A Review on the Catalytic Remediation of Dyes by Tailored Carbon Dots
Abstract
:1. Introduction
2. Carbon Dots
2.1. Synthesis of Carbon Dots Using Environmentally Friendly Resources as Precursors
2.2. Sustainable Synthesis of CDs Using Natural Material as Precursors
2.3. Properties of Carbon Dots
2.3.1. Size
2.3.2. Surface Functional Groups
2.3.3. Charge Transfer Properties (Electron-Donating/Accepting Properties)
2.3.4. Water Solubility and Dispersibility
2.4. Characterisation Techniques Used to Analyse the Properties of Carbon Dots
2.5. Mechanism Involved in the Catalytic Remediation of Dyes by Carbon Dots in the Presence of H2O2
2.6. Factors Influencing the Performance of Carbon Dots on the Activation of H2O2
2.6.1. Effect of pH
2.6.2. Effect of Temperature
2.6.3. Effect of Carbon Dots Concentration
2.7. Comparison with Current Treatments Using Fenton-like Approach
2.8. Environmental Impacts of Carbon Dots
2.8.1. Ecotoxicological Impacts of Carbon Dots
2.8.2. Microbial Responses to Carbon Dots in Soil and Water
2.9. Challenges and Future Work of Carbon Dots in the Remediation of Dye-Containing Water
2.10. Conclusions and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations. The Sustainable Development Goals Report; United Nations: New York, NY, USA, 2020; ISSN 2521–6899. [Google Scholar]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Behera, M.; Nayak, J.; Banerjee, S.; Chakrabortty, S.; Tripathy, S.K. A review on the treatment of textile industry waste effluents towards the development of efficient mitigation strategy: An integrated system design approach. J. Environ. Chem. Eng. 2021, 9, 105277. [Google Scholar] [CrossRef]
- Mani, S.; Bharagava, R.N. Exposure to Crystal Violet, Its Toxic, Genotoxic and Carcinogenic Effects on Environment and Its Degradation and Detoxification for Environmental Safety. Rev. Environ. Contam. Toxicol. 2016, 237, 71–104. [Google Scholar] [CrossRef]
- Rani, M.; Shanker, U. Sun-light driven rapid photocatalytic degradation of methylene blue by poly(methyl methacrylate)/metal oxide nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 136–147. [Google Scholar] [CrossRef]
- Bessashia, W.; Berredjem, Y.; Hattab, Z.; Bououdina, M. Removal of Basic Fuchsin from water by using mussel powdered eggshell membrane as novel bioadsorbent: Equilibrium, kinetics, and thermodynamic studies. Environ. Res. 2020, 186, 109484. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Asghar, A.; Raman, A.A.A.; Daud, W.M.A.W. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: A review. J. Clean. Prod. 2015, 87, 826–838. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Zheng, T.; Zhang, G.; Wang, P. A review on Fenton-like processes for organic wastewater treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, C.S.D.; Soares, O.S.G.P.; Pinho, M.T.; Pereira, M.F.R.; Madeira, L.M. p-Nitrophenol degradation by heterogeneous Fenton’s oxidation over activated carbon-based catalysts. Appl. Catal. B Environ. 2017, 219, 109–122. [Google Scholar] [CrossRef]
- Wang, S. A Comparative study of Fenton and Fenton-like reaction kinetics in decolourisation of wastewater. Dyes Pigment. 2008, 76, 714–720. [Google Scholar] [CrossRef]
- Arshadi, M.; Abdolmaleki, M.; Mousavinia, F.; Khalafi-Nezhad, A.; Firouzabadi, H.; Gil, A. Degradation of methyl orange by heterogeneous Fenton-like oxidation on a nano-organometallic compound in the presence of multi-walled carbon nanotubes. Chem. Eng. Res. Des. 2016, 112, 113–121. [Google Scholar] [CrossRef]
- Liu, X.; Sun, C.; Chen, L.; Yang, H.; Ming, Z.; Bai, Y.; Feng, S.; Yang, S.-T. Decoloration of methylene blue by heterogeneous Fenton-like oxidation on Fe3O4/SiO2/C nanospheres in neutral environment. Mater. Chem. Phys. 2018, 213, 231–238. [Google Scholar] [CrossRef]
- Su, Z.; Li, J.; Zhang, D.; Ye, P.; Li, H.; Yan, Y. Novel flexible Fenton-like catalyst: Unique CuO nanowires arrays on copper mesh with high efficiency across a wide pH range. Sci. Total Environ. 2019, 647, 587–596. [Google Scholar] [CrossRef]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Mella, B.; de Carvalho Barcellos, B.S.; da Silva Costa, D.E.; Gutterres, M. Treatment of Leather Dyeing Wastewater with Associated Process of Coagulation-Flocculation/Adsorption/Ozonation. Ozone Sci. Eng. 2018, 40, 133–140. [Google Scholar] [CrossRef]
- Mousazadeh, M.; Alizadeh, S.; Frontistis, Z.; Kabdaşlı, I.; Niaragh, E.K.; Al Qodah, Z.; Naghdali, Z.; Mahmoud, A.; Sandoval, M.; Butler, E.; et al. Electrocoagulation as a Promising Defluoridation Technology from Water: A Review of State of the Art of Removal Mechanisms and Performance Trends. Water 2021, 13, 656. [Google Scholar] [CrossRef]
- Ağtaş, M.; Yılmaz, Ö.; Dilaver, M.; Alp, K.; Koyuncu, I. Hot water recovery and reuse in textile sector with pilot scale ceramic ultrafiltration/nanofiltration membrane system. J. Clean. Prod. 2020, 256, 120359. [Google Scholar] [CrossRef]
- Mahmoud, A.E.D.; Fawzy, M.; Abdel-Fatah, M.M.A. Technical Aspects of Nanofiltration for Dyes Wastewater Treatment. In Membrane Based Methods for Dye Containing Wastewater. Sustainable Textiles: Production, Processing, Manufacturing and Chemistry; Muthu, S.S., Khadir, A., Eds.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Cetinkaya, A.Y.; Bilgili, L. Life Cycle Comparison of Membrane Capacitive Deionization and Reverse Osmosis Membrane for Textile Wastewater Treatment. Water Air Soil Pollut. 2019, 230, 149. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, L.; Liu, J.; Logan, B.E. Electrochemical technologies for wastewater treatment and resource reclamation. Environ. Sci. Water Res. Technol. 2016, 2, 800–831. [Google Scholar] [CrossRef]
- Marson, E.O.; de Paiva, V.A.B.; Gonçalves, B.R.; Júnior, O.G.; Neto, W.B.; Machado, A.E.H.; Trovó, A.G. Degradation of Direct Red 81 mediated by Fenton reactions: Multivariate optimization, effect of chloride and sulfate, and acute ecotoxicity assessment. Environ. Sci. Pollut. Res. 2017, 24, 6176–6186. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.M.; Gao, M.X.; Gao, P.F.; Yang, H.; Huang, C.Z. Carbon Nanodots-Catalyzed Chemiluminescence of Luminol: A Singlet Oxygen-Induced Mechanism. J. Phys. Chem. C 2013, 117, 19219–19225. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, A.; Gao, N.; Li, K.; Ren, J.; Qu, X. Deciphering a Nanocarbon-Based Artificial Peroxidase: Chemical Identification of the Catalytically Active and Substrate-Binding Sites on Graphene Quantum Dots. Angew. Chem. Int. Ed. 2015, 54, 7176–7180. [Google Scholar] [CrossRef] [PubMed]
- Cadranel, A.; Margraf, J.T.; Strauss, V.; Clark, T.; Guldi, D.M. Carbon Nanodots for Charge-Transfer Processes. Acc. Chem. Res. 2019, 52, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Dhenadhayalan, N.; Lin, K.-C.; Saleh, T.A. Recent Advances in Functionalized Carbon Dots toward the Design of Efficient Materials for Sensing and Catalysis Applications. Small 2020, 16, e1905767. [Google Scholar] [CrossRef]
- Chen, P.; Wang, F.; Chen, Z.-F.; Zhang, Q.; Su, Y.; Shen, L.; Yao, K.; Liu, Y.; Cai, Z.; Lv, W.; et al. Study on the photocatalytic mechanism and detoxicity of gemfibrozil by a sunlight-driven TiO2/carbon dots photocatalyst: The significant roles of reactive oxygen species. Appl. Catal. B Environ. 2017, 204, 250–259. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, Q.; Su, Y.; Shen, L.; Wang, F.; Liu, H.; Liu, Y.; Cai, Z.; Lv, W.; Liu, G. Accelerated photocatalytic degradation of diclofenac by a novel CQDs/BiOCOOH hybrid material under visible-light irradiation: Dechloridation, detoxicity, and a new superoxide radical model study. Chem. Eng. J. 2018, 332, 737–748. [Google Scholar] [CrossRef]
- Habibi-Yangjeh, A.; Feizpoor, S.; Seifzadeh, D.; Ghosh, S. Improving visible-light-induced photocatalytic ability of TiO2 through coupling with Bi3O4Cl and carbon dot nanoparticles. Sep. Purif. Technol. 2020, 238, 116404. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Li, W.; Zhao, C.; Zhang, Q. Synthesis of Bi/BiOCl-TiO2-CQDs quaternary photocatalyst with enhanced visible-light photoactivity and fast charge migration. Catal. Commun. 2018, 107, 74–77. [Google Scholar] [CrossRef]
- Huang, C.-C.; Hung, Y.-S.; Weng, Y.-M.; Chen, W.; Lai, Y.-S. Sustainable development of carbon nanodots technology: Natural products as a carbon source and applications to food safety. Trends Food Sci. Technol. 2019, 86, 144–152. [Google Scholar] [CrossRef]
- Kang, Z.; Lee, S.-T. Carbon dots: Advances in nanocarbon applications. Nanoscale 2019, 11, 19214–19224. [Google Scholar] [CrossRef] [PubMed]
- Bin Chen, B.; Liu, M.L.; Huang, C.Z. Carbon dot-based composites for catalytic applications. Green Chem. 2020, 22, 4034–4054. [Google Scholar] [CrossRef]
- Desmond, L.J.; Phan, A.N.; Gentile, P. Critical overview on the green synthesis of carbon quantum dots and their application for cancer therapy. Environ. Sci. Nano 2021, 8, 848–862. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Huang, H.; Liu, Y.; Kang, Z. Carbon Dots: A Small Conundrum. Trends Chem. 2019, 1, 235–246. [Google Scholar] [CrossRef]
- Yan, F.; Jiang, Y.; Sun, X.; Bai, Z.; Zhang, Y.; Zhou, X. Surface modification and chemical functionalization of carbon dots: A review. Mikrochim. Acta 2018, 185, 424. [Google Scholar] [CrossRef]
- Hu, S.-L.; Niu, K.-Y.; Sun, J.; Yang, J.; Zhao, N.-Q.; Du, X.-W. One-step synthesis of fluorescent carbon nanoparticles by laser irradiation. J. Mater. Chem. 2009, 19, 484–488. [Google Scholar] [CrossRef]
- Xiao, L.; Sun, H. Novel properties and applications of carbon nanodots. Nanoscale Horiz. 2018, 3, 565–597. [Google Scholar] [CrossRef]
- Choi, Y.; Choi, Y.; Kwon, O.-H.; Kim, B.-S. Carbon Dots: Bottom-Up Syntheses, Properties, and Light-Harvesting Applications. Chem. Asian J. 2018, 13, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Rossini, E.; Milani, M.I.; Pezza, H.R. Green synthesis of fluorescent carbon dots for determination of glucose in biofluids using a paper platform. Talanta 2019, 201, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Truskewycz, A.; Beker, S.; Ball, A.S.; Cole, I. Photoluminescence measurements of carbon quantum dots within three-dimensional hydrogel matrices using a high throughput 96 well plate method. MethodsX 2019, 6, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-B.; Jin, J.-C.; Xu, Z.-Q.; Jiang, Z.-W.; Li, X.; Jiang, F.-L.; Liu, Y. Single-step synthesis of highly photoluminescent carbon dots for rapid detection of Hg2+ with excellent sensitivity. J. Colloid Interface Sci. 2019, 551, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Ye, Y.; Su, Y.; Liu, S.; Gong, D.; Zhao, H. Functionalization of citric acid-based carbon dots by imidazole toward novel green corrosion inhibitor for carbon steel. J. Clean. Prod. 2019, 229, 180–192. [Google Scholar] [CrossRef]
- Yang, Z.-C.; Wang, M.; Yong, A.M.; Wong, S.Y.; Zhang, X.-H.; Tan, H.; Chang, A.Y.; Li, X.; Wang, J. Intrinsically fluorescent carbon dots with tunable emission derived from hydrothermal treatment of glucose in the presence of monopotassium phosphate. Chem. Commun. 2011, 47, 11615–11617. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yang, J.; Tian, J.; Yu, J.-S. How do nitrogen-doped carbon dots generate from molecular precursors? An investigation of the formation mechanism and a solution-based large-scale synthesis. J. Mater. Chem. B 2015, 3, 5608–5614. [Google Scholar] [CrossRef]
- Papaioannou, N.; Titirici, M.-M.; Sapelkin, A. Investigating the Effect of Reaction Time on Carbon Dot Formation, Structure, and Optical Properties. ACS Omega 2019, 4, 21658–21665. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.L.; Chen, B.B.; Li, C.M.; Huang, C.Z. Carbon dots: Synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem. 2019, 21, 449–471. [Google Scholar] [CrossRef]
- Das, R.; Bandyopadhyay, R.; Pramanik, P. Carbon quantum dots from natural resource: A review. Mater. Today Chem. 2018, 8, 96–109. [Google Scholar] [CrossRef]
- Yang, X.; Zhuo, Y.; Zhu, S.; Luo, Y.; Feng, Y.; Dou, Y. Novel and green synthesis of high-fluorescent carbon dots originated from honey for sensing and imaging. Biosens. Bioelectron. 2014, 60, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, H.S. Green Synthesis of Luminescent Nitrogen-Doped Carbon Dots from Milk and Its Imaging Application. Anal. Chem. 2014, 86, 8902–8905. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Dong, Y.; Sun, H.; Chen, N. Highly fluorescent carbon dots from peanut shells as potential probes for copper ion: The optimization and analysis of the synthetic process. Mater. Today Chem. 2017, 5, 1–10. [Google Scholar] [CrossRef]
- Razmi, H.; Abdollahi, V.; Mohammad-Rezaei, R. Graphene quantum dots—Eggshell nanocomposite to extract polycyclic aromatic hydrocarbons in water. Environ. Chem. Lett. 2016, 14, 521–526. [Google Scholar] [CrossRef]
- Thambiraj, S.; Shankaran, D.R. Green synthesis of highly fluorescent carbon quantum dots from sugarcane bagasse pulp. Appl. Surf. Sci. 2016, 390, 435–443. [Google Scholar] [CrossRef]
- Varisco, M.; Zufferey, D.; Ruggi, A.; Zhang, Y.; Erni, R.; Mamula, O. Synthesis of hydrophilic and hydrophobic carbon quantum dots from waste of wine fermentation. R. Soc. Open Sci. 2017, 4, 170900. [Google Scholar] [CrossRef] [Green Version]
- Pooja, D.; Singh, L.; Thakur, A.; Kumar, P. Green synthesis of glowing carbon dots from Carica papaya waste pulp and their application as a label-freechemo probe for chromium detection in water. Sens. Actuators B Chem. 2019, 283, 363–372. [Google Scholar] [CrossRef]
- Bandi, R.; Gangapuram, B.R.; Dadigala, R.; Eslavath, R.; Singh, S.S.; Guttena, V. Facile and green synthesis of fluorescent carbon dots from onion waste and their potential applications as sensor and multicolour imaging agents. RSC Adv. 2016, 6, 28633–28639. [Google Scholar] [CrossRef]
- Tyagi, A.; Tripathi, K.M.; Singh, N.; Choudhary, S.; Gupta, R.K. Green synthesis of carbon quantum dots from lemon peel waste: Applications in sensing and photocatalysis. RSC Adv. 2016, 6, 72423–72432. [Google Scholar] [CrossRef]
- Bhamore, J.R.; Jha, S.; Park, T.J.; Kailasa, S.K. Green synthesis of multi-color emissive carbon dots from Manilkara zapota fruits for bioimaging of bacterial and fungal cells. J. Photochem. Photobiol. B Biol. 2019, 191, 150–155. [Google Scholar] [CrossRef]
- Ahmadian-Fard-Fini, S.; Salavati-Niasari, M.; Safardoust-Hojaghan, H. Hydrothermal green synthesis and photocatalytic activity of magnetic CoFe2O4—carbon quantum dots nanocomposite by turmeric precursor. J. Mater. Sci. Mater. Electron. 2017, 28, 16205–16214. [Google Scholar] [CrossRef]
- Arumugam, N.; Kim, J. Synthesis of carbon quantum dots from Broccoli and their ability to detect silver ions. Mater. Lett. 2018, 219, 37–40. [Google Scholar] [CrossRef]
- De, B.; Karak, N. A green and facile approach for the synthesis of water soluble fluorescent carbon dots from banana juice. RSC Adv. 2013, 3, 8286–8290. [Google Scholar] [CrossRef]
- Cailotto, S.; Mazzaro, R.; Enrichi, F.; Vomiero, A.; Selva, M.; Cattaruzza, E.; Cristofori, D.; Amadio, E.; Perosa, A. Design of Carbon Dots for Metal-free Photoredox Catalysis. ACS Appl. Mater. Interfaces 2018, 10, 40560–40567. [Google Scholar] [CrossRef] [PubMed]
- Arcudi, F.; Đorđević, L.; Prato, M. Design, Synthesis, and Functionalization Strategies of Tailored Carbon Nanodots. Acc. Chem. Res. 2019, 52, 2070–2079. [Google Scholar] [CrossRef]
- Zhou, Y.; Zahran, E.M.; Quiroga, B.A.; Perez, J.; Mintz, K.J.; Peng, Z.; Liyanage, P.Y.; Pandey, R.R.; Chusuei, C.C.; Leblanc, R.M. Size-dependent photocatalytic activity of carbon dots with surface-state determined photoluminescence. Appl. Catal. B Environ. 2019, 248, 157–166. [Google Scholar] [CrossRef]
- Wu, Q.; Li, W.; Wu, P.; Li, J.; Liu, S.; Jin, C.; Zhan, X. Effect of reaction temperature on properties of carbon nanodots and their visible-light photocatalytic degradation of tetracyline. RSC Adv. 2015, 5, 75711–75721. [Google Scholar] [CrossRef]
- Cao, S.; Tao, F.F.; Tang, Y.; Li, Y.; Yu, J. Size- and shape-dependent catalytic performances of oxidation and reduction reactions on nanocatalysts. Chem. Soc. Rev. 2016, 45, 4747–4765. [Google Scholar] [CrossRef]
- Das, S.; Ngashangva, L.; Mog, H.; Gogoi, S.; Goswami, P. An insight into the mechanism of peroxidase-like activity of carbon dots. Opt. Mater. 2021, 115, 111017. [Google Scholar] [CrossRef]
- Long, Y.; Wang, X.; Shen, D.; Zheng, H. Detection of glucose based on the peroxidase-like activity of reduced state carbon dots. Talanta 2016, 159, 122–126. [Google Scholar] [CrossRef]
- Bin Chen, B.; Liu, M.L.; Li, C.M.; Huang, C.Z. Fluorescent carbon dots functionalization. Adv. Colloid Interface Sci. 2019, 270, 165–190. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Sun, Z.; Zhang, H.; Sun, X.; Jiang, Y.; Bai, Z. The fluorescence mechanism of carbon dots, and methods for tuning their emission color: A review. Mikrochim. Acta 2019, 186, 583. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Kuang, T.; Liu, Y.; Cai, L.; Peng, X.; Sreeprasad, T.S.; Zhao, P.; Yu, Z.; Li, N. Heteroatom-doped carbon dots: Synthesis, characterization, properties, photoluminescence mechanism and biological applications. J. Mater. Chem. B 2016, 4, 7204–7219. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, F.; Li, H.; Wang, Y. Nitrogen-doped porous carbon materials: Promising catalysts or catalyst supports for heterogeneous hydrogenation and oxidation. Catal. Sci. Technol. 2016, 6, 3670–3693. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, Z.; Niu, Y.; Zhang, B.; Lu, Q.; Wu, S.; Centi, G.; Perathoner, S.; Heumann, S.; Yu, L.; et al. Highly Efficient Metal-Free Nitrogen-Doped Nanocarbons with Unexpected Active Sites for Aerobic Catalytic Reactions. ACS Nano 2019, 13, 13995–14004. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Zhou, L.; Duan, X.; Sun, H.; Wang, S. Catalytic degradation of antibiotics by metal-free catalysis over nitrogen-doped graphene. Catal. Today 2020, 357, 341–349. [Google Scholar] [CrossRef]
- Zhang, Z.; Yi, G.; Li, P.; Zhang, X.; Fan, H.; Zhang, Y.; Wang, X.; Zhang, C. A minireview on doped carbon dots for photocatalytic and electrocatalytic applications. Nanoscale 2020, 12, 13899–13906. [Google Scholar] [CrossRef]
- Li, X.; Fu, Y.; Zhao, S.; Xiao, J.; Lan, M.; Wang, B.; Zhang, K.; Song, X.; Zeng, L. Metal ions-doped carbon dots: Synthesis, properties, and applications. Chem. Eng. J. 2021, 430, 133101. [Google Scholar] [CrossRef]
- Lin, L.; Xiao, Y.; Wang, Y.; Zeng, Y.; Lin, Z.; Chen, X. Hydrothermal synthesis of nitrogen and copper co-doped carbon dots with intrinsic peroxidase-like activity for colorimetric discrimination of phenylenediamine isomers. Mikrochim. Acta 2019, 186, 288. [Google Scholar] [CrossRef]
- Lin, L.; Luo, Y.; Tsai, P.; Wang, J.; Chen, X. Metal ions doped carbon quantum dots: Synthesis, physicochemical properties, and their applications. TrAC Trends Anal. Chem. 2018, 103, 87–101. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Rathi, A.K.; Gawande, M.B.; Hola, K.; Goswami, A.; Kalytchuk, S.; Karakassides, M.A.; Kouloumpis, A.; Gournis, D.; Deligiannakis, Y.; et al. Fe(III)-functionalized carbon dots—Highly efficient photoluminescence redox catalyst for hydrogenations of olefins and decomposition of hydrogen peroxide. Appl. Mater. Today 2017, 7, 179–184. [Google Scholar] [CrossRef]
- Hu, R.; Li, L.; Jin, W.J. Controlling speciation of nitrogen in nitrogen-doped carbon dots by ferric ion catalysis for enhancing fluorescence. Carbon 2017, 111, 133–141. [Google Scholar] [CrossRef]
- Guan, Y.-F.; Zhang, F.; Huang, B.-C.; Yu, H.-Q. Enhancing electricity generation of microbial fuel cell for wastewater treatment using nitrogen-doped carbon dots-supported carbon paper anode. J. Clean. Prod. 2019, 229, 412–419. [Google Scholar] [CrossRef]
- Ma, Z.; Ming, H.; Huang, H.; Liu, Y.; Kang, Z. One-step ultrasonic synthesis of fluorescent N-doped carbon dots from glucose and their visible-light sensitive photocatalytic ability. New J. Chem. 2012, 36, 861–864. [Google Scholar] [CrossRef]
- Gao, S.; Chen, Y.; Fan, H.; Wei, X.; Hu, C.; Wang, L.; Qu, L. A green one-arrow-two-hawks strategy for nitrogen-doped carbon dots as fluorescent ink and oxygen reduction electrocatalysts. J. Mater. Chem. A 2014, 2, 6320–6325. [Google Scholar] [CrossRef]
- Hu, C.; Yu, C.; Li, M.; Wang, X.; Dong, Q.; Wang, G.; Qiu, J. Nitrogen-doped carbon dots decorated on graphene: A novel all-carbon hybrid electrocatalyst for enhanced oxygen reduction reaction. Chem. Commun. 2015, 51, 3419–3422. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Lee, Y.R. Nitrogen-doped carbon dots originating from unripe peach for fluorescent bioimaging and electrocatalytic oxygen reduction reaction. J. Colloid Interface Sci. 2016, 482, 8–18. [Google Scholar] [CrossRef]
- Miao, X.; Yue, X.; Ji, Z.; Shen, X.; Zhou, H.; Liu, M.; Xu, K.; Zhu, J.; Zhu, G.; Kong, L.; et al. Nitrogen-doped carbon dots decorated on g-C3N4/Ag3PO4 photocatalyst with improved visible light photocatalytic activity and mechanism insight. Appl. Catal. B Environ. 2018, 227, 459–469. [Google Scholar] [CrossRef]
- Lin, L.; Song, X.; Chen, Y.; Rong, M.; Zhao, T.; Wang, Y.; Jiang, Y.; Chen, X. Intrinsic peroxidase-like catalytic activity of nitrogen-doped graphene quantum dots and their application in the colorimetric detection of H2O2 and glucose. Anal. Chim. Acta 2015, 869, 89–95. [Google Scholar] [CrossRef]
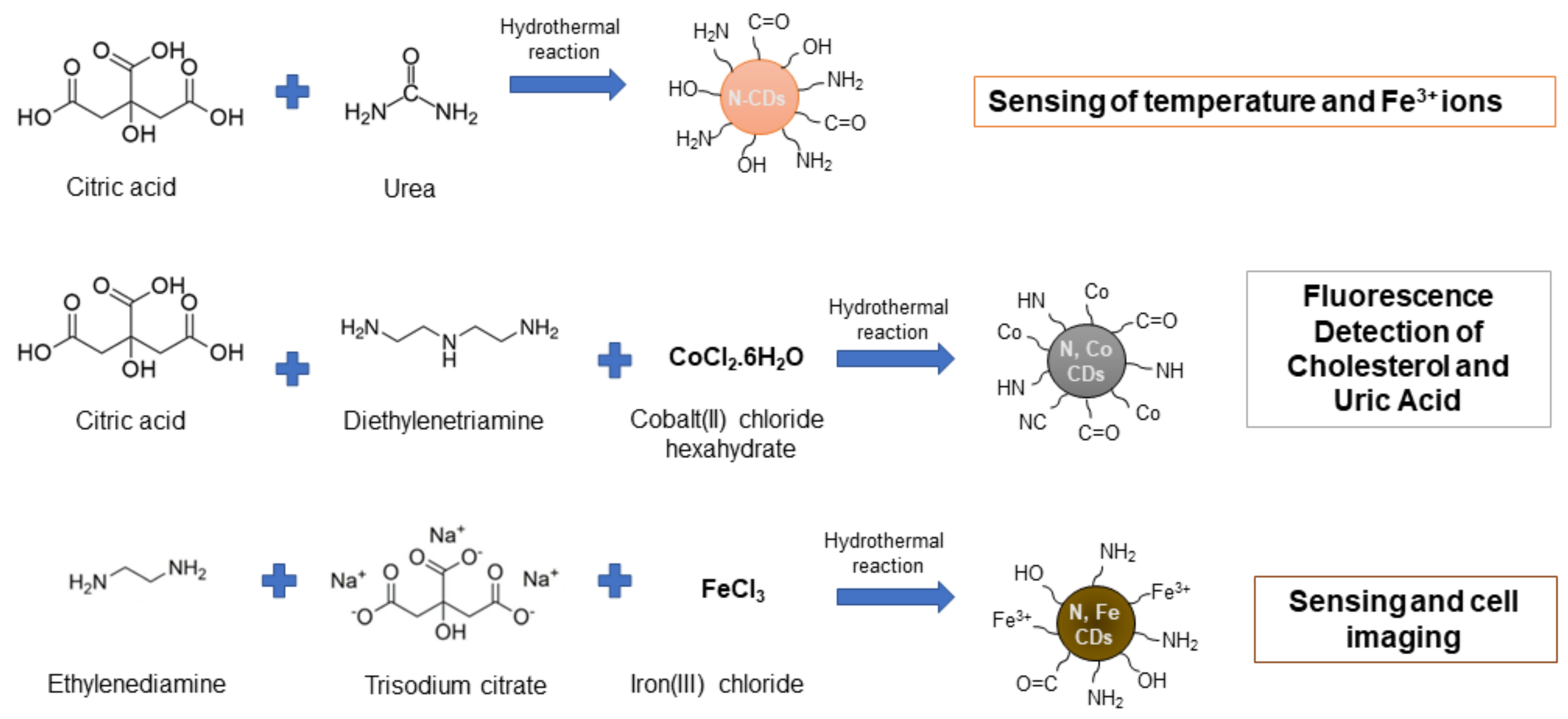
- Wang, C.; Jiang, K.; Xu, Z.; Lin, H.; Zhang, C. Glutathione modified carbon-dots: From aggregation-induced emission enhancement properties to a “turn-on" sensing of temperature/Fe3+ ions in cells. Inorg. Chem. Front. 2016, 3, 514–522. [Google Scholar] [CrossRef]
- Huang, S.; Yang, E.; Yao, J.; Chu, X.; Liu, Y.; Zhang, Y.; Xiao, Q. Nitrogen, Cobalt Co-doped Fluorescent Magnetic Carbon Dots as Ratiometric Fluorescent Probes for Cholesterol and Uric Acid in Human Blood Serum. ACS Omega 2019, 4, 9333–9342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Cao, L.; Zan, M.; Hou, Z.; Ge, M.; Dong, W.-F.; Li, L. Iron and nitrogen-co-doped carbon quantum dots for the sensitive and selective detection of hematin and ferric ions and cell imaging. Analyst 2021, 146, 4954–4963. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Jiang, Z.; Shangguan, J.; Qing, T.; Zhang, P.; Feng, B. Applications of carbon dots in environmental pollution control: A review. Chem. Eng. J. 2021, 406, 126848. [Google Scholar] [CrossRef]
- Al Ja’Farawy, M.S.; Kusumandari; Purwanto, A.; Widiyandari, H. Carbon quantum dots supported zinc oxide (ZnO/CQDs) efficient photocatalyst for organic pollutant degradation—A systematic review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100681. [Google Scholar] [CrossRef]
- Behnood, R.; Sodeifian, G. Synthesis of N doped-CQDs/Ni doped-ZnO nanocomposites for visible light photodegradation of organic pollutants. J. Environ. Chem. Eng. 2020, 8, 103821. [Google Scholar] [CrossRef]
- Chen, J.; Shu, J.; Chen, J.; Cao, Z.; Xiao, A.; Yan, Z. Highly luminescent S,N co-doped carbon quantum dots-sensitized chemiluminescence on luminol-H2O2 system for the determination of ranitidine. Luminescence 2016, 32, 277–284. [Google Scholar] [CrossRef]
- Zhao, P.; Zhu, L. Dispersibility of carbon dots in aqueous and/or organic solvents. Chem. Commun. 2018, 54, 5401–5406. [Google Scholar] [CrossRef]
- Shi, J.; Yin, T.; Shen, W. Effect of surface modification on the peroxidase-like behaviors of carbon dots. Colloids Surf. B Biointerfaces 2019, 178, 163–169. [Google Scholar] [CrossRef]
- Yin, K.; Lu, D.; Wang, L.-P.; Zhang, Q.; Hao, J.; Li, G.; Li, H. Hydrophobic Carbon Dots from Aliphatic Compounds with One Terminal Functional Group. J. Phys. Chem. C 2019, 123, 22447–22456. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, J. A review of carbon dots in biological applications. J. Mater. Sci. 2016, 51, 4728–4738. [Google Scholar] [CrossRef]
- Zuo, P.; Lu, X.; Sun, Z.; Guo, Y.; He, H. A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Mikrochim. Acta 2015, 183, 519–542. [Google Scholar] [CrossRef]
- Rosso, C.; Filippini, G.; Prato, M. Carbon Dots as Nano-Organocatalysts for Synthetic Applications. ACS Catal. 2020, 10, 8090–8105. [Google Scholar] [CrossRef]
- Tang, D.; Liu, J.; Wu, X.; Liu, R.; Han, X.; Han, Y.; Huang, H.; Liu, Y.; Kang, Z. Carbon Quantum Dot/NiFe Layered Double-Hydroxide Composite as a Highly Efficient Electrocatalyst for Water Oxidation. ACS Appl. Mater. Interfaces 2014, 6, 7918–7925. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.; Rauscher, H.; Mech, A.; Sintes, J.R.; Gilliland, D.; González, M.; Kearns, P.; Moss, K.; Visser, M.; Groenewold, M.; et al. Physico-chemical properties of manufactured nanomaterials-Characterisation and relevant methods. An outlook based on the OECD Testing Programme. Regul. Toxicol. Pharmacol. 2018, 92, 8–28. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Huang, H.; Wang, C.; Long, R.; Xiong, Y. Engineering the surface charge states of nanostructures for enhanced catalytic performance. Mater. Chem. Front. 2017, 1, 1951–1964. [Google Scholar] [CrossRef]
- Arcudi, F.; Đorđević, L.; Prato, M. Synthesis, Separation, and Characterization of Small and Highly Fluorescent Nitrogen-Doped Carbon NanoDots. Angew. Chem. 2016, 128, 2147–2152. [Google Scholar] [CrossRef]
- Majumdar, B.; Mandani, S.; Bhattacharya, T.; Sarma, D.; Sarma, T.K. Probing Carbocatalytic Activity of Carbon Nanodots for the Synthesis of Biologically Active Dihydro/Spiro/Glyco Quinazolinones and Aza-Michael Adducts. J. Org. Chem. 2017, 82, 2097–2106. [Google Scholar] [CrossRef]
- Attri, P.; Kim, Y.H.; Park, D.H.; Park, J.H.; Hong, Y.J.; Uhm, H.S.; Kim, K.-N.; Fridman, A.; Choi, E.H. Generation mechanism of hydroxyl radical species and its lifetime prediction during the plasma-initiated ultraviolet (UV) photolysis. Sci. Rep. 2015, 5, 9332. [Google Scholar] [CrossRef]
- Wang, K.-K.; Song, S.; Jung, S.-J.; Hwang, J.-W.; Kim, M.-G.; Kim, J.-H.; Sung, J.; Lee, J.-K.; Kim, Y.-R. Lifetime and diffusion distance of singlet oxygen in air under everyday atmospheric conditions. Phys. Chem. Chem. Phys. 2020, 22, 21664–21671. [Google Scholar] [CrossRef]
- Zheng, C.; An, X.; Yin, T. New metal-free catalytic degradation systems with carbon dots for thymol blue. New J. Chem. 2017, 41, 13365–13369. [Google Scholar] [CrossRef]
- Beker, S.A.; Khudur, L.S.; Cole, I.S.; Ball, A.S. Catalytic degradation of methylene blue using iron and nitrogen-containing carbon dots as Fenton-like catalysts. New J. Chem. 2022, 46, 263–275. [Google Scholar] [CrossRef]
- Beker, S.A.; Truskewycz, A.; Cole, I.; Ball, A.S. Green synthesis of Opuntia-derived carbon nanodots for the catalytic decolourization of cationic dyes. New J. Chem. 2020, 44, 20001–20012. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Moradi, S.; Shahlaei, M.; Farhadian, N. Fenton-like removal of tetracycline from aqueous solution using iron-containing carbon dot nanocatalysts. New J. Chem. 2020, 44, 17735–17743. [Google Scholar] [CrossRef]
- Dou, X.; Lin, Z.; Chen, H.; Zheng, Y.; Lu, C.; Lin, J.-M. Production of superoxide anion radicals as evidence for carbon nanodots acting as electron donors by the chemiluminescence method. Chem. Commun. 2013, 49, 5871–5873. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Liu, Z.; Zhou, C.; Guo, Y.; Feng, Y.; Yang, M. Degradation Mechanism of Methylene Blue by H2O2 and Synthesized Carbon Nanodots/Graphitic Carbon Nitride/Fe(II) Composite. J. Phys. Chem. C 2019, 123, 26921–26931. [Google Scholar] [CrossRef]
- Yang, W.; Huang, T.; Zhao, M.; Luo, F.; Weng, W.; Wei, Q.; Lin, Z.; Chen, G. High peroxidase-like activity of iron and nitrogen co-doped carbon dots and its application in immunosorbent assay. Talanta 2017, 164, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadpour, Z.; Safavi, A.; Shamsipur, M. A new label free colorimetric chemosensor for detection of mercury ion with tunable dynamic range using carbon nanodots as enzyme mimics. Chem. Eng. J. 2014, 255, 1–7. [Google Scholar] [CrossRef]
- Wolski, L.; Walkowiak, A.; Ziolek, M. Formation of reactive oxygen species upon interaction of Au/ZnO with H2O2 and their activity in methylene blue degradation. Catal. Today 2019, 333, 54–62. [Google Scholar] [CrossRef]
- Luo, X.; Hu, H.; Pan, Z.; Pei, F.; Qian, H.; Miao, K.; Guo, S.; Wang, W.; Feng, G. Efficient and stable catalysis of hollow Cu9S5 nanospheres in the Fenton-like degradation of organic dyes. J. Hazard. Mater. 2020, 396, 122735. [Google Scholar] [CrossRef]
- Safavi, A.S.F.; Shahbaazi, H.; Farjami, E. Facile approach to the synthesis of carbon nanodots and their peroxidase mimetic function in azo dyes degradation. RSC Adv. 2012, 2, 7367–7370. [Google Scholar] [CrossRef]
- Ashraf, G.A.; Hassan, M.; Rasool, R.T.; Abbas, W.; Zhang, L. Mesoporous SnMgNd substituted M-hexaferrite catalyzed heterogeneous photo-Fenton-like activity for degradation of methylene blue. J. Colloid Interface Sci. 2019, 557, 408–422. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.G.L.; Mendes, H.M.; Bastos, S.L.; D’Agostino, L.C.; Tronto, J.; Pulcinelli, S.H.; Santilli, C.V.; Neto, J.L. Fenton-like degradation of methylene blue using Mg/Fe and MnMg/Fe layered double hydroxides as reusable catalysts. Appl. Clay Sci. 2020, 187, 105477. [Google Scholar] [CrossRef]
- Guo, X.; Wang, K.; Xu, Y. Tartaric acid enhanced CuFe2O4-catalyzed heterogeneous photo-Fenton-like degradation of methylene blue. Mater. Sci. Eng. B 2019, 245, 75–84. [Google Scholar] [CrossRef]
- Rivera, F.; Recio, F.; Palomares, F.; Sánchez-Marcos, J.; Menéndez, N.; Mazarío, E.; Herrasti, P. Fenton-like degradation enhancement of methylene blue dye with magnetic heating induction. J. Electroanal. Chem. 2020, 879, 114773. [Google Scholar] [CrossRef]
- Xiao, A.; Wang, C.; Chen, J.; Guo, R.; Yan, Z.; Chen, J. Carbon and Metal Quantum Dots toxicity on the microalgae Chlorella pyrenoidosa. Ecotoxicol. Environ. Saf. 2016, 133, 211–217. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, properties, and environmental toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, S.; Zhu, Y.; Sun, Y.; Zeng, G.; Yang, C.; Xu, P.; Yan, M.; Liu, Z.; Zhang, W. Toxicity of carbon nanomaterials to plants, animals and microbes: Recent progress from 2015-present. Chemosphere 2018, 206, 255–264. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, H.; Liu, P.; Song, Y.; Huang, H.; Shao, M.; Liu, Y.; Li, H.; Kang, Z. Biotoxicity of degradable carbon dots towards microalgae Chlorella vulgaris. Environ. Sci. Nano 2019, 6, 3316–3323. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, H.; Song, Y.; Huang, H.; Shao, M.; Liu, Y.; Li, H.; Kang, Z. Pristine Carbon Dots Boost the Growth of Chlorella vulgaris by Enhancing Photosynthesis. ACS Appl. Bio Mater. 2018, 1, 894–902. [Google Scholar] [CrossRef]
- Barrientos, K.; Gaviria, M.; Arango, J.; Placido, J.; Bustamante, S.; Londoño, M.; Jaramillo, M. Synthesis, Characterization and Ecotoxicity Evaluation of Biochar-Derived Carbon Dots from Spruce Tree, Purple Moor-Grass and African Oil Palm. Processes 2021, 9, 1095. [Google Scholar] [CrossRef]
- Alas, M.O.; Alkas, F.B.; Sukuroglu, A.A.; Alturk, R.G.; Battal, D. Fluorescent carbon dots are the new quantum dots: An overview of their potential in emerging technologies and nanosafety. J. Mater. Sci. 2020, 55, 15074–15105. [Google Scholar] [CrossRef]
- Yan, X.; Xu, Q.; Li, D.; Wang, J.; Han, R. Carbon dots inhibit root growth by disrupting auxin biosynthesis and transport in Arabidopsis. Ecotoxicol. Environ. Saf. 2021, 216, 112168. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, B.; Man, H.; Chen, L.; Wang, X.; Tu, J.; Guo, Z.; Jin, G.; Lou, J.; Ci, L. Enhanced bioaccumulation efficiency and tolerance for Cd (II) in Arabidopsis thaliana by amphoteric nitrogen-doped carbon dots. Ecotoxicol. Environ. Saf. 2020, 190, 110108. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dou, R.; Yang, Z.; Wang, X.; Mao, C.; Gao, X.; Wang, L. The effect and fate of water-soluble carbon nanodots in maize (Zea mays L.). Nanotoxicology 2015, 10, 818–828. [Google Scholar] [CrossRef]
- Rizzo, L. Bioassays as a tool for evaluating advanced oxidation processes in water and wastewater treatment. Water Res. 2011, 45, 4311–4340. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, P.; Feng, Y.; Xie, Z.; Liu, Y.; Su, Y.; Zhang, Q.; Wang, Y.; Yao, K.; Lv, W.; et al. Facile synthesis of N-doped carbon dots/g-C3N4 photocatalyst with enhanced visible-light photocatalytic activity for the degradation of indomethacin. Appl. Catal. B Environ. 2017, 207, 103–113. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, P.; Zhuo, M.; Wang, F.; Su, Y.; Chen, T.; Yao, K.; Cai, Z.; Lv, W.; Liu, G. Degradation of indometacin by simulated sunlight activated CDs-loaded BiPO4 photocatalyst: Roles of oxidative species. Appl. Catal. B Environ. 2018, 221, 129–139. [Google Scholar] [CrossRef]
- Liu, W.; Yao, J.; Chai, H.; Zhao, Z.; Zhang, C.; Jin, J.; Choi, M.M. Concentration-dependent effect of photoluminescent carbon dots on the microbial activity of the soil studied by combination methods. Environ. Toxicol. Pharmacol. 2015, 39, 857–863. [Google Scholar] [CrossRef]





| Name | Description | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Physical treatment | ||||
| Adsorption | The method comprises adsorbents made of highly adsorptive materials, usually porous materials that can adsorb dye molecules. | Effective for a wide variety of dyes. Recyclable. Environmentally friendly. Easy operation. | Expensive. Difficult to separate the adsorbent from dye. | [16] |
| Flocculation/Coagulation | Various inducing agents are added to dye wastewater to destabilise the charged dye particles which will clump together. The clusters are removed following filtration. | Economical. Robust method. Easy to conduct. | Significant sludge generation which will need correct disposal. Not suitable for all types of dye effluents. High maintenance as it requires expensive chemicals and optimum pH conditions. | [17] |
| Electrocoagulation | The electrocoagulation of dyes process includes electrochemistry, coagulation, and flotation; thereby involving three distinct stages that incorporate chemical and physical phenomena. | Economical. Effective. Environmentally versatile. Low sludge production. | High operating and maintenance costs. High energy consumption. | [18] |
| Nanofiltration/Ultrafiltration | The filtration process can separate dye from clean water by filtering the dye wastewater through a nano (0.001 micron) or ultra-membrane (approximately from 0.002 to 0.1 microns) which retains dye particles. | High efficiency. Effective for any type of dye. | Costly. High energy consumption. High maintenance due to the constant membrane clogging problem. | [19,20] |
| Reverse osmosis | Reverse osmosis is a system where the dye wastewater is passed through an extremely thin membrane (0.0001 micron) under high pressure retaining the dye on one side and clean water on the other. | Very effective for decolourising and desalting dye wastewater. | Costly. Requires high pressure. | [21] |
| Chemical treatment | ||||
| Electrochemical oxidation | There are two different pathways in which electrochemical oxidation operates: via direct oxidation where the contaminant is directly oxidised on the anodic surface; and via indirect oxidation where the transfer of electrons is mediated by oxidant species such as the hydroxyl radicals. | No sludge formation. Suitable for soluble and insoluble dye removal. | High electricity cost. Additional hazardous material production. | [22] |
| Ion exchange | The ion-exchange process comprises the removal of dyes via the strong affinities between the functional groups on ion exchange resins and charged dye molecules. The dyes are separated from wastewater due to the formation of strong linkages between the resins and the dye molecules. | Reusable method. | Selective to a limited number of dyes. | [16] |
| Fenton reaction | This reaction involves the formation of hydroxyl radicals by a reaction between iron (II) and hydrogen peroxide (H2O2). The free radicals formed can fully oxidise dye molecules. | Effective for a wide range of dyes. | High iron sludge generation. Limited to acidic pH. Short lifetime of the free radicals. | [23] |
| Biological treatment | ||||
| Bioremediation | Dye degradation by metabolic pathways or adsorption by living/dead biomass including bacteria, fungi, yeasts, algae, and plants. | Flexible method. Economical. Environmentally friendly process. | Not effective for non-biodegradable dyes. Lengthy process. Unstable system. | [3] |
| Experimental Conditions | |||||||
|---|---|---|---|---|---|---|---|
| Dye | Catalyst Dose | H2O2 Dose | pH | Temperature (°C) | Reaction Time (min) | Treatment Efficiency (%) | Ref. |
| Rhodamine B, crystal violet, methylene blue, and rhodamine 6G at 2.5 × 10−5 M | CuO nanowire arrays on copper mesh | 10% | Neutral | Room temperature | 16, 20, 20 and 90, respectively | 100 | [15] |
| Methylene blue at 100 mg L−1 | Fe3O4/SiO2/C nanospheres at 100 mg L−1 | 5% | 6.0 | 40 | 60 | 90 | [14] |
| Methylene blue at 15 mg L−1 | Au/ZnO nanoparticles at 0.36 mg mL−1 | 5% | 7.5 | Room temperature | 120 | 90 | [120] |
| Methyl red and methyl orange 10 mg L−1 | CDs | 160 mM | 10 | 25 | 200 | 83 | [122] |
| Methylene blue at 10 mg L−1 | Photo-Fenton-like Barium M-hexaferrites nanoparticles SMN-NP (0.75 mg mL−1) | 8 mM | 3 | Room temperature | 140 | 98.9 | [123] |
| Methylene blue at 20 mg L−1 | Fenton-like MnMgFe-layered double hydroxide (1.0 mg mL−1) | ~1 M | 7 | 25 | 300 | 68 | [124] |
| Methylene blue at 50 mg L−1 | Photo-Fenton-like CuFe2O4 nanoparticles (0.2 mg mL−1) | 20 mM | 5 | 25 | 80 | 52 | [125] |
| Methylene blue at 20 mg L−1 | Fenton-like Cu9S5 nanospheres (0.2 mg mL−1) | ~60 mM | From 5.0 to 9.0 | 50 | 21 | 98 | [121] |
| Methylene blue at 100 mg L−1 | Fenton-like Iron oxide nanoparticles (M-NPs) (2.0 mg mL−1) | 560 mM | 3.5 | 90 | 90 | 100 | [126] |
| Methylene blue at 15 mg L−1 | Fenton-like Gold nanoparticles deposited on ZnO (Au/ZnO) (1.05 mg mL−1) | ~500 mM | 7.5 | Room temperature | 120 | 90 | [120] |
| Methylene blue at 20 mg L−1 | Fenton-like Fe, N–CDs (0.5 mg mL−1) | 147 mM | 8 | 50 | 60 | 97.5 | [113] |
| Mix of crystal violet, methyl green, basic fuchsin dyes at 15 mg L−1 | Opuntia derived carbon nanodots (0.25 mg mL−1) | 0.5% | 7.5 | 20 | 60 | 90 | [114] |
| Carbon Dots | Ecotoxicological Effects | Reference |
|---|---|---|
| CDs made by electrochemical etching | Chlorella vulgaris biomass and growth rate increased by 17 and 21%, respectively. | [131] |
| CDs made by electrochemical oxidation | EC50 for the growth of Chlorella vulgaris after 4 days of cultivation, based on the growth rate, was 70 μg mL−1. | [130] |
| Biochar-derived CDs from Picea, Molinia caerulea and Elaeis guineensis | EC50 (15 min) of 23.5 mg/L, 419.2 mg/L, and 8586 mg/L for Picea, Molinia caerulea, and Elaeis guineensis CDs, respectively. | [132] |
| NaNO3/KNO3/NaNO2− and citric-acid-based CDs | 40 mg/L CDs treatment inhibited the growth of the primary root of Arabidopsis greatly. | [134] |
| Amphoteric nitrogen-doped carbon dots (N–CDs) | N–CDs significantly alleviated the toxicity caused by high Cd stress on Arabidopsis thaliana seedlings growth. N–CDs induced higher germination rate (maximum: 2.5-fold), higher biomass (maximum: 3.7-fold), and better root development (maximum: 1.4-fold). | [135] |
| Citric-acid- and urea-based CDs | CDs at 250 and 500 mg/L showed no toxicity to maize (Zea mays L.). However, 1000 and 2000 mg/L CDs significantly reduced the fresh weight of root by 57% and 68% and decreased shoot fresh weight by 38% and 72%, respectively. | [136] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beker, S.A.; Cole, I.; Ball, A.S. A Review on the Catalytic Remediation of Dyes by Tailored Carbon Dots. Water 2022, 14, 1456. https://doi.org/10.3390/w14091456
Beker SA, Cole I, Ball AS. A Review on the Catalytic Remediation of Dyes by Tailored Carbon Dots. Water. 2022; 14(9):1456. https://doi.org/10.3390/w14091456
Chicago/Turabian StyleBeker, Sabrina A., Ivan Cole, and Andrew S. Ball. 2022. "A Review on the Catalytic Remediation of Dyes by Tailored Carbon Dots" Water 14, no. 9: 1456. https://doi.org/10.3390/w14091456
APA StyleBeker, S. A., Cole, I., & Ball, A. S. (2022). A Review on the Catalytic Remediation of Dyes by Tailored Carbon Dots. Water, 14(9), 1456. https://doi.org/10.3390/w14091456






