Gas Hydrate-Based Heavy Metal Ion Removal from Industrial Wastewater: A Review
Abstract
:1. Introduction
1.1. Wastewater
1.2. Heavy Metals
| Metals | Al | As | Cd | Cr | Co | Cu | Fe | Hg | Mn | Ni | Pb | Zn | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Industries | |||||||||||||
| Aviation | X | X | X | X | X | X | X | X | [17] | ||||
| Allies, Chlorine | X | X | X | X | X | X | |||||||
| Urea/Fertilizers | X | X | X | X | X | X | X | X | X | ||||
| Glass | X | X | X | X | X | X | |||||||
| Cement | X | X | X | X | X | X | |||||||
| Organic chemistry | X | X | X | X | X | X | X | ||||||
| Paper manufacturing | X | X | X | X | X | X | X | ||||||
| Petroleum refiner | X | X | X | X | X | X | X | X | |||||
| Power plants | X | ||||||||||||
| Steel works | X | X | X | X | X | X | X | X | X | ||||
| Tanning | X | ||||||||||||
| Textile mills | X | ||||||||||||
| Pharmaceutical | X | X | X | X | X | X | X | [21] | |||||
| Dyes | X | X | X | X | X | X | X | [22] | |||||
| Engineering | X | X | X | X | X | X | X | ||||||
| Fine chemicals | X | X | X | X | X | X | X | ||||||
| Batteries | X | [23] | |||||||||||
| Brass manufacture | X | ||||||||||||
| Electroplating | X | X | X | X | |||||||||
| Ferromanganese alloy production | X | X | |||||||||||
| Fungicides | X | ||||||||||||
| Metal smelters | X | ||||||||||||
| Mining | X | ||||||||||||
| Nuclear fission | X | ||||||||||||
| Pesticides | X | X | X | X | X | ||||||||
| Welding | X | X |
1.3. Various Heavy Metals and Their Effects
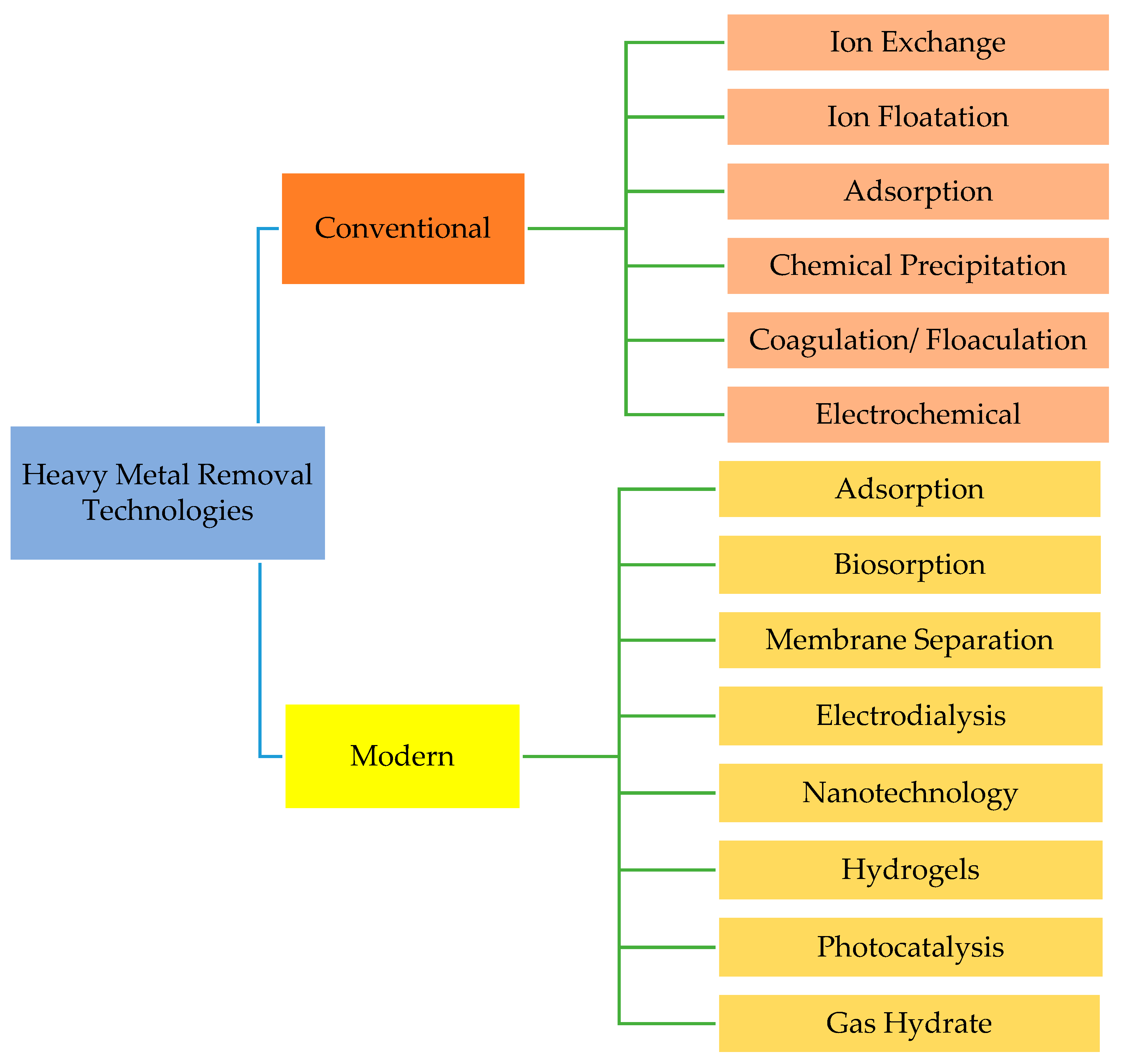
2. Conventional Technologies for Heavy Metal Removal in Wastewater
2.1. Coagulation/Flocculation
2.2. Ion Exchange
2.3. Flotation
2.4. Membrane Filtration
2.5. Chemical Precipitation
2.6. Electrochemical Treatment
2.7. Adsorption
2.8. Gas Hydrate-Based Mechanism in Eliminating Heavy Metal Ions
3. Overview of Gas Hydrate Technology
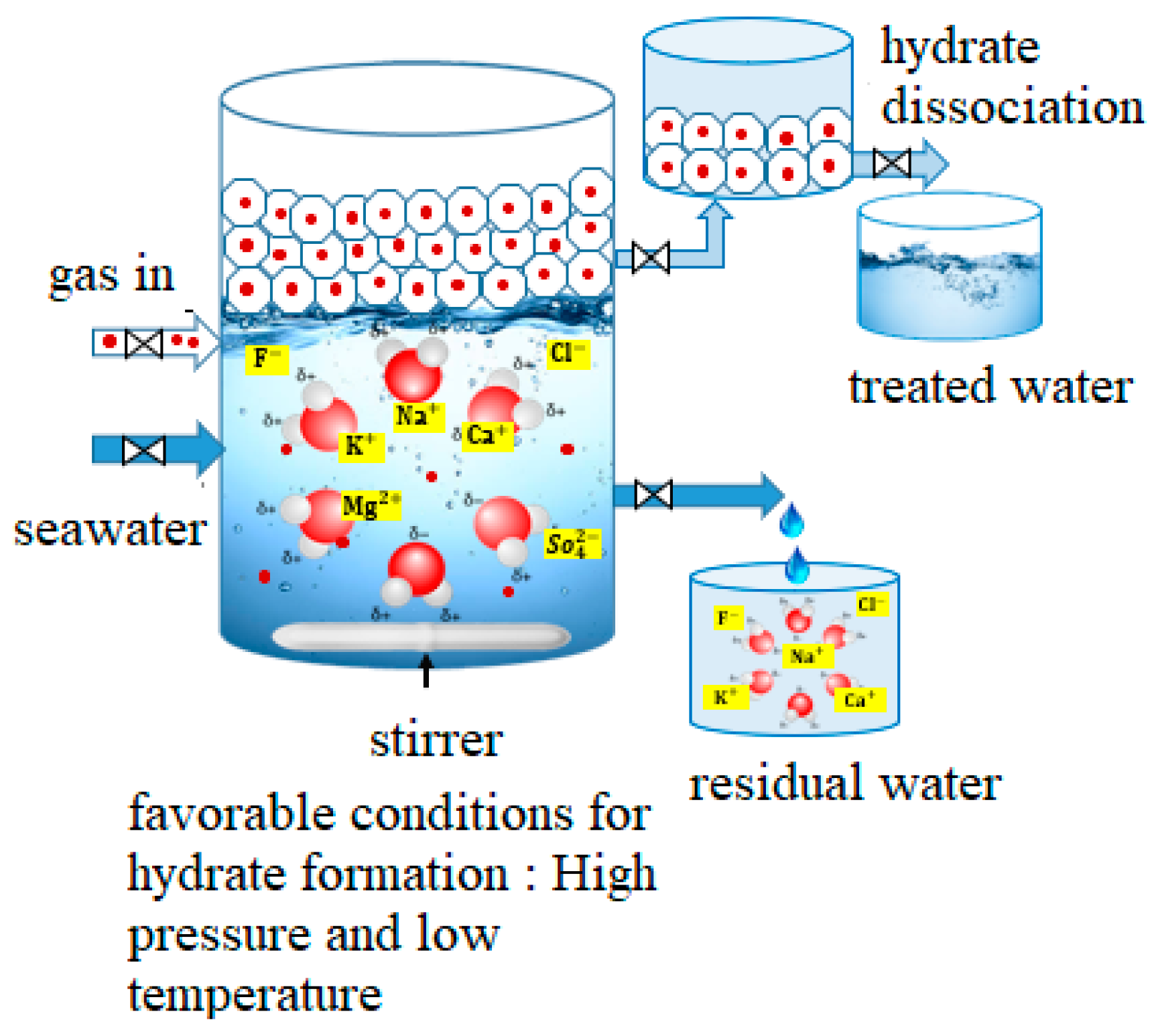
3.1. Gas Hydrate-Based Desalination
3.2. Gas Hydrate Desalination Reactor Design Innovations
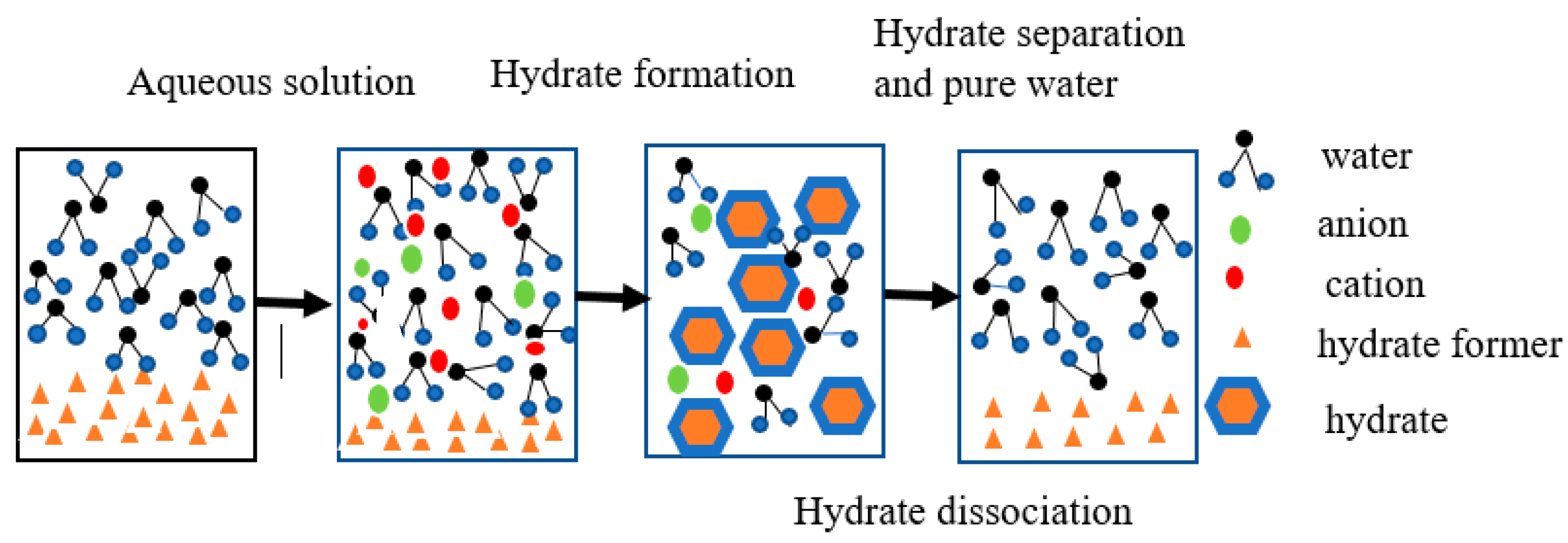
3.3. Heavy Metal Separation Mechanism Based on Gas Hydrates

| System and Concentration | Hydrate Former | Metals Removed | Removal Efficiency | Remarks | Reference |
|---|---|---|---|---|---|
| NaCl 3.2 wt% | CO2 | K+, Na+, Mg2+, B3+, Ca2+ | K+ (80.4%), Na+ (78.7%), Mg2+ (76.6%), B3+ (73.3%), Ca2+ (72%) | Hydraulic pressure is applied to make the hydrate into the form of pellets uisng piston which is energy intensive. 1 stage operation | [162] |
| Saline solution of 3.0 wt% | CO2 | - | 20.26% | Semi batch reactor system carried out at 3.0–3.5 MPa and 6 °C. The efficiency based on individual metal ion is not discussed | [142] |
| Nacl 3.5 wt% | CP | - | (49–72%). | Post hydrate formation series of unit operations were carried to enhance efficiency. Centrifuging provided a high removal efficiency of 96%. Post-treatment is expensive as CP leaves a suspension and is also not environemnt friendly. | [178] |
| Synthetic produced water 8.9 wt% | CO2 CO2+CP CO2+CH | Na+, Mg2+, K+, Ca2+ | 74 91 95 | Addition of CP and CH to CO2 enhance the hydrate formation temperature. Post-treatment is required as they form a suspension at interface | [179] |
| Synthetic seawater 3.5 wt% | CO2 CH4 | Na+, K+, Mg2+, Ca2+, B3+, Cl−, SO42− | Cations 71–94% Anions 73–83% 68.86% | CO2 based hydrate based desalaintion is better compared to CH4 and might be as CO2 is more soluble in water comapred to CH4 | [118] |
| NaCl 4 wt% at 4 °C | R 141 b | NaCl | 61.46% | Better at removing alkaline metals than it is at removing alkaline earth metals. Not environment friendly have higher global warming potential. | [121] |
| Seawater 3.5 wt% | Not available | K+ Na+, Mg2+, B3+, Ca2+ | 80.4% 78.7% 76.6% 73.3% 72% | The reverese osmosis recovery is inversely related to the gas hydrate energy consumption. With the increase in energy consumption it was founf that the efficienccy of metal ion removal increased. | [180] |
| CuSO4·5H2O Coppersulphate pentahydrate | R141b | Cu2+ | 90.82% | Optimumratio of waterto R141b is found to be 1:5 Involves post-treatment techniques due to usgae of R141b and is not environment friendly | [181] |
| NaCl | R141 b | Cr3+, Cu2+, Ni2+, Zn2+ | 70.02%, 71.87%, 71.79%, 67.82% | Lower effluent volume ratio yielded higher removal efficiency. R141B is likewise extremely flammable and when discharged into the atmosphere, this causes ozone depletion. | [170] |
| NaCl 3–5 wt% | CP | - | Removal efficiency increased from 50 to 79% by washing the hydrate. | Higher frequency, lower temperature (274.1 K), lower salinity could all help to form more hydrates, albeit at the expense of removal efficiency. Post-treatment of seperating the suspension is expensive | [107] |
| Produced Water 8.6 wt% | Compressed natural gas | Mg2+, Na+. Ca2+. K+, HCO3−, Cl−, SO42− | 79.5–84.3% 3 stage process | The number of water molecules in the hydrate structure decreased, resulting in powerful electrostatic interactions that cause the hydration of salt ions and ion clustering also decreased the solubility of gas | [182] |
| Brine 3.5 wt% | CP | - | 81% 3 step process, gravity seperation, flitration and washing | Because of the fine cyclopentane droplet sizes formed by the spray injection approach, more water can be turned into hydrates. | [183] |
| Seawater 3.4 wt% | CP | - | 63% | Washing the hydrate enhanced removal efficiency by 42%. Emulsion formed could be difficult to separate from treated water. | [184] |
| PW 8.6 wt% | CO2 | - | 82–89.2% In 3 stage process | Application of hydrate-based desalination of produced water. Removal efficiency of each metal ion is not listed. | [185] |
| Seawater 3.42 wt% | CO2+CP | Na+, K+, Mg2+, Ca2+, Cl−, SO42− | 85.52%, 83.93%, 80.73%, 78.21%, 55.72%, 62.09% | A piston is used to separate the solid from liquid stream hence higher energy requirement. | [130] |
| Aqueous NiCl2 solution 200–10,000 ppm | CP | Ni2+ | 62–88% | Water recovery of 43% is attained. The water recovery and enrichment factory decreases with increase in concentration whereas removal efficiency increased with increased in aqueous concentration. | [171] |
| Saline solution 3.3 wt% + dodecane | CH4+C2H6 | - | 80% | The decreased viscosity of the dodecane system allows the hydrate crystals to move through the oil layer quicker, resulting in better desalination efficiencies. | [186] |
| CuSO4 aqueous solution | R141b | Cu2+ | 44.79–90.82% | efficient approach was discovered to be vacuum filtration and centrifugation | [176] |
| PW 8.6 wt% | CO2/ Natural gas | - | 73% 74% | CO2/NG hydrate formers can be utilized to desalinate produced waters. Post-treatment/separation is not listed | [135] |
| NaCl 3.5 wt% | Graphite +CP | 99.76% in 4th stage | The hydrate process with graphite particles is a viable desalination technology, according to the research, with advantages such as quick nucleation, a high conversion ratio, and a fair desalting efficiency. | [187] | |
| CuSO4 3 wt% | R141b | Cu2+ | 84% | Post-treatment by vacuum filtration combined with washing produced maximum removal efficiency Water yield and enrichment factor decreased with increase in concertation | [188] |
| NaCl 3 wt% | CO2+C3H8 (90:10) | Na+ Cl− | 87.5% 84% | Novel reactor design of flat bed reactor was used for hydrate-based desalination. The influence of salts on water recovery and salt rejection rate was not discussed | [177] |
| Coca cola NaCl 15 wt% | HFC134a | Na+, Mg2+, Ca2+, K+, B3+, Cl−, SO42− | 75.72% 80% | With HFC134a in coca cola formed sII hydrate and ions did not effect the structure of hydrate | [189] |
| LiCl, LiBr, LiI | CP+graphite | I− > Br− > Cl− | 70% | The efficiency of desalination was enhanced by increasing lithium halides, but salts restrict induction time and water recovery. | [190] |
3.4. Water Recovery
3.5. Removal Efficiency
| Removal Efficiency (%) | |||||
|---|---|---|---|---|---|
| Metal Ion | Hydrate Former | Effluent to Former Ratio | With Washing | Without Washing | Reference |
| Zn | CP | 1:6 | 85 | [193] | |
| Cu | 92 | ||||
| Cr | 50 | ||||
| Ni | 80 | ||||
| Cr | R141b | 1:6 | 89.72 | 70.02 | [170] |
| Cu | 90.82 | 71.87 | |||
| Ni | 89.93 | 71.79 | |||
| Zn | 88.01 | 67.82 | |||
| Cu | R141b | 44.7 | [176] | ||
| 90.82 VF | 71.87 | ||||
| 87.69 WHVF | |||||
| 90.82 WFVF | |||||
| 90.46 VCF | |||||
| Cu | R141b | 1:5 | [192] | ||
| Cu | R141b | 1:4 | 51.8 VF 83.80 VFC 87.42 VFWW | [192] | |
| Ni | Cyclopentane | 1:3 | 84 (1-stage) 96 (2-stage) 99.2 (3-stage) | [171] | |
3.6. Enrichment Factor (Ef)
3.7. Gas Hydrate and Hybrid Technologies
3.8. Limitations in Adopting Gas Hydrate Technique
3.9. Opportunities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosado, D.; Usero, J.; Morillo, J. Assessment of heavy metals bioavailability and toxicity toward Vibrio fischeri in sediment of the Huelva estuary. Chemosphere 2016, 153, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Azetsu-Scott, K.; Yeats, P.; Wohlgeschaffen, G.; Dalziel, J.; Niven, S.; Lee, K. Precipitation of heavy metals in produced water: Influence on contaminant transport and toxicity. Mar. Environ. Res. 2007, 63, 146–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, I.; Alharbi, O.M.; Alothman, Z.A.; Badjah, A.Y. Kinetics, thermodynamics, and modeling of amido black dye photodegradation in water using Co/TiO2 nanoparticles. Photochem. Photobiol. 2018, 94, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Alharbi, O.M.; ALOthman, Z.A.; Alwarthan, A.; Al-Mohaimeed, A.M. Preparation of a carboxymethylcellulose-iron composite for uptake of atorvastatin in water. Int. J. Biol. Macromol. 2019, 132, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Ahmed, S.; Farooqi, I.H.; Ali, I.; Vambol, V.; Changani, F.; Khan, A.H. Occurrence, sources and conventional treatment techniques for various antibiotics present in hospital wastewaters: A critical review. TrAC Trends Anal. Chem. 2020, 129, 115921. [Google Scholar] [CrossRef]
- Igunnu, E.T.; Chen, G.Z. Produced water treatment technologies. Int. J. Low-Carbon Technol. 2014, 9, 157–177. [Google Scholar] [CrossRef] [Green Version]
- Sloan, E.D., Jr.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 116–117. [Google Scholar]
- Gonçalves, A.L.; Pires, J.C.M.; Simões, M. A review on the use of microalgal consortia for wastewater treatment. Algal Res. 2017, 24, 403–415. [Google Scholar] [CrossRef]
- Renuka, N.; Sood, A.; Ratha, S.K.; Prasanna, R.; Ahluwalia, A.S. Evaluation of microalgal consortia for treatment of primary treated sewage effluent and biomass production. J. Appl. Phycol. 2013, 25, 1529–1537. [Google Scholar] [CrossRef]
- Tee, P.F.; Abdullah, M.O.; Tan, I.A.W.; Rashid, N.K.A.; Amin, M.A.M.; Nolasco-Hipolito, C.; Bujang, K. Review on hybrid energy systems for wastewater treatment and bio-energy production. Renew. Sustain. Energy Rev. 2016, 54, 235–246. [Google Scholar] [CrossRef]
- Ngah, W.S.W.; Teong, L.C.; Hanafiah, M.A.K.M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Quero, G.M.; Cassin, D.; Botter, M.; Perini, L.; Luna, G.M. Patterns of benthic bacterial diversity in coastal areas contaminated by heavy metals, polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs). Front. Microb. 2015, 6, 1053. [Google Scholar] [CrossRef] [PubMed]
- Finizio, A.; Azimonti, G.; Villa, S. Occurrence of pesticides in surface water bodies: A critical analysis of the Italian national pesticide survey programs. J. Environ. Monit. 2011, 13, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Zhao, X.; Cai, Z.; O’reilly, S.E.; Hao, X.; Zhao, D. A review of oil, dispersed oil and sediment interactions in the aquatic environment: Influence on the fate, transport and remediation of oil spills. Mar. Pollut. Bull. 2014, 79, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.F.; Manjreker, T.G.; Halligan, J.E. Removal of oil from water surfaces by sorption on unstructured fibers. Environ. Sci. Technol. 1973, 7, 439–443. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Liu, Z.F. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef]
- Srivastava, N.K.; Majumder, C.B. Novel biofiltration methods for the treatment of heavy metals from industrial wastewater. J. Hazard. Mater. 2008, 151, 1–8. [Google Scholar] [CrossRef]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Joshi, M.K. Technological Trends in Heavy Metals Removal from Industrial Wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Tran, T.; Leu, H.; Chiu, K.; Lin, C. Electrochemical Treatment of Heavy Metal-containing Wastewater with the Removal of COD and Heavy Metal Ions. J. Chin. Chem. Soc. 2017, 64, 493–502. [Google Scholar] [CrossRef]
- Bielen, A.; Šimatović, A.; Kosić-Vukšić, J.; Senta, I.; Ahel, M.; Babić, S.; Udiković-Kolić, N. Negative environmental impacts of antibiotic-contaminated effluents from pharmaceutical industries. Water Res. 2017, 126, 79–87. [Google Scholar] [CrossRef]
- Lokhande, R.S.; Singare, P.U.; Pimple, D.S. Toxicity study of heavy metals pollutants in waste water effluent samples collected from Taloja industrial estate of Mumbai. Resour. Environ. 2011, 1, 13–19. [Google Scholar]
- Alluri, H.K.; Ronda, S.R.; Settalluri, V.S.; Bondili, J.S.; Suryanarayana, V.; Venkateshwar, P. Biosorption: An eco-friendly alternative for heavy metal removal. Afr. J. Biotechnol. 2007, 6, 2924–2931. [Google Scholar]
- Madu, P.C.; Akpaiyo, G.D.; Ikoku, P. Biosorption of Cr3+, Pb2+, and Cd2+ ions from aqueous solution using modified and unmodified millet chaff. J. Chem. Pharm. Res. 2011, 3, 467–477. [Google Scholar]
- Dhir, B.; Sharmila, P.; Saradhi, P.P. Potential of aquatic macrophytes for removing contaminants from the environment. Crit. Rev. Environ. Sci. Technol. 2009, 39, 754–781. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Crisponi, G.; Villaescusa, I. Chemical equilibria in wastewaters during toxic metal ion removal by agricultural biomass. Coord. Chem. Rev. 2010, 254, 2181–2192. [Google Scholar] [CrossRef]
- Simate, G.S.; Maledi, N.; Ochieng, A.; Ndlovu, S.; Zhang, J.; Walubita, L.F. Coal-based adsorbents for water and wastewater treatment. J. Environ. Chem. Eng. 2016, 4, 2291–2312. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Guieysse, B.; Norvill, Z.N. Sequential chemical–biological processes for the treatment of industrial wastewaters: Review of recent progresses and critical assessment. J. Hazard. Mater. 2014, 267, 142–152. [Google Scholar] [CrossRef]
- Boamah, P.O.; Huang, Y.; Hua, M.; Zhang, Q.; Wu, J.; Onumah, J. Sorption of heavy metal ions onto carboxylate chitosan derivatives—A mini-review. Ecotoxicol. Environ. Saf. 2015, 116, 113–120. [Google Scholar] [CrossRef]
- Lesmana, S.O.; Febriana, N.; Soetaredjo, F.E.; Sunarso, J.; Ismadji, S. Studies on potential applications of biomass for the separation of heavy metals from water and wastewater. Biochem. Eng. J. 2009, 44, 19–41. [Google Scholar] [CrossRef]
- Ahmed, M.J.K.; Ahmaruzzaman, M. A review on potential usage of industrial waste materials for binding heavy metal ions from aqueous solutions. J. Water Proc. Eng. 2016, 10, 39–47. [Google Scholar] [CrossRef]
- Ruihua, L.; Lin, Z.; Tao, T.; Bo, L. Phosphorus removal performance of acid mine drainage from wastewater. J. Hazard. Mater. 2011, 90, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.A.H.; Ngo, H.H.; Guo, W.S.; Zhang, J.; Liang, S.; Yue, Q.Y.; Nguyen, T.V. Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. Bioresour. Technol. 2013, 148, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Farooq, U.; Kozinski, J.A.; Khan, M.A.; Athar, M. Biosorption of heavy metal ions using wheat based biosorbents—A review of the recent literature. Bioresour. Technol. 2010, 101, 5043–5053. [Google Scholar] [CrossRef] [PubMed]
- Ahmaruzzaman, M. Role of fly ash in the removal of organic pollutants from wastewater. Energy Fuels 2009, 23, 1494–1511. [Google Scholar] [CrossRef]
- Visa, M. Synthesis and characterization of new zeolite materials obtained from fly ash for heavy metals removal in advanced wastewater treatment. Powder Technol. 2016, 294, 338–347. [Google Scholar] [CrossRef]
- Ghernaout, D.; Al-Ghonamy, A.I.; Boucherit, A.; Ghernaout, B.; Naceur, M.W.; Messaoudene, N.A.; Elboughdiri, N.A. Brownian motion and coagulation process. Am. J. Environ. Prot. 2015, 4, 1–15. [Google Scholar] [CrossRef]
- Chang, Q.; Zhang, M.; Wang, J. Removal of Cu2+ and turbidity from wastewater by mercaptoacetyl chitosan. J. Hazard. Mater. 2009, 169, 621–625. [Google Scholar] [CrossRef]
- Sakhi, D.; Rakhila, Y.; Elmchaouri, A.; Abouri, M.; Souabi, S.; Jada, A. Optimization of coagulation flocculation process for the removal of heavy metals from real textile wastewater. Adv. Intell. Syst. Comput. 2019, 913, 257–266. [Google Scholar]
- Guo, W.; Fu, Z.; Wang, H.; Liu, S.; Wu, F.; Giesy, J.P. Removal of antimonate (Sb(V)) and antimonite (Sb(III)) from aqueous solutions by coagulation-flocculation-sedimentation (CFS): Dependence on influencing factors and insights into removal mechanisms. Sci. Total Environ. 2018, 644, 1277–1285. [Google Scholar] [CrossRef]
- Yan, L.; Yin, H.; Zhang, S.; Leng, F.; Nan, W.; Li, H. Biosorption of inorganic and organic arsenic from aqueous solution by Acidithiobacillus ferrooxidans BY-3. J. Hazard. Mater. 2010, 178, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Łukasiewicz, E. Post-coagulation sludge management for water and wastewater treatment with focus on limiting its impact on the environment. Econ. Environ. Stud. 2016, 16, 831–841. [Google Scholar]
- Ahmad, T.; Ahmad, K.; Alam, M. Sustainable management of water treatment sludge through 3 ‘R’concept. J. Clean. Prod. 2016, 124, 1–13. [Google Scholar] [CrossRef]
- Bilal, M.; Shah, J.A.; Ashfaq, T.; Gardazi, S.M.H.; Tahir, A.A.; Pervez, A.; Haroon, H.; Mahmood, Q. Waste biomass adsorbents for copper removal from industrial wastewater—A review. J. Hazard. Mater. 2013, 263, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Hubicki, Z.; Kołodyńska, D. Selective removal of heavy metal ions from waters and waste waters using ion exchange methods. Ion Exch. Technol. 2012, 7, 193–240. [Google Scholar]
- An, B.; Liang, Q.; Zhao, D. Removal of arsenic (V) from spent ion exchange brine using a new class of starch-bridged magnetite nanoparticles. Water Res. 2011, 45, 1961–1972. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Motsi, T.; Rowson, N.A.; Simmons, M.J.H. Adsorption of heavy metals from acid mine drainage by natural zeolite. Int. J. Miner. Process. 2009, 92, 42–48. [Google Scholar] [CrossRef]
- Figueiredo, H.; Quintelas, C. Tailored zeolites for the removal of metal oxyanions: Overcoming intrinsic limitations of zeolites. J. Hazard. Mater. 2014, 274, 287–299. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, H.S.; Jamil, T.S.; Hegazy, E.Z. Application of zeolite prepared from Egyptian kaolin for the removal of heavy metals: II. Isotherm models. J. Hazard. Mater. 2010, 182, 842–847. [Google Scholar] [CrossRef]
- Kononova, O.N.; Bryuzgina, G.L.; Apchitaeva, O.V.; Kononov, Y.S. Ion exchange recovery of chromium (VI) and manganese (II) from aqueous solutions. Arab. J. Chem. 2019, 12, 2713–2720. [Google Scholar] [CrossRef]
- Mahmoud, M.R.; Lazaridis, N.K.; Matis, K.A. Study of flotation conditions for cadmium (II) removal from aqueous solutions. Process Saf. Environ. Prot. 2015, 94, 203–211. [Google Scholar] [CrossRef]
- Patil, D.S.; Chavan, S.M.; Oubagaranadin, J.U.K. A review of technologies for manganese removal from wastewaters. J. Environ. Chem. Eng. 2016, 4, 468–487. [Google Scholar] [CrossRef]
- Hoseinian, F.S.; Rezai, B.; Kowsari, E.; Chinnappan, A.; Ramakrishna, S. Synthesis and characterization of a novel nanocollector for the removal of nickel ions from synthetic wastewater using ion flotation. Sep. Purif. Technol. 2020, 240, 116639. [Google Scholar] [CrossRef]
- Mahne, E.J.; Pinfold, T.A. Precipitate flotation I. Removal of nickel from dilute aqueous solutions and its separation from cobalt. J. Appl. Chem. 1968, 18, 52–54. [Google Scholar] [CrossRef]
- Peng, W.; Chang, L.; Li, P.; Han, G.; Huang, Y.; Cao, Y. An overview on the surfactants used in ion flotation. J. Mol. Liq. 2019, 286, 110955. [Google Scholar] [CrossRef]
- Lyu, S.; Chen, W.; Zhang, W.; Fan, Y.; Jiao, W. Wastewater reclamation and reuse in China: Opportunities and challenges. J. Environ. Sci. 2016, 39, 86–96. [Google Scholar] [CrossRef]
- Mutamim, N.S.A.; Noor, Z.Z.; Hassan, M.A.A.; Olsson, G. Application of membrane bioreactor technology in treating high strength industrial wastewater: A performance review. Desalination 2012, 305, 1–11. [Google Scholar] [CrossRef]
- Wang, L.K.; Chen, J.P.; Hung, Y.T.; Shammas, N.K. Membrane and Desalination Technologies, 1st ed.; Humana Press: Totowa, NJ, USA, 2008; pp. 1–45. [Google Scholar]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Li, W.; Zheng, P.; Guo, J.; Ji, J.; Zhang, M.; Zhang, Z.; Zhan, E.; Abbas, G. Characteristics of self-alkalization in high-rate denitrifying automatic circulation (DAC) reactor fed with methanol and sodium acetate. Bioresour. Technol. 2014, 154, 44–50. [Google Scholar] [CrossRef]
- Hashim, M.A.; Mukhopadhyay, S.; Sahu, J.N.; Sengupta, B. Remediation technologies for heavy metal contaminated groundwater. J. Environ. Manag. 2011, 92, 2355–2388. [Google Scholar] [CrossRef] [PubMed]
- Tanong, K.; Tran, L.H.; Mercier, G.; Blais, J.F. Recovery of Zn (II), Mn (II), Cd (II) and Ni (II) from the unsorted spent batteries using solvent extraction, electrodeposition and precipitation methods. J. Clean. Prod. 2017, 148, 233–244. [Google Scholar] [CrossRef] [Green Version]
- Kuan, Y.C.; Lee, L.H.; Chern, J.M. Heavy metal extraction from PCB wastewater treatment sludge by sulfuric acid. J. Hazard. Mater. 2010, 177, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Gupta, R.; Sharma, R.K. Green and sustainable pathways for wastewater purification. In Advances in Water Purification Techniques; Elsevier: Amsterdam, The Netherlands, 2019; pp. 355–383. [Google Scholar]
- Ain Zainuddin, N.; Azwan Raja Mamat, T.; Imam Maarof, H.; Wahidah Puasa, S.; Rohana Mohd Yatim, S. Removal of Nickel, Zinc and Copper from Plating Process Industrial Raw Effluent Via Hydroxide Precipitation Versus Sulphide Precipitation. IOP Conf. Ser. Mater. Sci. Eng. 2019, 551, 12122. [Google Scholar] [CrossRef] [Green Version]
- Vu, H.H.T.; Gu, S.; Thriveni, T.; Khan, M.D.; Tuan, L.Q.; Ahn, J.W. Sustainable treatment for sulfate and lead removal from battery wastewater. Sustainability 2019, 11, 3497. [Google Scholar] [CrossRef] [Green Version]
- Elsheikh, M.A.; Al-Hemaidi, W.K. Approach in choosing suitable technology for industrial wastewater treatment. J. Civ. Environ. Eng. 2012, 2, 5. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, J.; Hu, Y.; Han, H.; Luo, X.; Sun, W.; Zhou, H. Selective sulfide precipitation of copper ions from arsenic wastewater using monoclinic pyrrhotite. Sci. Total Environ. 2020, 705, 135816. [Google Scholar] [CrossRef]
- Elabbas, S.; Ouazzani, N.; Mandi, L.; Berrekhis, F.; Perdicakis, M.; Pontvianne, S.; Pons, M.N.; Lapicque, F.; Leclerc, J.P. Treatment of highly concentrated tannery wastewater using electrocoagulation: Influence of the quality of aluminium used for the electrode. J. Hazard. Mater. 2016, 319, 69–77. [Google Scholar] [CrossRef]
- de Almeida, C.C.; da Costa, P.R.F.; Melo, M.J.D.M.; dos Santos, E.V.; Martínez-Huitle, C.A. Application of electrochemical technology for water treatment of Brazilian industry effluents. J. Mex. Chem. Soc. 2014, 58, 276–286. [Google Scholar]
- Liu, C.; Wu, T.; Hsu, P.C.; Xie, J.; Zhao, J.; Liu, K.; Cui, Y. Direct/alternating current electrochemical method for removing and recovering heavy metal from water using graphene oxide electrode. ACS Nano 2019, 13, 6431–6437. [Google Scholar] [CrossRef]
- Yang, X.; Liu, L.; Zhang, M.; Tan, W.; Qiu, G.; Zheng, L. Improved removal capacity of magnetite for Cr (VI) by electrochemical reduction. J. Hazard. Mater. 2019, 374, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jiang, Y.; Li, Y.; Hu, Z.; Zhou, L.; Zhou, M. Three-dimensional electrochemical process for wastewater treatment: A general review. Chem. Eng. J. 2013, 228, 455–467. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Babel, S. A research study on Cr (VI) removal from contaminated wastewater using low-cost adsorbents and commercial activated carbon. In Proceedings of the 2nd International Conference on Energy Technology Towards a Clean Environment (RCETE), Phuket, Thailand, 12–14 February 2003; Volume 2, pp. 1110–1117. [Google Scholar]
- Nallakukkala, S.; Lal, B.; Shaik, F. Kinetic and isothermal investigations in elimination of iron metal from aqueous mixture by using natural adsorbent. Int. J. Environ. Sci. Technol. 2021, 18, 1761–1772. [Google Scholar] [CrossRef]
- Bose, P.; Bose, M.A.; Kumar, S. Critical evaluation of treatment strategies involving adsorption and chelation for wastewater containing copper, zinc and cyanide. Adv. Environ. Res. 2002, 7, 179–195. [Google Scholar] [CrossRef]
- Vunain, E.; Mishra, A.K.; Mamba, B.B. Dendrimers, mesoporous silicas and chitosan-based nanosorbents for the removal of heavy-metal ions: A review. Int. J. Biol. Macromol. 2016, 86, 570–586. [Google Scholar] [CrossRef]
- Ewecharoen, A.; Thiravetyan, P.; Wendel, E.; Bertagnolli, H. Nickel adsorption by sodium polyacrylate-grafted activated carbon. J. Hazard. Mater. 2009, 171, 335–339. [Google Scholar] [CrossRef]
- Li, Y.; Du, Q.; Liu, T.; Peng, X.; Wang, J.; Sun, J.; Xia, L. Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes. Chem. Eng. Res. Des. 2013, 91, 361–368. [Google Scholar] [CrossRef]
- El-Sikaily, A.; El Nemr, A.; Khaled, A.; Abdelwehab, O. Removal of toxic chromium from wastewater using green alga Ulva lactuca and its activated carbon. J. Hazard. Mater. 2007, 148, 216–228. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Arulkumar, M.; Palvannan, T. Utilization of agro-industrial waste Jatropha curcas pods as an activated carbon for the adsorption of reactive dye Remazol Brilliant Blue R (RBBR). J. Clean. Prod. 2012, 22, 67–75. [Google Scholar] [CrossRef]
- Qdais, H.A.; Moussa, H. Removal of heavy metals from wastewater by membrane processes: A comparative study. Desalination 2004, 164, 105–110. [Google Scholar] [CrossRef]
- Tzanetakis, N.; Taama, W.M.; Scott, K.; Jachuck, R.J.J.; Slade, R.S.; Varcoe, J. Comparative performance of ion exchange membranes for electrodialysis of nickel and cobalt. Sep. Purif. Technol. 2003, 30, 113–127. [Google Scholar] [CrossRef]
- Christensen, I.V.; Pedersen, A.J.; Ottosen, L.M.; Ribeiro, A.B. Electrodialytic remediation of CCA-treated waste wood in a 2 m3 pilot plant. Sci. Total Environ. 2006, 364, 45–54. [Google Scholar] [CrossRef] [PubMed]
- BrbootI, M.M.; AbiD, B.A.; Al-ShuwaikI, N.M. Removal of heavy metals using chemicals precipitation. Eng. Technol. J. 2011, 29, 595–612. [Google Scholar]
- Al-Enezi, G.; Hamoda, M.F.; Fawzi, N. Ion exchange extraction of heavy metals from wastewater sludges. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2004, 39, 455–464. [Google Scholar] [CrossRef]
- Babu, P.; Nambiar, A.; He, T.; Karimi, I.A.; Lee, J.D.; Englezos, P.; Linga, P. A review of clathrate hydrate based desalination to strengthen energy—Water nexus. ACS Sustain. Chem. Eng. 2018, 6, 8093–8107. [Google Scholar] [CrossRef]
- Nallakukkala, S.; Lal, B. Seawater and produced water treatment via gas hydrate: Review. J. Environ. Chem. Eng. 2021, 9, 105053. [Google Scholar] [CrossRef]
- Rehman Ur, A.; Zaini, D.B.; Lal, B. Application of Gas Hydrate Based Technique in Wastewater Treatment—A Mini Review. In Proceedings of the Third International Conference on Separation Technology 2020 (ICoST 2020), Kelantan, Malaysia, 15 August 2020; pp. 249–254. [Google Scholar]
- Nallakukkala, S.; Kassim, Z.; Othman, N.A.; Lal, B. Advancement in Gas Hydrate Water Based Produced Water Desalination: An Overview. In Proceedings of the Third International Conference on Separation Technology 2020 (ICoST 2020), Kelantan, Malaysia, 15 August 2020. [Google Scholar]
- Lal, B.; Nashed, O. Chemical Additives for Gas Hydrates, 1st ed.; Springer Publication: Cham, Switzerland, 2020; pp. 7–14. [Google Scholar]
- Leopercio, B.C. Kinetics of Cyclopentane Hydrate Formation—An Interfacial Rheology Study. Master’s Thesis, Pontifical Catholic University of Rio de Janeiro, PUC-Rio, Brazil, 2016. [Google Scholar]
- Sangwai, J.S.; Patel, R.S.; Mekala, P.; Mech, D.; Busch, M. Desalination of seawater using gas hydrate technology-current status and future direction. In Proceedings of the 18th International Conference on Hydraulics, Water Resources, Coastal and Environmental Engineering, HYDRO, Madras, India, 4–6 December 2013. [Google Scholar]
- Kang, S.P.; Lee, H. Recovery of CO2 from flue gas using gas hydrate: Thermodynamic verification through phase equilibrium measurements. Environ. Sci. Technol. 2000, 34, 4397–4400. [Google Scholar] [CrossRef]
- Kezirian, M.T.; Phoenix, S.L. Natural gas hydrate as a storage mechanism for safe, sustainable and economical production from offshore petroleum reserves. Energies 2017, 10, 828. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H. Hydrate Slurry as Cold Energy Storage and Distribution Medium: Enhancing the Performance of Refrigeration Systems. Master’s Thesis, Guangzhou Institute of Energy Conversion, Guangzhou, China, 2017. [Google Scholar]
- Aregbe, A.G. Gas hydrate—Properties, formation and benefits. Open J. Yangtze Oil Gas 2017, 2, 27–44. [Google Scholar] [CrossRef] [Green Version]
- Subramani, A.; Jacangelo, J.G. Emerging desalination technologies for water treatment: A critical review. Water Res. 2015, 75, 64–187. [Google Scholar] [CrossRef]
- Ghaffour, N.; Missimer, T.M.; Amy, G.L. Technical review and evaluation of the economics of water desalination: Current and future challenges for better water supply sustainability. Desalination 2013, 309, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Cheng, F.; Li, Y.; Lü, X.; Yang, M. Progress and trends in hydrate based desalination (HBD) technology: A review. Chin. J. Chem. Eng. 2019, 27, 2037–2043. [Google Scholar] [CrossRef]
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Klemeš, J.J.; Varbanov, P.S.; Alwi, S.R.W. Analyzing the energy consumption, GHG emission, and cost of seawater desalination in China. Energies 2019, 12, 463. [Google Scholar] [CrossRef] [Green Version]
- Nallakukkala, S.; Lal, B.; Shariff, M.A. Influence of water volume on CO2 hydrate-based desalination of brine solution. Mater. Today Proc. 2021, 56, 2172–2177. [Google Scholar] [CrossRef]
- He, T.; Nair, S.K.; Babu, P.; Linga, P.; Karimi, I.A. A novel conceptual design of hydrate-based desalination (HyDesal) process by utilizing LNG cold energy. Appl. Energy 2018, 222, 13–24. [Google Scholar] [CrossRef]
- Lv, Y.N.; Wang, S.S.; Sun, C.Y.; Gong, J.; Chen, G.J. Desalination by forming hydrate from brine in cyclopentane dispersion system. Desalination 2017, 413, 217–222. [Google Scholar] [CrossRef]
- Xu, C.; Li, X.; Yan, K.; Ruan, X.; Chen, Z.; Xia, Z. Research progress in hydrate-based technologies and processes in China: A review. Chin. J. Chem. Eng. 2019, 27, 1998–2013. [Google Scholar] [CrossRef]
- Sahu, P.; Krishnaswamy, S.; Ponnani, K.; Pande, N.K. A thermodynamic approach to selection of suitable hydrate formers for seawater desalination. Desalination 2018, 436, 144–151. [Google Scholar] [CrossRef]
- Knox, W.G.; Hess, M.; Jones, G.E.; Smith, H.B. The clathrate process. Chem. Eng. Prog. 1961, 57, 66–71. [Google Scholar]
- Ngan, Y.T.; Englezos, P. Concentration of mechanical pulp mill effluents and NaCl solutions through propane hydrate formation. Ind. Eng. Chem. Res. 1996, 35, 1894–1900. [Google Scholar] [CrossRef]
- Nakajima, M.; Ohmura, R.; Mori, Y.H. Clathrate hydrate formation from cyclopentane-in-water emulsions. Ind. Eng. Chem. Res. 2008, 47, 8933–8939. [Google Scholar] [CrossRef]
- Corak, D.; Barth, T.; Høiland, S.; Skodvin, T.; Larsen, R.; Skjetne, T. Effect of subcooling and amount of hydrate former on formation of cyclopentane hydrates in brine. Desalination 2011, 278, 268–274. [Google Scholar] [CrossRef]
- Cai, L.; Pethica, B.A.; Debenedetti, P.G.; Sundaresan, S. Formation kinetics of cyclopentane–methane binary clathrate hydrate. Chem. Eng. Sci. 2014, 119, 147–157. [Google Scholar] [CrossRef]
- Cai, L.; Pethica, B.A.; Debenedetti, P.G.; Sundaresan, S. Formation of cyclopentane methane binary clathrate hydrate in brine solutions. Chem. Eng. Sci. 2016, 141, 125–132. [Google Scholar] [CrossRef]
- Misyura, S.Y.; Manakov, A.Y.; Morozov, V.S.; Nyashina, G.S.; Gaidukova, O.S.; Skiba, S.S.; Volkov, R.S.; Voytkov, I.S. The influence of key parameters on combustion of double gas hydrate. J. Nat. Gas Sci. Eng. 2020, 80, 103396. [Google Scholar] [CrossRef]
- Yang, M.; Song, Y.; Jiang, L.; Liu, W.; Dou, B.; Jing, W. Effects of operating mode and pressure on hydrate-based desalination and CO2 capture in porous media. Appl. Energy 2014, 135, 504–511. [Google Scholar] [CrossRef]
- Kang, K.C.; Linga, P.; Park, K.; Choi, S.J.; Lee, J.D. Seawater desalination by gas hydrate process and removal characteristics of dissolved ions (Na+, K+, Mg2+, Ca2+, B3+, Cl−, SO42−). Desalination 2014, 353, 84–90. [Google Scholar] [CrossRef]
- McCormack, R.A.; Niblock, G.A. Investigation of High Freezing Temperature, Zero Ozone, and Zero Global Warming Potential, Clathrate Formers for Desalination; US Department of the Interior, Bureau of Reclamation, Technical Service: Denver, CO, USA, 2000.
- Simmons, B.A.; Bradshaw, R.W.; Dedrick, D.E.; Cygan, R.T.; Greathouse, J.A.; Majzoub, E.H. Desalination Utilizing Clathrate Hydrates (LDRD Final Report); National Technical Information Service: Livermore, CA, USA, 2008.
- Karamoddin, M.; Varaminian, F. Water desalination using R141b gas hydrate formation. Desalin. Water Treat. 2014, 52, 2450–2456. [Google Scholar] [CrossRef]
- Ngema, P.T.; Petticrew, C.; Naidoo, P.; Mohammadi, A.H.; Ramjugernath, D. Experimental measurements and thermodynamic modeling of the dissociation conditions of clathrate hydrates for (refrigerant + NaCl + water) systems. J. Chem. Eng. Data. 2014, 59, 466–475. [Google Scholar] [CrossRef]
- Seo, Y.; Moon, D.; Lee, C.; Park, J.W.; Kim, B.S.; Lee, G.W.; Yoon, J.H. Equilibrium, kinetics, and spectroscopic studies of SF6 hydrate in NaCl electrolyte solution. Environ. Sci. Technol. 2015, 49, 6045–6050. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Zhang, X.M.; Li, H.H.; Shao, L.; Zhang, D.; Jiao, L. Theory research on desalination of brackish water using gas hydrate method. Adv. Mater. Res. 2013, 616, 1202–1207. [Google Scholar] [CrossRef]
- Lee, D.; Lee, Y.; Lee, S.; Seo, Y. Accurate measurement of phase equilibria and dissociation enthalpies of HFC-134a hydrates in the presence of NaCl for potential application in desalination. Korean J. Chem. Eng. 2016, 33, 1425–1430. [Google Scholar] [CrossRef]
- Khan, M.N. Phase Equilibria Modeling of Inhibited Gas Hydrate Systems Including Salts: Applications in Flow Assurance, Seawater Desalination and Gas Separation. Ph.D. Thesis, Colorado School of Mines, Golden, CO, USA, 2016. [Google Scholar]
- Zhang, Y.; Sheng, S.M.; Shen, X.D.; Zhou, X.B.; Wu, W.Z.; Wu, X.P.; Liang, D.Q. Phase equilibrium of cyclopentane + carbon dioxide binary hydrates in aqueous sodium chloride solutions. J. Environ. Chem. Eng. 2017, 62, 2461–2465. [Google Scholar] [CrossRef]
- Ho-Van, S.; Bouillot, B.; Douzet, J.; Babakhani, S.M.; Herri, J.M. Implementing cyclopentane hydrates phase equilibrium data and simulations in brine solutions. Ind. Eng. Chem. Res. 2018, 43, 14774–14783. [Google Scholar] [CrossRef]
- Ho-Van, S.; Bouillot, B.; Douzet, J.; Babakhani, S.M.; Herri, J.M. Cyclopentane hydrates–A candidate for desalination? J. Environ. Chem. Eng. 2019, 7, 103359. [Google Scholar] [CrossRef]
- Lv, Q.; Li, X.; Li, G. Seawater desalination by hydrate formation and pellet production process. Energy Procedia 2019, 158, 5144–5148. [Google Scholar] [CrossRef]
- Choi, W.; Lee, Y.; Mok, J.; Lee, S.; Lee, J.D.; Seo, Y. Thermodynamic and kinetic influences of NaCl on HFC-125a hydrates and their significance in gas hydrate-based desalination. Chem. Eng. J. 2019, 358, 598–605. [Google Scholar] [CrossRef]
- Seo, S.D.; Hong, S.Y.; Sum, A.K.; Lee, K.H.; Lee, J.D.; Lee, B.R. Thermodynamic and kinetic analysis of gas hydrates for desalination of saturated salinity water. Chem. Eng. J. 2019, 370, 980–987. [Google Scholar] [CrossRef]
- Han, S.; Shin, J.Y.; Rhee, Y.W.; Kang, S.P. Enhanced efficiency of salt removal from brine for cyclopentane hydrates by washing, centrifuging, and sweating. Desalination 2014, 354, 17–22. [Google Scholar] [CrossRef]
- Karamoddin, M.; Varaminian, F. Water purification by freezing and gas hydrate processes, and removal of dissolved minerals (Na+, K+, Mg2+, Ca2+). J. Mol. Liq. 2016, 223, 1021–1031. [Google Scholar] [CrossRef]
- Fakharian, H.; Ganji, H.; Naderifar, A.; Mofrad, H.R.; Kakavand, M. Effect of gas type and salinity on performance of produced water desalination using gas hydrates. J. Water Reuse Desalin. 2019, 9, 396–404. [Google Scholar] [CrossRef]
- Kang, K.C.; Hong, S.Y.; Cho, S.J.; Kim, D.H.; Lee, J.D. Evaluation of desalination by nanostructured hydrate formation and pellet production process. J. Nanosci. Nanotechnol. 2017, 17, 4059–4062. [Google Scholar] [CrossRef]
- Javanmardi, J.; Moshfeghian, M. Energy consumption and economic evaluation of water desalination by hydrate phenomenon. Appl. Therm. Eng. 2003, 23, 845–857. [Google Scholar] [CrossRef]
- Ghalavand, Y.; Hatamipour, M.S.; Rahimi, A. A review on energy consumption of desalination processes. Desalin. Water Treat. 2015, 54, 1526–1541. [Google Scholar] [CrossRef]
- Singh, J.; Lal, B. Prospectives on gas hydrates-based desalination. In Gas Hydrate in Water Treatment, 1st ed.; Lal, B., Nallakukkala, S., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; Volume 1, pp. 31–53. [Google Scholar]
- Chong, Z.R.; He, T.; Babu, P.; Zheng, J.; Linga, P. Economic evaluation of energy efficient hydrate-based desalination utilizing cold energy from liquefied natural gas (LNG). Desalination 2019, 463, 69–80. [Google Scholar] [CrossRef]
- He, T.; Chong, Z.R.; Zheng, J.; Ju, Y.; Linga, P. LNG cold energy utilization: Prospects and challenges. Energy 2019, 170, 557–568. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, M. Experimental investigation on novel desalination system via gas hydrate. Desalination 2020, 478, 114284. [Google Scholar] [CrossRef]
- Sarshar, M.; Sharafi, A.H. Simultaneous water desalination and CO2 capturing by hydrate formation. Desalin. Water Treat. 2011, 28, 59–64. [Google Scholar] [CrossRef]
- Babu, P.; Kumar, R.; Linga, P. Unusual behavior of propane as a co-guest during hydrate formation in silica sand: Potential application to seawater desalination and carbon dioxide capture. Chem. Eng. Sci. 2014, 117, 342–351. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, J.; Liu, W.; Liu, Y.; Song, Y. Effects of C3H8 on hydrate formation and dissociation for integrated CO2 capture and desalination technology. Energy 2015, 93, 1971–1979. [Google Scholar] [CrossRef]
- Donath, W.E. Method and Apparatus for Producing Purified Water from Aqueous Saline Solutions. U.S. Patent No. 2,904,511, 15 September 1959. [Google Scholar]
- Buchanan, B.B. Removing Salt from Sea Water. U.S. Patent 3,027,320, 27 March 1962. [Google Scholar]
- Walton, P.R. Continuous Saline Water Purification. U.S. Patent 3,132,096, 5 May 1964. [Google Scholar]
- Klass, D. Hydrate Forming in Water Desalination. U.S. Patent No 3,856,492, 24 December 1974. [Google Scholar]
- Guo, B.; Bretz, R.E.; Lee, R.L. Method and Apparatus for Generating, Transporting and Dissociating Gas Hydrates. U.S. Patent No. 5,473,904, 12 December 1995. [Google Scholar]
- McCormack, R.A. Clathrate Freeze Desalination Apparatus and Method. U.S. Patent No. 5,553,456, 10 September 1996. [Google Scholar]
- Max, M.D.; Pellenbarg, R.E. Desalination through Methane Hydrate. U.S. Patent No. 5,873,262, 23 February 1999. [Google Scholar]
- Heinemann, R.F.; Huang, D.D.T.; Long, J.; Saeger, R.B. Process for Making Gas Hydrates. U.S. Patent No. 6,028,234, 22 February 2000. [Google Scholar]
- Heinemann, R.F.; Huang, D.D.T.; Long, J.; Saeger, R.B. Method for Producing Gas Hydrates Utilizing a Fluidized Bed. U.S. Patent No. 6,180,843, 30 January 2001. [Google Scholar]
- Max, M.D. Hydrate Formation and Growth for Hydrate-Based Desalination by Means of Enriching Water to Be Treated. U.S. Patent No. 6,890,444, 10 May 2005. [Google Scholar]
- Max, M.D.; Korsgaard, J. Hydrate-Based Desalination with Hydrate-Elevating Density-Driven Circulation. U.S. Patent No. 6,969,467, 10 November 2005. [Google Scholar]
- Simmons, B.A.; Bradshaw, R.W.; Dedrick, D.E.; Anderson, D.W. Complex Admixtures of Clathrate Hydrates in a Water Desalination Method. U.S. Patent 7560028B1, 14 July 2009. [Google Scholar]
- Phelps, T.J.; Tsouris, C.; Palumbo, A.V.; Riestenberg, D.E.; McCallum, S.D. Method for Excluding Salt and Other Soluble Materials from Produced Water. U.S. Patent No. 7,569,737, 4 August 2009. [Google Scholar]
- Li, D.; Liang, D.; Tang, C. Test Device for Desalination of Sea Water by Hydrate Method. CN Patent CN101289231B, 6 June 2010. [Google Scholar]
- Osegovic, J.P.; Max, M.D.; Tatro, S.R. Seawater-Based Carbon Dioxide Disposal. U.S. Patent No. 8,048,309, 1 November 2011. [Google Scholar]
- Carstens, C.; Dickinson, W.; Dickinson, W.; Myers, J. Clathrate Hydrate Modular Storage, Applications and Utilization Processes. U.S. Patent No. 7914749, 29 March 2011. [Google Scholar]
- Park, K.N.; Hong, S.Y.; Lee, J.W.; Kang, K.C.; Lee, Y.C.; Ha, M.G.; Lee, J.D. A new apparatus for seawater desalination by gas hydrate process and removal characteristics of dissolved minerals (Na+, Mg2+, Ca2+, K+, B3+). Desalination 2011, 274, 91–96. [Google Scholar] [CrossRef]
- Katyal, A.A. System and Method for Hydrate-Based Desalination. U.S. Patent No. 9,643,860, 9 May 2017. [Google Scholar]
- McCormack, R.A.; Ripmeester, J.A. Clathrate Desalination Process Using an Ultrasonic Actuator. U.S. Patent 20140223958A1, 14 August 2014. [Google Scholar]
- Parker, A. Potable water from sea-water. Nature 1942, 149, 184–186. [Google Scholar] [CrossRef]
- Hesse, R.; Harrison, W.E. Gas hydrates (clathrates) causing pore-water freshening and oxygen isotope fractionation in deep-water sedimentary sections of terrigenous continental margins. Earth Planet. Sci. Lett. 1981, 55, 453–462. [Google Scholar] [CrossRef]
- Willson III, R.C.; Bulot, E.; Cooney, C. L Use of Hydrates for Aqueous Solution Treatment. U.S. Patent No. 4,678,583, 7 July 1987. [Google Scholar]
- Huang, C.P.; Fennema, O.; Powrie, W.D. Gas hydrates in aqueous-organic systems: II. Concentration by gas hydrate formation. Cryobiology 1966, 2, 240–245. [Google Scholar] [CrossRef]
- Truong-Lam, H.S.; Kim, S.; Seo, S.D.; Jeon, C.; Lee, J.D. Water purifying by gas hydrate: Potential applications to desalination and wastewater treatments. Chem. Eng. Trans. 2020, 78, 67–72. [Google Scholar]
- Song, Y.; Dong, H.; Yang, L.; Yang, M.; Li, Y.; Ling, Z.; Zhao, J. Hydrate-based heavy metal separation from aqueous solution. Sci. Rep. 2016, 6, 21389. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, H.; Li, F.; Shi, C.; Wang, S.; Ling, Z. Cyclopentane hydrate-based processes for treating heavy metal containing wastewater. E3S Web Conf. 2019, 118, 10–13. [Google Scholar] [CrossRef]
- Li, X.S.; Yang, B.; Zhang, Y.; Li, G.; Duan, L.P.; Wang, Y.; Wu, H.J. Experimental investigation into gas production from methane hydrate in sediment by depressurization in a novel pilot-scale hydrate simulator. Appl. Energy 2012, 93, 722–732. [Google Scholar] [CrossRef]
- Ohmura, R.; Ogawa, M.; Yasuoka, K.; Mori, Y.H. Statistical study of clathrate-hydrate nucleation in a water/hydrochlorofluorocarbon system: Search for the nature of the memory effect. J. Phys. Chem. B 2003, 107, 5289–5293. [Google Scholar] [CrossRef]
- Murshed, M.M.; Faria, S.H.; Kuhs, W.F.; Kipfstuhl, S.; Wilhelms, F. The role of hydrochlorofluorocarbon densifiers in the formation of clathrate hydrates in deep boreholes and subglacial environments. Ann. Glaciol. 2007, 47, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Kato, M.; Iida, T.; Mori, Y.H. Drop formation behaviour of a hydrate-forming liquid in a water stream. J. Fluid Mech. 2000, 414, 367–378. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, L.; Ling, Z.; Zhao, J.; Song, Y. The Controlling Factors and Ion Exclusion Mechanism of Hydrate-Based Pollutant Removal. ACS Sustain. Chem. Eng. 2019, 7, 7932–7940. [Google Scholar] [CrossRef]
- Gaikwad, N.; Nakka, R.; Khavala, V.; Bhadani, A.; Mamane, H.; Kumar, R. Gas Hydrate-Based Process for Desalination of Heavy Metal Ions from an Aqueous Solution: Kinetics and Rate of Recovery. ACS ES&T Water 2021, 1, 134–144. [Google Scholar]
- Nallakukkala, S.; Lal, B. Waste brine management. In Gas Hydrate in Water Treatment, 1st ed.; Lal, B., Nallakukkala, S., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; Volume 1, pp. 31–53. [Google Scholar]
- Lee, H.; Ryu, H.; Lim, J.H.; Kim, J.O.; Dong Lee, J.; Kim, S. An optimal design approach of gas hydrate and reverse osmosis hybrid system for seawater desalination. Desalin. Water Treat. 2016, 57, 9009–9017. [Google Scholar] [CrossRef]
- Dong, H.; Fan, Z.; Wang, B.; Xue, S.; Zhao, J.; Song, Y. Hydrate-based reduction of heavy metal ion from aqueous solution. Energy Procedia 2017, 105, 4706–4712. [Google Scholar] [CrossRef]
- Fakharian, H.; Ganji, H.; Naderifar, A. Desalination of high salinity produced water using natural gas hydrate. J. Taiwan Inst. Chem. Eng. 2017, 72, 157–162. [Google Scholar] [CrossRef]
- Xu, H.; Khan, M.N.; Peters, C.J.; Sloan, E.D.; Koh, C.A. Hydrate-based desalination using cyclopentane hydrates at atmospheric pressure. J. Chem. Eng. Data 2018, 63, 1081–1087. [Google Scholar] [CrossRef]
- Han, S.; Rhee, Y.-W.; Kang, S.-P. Investigation of salt removal using cyclopentane hydrate formation and washing treatment for seawater desalination. Desalination 2017, 404, 132–137. [Google Scholar] [CrossRef]
- Fakharian, H.; Ganji, H.; Naderifar, A. Naderifar, Saline produced water treatment using gas hydrates. J. Environ. Chem. Eng. 2017, 5, 4269–4273. [Google Scholar] [CrossRef]
- Khan, M.N.; Peters, C.J.; Koh, C.A. Desalination using gas hydrates: The role of crystal nucleation, growth and separation. Desalination 2019, 468, 114049. [Google Scholar] [CrossRef]
- Li, F.; Yang, Y.; Shi, C.; Yang, S.; Chen, Z.; Yang, S.; Song, Y. Hydrate-based desalination process enhanced via graphite. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 295, p. 042028. [Google Scholar]
- Sun, H.; Sun, L.; Zhao, Y.; Yang, S.; Zhang, L.; Dong, H.; Song, Y. A combined hydrate-based method for removing heavy metals from simulated wastewater with high concentrations. J. Environ. Chem. Eng. 2021, 9, 106633. [Google Scholar] [CrossRef]
- Truong-Lam, H.S.; Seo, S.D.; Jeon, C.; Lee, G.P.; Lee, J.D. A gas hydrate process for high-salinity water and wastewater purification. Desalination 2022, 529, 115651. [Google Scholar] [CrossRef]
- Ling, Z.; Shi, C.; Li, F.; Fu, Y.; Zhao, J.; Dong, H.; Song, Y. Desalination and Li+ enrichment via formation of cyclopentane hydrate. Sep. Purif. Technol. 2020, 231, 115921. [Google Scholar] [CrossRef]
- Nambiar, A.; Babu, P.; Linga, P. Improved kinetics and water recovery with propane as co-guest gas on the hydrate-based desalination (hydesal) process. ChemEngineering 2019, 3, 31. [Google Scholar] [CrossRef] [Green Version]
- Babu, P.; Nambiar, A.; Chong, Z.R.; Daraboina, N.; Albeirutty, M.; Bamaga, O.A.; Linga, P. Hydrate-based desalination (HyDesal) process employing a novel prototype design. Chem. Eng. Sci. 2020, 218, 115563. [Google Scholar] [CrossRef]
- Al-Hemeri, S.T.; Al-Mukhtar, R.S.; Hussine, M.N. Removal of heavy metals from industrial wastewater by use of Cyclopentane-Clathrate Hydrate formation technology. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Istanbul, Turkey, 2020; Volume 737, p. 012178. [Google Scholar]
- Johannsen, P.; Karlapudi, R.; Reinhold, G. High pressure reverse osmosis for wastewater minimization and zero liquid discharge applications. Desalination 2006, 199, 84–85. [Google Scholar] [CrossRef]
- Nallakukkala, S.; Abulkhair, H.; Alsaiari, A.; Ahmad, I.; Almatrafi, E.; Bamaga, O.; Mohd Shariff, A. Suitable Binary and Ternary Thermodynamic Conditions for Hydrate Mixtures of CH4, CO2, and C3H8 for Gas Hydrate-Based Applications. ACS Omega 2022, 7, 10877–10889. [Google Scholar] [CrossRef]
- Falahieh, M.M.; Bonyadi, M.; Lashanizadegan, A. A new hybrid desalination method based on the CO2 gas hydrate and capacitive deionization processes. Desalination 2021, 502, 114932. [Google Scholar] [CrossRef]
- Youssef, P.; Al-Dadah, R.; Mahmoud, S. Comparative Analysis of Desalination Technologies. Energy Procedia 2014, 61, 2604–2607. [Google Scholar] [CrossRef] [Green Version]
- Yun, S.H.; Woo, D.S. Analysis of seawater desalination energy consumption based on changes in raw water characteristics and operating condition. J. Korean Soc. Water Wastewater 2019, 33, 281–289. [Google Scholar] [CrossRef]
- Lal, B.; Nallakukkala, S. Gas Hydrate in Water Treatment: Technological, Economic, and Industrial Aspects, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 14–301. [Google Scholar]
| Metal | Route of Entry | Toxicity Effect | Disposal Limit Recommended by WHO * (mg/L) |
|---|---|---|---|
| Copper | Ingestion and inhalation | intestinal irritation, liver illness, anemia, and cancer in respiratory tract | 0.02 |
| Cadmium | Inhalation and ingestion | lung damage and limits the respiratory system | 0.06 |
| Chromium | Inhalation, ingestion, and absorption through skin | damage the lungs and limits the respiratory system | 0.05 |
| Mercury | Inhalation, ingestion, and absorption through skin | imitation of respiratory system, liver and kidney damage and loss of hearing | 0.01 (vapor) |
| Lead | Inhalation and ingestion | lungs and kidney damage | 0.15 |
| Nickel | Inhalation | lung, liver kidney damage | 0.1 |
| Zinc | Inhalation and ingestion | It causes a number of health problems, including fever, nausea, vomiting, skin irritation and anemia, despite the fact that it is required by humans at a trace amount. | 0.05 |
| S. No | Techniques | Merits | Demerits | Reference |
|---|---|---|---|---|
| 1. | Coagulation | cheap, dewatering mechanism | Production of sludge, Chemicals are utilized extensively. | [32] |
| 2. | Filtration through membrane | Heavy metals exclusion at a rapid rate demands less room. | Extremely costly, membrane fouling, and complicated procedure. | [32] |
| 3. | Adsorption | Simple operation, minimal sludge formation, and the utilization of low-cost adsorbents | Desorption cost for regenerable adsorbent is high. | [33] |
| 4. | Electrochemical treatment | Effective in eliminating metal ions with slight chemical use | The initial expenditure is significant, and a large quantity of electrical power is essential | [32] |
| 5. | Electrodialysis | Metals are separated to a higher degree. | Clogging and loss of energy | [34] |
| 6. | Ion exchange | High transformation of components | removes a little amount of metal ions, and the operational cost is significant. | [35] |
| 7. | Oxidation | No requirement for electricity | oxidation process causes rusting in the system. | [36] |
| Technique | Material Used | Metals | Removal % | Remarks | Reference |
|---|---|---|---|---|---|
| Adsorption | Modified graphene (GN) with cetyltrimethylammonium bromide | Cr | 98.2 | Low selectivity, production of waste. It is difficult to synthesize. The cost of adsorbent is too high. | [81] |
| Activated Carbon from Prawn shell and green alga Ulva lactuca | Cr, Cd | 98, 95 | Higher quantities on larger scales are difficult to manage | [82,83] | |
| Membrane Filtration | RO | Cr, Cu, Cd | 98, 99, 90 | High operational cost due to membrane fouling | [84] |
| Ultrafiltration | Cu | 90 | |||
| Electrodialysis principles | perfluorosulfonic Nafion 117 | Co, Ni | 90, 69 | Clogging and energy loss | [85] |
| HNO3 | Cd | 70 | [86] | ||
| Chemical Precipitation | Magnesia and lime-water | Fe(III), Cr(III), Cu(II), Pb(II), Ni(II) & Cd(II) | 97 | The cost of producing high-water-content sludge and disposing of it has increased. Precipitation with lime and bisulphide, lacks specificity. When it comes to removing metal ions of low concentration, this method can be useless. | [87] |
| Ion Exchange | Magnetic ion exchange | Cr, Cu, Cd, Hg | 99.9 | Not effective when employing concentrated metal solutions since the exchange matrix is easily fouled by organic/other wastes and is the major limitation. | [88] |
| Floatation | potassium ethyl xanthate (KEtX) | Ni, Cd, Co | 98.3, 97.5 and 94.7 | High concentration of floatation agents are employed which affects the economy of the process. | [53] |
| Method | Principle | T (°C) | P (MPa) | Water Recovery | Total Average Specific Energy Consumption (kWh/m3 of Water) | Maintainance | Advanatge/Constraints |
|---|---|---|---|---|---|---|---|
| Distillation | Flash process | 90–120 | Less than 0.1 | 20% [3] | 23.4 [104] | Corrosion/ scaling | Used for high TDS (total dissolved solid) concentration/High energy, less water recovery |
| Reverse Osmosis | Solute diffusion | 20–35 | 5.5–7.0 | 55% | 5 [104] | Sludge generation/membrane replacement | Requires pretreatment, less water recovery, resistant to impurities |
| Hydrate desalination | Phase change | Near to 0 | 0.45–0.65 | 58.6% [105] | 0.6 [106] | No maintainance | suitable for greater TDS concentrations/higher water recovery |
| Metal Ion | Hydrate Former | Conc. of Former | Water Recovery | Reference | |
|---|---|---|---|---|---|
| With Additive | Without Additive | ||||
| As | CP | 1:6 | 28.75 | 25.72 | |
| Cd | 24.51 | 19.76 | [177] | ||
| Cr | 23.33 | 14.97 | |||
| Pb | 27.92 | 15.51 | |||
| Cr | R141b | 1:4 | 80 | ||
| Cu | 80 | [170] | |||
| Ni | 80 | ||||
| Zn | 80 | ||||
| Ni | CP | 1:3 | 43 | [176] | |
| Cu | R141b | 1:5 | 80.83 | ||
| Cu | CP | 1:6 1:2 | 76 | [170] | |
| Ni | 75 | ||||
| Zn | 70 | [171] | |||
| Cr | 74 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nallakukkala, S.; Rehman, A.u.; Zaini, D.B.; Lal, B. Gas Hydrate-Based Heavy Metal Ion Removal from Industrial Wastewater: A Review. Water 2022, 14, 1171. https://doi.org/10.3390/w14071171
Nallakukkala S, Rehman Au, Zaini DB, Lal B. Gas Hydrate-Based Heavy Metal Ion Removal from Industrial Wastewater: A Review. Water. 2022; 14(7):1171. https://doi.org/10.3390/w14071171
Chicago/Turabian StyleNallakukkala, Sirisha, Adeel ur Rehman, Dzulkarnain B. Zaini, and Bhajan Lal. 2022. "Gas Hydrate-Based Heavy Metal Ion Removal from Industrial Wastewater: A Review" Water 14, no. 7: 1171. https://doi.org/10.3390/w14071171
APA StyleNallakukkala, S., Rehman, A. u., Zaini, D. B., & Lal, B. (2022). Gas Hydrate-Based Heavy Metal Ion Removal from Industrial Wastewater: A Review. Water, 14(7), 1171. https://doi.org/10.3390/w14071171






