | |
| RSI720,580 | R720/R580 | |
| RSI776,490 | R776/R490 | |
| RSI686,570 | R686/R570 | |
| RSI780,514 | R780/R514 | |
| RSI730,540 | R730/R540 | |
| RSI590,540 | R590/R640 |
| Physicochemical Parameters | Rosetta Branch, Nile River (n = 21) | Damietta Branch, Nile River (n = 30) | Data across Two Branches (n = 51) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Min. | Max. | Mean | Min. | Max. | Mean | Min. | Max. | Mean | |
| T °C | 27.0 | 33.7 | 30.0 | 27.1 | 28.1 | 27.4 | 27.0 | 33.7 | 28.4 |
| pH | 7.40 | 8.10 | 7.80 | 7.40 | 8.40 | 7.89 | 7.40 | 8.40 | 7.85 |
| Turb. | 0.69 | 7.62 | 3.26 | 0.69 | 7.44 | 2.76 | 0.69 | 7.62 | 2.97 |
| TSS | 9.20 | 45.20 | 21.17 | 8.69 | 41.2 | 17.41 | 8.69 | 45.2 | 18.96 |
| EC | 341.00 | 544.00 | 404.00 | 328.00 | 703.00 | 385.40 | 328.00 | 703.00 | 393.06 |
| TDS | 218.00 | 348.00 | 258.52 | 210.00 | 450.00 | 246.63 | 210.00 | 450.00 | 251.53 |
| K+ | 3.11 | 16.81 | 9.01 | 5.21 | 14.41 | 8.15 | 3.11 | 16.81 | 8.50 |
| Na+ | 20.70 | 38.91 | 28.91 | 16.29 | 46.05 | 22.36 | 16.29 | 46.05 | 25.06 |
| Mg2− | 8.20 | 19.00 | 13.17 | 3.40 | 22.80 | 12.15 | 3.40 | 22.80 | 12.57 |
| Ca2+ | 16.00 | 36.00 | 23.24 | 22.72 | 44.00 | 28.06 | 16.00 | 44.00 | 26.07 |
| Cl− | 35.50 | 88.70 | 54.13 | 23.00 | 61.00 | 40.10 | 23.00 | 88.70 | 45.88 |
| SO42− | 11.00 | 22.00 | 14.90 | 12.00 | 33.00 | 16.80 | 11.00 | 33.00 | 16.02 |
| HCO32− | 88.40 | 134.00 | 108.88 | 60.80 | 208.60 | 104.17 | 60.80 | 208.60 | 106.11 |
| CO3− | N.D. | N.D. | N.D. | 5.00 | 19.00 | 8.87 | N.D. | 19.00 | 5.22 |
| NO3− | 3.68 | 12.53 | 6.11 | 2.25 | 8.51 | 5.48 | 2.25 | 12.53 | 5.74 |
| Al | 0.0161 | 1.7248 | 0.4070 | 0.1825 | 1.8854 | 0.6096 | 0.0161 | 1.8854 | 0.5262 |
| Ba | 0.0439 | 0.0675 | 0.0519 | 0.0341 | 0.0713 | 0.0402 | 0.0341 | 0.0713 | 0.0451 |
| Cr | 0.0056 | 0.0141 | 0.0093 | 0.0001 | 0.0037 | 0.0013 | 0.0001 | 0.0141 | 0.0046 |
| Cu | 0.0061 | 0.0260 | 0.0094 | 0.0001 | 0.0058 | 0.0013 | 0.0001 | 0.0260 | 0.0046 |
| Fe | 0.0873 | 2.2767 | 0.5954 | 0.0003 | 2.4102 | 0.3194 | 0.0003 | 2.4102 | 0.4330 |
| Mn | 0.0368 | 0.1537 | 0.0738 | 0.00806 | 0.1035 | 0.0284 | 0.0080 | 0.1537 | 0.0471 |
| Mo | 0.0003 | 0.0041 | 0.00211 | 0.0055 | 0.0325 | 0.0142 | 0.0003 | 0.0325 | 0.0092 |
| Ni | 0.0080 | 0.0175 | 0.0128 | 0.0140 | 0.0357 | 0.0222 | 0.0080 | 0.0357 | 0.0183 |
| Zn | 0.0145 | 0.0394 | 0.0218 | 0.0008 | 0.0329 | 0.0120 | 0.0008 | 0.0394 | 0.0161 |
| WQIs | Range | Water Category | Number of Samples (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rosetta Branch (n = 21) | Damietta Branch (n = 30) | Across Two Branches (n = 51) | |||||||
| Min. | Max. | Mean | SD | ||||||
| DWQI | 35.22 | 239.68 | 100.32 | 45.37 | <50 | Excellent | 6 (28.0%) | 0 (0.0%) | 6 (12%) |
| 50–100 | Good | 9 (43%) | 12 (41%) | 21 (41%) | |||||
| 100–150 | Poor | 5 (24.0%) | 13 (43.0%) | 18 (35%) | |||||
| 150–200 | Very poor | 0 (0.0%) | 4 (13.0%) | 4 (8%) | |||||
| >200 | Unsuitable | 1 (5.0%) | 1 (3.0%) | 2 (4%) | |||||
| MI | 1.81 | 29.00 | 8.48 | 5.67 | <0.3 | Very pure | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 0.3–1.0 | Pure | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |||||
| 1.0–2.0 | Slightly affected | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |||||
| 2.0–3.0 | Moderately affected | 3 (14%) | 0 (0.0%) | 3 (6%) | |||||
| 3.0–6.0 | Strongly affected | 9 (43%) | 9 (30%) | 18 (35%) | |||||
| >6.0 | Seriously affected | 9 (43%) | 21 (70%) | 30 (59%) | |||||
| Metals | PI | Class | Effect | ||
|---|---|---|---|---|---|
| Rosetta Branch | Damietta Branch | Across Two Branches | |||
| Al | 8.624375702 | 9.4710604 | 9.43 | V | Seriously affected |
| Ba | 0.134199892 | 0.1317247 | 0.13 | I | No effect |
| Cr | 0.151713546 | 0.0370135 | 0.14 | I | No effect |
| Cu | 0.006676498 | 0.0014502 | 0.01 | I | No effect |
| Fe | 3.797288572 | 4.0166667 | 4.02 | IV | Moderately affected |
| Mn | 0.790220381 | 0.5190703 | 0.77 | I | No effect |
| Mo | 0.029364007 | 0.2354436 | 0.23 | I | No effect |
| Ni | 0.481047035 | 0.9606389 | 0.92 | I | No effect |
| Zn | 0.006997242 | 0.005485 | 0.01 | I | No effect |
| Rosetta Branch | Damietta Branch | Rosetta and Damietta Branches | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DWQI | MI | Turb. | TSS | DWQI | MI | Turb. | TSS | DWQI | MI | Turb. | TSS | |
| Blue/Red | 0.63 | 0.66 | 0.59 | 0.64 | 0.68 | 0.51 | 0.44 | 0.43 | 0.58 | 0.57 | 0.48 | 0.46 |
| Green/Red | 0.76 | 0.79 | 0.64 | 0.72 | 0.77 | 0.64 | 0.54 | 0.56 | 0.70 | 0.70 | 0.54 | 0.54 |
| NIR/Red | 0.41 | 0.41 | 0.19 | 0.29 | 0.79 | 0.74 | 0.70 | 0.79 | 0.53 | 0.55 | 0.37 | 0.40 |
| NDI704,698 | 0.60 | 0.61 | 0.36 | 0.49 | 0.75 | 0.70 | 0.58 | 0.68 | 0.58 | 0.65 | 0.46 | 0.52 |
| RSI717,630 | 0.67 | 0.70 | 0.45 | 0.54 | 0.74 | 0.73 | 0.60 | 0.71 | 0.64 | 0.71 | 0.49 | 0.53 |
| RSI620,608 | 0.80 | 0.81 | 0.74 | 0.75 | 0.64 | 0.62 | 0.46 | 0.50 | 0.69 | 0.70 | 0.53 | 0.51 |
| RSI670,470 | 0.76 | 0.79 | 0.62 | 0.66 | 0.76 | 0.70 | 0.54 | 0.59 | 0.69 | 0.73 | 0.54 | 0.53 |
| RSI806,670 | 0.48 | 0.49 | 0.24 | 0.35 | 0.74 | 0.76 | 0.64 | 0.76 | 0.53 | 0.61 | 0.40 | 0.46 |
| RSI850,550 | 0.61 | 0.62 | 0.35 | 0.45 | 0.80 | 0.85 | 0.70 | 0.83 | 0.64 | 0.72 | 0.48 | 0.52 |
| RSI700,670 | 0.54 | 0.57 | 0.37 | 0.44 | 0.60 | 0.53 | 0.41 | 0.50 | 0.48 | 0.54 | 0.39 | 0.43 |
| RSI584,628 | 0.79 | 0.82 | 0.66 | 0.73 | 0.77 | 0.70 | 0.58 | 0.61 | 0.72 | 0.75 | 0.57 | 0.57 |
| RSI530,680 | 0.77 | 0.79 | 0.65 | 0.73 | 0.76 | 0.64 | 0.53 | 0.56 | 0.71 | 0.71 | 0.54 | 0.54 |
| RSI640,590 | 0.77 | 0.79 | 0.60 | 0.68 | 0.79 | 0.73 | 0.60 | 0.65 | 0.71 | 0.75 | 0.56 | 0.57 |
| RSI760,560 | 0.65 | 0.67 | 0.40 | 0.50 | 0.79 | 0.83 | 0.67 | 0.79 | 0.66 | 0.74 | 0.49 | 0.54 |
| RSI720,580 | 0.72 | 0.75 | 0.50 | 0.59 | 0.79 | 0.79 | 0.63 | 0.73 | 0.69 | 0.76 | 0.53 | 0.56 |
| RSI776,490 | 0.73 | 0.75 | 0.50 | 0.57 | 0.81 | 0.82 | 0.63 | 0.74 | 0.69 | 0.78 | 0.53 | 0.56 |
| RSI686,570 | 0.79 | 0.82 | 0.61 | 0.68 | 0.74 | 0.73 | 0.57 | 0.65 | 0.72 | 0.77 | 0.54 | 0.55 |
| RSI780,514 | 0.71 | 0.73 | 0.47 | 0.56 | 0.81 | 0.82 | 0.64 | 0.75 | 0.69 | 0.77 | 0.53 | 0.56 |
| RSI730,540 | 0.73 | 0.75 | 0.50 | 0.59 | 0.82 | 0.81 | 0.64 | 0.74 | 0.71 | 0.78 | 0.53 | 0.56 |
| RSI590,540 | 0.76 | 0.79 | 0.61 | 0.69 | 0.79 | 0.70 | 0.59 | 0.63 | 0.71 | 0.74 | 0.56 | 0.56 |
| Parameters | Calibration Dataset from Two River Branches | Validation Dataset for Rosetta Branch | Validation Dataset for Damietta Branch | |||||
|---|---|---|---|---|---|---|---|---|
| ONLFs | Equation | R2 | RMSE | R2 | RMSE | R2 | RMSE | |
| DWQI | 6 | y = 0.8459x + 14.707 | 0.85 *** | 16.32 | 0.82 *** | 20.82 | 0.93 *** | 11.67 |
| MI | 1 | y = 0.7726x + 1.7905 | 0.77 *** | 2.47 | 0.78 *** | 2.77 | 0.78 *** | 2.22 |
| Turb. | 1 | y = 0.5484x + 1.3048 | 0.55 *** | 1.03 | 0.55 *** | 1.18 | 0.62 *** | 0.89 |
| TSS | 1 | y = 0.5661x + 8.0024 | 0.57 *** | 5.31 | 0.62 *** | 6.60 | 0.70 *** | 4.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gad, M.; Saleh, A.H.; Hussein, H.; Farouk, M.; Elsayed, S. Appraisal of Surface Water Quality of Nile River Using Water Quality Indices, Spectral Signature and Multivariate Modeling. Water 2022, 14, 1131. https://doi.org/10.3390/w14071131
Gad M, Saleh AH, Hussein H, Farouk M, Elsayed S. Appraisal of Surface Water Quality of Nile River Using Water Quality Indices, Spectral Signature and Multivariate Modeling. Water. 2022; 14(7):1131. https://doi.org/10.3390/w14071131
Chicago/Turabian StyleGad, Mohamed, Ali H. Saleh, Hend Hussein, Mohamed Farouk, and Salah Elsayed. 2022. "Appraisal of Surface Water Quality of Nile River Using Water Quality Indices, Spectral Signature and Multivariate Modeling" Water 14, no. 7: 1131. https://doi.org/10.3390/w14071131
APA StyleGad, M., Saleh, A. H., Hussein, H., Farouk, M., & Elsayed, S. (2022). Appraisal of Surface Water Quality of Nile River Using Water Quality Indices, Spectral Signature and Multivariate Modeling. Water, 14(7), 1131. https://doi.org/10.3390/w14071131










