Assessing Surface Water Quality for Irrigation Purposes in Some Dams of Asir Region, Saudi Arabia Using Multi-Statistical Modeling Approaches
Abstract
:1. Introduction
2. Materials and Methods
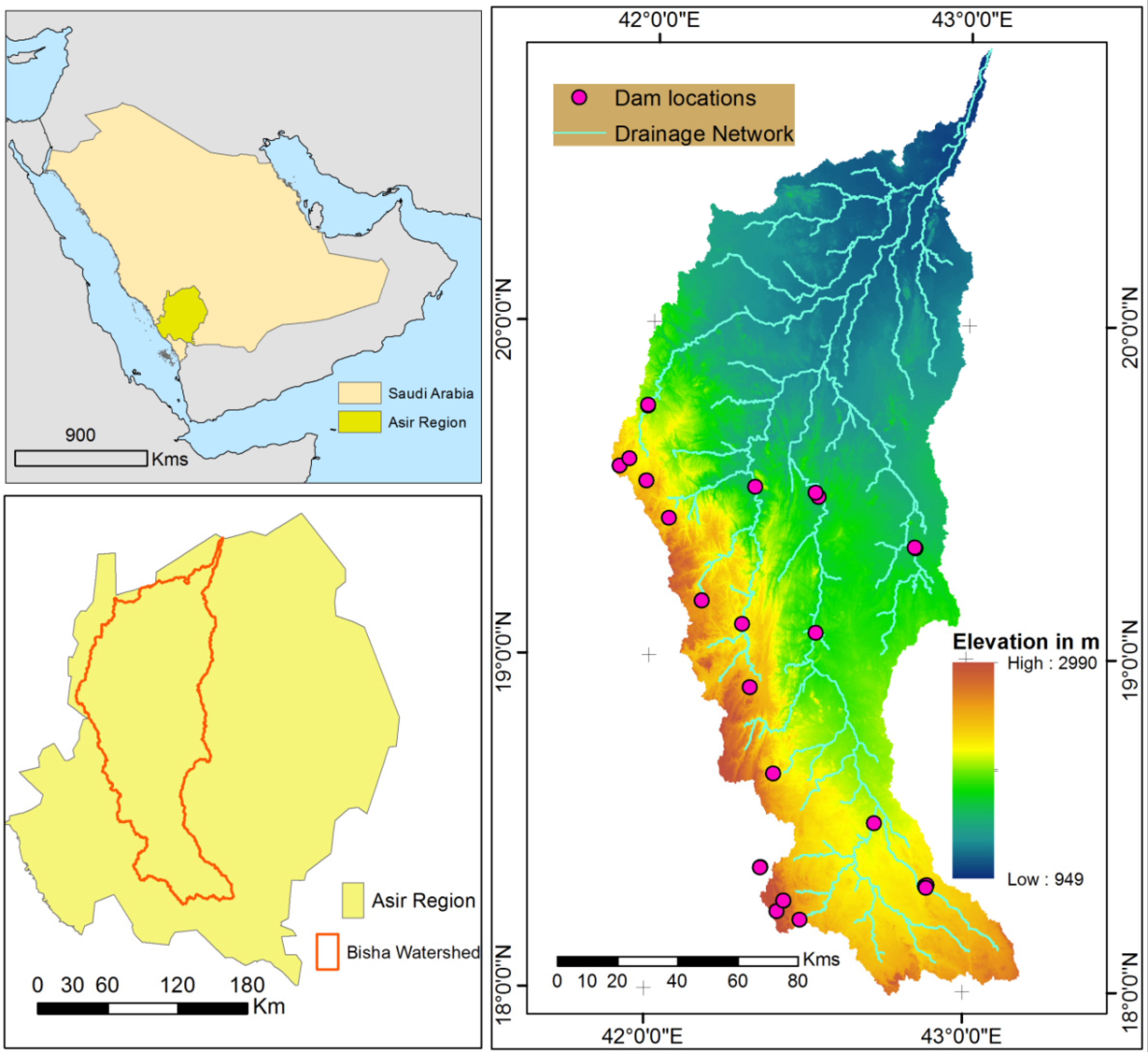
2.1. Study Area Description
2.2. Data Collection and Analytical Design
2.3. Irrigation Water Quality Parameters
2.4. Statistical Analyses
3. Results
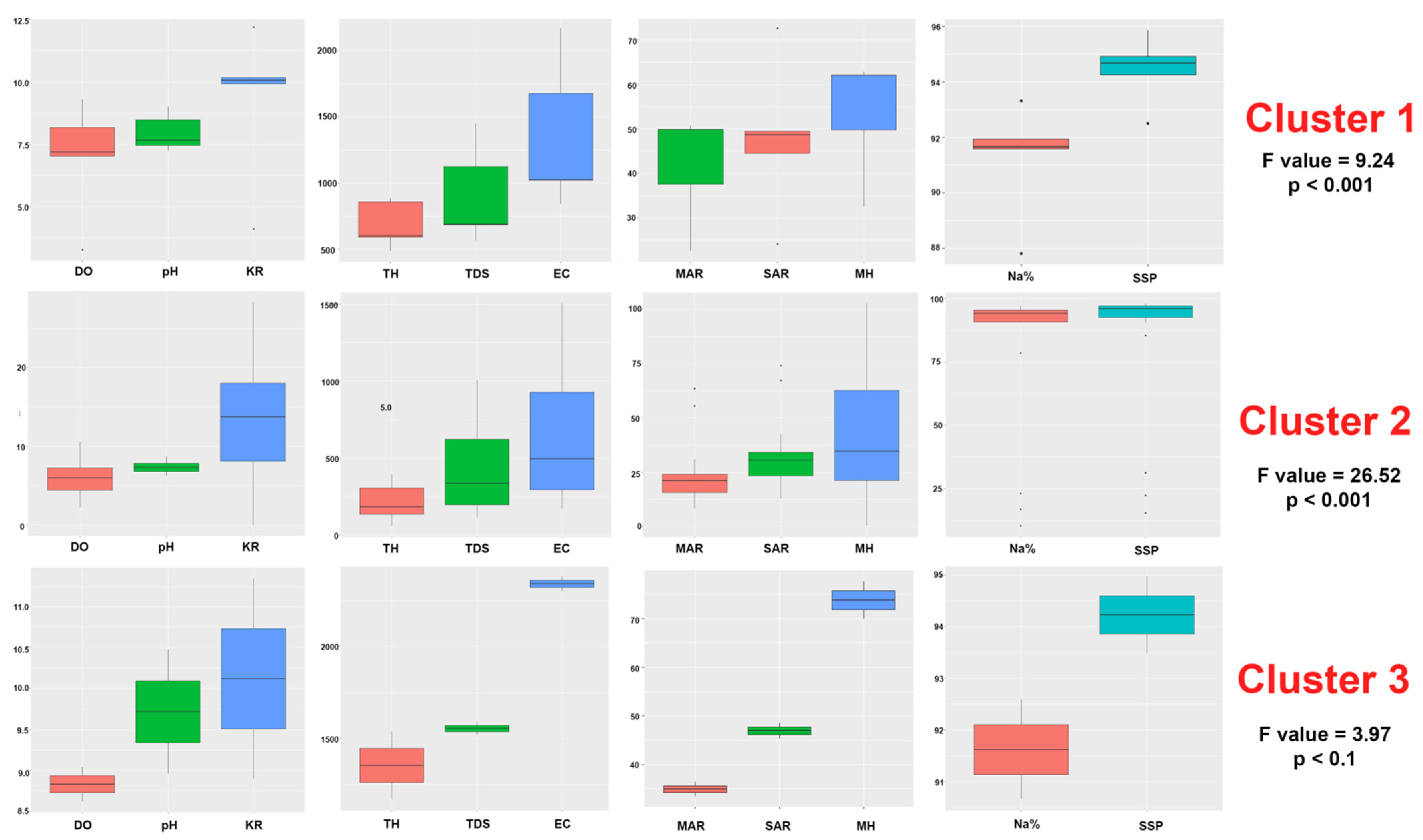
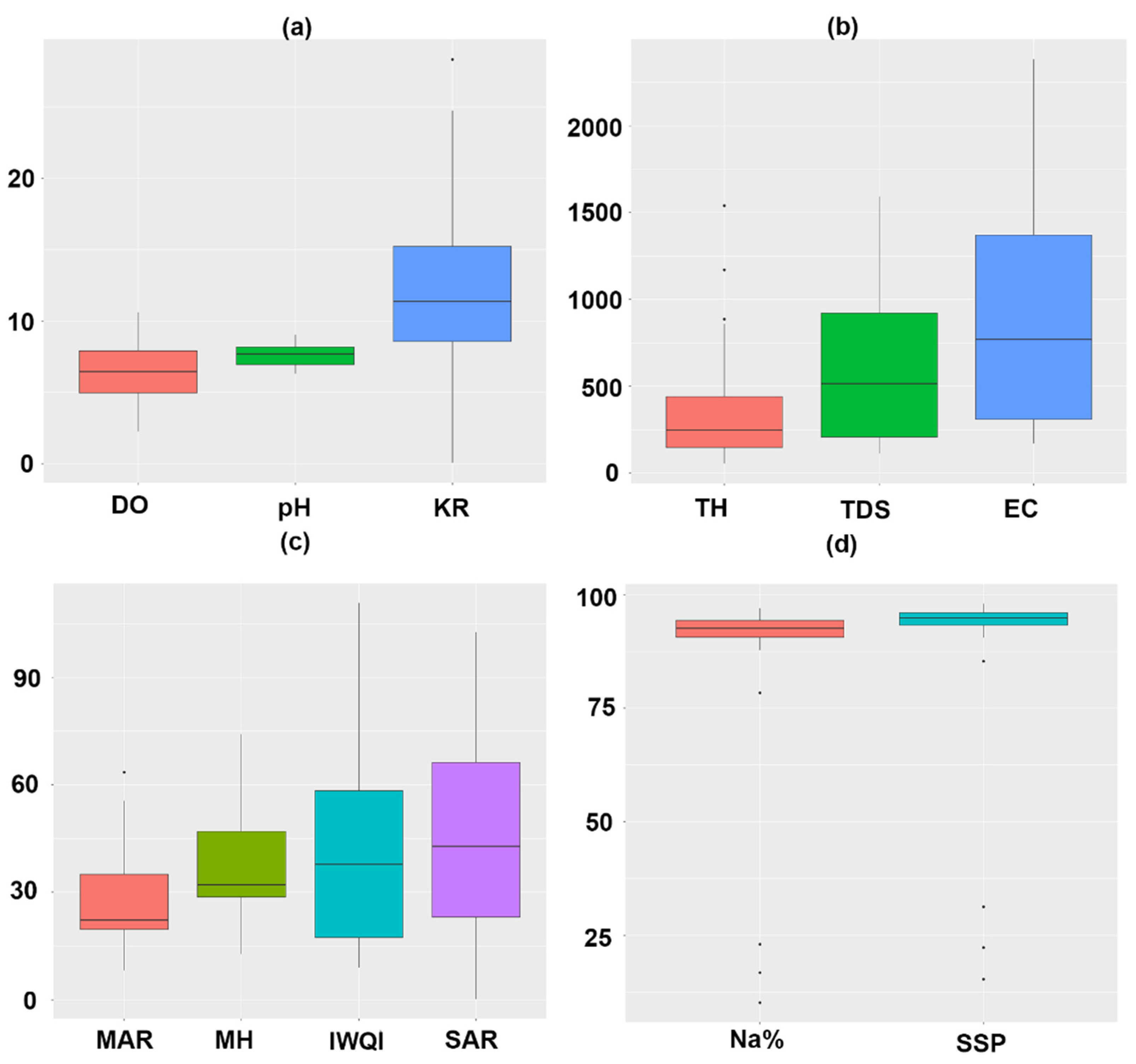
3.1. Variation of Physicochemical Parameters
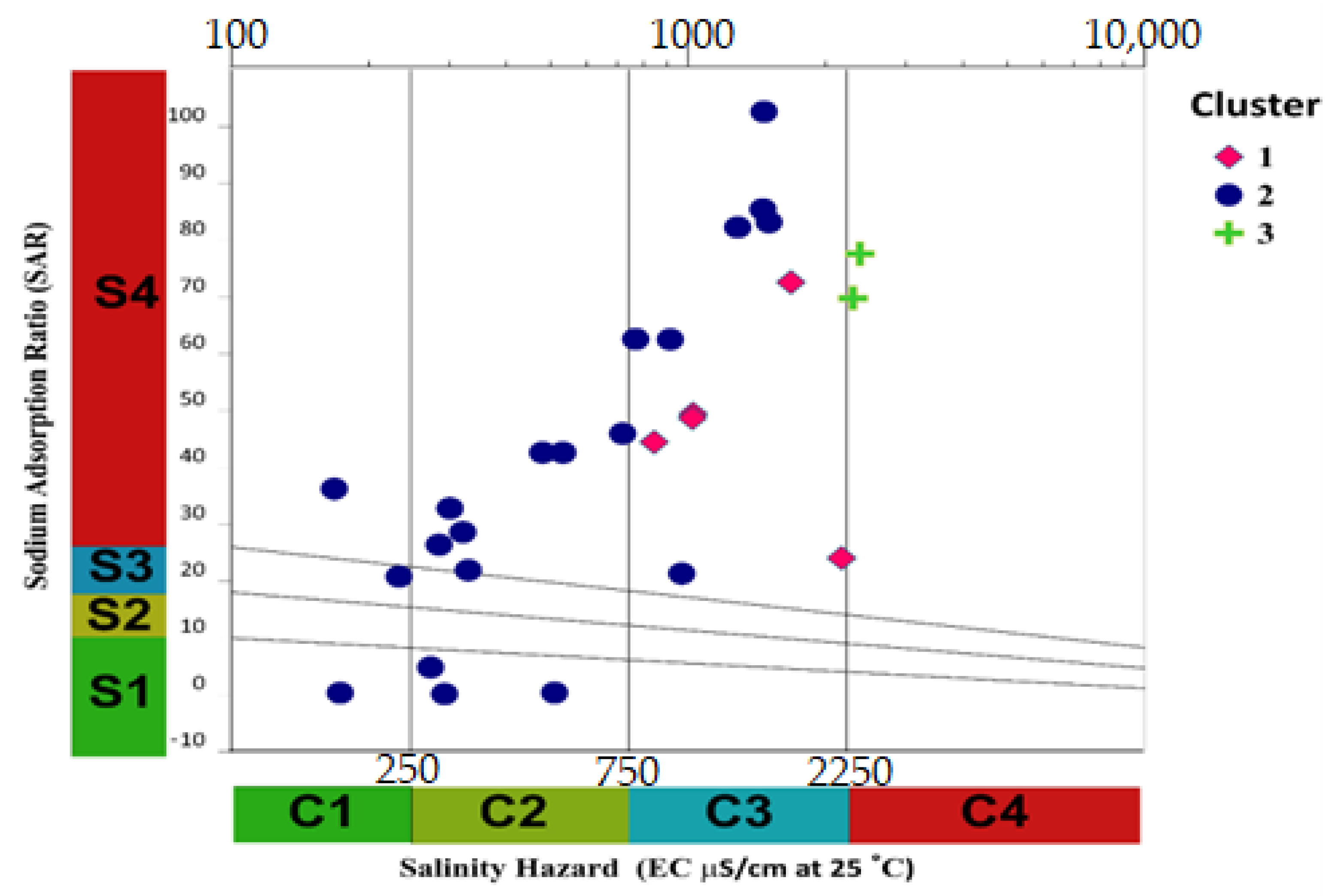
3.2. Indices of Irrigation Water Quality
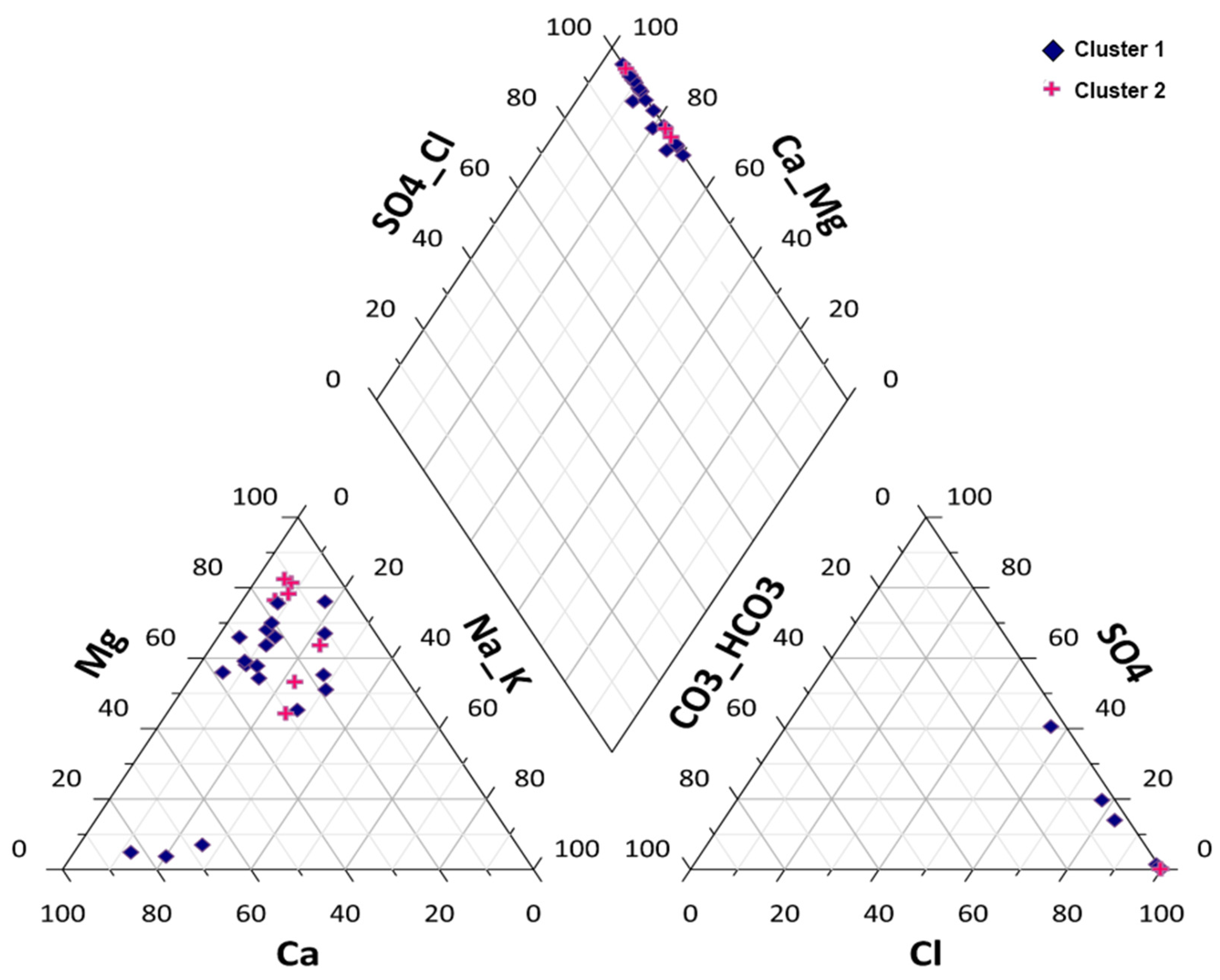
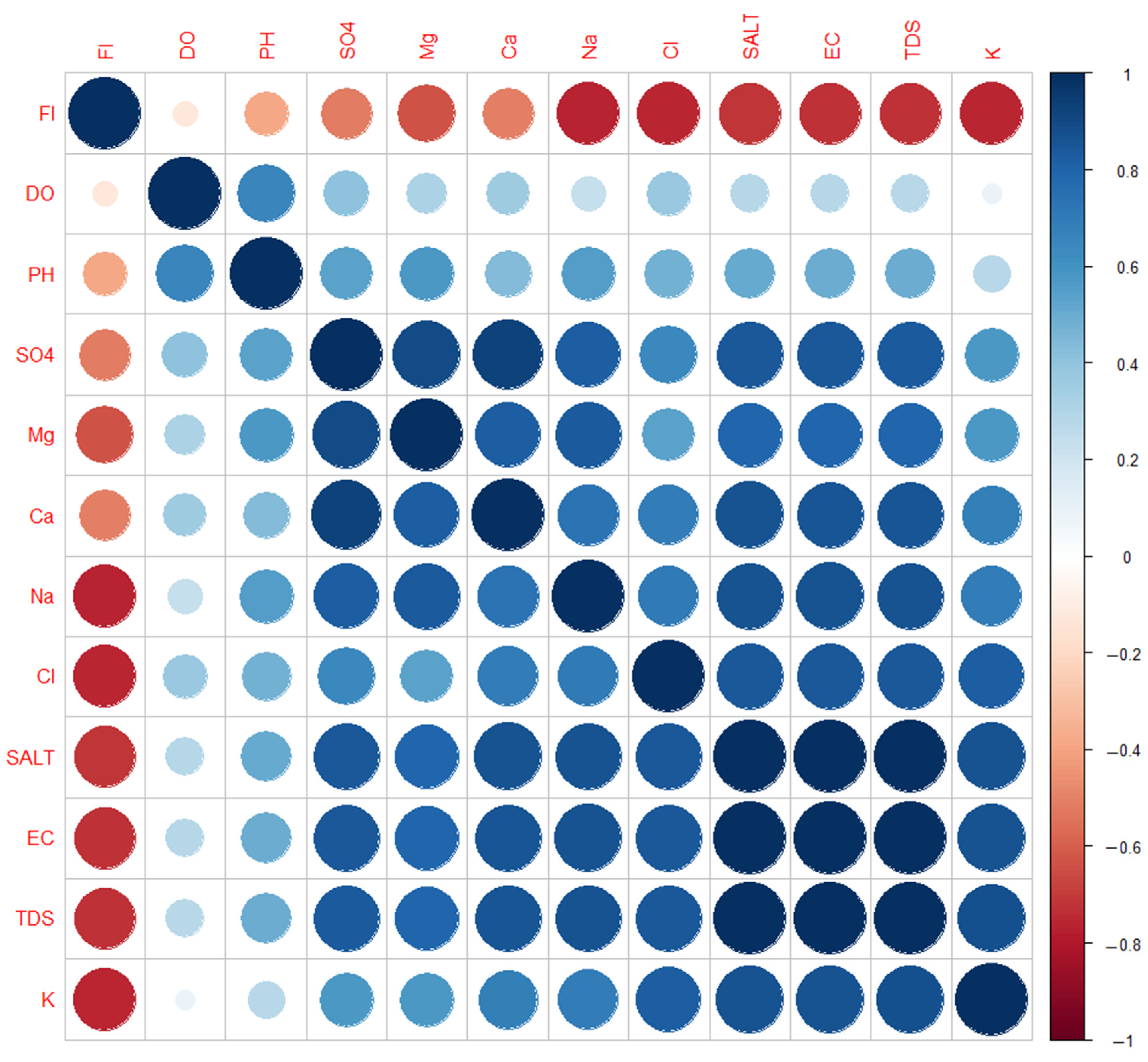
3.3. Associations between Irrigation Water Parameters
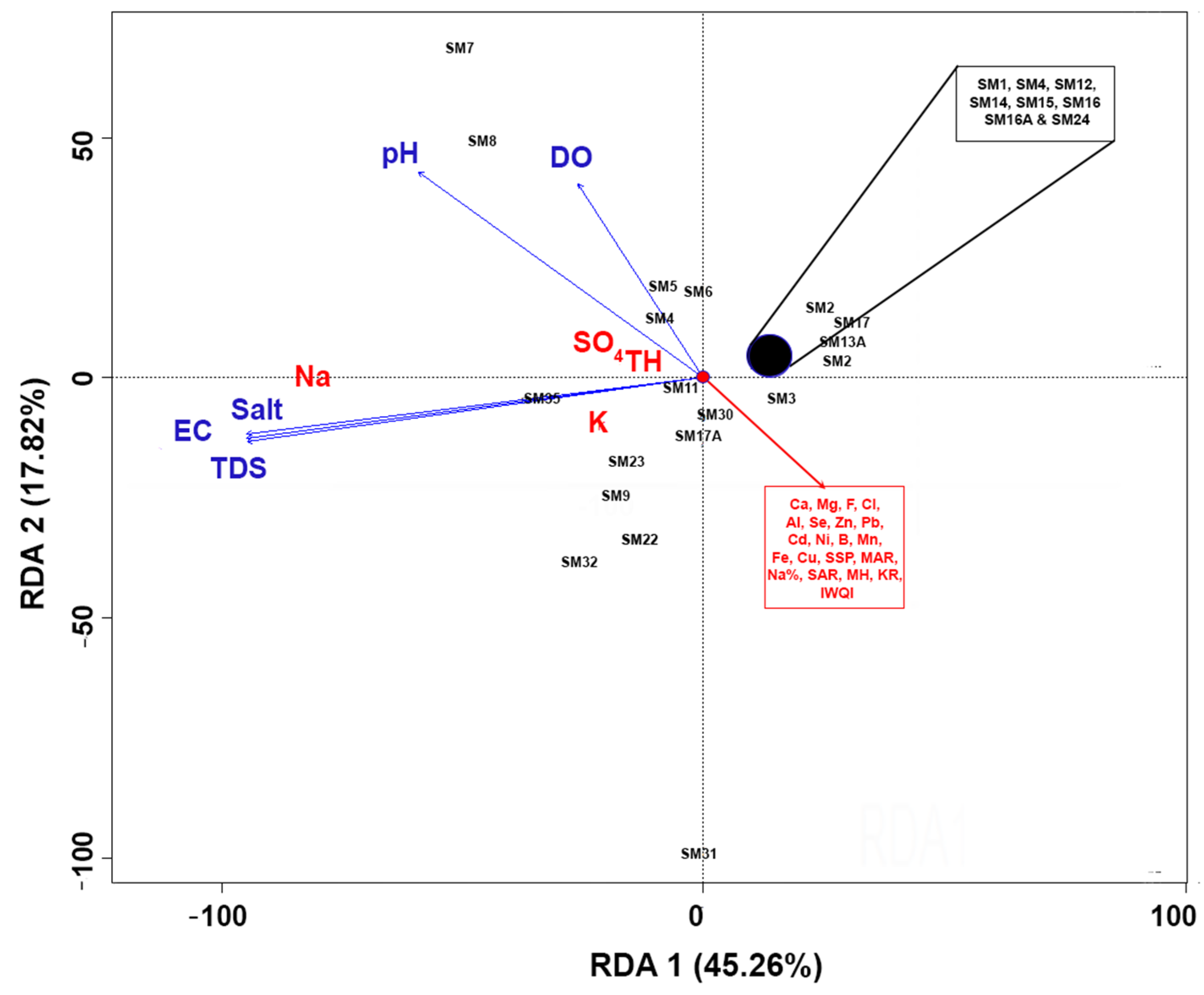
3.4. Irrigation Water and Physicochemical Parameter Associations
3.5. Impacts of Irrigation Water Variables on Physicochemical Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddique, M.A.B.; Khan, R.; Islam, A.R.M.T.; Alam, M.K.; Islam, M.S.; Hossain, M.S.; Habib, M.A.; Akbor, M.A.; Bithi, U.H.; Rashid, M.B.; et al. Quality assessment of freshwaters from a coastal city of southern Bangladesh: Irrigation feasibility and preliminary health risks appraisal. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100524. [Google Scholar] [CrossRef]
- Safiur Rahman, M.; Saha, N.; Islam, A.R.M.T.; Shen, S.; Bodrud-Doza, M. Evaluation of Water Quality for Sustainable Agriculture in Bangladesh. Water Air Soil Pollut. 2017, 228, 385. [Google Scholar] [CrossRef]
- Bouaroudj, S.; Menad, A.; Bounamous, A.; Ali-Khodja, H.; Gherib, A.; Weigel, D.E.; Chenchouni, H. Assessment of water quality at the largest dam in Algeria (Beni Haroun Dam) and effects of irrigation on soil characteristics of agricultural lands. Chemosphere 2019, 219, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Khalaf Ahmed Albaggar, A. Investigation of some physical, chemical, and bacteriological parameters of water quality in some dams in Albaha region, Saudi Arabia. Saudi J. Biol. Sci. 2021, 28, 4605–4612. [Google Scholar] [CrossRef]
- Mallick, J.; Kumar, A.; Almesfer, M.K.; Alsubih, M.; Singh, C.K.; Ahmed, M.; Khan, R.A. An index-based approach to assess groundwater quality for drinking and irrigation in Asir region of Saudi Arabia. Arab. J. Geosci. 2021, 14, 157. [Google Scholar] [CrossRef]
- Mallick, J. Hydrogeochemical characteristics and assessment of water quality in the Al-Saad Lake, Abha Saudi Arabia. Appl. Water Sci. 2017, 7, 2869–2882. [Google Scholar] [CrossRef] [Green Version]
- Islam, A.R.M.T.; Shen, S.; Haque, M.A.; Bodrud-Doza, M.; Maw, K.W.; Habib, M.A. Assessing groundwater quality and its sustainability in Joypurhat district of Bangladesh using GIS and multivariate statistical approaches. Environ. Dev. Sustain. 2017, 20, 1935–1959. [Google Scholar] [CrossRef]
- Towfiqul Islam, A.R.M.; Shen, S.; Bodrud-Doza, M.; Safiur Rahman, M. Assessing irrigation water quality in Faridpur district of Bangladesh using several indices and statistical approaches. Arab. J. Geosci. 2017, 10, 418. [Google Scholar] [CrossRef]
- Ouali, N.; Belabed, B.E.; Chenchouni, H. Modelling environment contamination with heavy metals in flathead grey mullet Mugil cephalus and upper sediments from north African coasts of the Mediterranean Sea. Sci. Total Environ. 2018, 639, 156–174. [Google Scholar] [CrossRef]
- Islam, A.R.M.T.; Islam, H.M.T.; Mia, M.U.; Khan, R.; Habib, M.A.; Bodrud-Doza, M.; Siddique, M.A.B.; Chu, R. Co-distribution, possible origins, status and potential health risk of trace elements in surface water sources from six major river basins, Bangladesh. Chemosphere 2020, 249, 126180. [Google Scholar] [CrossRef]
- Bodrud-Doza, M.; Islam, A.R.M.T.; Ahmed, F.; Das, S.; Saha, N.; Rahman, M.S. Characterization of groundwater quality using water evaluation indices, multivariate statistics and geostatistics in central Bangladesh. Water Sci. 2016, 30, 19–40. [Google Scholar] [CrossRef] [Green Version]
- Siddique, M.A.B.; Islam, A.R.M.T.; Hossain, M.S.; Khan, R.; Akbor, M.A.; Hasanuzzaman, M.; Sajid, M.W.M.; Mia, M.Y.; Mallick, J.; Rahman, M.S.; et al. Multivariate statistics and entropy theory for irrigation water quality and entropy-weighted index development in a subtropical urban river, Bangladesh. Environ. Sci. Pollut. Res. 2021, 29, 8577–8596. [Google Scholar] [CrossRef] [PubMed]
- Merouche, A.; Selvam, S.; Imessaoudene, Y.; Maten, C.N. Assessment of dam water quality for irrigation in the northeast of catchment Cheliff-Zahrez, Central Algeria. Environ. Dev. Sustain. 2020, 22, 5709–5730. [Google Scholar] [CrossRef]
- Etteieb, S.; Cherif, S.; Tarhouni, J. Hydrochemical assessment of water quality for irrigation: A case study of the Medjerda River in Tunisia. Appl. Water Sci. 2017, 7, 469–480. [Google Scholar] [CrossRef] [Green Version]
- Soltani, A.A.; Bermad, A.; Boutaghane, H.; Oukil, A.; Abdalla, O.; Hasbaia, M.; Oulebsir, R.; Zeroual, S.; Lefkir, A. An integrated approach for assessing surface water quality: Case of Beni Haroun dam (Northeast Algeria). Environ. Monit. Assess. 2020, 192, 630. [Google Scholar] [CrossRef]
- Apollaro, C.; Fuoco, I.; Bloise, L.; Calabrese, E.; Marini, L.; Vespasiano, G.; Muto, F. Geochemical modeling of water-rock interaction processes in the Pollino National Park. Geofluids 2021, 2021, 1–17. [Google Scholar] [CrossRef]
- Wu, J.; Li, P.; Wang, D.; Ren, X.; Wei, M. Statistical and multivariate statistical techniques to trace the sources and affecting factors of groundwater pollution in a rapidly growing city on the Chinese Loess Plateau. Hum. Ecol. Risk Assess. Int. J. 2019, 26, 1603–1621. [Google Scholar] [CrossRef]
- Fuoco, I.; Apollaro, C.; Criscuoli, A.; De Rosa, R.; Velizarov, S.; Figoli, A. Fluoride Polluted Groundwaters in Calabria Region (Southern Italy): Natural Source and Remediation. Water 2021, 13, 1626. [Google Scholar] [CrossRef]
- Roy, K. Irrigation Water Quality Assessment and Identification of River Pollution Sources in Bangladesh: Implications in Policy and Management. J. Water Resour. Hydraul. Eng. 2015, 4, 303–317. [Google Scholar] [CrossRef]
- Sarkar, S.K.; Talukdar, S.; Rahman, A.; Shahfahad; Roy, S.K. Groundwater potentiality mapping using ensemble machine learning algorithms for sustainable groundwater management. Front. Eng. Built Environ. 2021, 2, 43–54. [Google Scholar] [CrossRef]
- Batarseh, M.; Imreizeeq, E.; Tilev, S.; Al Alaween, M.; Suleiman, W.; Al Remeithi, A.M.; Al Tamimi, M.K.; Al Alawneh, M. Assessment of groundwater quality for irrigation in the arid regions using irrigation water quality index (IWQI) and GIS-Zoning maps: Case study from Abu Dhabi Emirate, UAE. Groundw. Sustain. Dev. 2021, 14, 100611. [Google Scholar] [CrossRef]
- El Osta, M.; Masoud, M.; Alqarawy, A.; Elsayed, S.; Gad, M.; Sa, A.A. Groundwater Suitability for Drinking and Irrigation Using Water Quality Indices and Multivariate Modeling in Makkah Al-Mukarramah Province, Saudi Arabia. Water 2022, 14, 483. [Google Scholar] [CrossRef]
- Mallick, J.; Singh, C.K.; AlMesfer, M.K.; Kumar, A.; Khan, R.A.; Islam, S.; Rahman, A. Hydro-Geochemical Assessment of Groundwater Quality in Aseer Region, Saudi Arabia. Water 2018, 10, 1847. [Google Scholar] [CrossRef] [Green Version]
- Mora, A.; Mahlknecht, J.; Rosales-Lagarde, L.; Hernández-Antonio, A. Assessment of major ions and trace elements in groundwater supplied to the Monterrey metropolitan area, Nuevo León, Mexico. Environ. Monit. Assess. 2017, 189, 394. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, I.A.; Shahfahad, S.; Baig, M.R.I.; Talukdar, S.; Asgher, M.S.; Usmani, T.M.; Ahmed, S.; Rahman, A. Lake water volume calculation using time series LANDSAT satellite data: A geospatial analysis of Deepor Beel Lake, Guwahati. Front. Eng. Built Environ. 2021, 1, 107–130. [Google Scholar] [CrossRef]
- An, N.N.; Nhut, H.S.; Phuong, T.A.; Huy, V.Q.; Hanh, N.C.; Thao, G.T.P.; Trinh, P.T.; Hoa, P.V.; Bình, N.A. Groundwater simulation in Dak Lak province based on MODFLOW model and climate change scenarios. Front. Eng. Built Environ. 2022, 2, 55–67. [Google Scholar] [CrossRef]
- Rodier, J.; Legube, B.; Merlet, N. L’analyse de L’eau. 2009. Available online: https://www.oieau.fr/ReFEA/fiches/AnalyseEau/AnalyseEau_PresGen.pdf (accessed on 12 January 2022).
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture, FAO Irrigation and Drainage Paper; Food and Agriculture Organization of the United Nations Rome: Rome, Italy, 1985. [Google Scholar]
- Todd, D.K. Groundwater Hydrology, 2nd ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 1980. [Google Scholar]
- Raghunath, H.M. Groundwater, 2nd ed.; Wiley Eastern Ltd.: New Delhi, India, 1990. [Google Scholar]
- Allison, L.; Bernstein, C.A.; Bower, J.W.; Brown, M.; Fireman, J.T.; Hatcher, H.E.; Hayward, G.A.; Pearson, R.C.; Reeve, L.E.; Richards Wilcox, L.A.; et al. Diagnosis and Improvement of United States Salinity Laboratory Staff. 1954. Available online: https://www.ars.usda.gov/ARSUserFiles/20360500/hb60_pdf/hb60complete.pdf (accessed on 10 January 2022).
- Kelley, W.P. Use of Saline Irrigation Watertitle. Soil Sci. Soc. Am. J. 1963, 95, 385–391. [Google Scholar] [CrossRef]
- Richards, L.A. Diagnosis and Improvement of Saline Alkali Soils, Agriculture, 160, Handbook 60; U.S. Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Wilcox, L.V. Classification and Use of Irrigation Waters, Circular No. 969; U.S. Department of Agriculture: Washington, DC, USA, 1955. [Google Scholar]
- Krishna kumar, S.; Logeshkumaran, A.; Magesh, N.S.; Godson, P.S.; Chandrasekar, N. Hydro-geochemistry and application of water quality index (WQI) for groundwater quality assessment, Anna Nagar, part of Chennai City, Tamil Nadu, India. Appl. Water Sci. 2015, 5, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Chandra, D.S.; Raju, M.V.S.; Asadi, S.S. Estimation of Water Quality Index by Weighted Arithmetic Water Quality Index Method: A Model Study. Int. J. Civ. Eng. Technol. 2017, 8, 1215–1222. [Google Scholar]
- Şimşek, C.; Gunduz, O. IWQ Index: A GIS-Integrated Technique to Assess Irrigation Water Quality. Environ. Monit. Assess. 2006, 128, 277–300. [Google Scholar] [CrossRef]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 19 February 2022).
- Islam, A.R.M.T.; Hasanuzzaman, M.; Jaman, M.; Alam, E.; Mallick, J.; Monirul Alam, G.M.; Sattar, M.A.; Techato, K. Assessing Farmers’ Typologies of Perception for Adopting Sustainable Adaptation Strategies in Bangladesh. Climate 2021, 9, 167. [Google Scholar] [CrossRef]
- Barakat, A.; El Baghdadi, M.; Rais, J.; Aghezzaf, B.; Slassi, M. Assessment of spatial and seasonal water quality variation of Oum Er Rbia River (Morocco) using multivariate statistical techniques. Int. Soil Water Conserv. Res. 2016, 4, 284–292. [Google Scholar] [CrossRef]
- Selvam, S.; Singaraja, C.; Venkatramanan, S.; Chung, S.Y. Geochemical Appraisal of Groundwater Quality in Ottapidaram Taluk, Thoothukudi District, Tamil Nadu using Graphical and Numerical Method. J. Geol. Soc. India 2018, 92, 313–320. [Google Scholar] [CrossRef]
- FAO. FAO Water Quality for Agriculture; FAO: Rome, Italy, 1985. [Google Scholar]
- Bassuony, M.A.; Ali, M.E.; Abdel Hameed, A.H.; Jahin, H.S. Evaluation of Irrigation Water Quality in Different Regions of North East Delta-Egypt. Int. J. Eng. Appl. Sci. 2014, 5, 10–15. [Google Scholar]
- Shammi, M.; Karmakar, B.; Rahman, M.M.; Islam, M.S.; Rahaman, R.; Uddin, K. Assessment of Salinity Hazard of Irrigation Water Quality in Monsoon Season of Batiaghata Upazila, Khulna District, Bangladesh and adaptation strategies. Pollution 2016, 2, 183–197. [Google Scholar]
- Guemmaz, F.; Neffar, S.; Chenchouni, H. Physicochemical and Bacteriological Quality of Surface Water Resources Receiving Common Wastewater Effluents in Drylands of Algeria. Handb. Environ. Chem. 2019, 98, 117–148. [Google Scholar]
- Guergazi, S.; Harrat, N.; Achour, S. Parametres Organiques et Potentiels de Formation du Chloroforme D’eaux de Surface de L’est Algerien. Courr. Savoir 2013, 7, 45–50. [Google Scholar]

| Potential Irrigation Problems | Unit | Restriction on Irrigation | ||
|---|---|---|---|---|
| No Problem | Growing Problem | Serious Problem | ||
| Salinity: EC | mS/cm | <0.7 | 0.7–3.0 | >3.0 |
| Total dissolved solids (TDS) | mg/L | <450 | 450–2000 | >2000 |
| Infiltration: | ||||
| EC (SAR=0–3) | mS/cm | >0.7 | 0.7–0.2 | <0.2 |
| EC (SAR=3–6) | mS/cm | >1.2 | 1.3–0.3 | <0.3 |
| EC (SAR=6–12) | mS/cm | >1.9 | 1.9–0.5 | <0.5 |
| EC (SAR=12–20) | mS/cm | >2.9 | 2.9–1.3 | <1.3 |
| EC (SAR=20–40) | mS/cm | >5.0 | 5.0–2.9 | <2.9 |
| Toxicity of certain ions (affecting sensitive crops) | ||||
| Sodium: surface irrigation Sodium: irrigation by sprinkling | SAR meq/L | 3 <3 | 3–9 >3 | >9 |
| Chloride: surface irrigation Chloride: irrigation by sprinkling | meq/L meq/L | <4 <3 | 4–10 >3 | >10 |
| Boron | mg/L | <0.7 | 0.7–3.0 | >3.0 |
| Various effects | ||||
| Nitrogen (NO3–N) | mg/L | <5 | 5–30 | >30 |
| Bicarbonate (HCO3) | meq/L | <1.5 | 1.5–8.5 | >8.5 |
| pH | unitless | Normal range: 6.5–8.4 | ||
| C1 | C2 | C3 | All Sites | Standard Values ([39]) | |
|---|---|---|---|---|---|
| Mean ± Std. Dev. | Mean ± Std. Dev. | Mean ± Std. Dev. | Mean ± Std. Dev. | ||
| DO | 6.99 ± 2.27 | 6 ± 1.99 | 9.72 ± 1.07 | 6.46 ± 2.19 | - |
| pH | 7.97 ± 0.75 | 7.39 ± 0.67 | 8.83 ± 0.3 | 7.61 ± 0.77 | 6.0–8.5 a |
| EC | 1348.2 ± 559.22 | 649.1 ± 463.01 | 2340 ± 56.57 | 903.81 ± 671.89 | 3000 a |
| TDS | 902.4 ± 373.21 | 434.9 ± 310.47 | 1555 ± 49.5 | 604.44 ± 447.94 | 2000 a |
| SALT | 674 ± 295.19 | 319.25 ± 234.43 | 1205 ± 21.21 | 450.56 ± 346.74 | - |
| Ca2+ | 128.61 ± 60.4 | 53.26 ± 23.95 | 288.96 ± 67.8 | 84.67 ± 74.15 | 400 a |
| Mg2+ | 88.31 ± 29.62 | 17.48 ± 14.09 | 153.7 ± 22.37 | 40.69 ± 46.18 | 60 a |
| K+ | 932.78 ± 725.2 | 390.06 ± 373.5 | 999.59 ± 60.43 | 535.72 ± 496.17 | 2 a |
| Na+ | 2872.6 ± 1262.74 | 1483.88 ± 1350.95 | 6203.53 ± 137.57 | 2090.65 ± 1811.56 | 900 a |
| F− | 0.42 ± 0.35 | 2.43 ± 1.72 | 0.43 ± 0.01 | 1.91 ± 1.73 | 1.5 b |
| Cl− | 146.14 ± 121.73 | 79.32 ± 88.03 | 271.98 ± 0.74 | 105.96 ± 104.5 | 250 b |
| SO42− | 348.44 ± 142.62 | 64.45 ± 81.62 | 1293.87 ± 1.62 | 208.11 ± 344.01 | 500 b |
| Al | 0.63 ± 0.55 | 1.65 ± 2.7 | 0.11 ± 0.02 | 1.34 ± 2.38 | 0.2 b |
| Se | 0.15 ± 0.09 | 0.3 ± 0.25 | 0.07 ± 0.09 | 0.26 ± 0.23 | 0.01 b |
| Zn | 0.14 ± 0.24 | 0.22 ± 0.49 | 0.06 ± 0 | 0.19 ± 0.44 | 3 b |
| Pb | 0.24 ± 0.23 | 0.23 ± 0.19 | 0.07 ± 0.06 | 0.22 ± 0.19 | 0.01 b |
| Cd | 0.04 ± 0.02 | 0.12 ± 0.08 | 0.02 ± 0.01 | 0.1 ± 0.08 | 0.003 b |
| Ni | 0.27 ± 0.2 | 0.29 ± 0.32 | 0.03 ± 0.01 | 0.27 ± 0.29 | 0.02 b |
| B | 28 ± 17.8 | 41.6 ± 57.2 | 34 ± 0.11 | 38.5 ± 49.7 | 0.3 b |
| Mn | 0.14 ± 0.08 | 8.96 ± 39.6 | 0.08 ± 0.04 | 6.67 ± 34 | 0.5 b |
| Fe | 0.41 ± 0.36 | 2.18 ± 6.25 | 0.09 ± 0 | 1.7 ± 5.41 | - |
| Cu | 3.86 ± 4.37 | 6.63 ± 3.08 | 0.4 ± 0.05 | 5.66 ± 3.66 | 2 b |
| TH | 684.89 ± 177.14 | 205.05 ± 94.39 | 1354.8 ± 261.55 | 379.07 ± 358.45 | - |
| SSP | 94.44 ± 1.23 | 84.3 ± 26.68 | 94.22 ± 1.05 | 86.92 ± 23.26 | - |
| MAR | 42.08 ± 12.21 | 23.49 ± 13.67 | 34.93 ± 2.06 | 27.78 ± 14.73 | - |
| Na% | 91.26 ± 2.05 | 80.32 ± 28.66 | 91.62 ± 1.37 | 83.18 ± 25.01 | - |
| SAR | 47.86 ± 17.27 | 39.68 ± 31.27 | 73.71 ± 5.52 | 43.71 ± 29.1 | - |
| MH | 53.81 ± 13.16 | 32.43 ± 15.2 | 46.9 ± 2.26 | 37.46 ± 16.54 | - |
| KR | 9.29 ± 3.04 | 12.62 ± 8.59 | 10.12 ± 1.73 | 11.82 ± 7.58 | - |
| Quality Indices [Unit] | Values | Classes of Quality or Suitability | Frequency of Water Samples [%] | |||
| C1 | C2 | C3 | All Sites | |||
| EC [uS/cm] | <250 | C1: Excellent | 0 | 15 | 0 | 11.11 |
| [250–750] | C2: Good | 0 | 50 | 0 | 37.04 | |
| [750–2250] | C3: Eligible | 100 | 35 | 0 | 44.44 | |
| [2250–5000] | C4: Not recommended | 0 | 0 | 100 | 7.41 | |
| >5000 | C5: Unsuitable (bad) | 0 | 0 | 0 | 0 | |
| Na [%] | <20 | Excellent | 0 | 5 | 0 | 3.7 |
| [20–40] | Good | 0 | 10 | 0 | 7.41 | |
| [40–60] | Eligible | 0 | 0 | 0 | 0 | |
| [60–80] | Not recommended | 0 | 0 | 0 | 0 | |
| >80 | Unsuitable (bad) | 100 | 85 | 100 | 88.89 | |
| SAR [meq/L] | <10 | Excellent | 0 | 20 | 0 | 14.81 |
| [10–18] | Good | 0 | 0 | 0 | 0 | |
| [18–26] | Uncertain | 20 | 15 | 0 | 14.81 | |
| >26 | Unsuitable | 80 | 65 | 100 | 70.37 | |
| KR [meq/L] | <1 | Suitable | 0 | 15 | 0 | 11.11 |
| >1 | Unsuitable | 100 | 85 | 100 | 88.89 | |
| MH [%] | <50 | Suitable | 40 | 90 | 100 | 100 |
| >50 | Unsuitable | 60 | 10 | 0 | 0 | |
| TH (ppm) | <75 | Soft | 0 | 5 | 0 | 3.7 |
| [75–150] | Moderately hard | 0 | 30 | 0 | 22.22 | |
| >150–300 | Hard | 0 | 40 | 0 | 29.63 | |
| >300 | Very hard | 100 | 25 | 100 | 44.44 | |
| SSP (meq/L) | <20 | Excellent | 0 | 5 | 0 | 3.7 |
| [20–40] | Good | 0 | 10 | 0 | 7.41 | |
| [40–80] | Fair | 0 | 0 | 0 | 0 | |
| >80 | Poor | 100 | 85 | 100 | 88.89 | |
| MAR (meq/L) | <50 | Excellent | 80 | 90 | 100 | 88.89 |
| >50 | Causes harmful effect to soil | 20 | 10 | 0 | 11.11 | |
| IWQI | <22 | Low Suitability | 0 | 50 | 0 | 37.04 |
| [22–37] | Medium Suitability | 0 | 15 | 0 | 11.11 | |
| >37 | High Suitability | 100 | 35 | 100 | 51.85 | |
| Water Quality Parameters | Metals | Est. | 2.5% CI | 97.5% CI | SE | t-Value | p | Sig. |
|---|---|---|---|---|---|---|---|---|
| DO | (Intercept) | 10.230 | 4.734 | 15.721 | 2.429 | 3.915 | 0.002 | ** |
| Pb | 500.700 | −564.758 | 1566.180 | 471.000 | 1.063 | 0.347 | n.s. | |
| SO42− | 0.007 | −0.002 | 0.016 | 0.004 | 1.840 | 0.099 | n.s. | |
| pH | (Intercept) | 9.556 | 7.814 | 11.298 | 0.770 | 12.409 | 0.000 | *** |
| Cu | −21.190 | −39.131 | −3.251 | 7.930 | −2.672 | 0.026 | * | |
| Na+ | −0.001 | −0.001 | 0.000 | 0.000 | −2.504 | 0.034 | * | |
| EC | (Intercept) | 0.271 | −0.016 | 0.177 | 0.394 | 0.690 | 0.508 | n.s. |
| K+ | 0.001 | 0.002 | 0.002 | 0.000 | 2.622 | 0.028 | * | |
| SO42− | 0.001 | 0.000 | 0.000 | 0.001 | 0.795 | 0.447 | n.s. | |
| B | 0.113 | −0.549 | 0.775 | 0.293 | 0.388 | 0.707 | n.s. | |
| Al | −1.934 | −43.900 | 40.032 | 18.550 | −0.104 | 0.919 | *** | |
| Zn | 21.750 | −94.933 | 138.432 | 51.580 | 0.422 | 0.683 | n.s. | |
| Ni | −68.930 | −270.050 | 132.183 | 88.900 | −0.775 | 0.458 | n.s. | |
| Se | −20.900 | −98.807 | 57.002 | 34.440 | −0.607 | 0.559 | n.s. | |
| Mn | 0.607 | −5.763 | 6.978 | 2.816 | 0.216 | 0.834 | n.s. | |
| TDS | (Intercept) | 194.200 | −4.044154e | 792.768 | 264.600 | 0.734 | 0.482 | n.s. |
| K+ | 0.459 | 6.815119e | 0.850 | 0.173 | 2.657 | 0.026 | * | |
| SO42− | 0.360 | −6.200 | 1.340 | 0.433 | 0.831 | 0.427 | n.s. | |
| B | 75.470 | −3.696 | 520.564 | 196.800 | 0.384 | 0.710 | n.s. | |
| Al | −1494.000 | −2.971 | 26,721.990 | 12,470.000 | 0.120 | 9073.000 | n.s. | |
| Zn | 14,640.000 | −6.381 | 93,093.970 | 34,680.000 | 0.422 | 6828.000 | n.s. | |
| Ni | −45,210.000 | −1.804 | 90,014.310 | 59,780.000 | 0.756 | 4688.000 | n.s. | |
| Se | −13,460.000 | −6.583685e | 38,923.220 | 23,150.000 | 0.581 | 5754.000 | n.s. | |
| Mn | 387.400 | −3.896 | 4670.692 | 1893.000 | 0.205 | 8424.000 | n.s. | |
| SALT | (Intercept) | 95.670 | −347.530 | 538.879 | 195.900 | 0.488 | 0.637 | n.s. |
| K+ | 0.352 | 0.641 | 0.641 | 0.128 | 2.753 | 0.022 | * | |
| SO42− | 0.244 | 0.970 | 0.970 | 0.321 | 0.760 | 0.467 | n.s. | |
| B | 31.920 | 361.471 | 361.471 | 145.700 | 0.219 | 0.832 | n.s. | |
| Al | −143.400 | 20,748.370 | 20,748.370 | 9235.000 | 0.016 | 0.988 | n.s. | |
| Fe | −1700.000 | 24,398.870 | 24,398.870 | 11,540.000 | 0.147 | 0.886 | n.s. | |
| Ni | −42,530.000 | 57,592.850 | 57,592.850 | 44,260.000 | 0.961 | 0.362 | n.s. | |
| Se | −10,020.000 | 3580.062 | 3580.062 | 7140.000 | 0.585 | 0.573 | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsubih, M.; Mallick, J.; Islam, A.R.M.T.; Almesfer, M.K.; Kahla, N.B.; Talukdar, S.; Ahmed, M. Assessing Surface Water Quality for Irrigation Purposes in Some Dams of Asir Region, Saudi Arabia Using Multi-Statistical Modeling Approaches. Water 2022, 14, 1439. https://doi.org/10.3390/w14091439
Alsubih M, Mallick J, Islam ARMT, Almesfer MK, Kahla NB, Talukdar S, Ahmed M. Assessing Surface Water Quality for Irrigation Purposes in Some Dams of Asir Region, Saudi Arabia Using Multi-Statistical Modeling Approaches. Water. 2022; 14(9):1439. https://doi.org/10.3390/w14091439
Chicago/Turabian StyleAlsubih, Majed, Javed Mallick, Abu Reza Md. Towfiqul Islam, Mohammed K. Almesfer, Nabil Ben Kahla, Swapan Talukdar, and Mohd. Ahmed. 2022. "Assessing Surface Water Quality for Irrigation Purposes in Some Dams of Asir Region, Saudi Arabia Using Multi-Statistical Modeling Approaches" Water 14, no. 9: 1439. https://doi.org/10.3390/w14091439
APA StyleAlsubih, M., Mallick, J., Islam, A. R. M. T., Almesfer, M. K., Kahla, N. B., Talukdar, S., & Ahmed, M. (2022). Assessing Surface Water Quality for Irrigation Purposes in Some Dams of Asir Region, Saudi Arabia Using Multi-Statistical Modeling Approaches. Water, 14(9), 1439. https://doi.org/10.3390/w14091439








