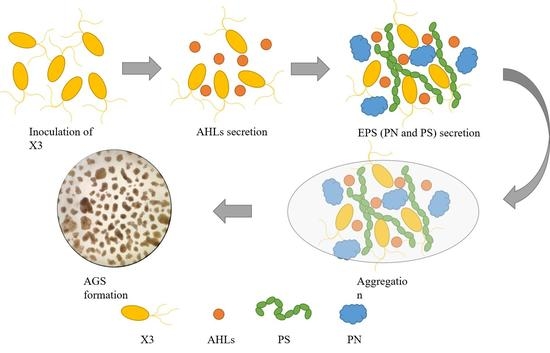
Self-Aggregation and Denitrifying Strains Accelerate Granulation and Enhance Denitrification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Calcium-Precipitating Strain
2.2. Auto-Aggregation and Coaggregation Indices
2.3. Aerobic Granules Cultivation in SBRs
2.4. Analytical Methods
2.5. EPS Extraction and Analysis
2.6. AHLs Extraction and Analysis
2.7. Microorganism Community Analysis
3. Results and Discussion
3.1. Characterization of Isolates
3.2. Characterization of Combined Strains
3.2.1. Removal and Aggregation Index of Combined Strains
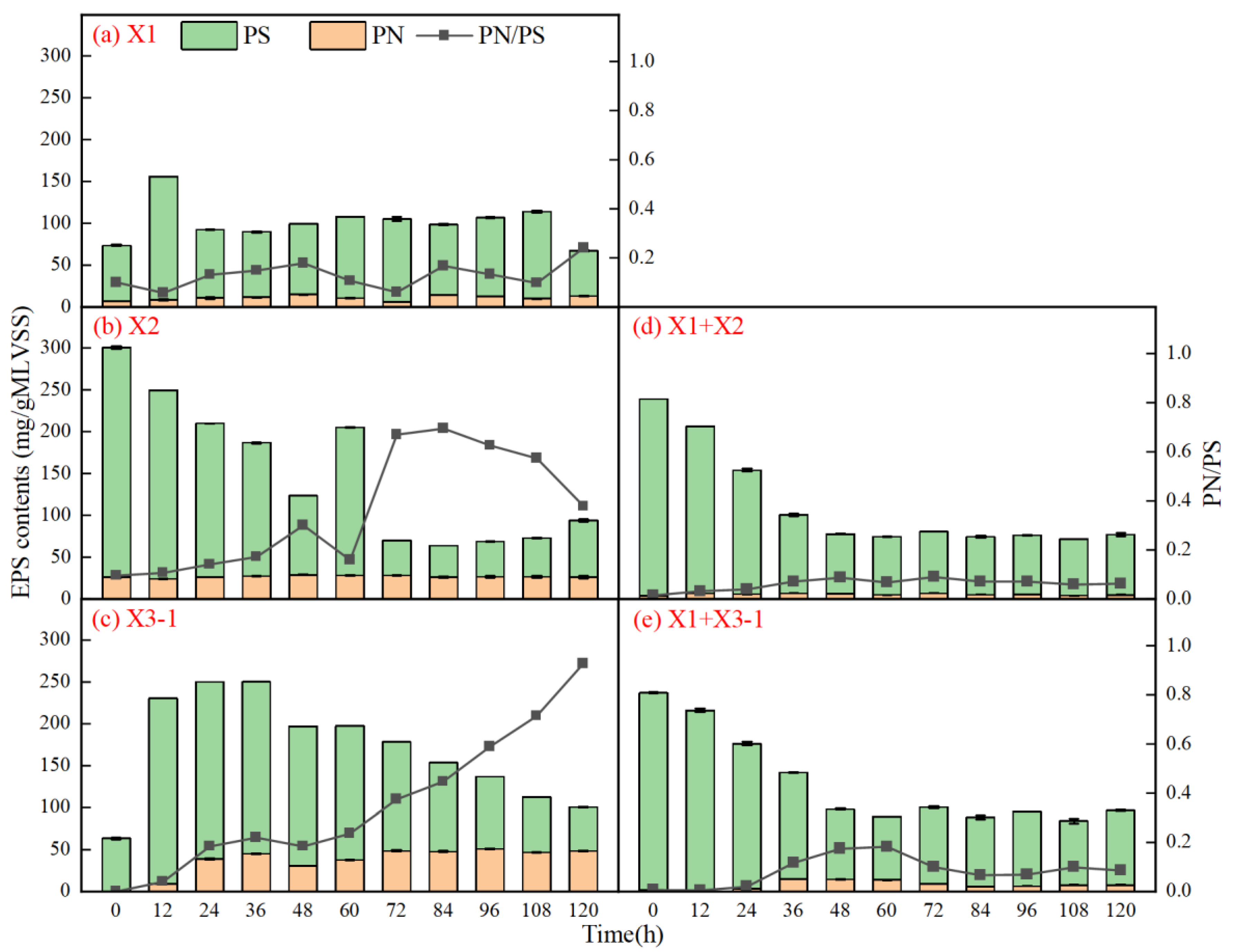
3.2.2. EPS Secretion of Combined Strains
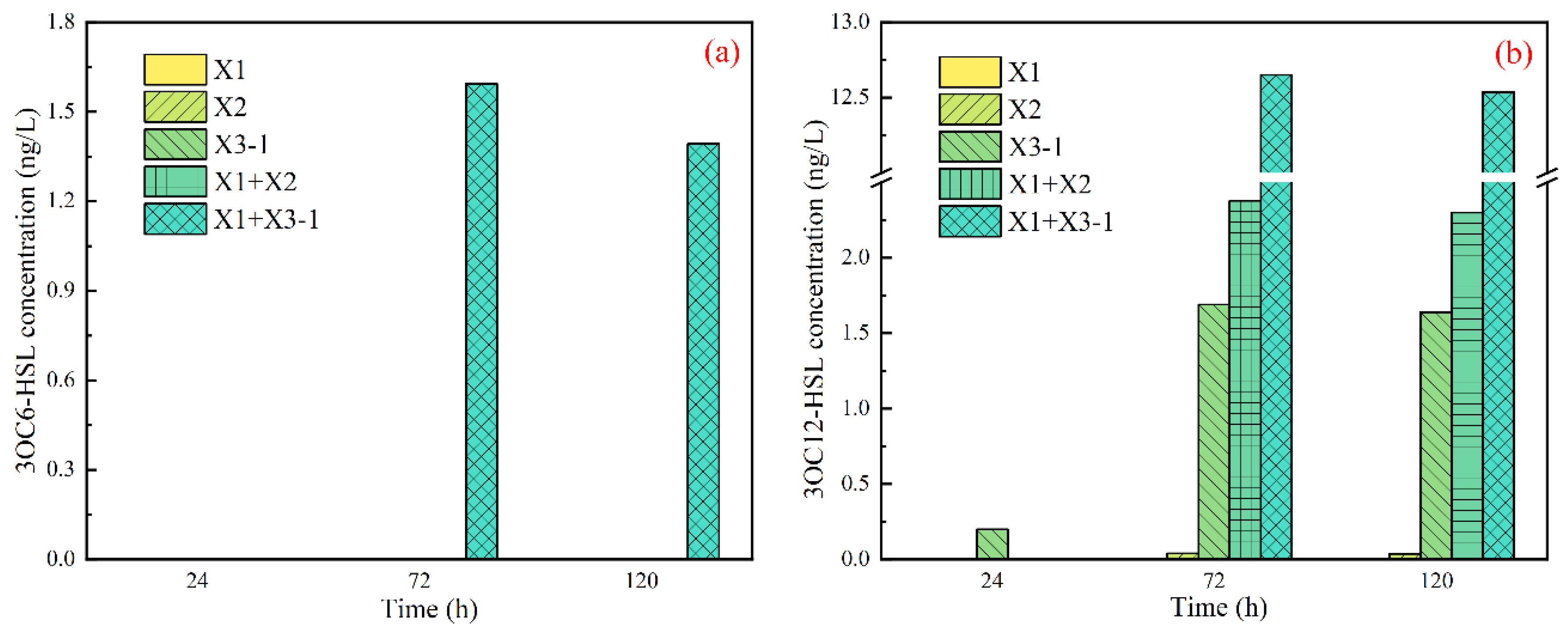
3.2.3. N-acyl-homoserine Lactones (AHLs) of Combined Strains
3.3. Performance of Granular Sludge in Sequencing Batch Reactors (SBRs)
3.3.1. Particle Size Distributions
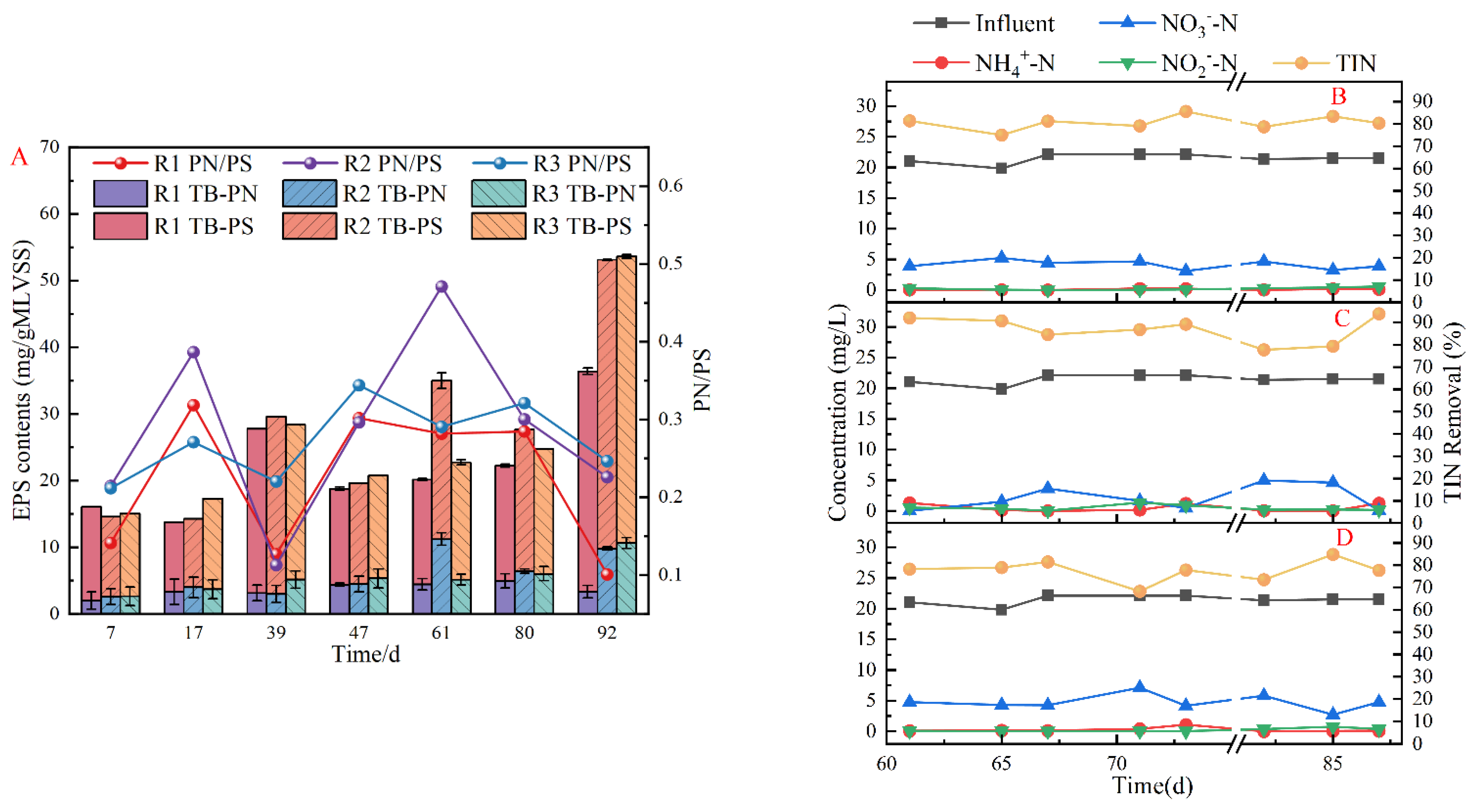
3.3.2. EPS Secretion
3.3.3. Denitrification Performance
3.3.4. Richness and Diversity of Bacteria Phylotype
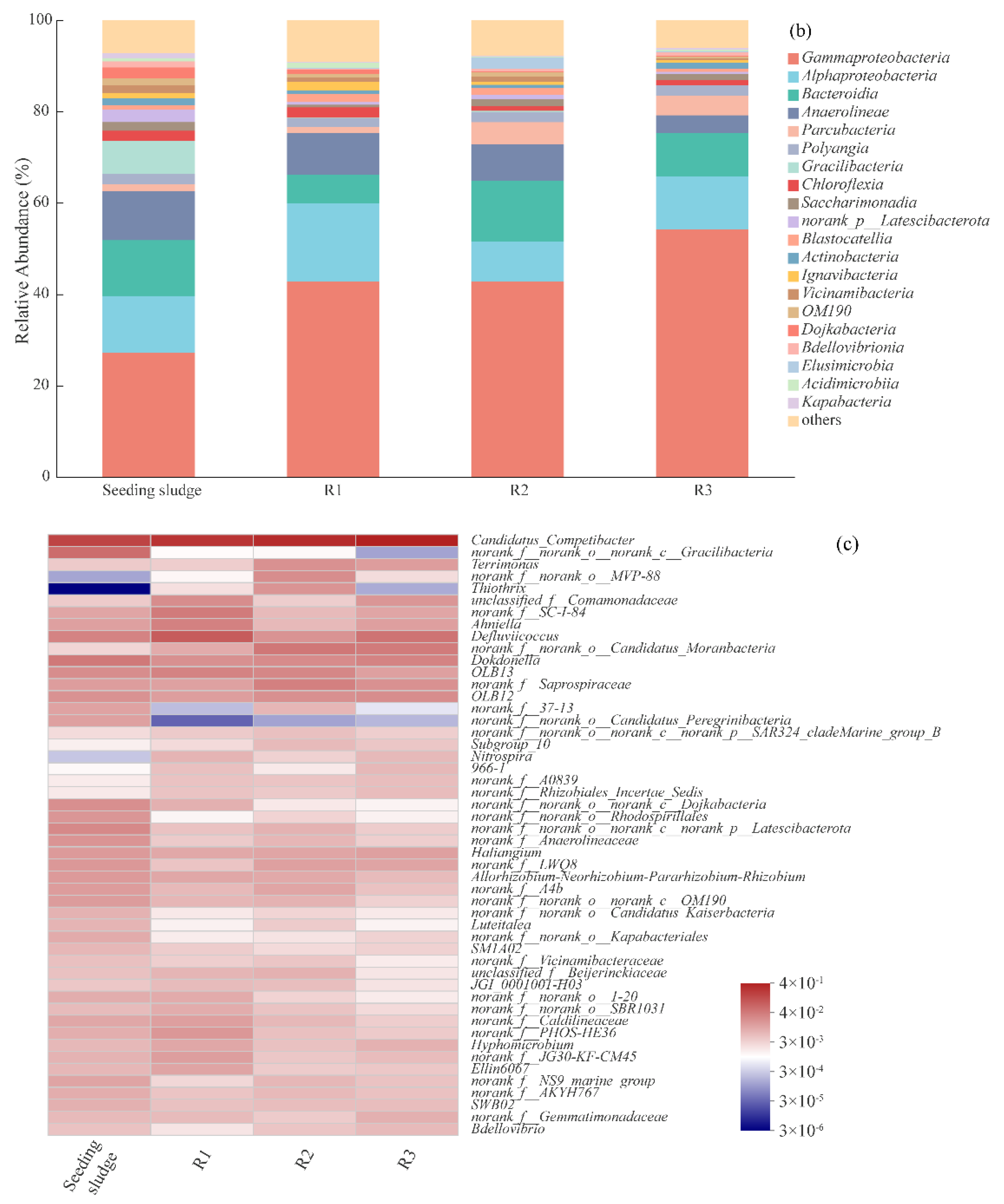
3.3.5. Microorganisms Community
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nancharaiah, Y.V.; Kiran Kumar Reddy, G. Aerobic granular sludge technology: Mechanisms of granulation and biotechnological applications. Bioresour. Technol. 2018, 247, 1128–1143. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Jin, M.; Zhao, Z.; Lei, Z.; Zhang, Z.; Adachi, Y.; Lee, D.-J. Influence of ferrous iron dosing strategy on aerobic granulation of activated sludge and bioavailability of phosphorus accumulated in granules. Bioresour. Technol. Rep. 2018, 2, 7–14. [Google Scholar] [CrossRef]
- Hailei, W.; Li, L.; Ping, L.; Hui, L.; Guosheng, L.; Jianming, Y. The acceleration of sludge granulation using the chlamydospores of Phanerochaete sp. HSD. J. Hazard. Mater. 2011, 192, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Shang, Y.; Wang, H.; Yang, K. Rapid formation and pollutant removal ability of aerobic granules in a sequencing batch airlift reactor at low temperature. Environ. Technol. 2016, 37, 3078–3085. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, X.; Nuramkhaan, M.; Lei, Z.; Shimizu, K.; Zhang, Z.; Adachi, Y.; Lee, D.-J.; Tay, J.H. Rapid granulation of aerobic granular sludge: A mini review on operation strategies and comparative analysis. Bioresour. Technol. Rep. 2019, 7, 100206. [Google Scholar] [CrossRef]
- Li, A.-J.; Li, X.-Y.; Yu, H. Aerobic sludge granulation facilitated by activated carbon for partial nitrification treatment of ammonia-rich wastewater. Chem. Eng. J. 2013, 218, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Wilderer, P.A.; McSwain, B.S. The SBR and its biofilm application potentials. Water Sci. Technol. 2004, 50, 1–10. [Google Scholar] [CrossRef]
- Ivanov, V.; Wang, X.-H.; Stabnikova, O. Starter culture of Pseudomonas veronii strain B for aerobic granulation. World J. Microbiol. Biotechnol. 2007, 24, 533–539. [Google Scholar] [CrossRef]
- Jiang, H.L.; Tay, J.H.; Maszenan, A.M.; Tay, S.T.L. Enhanced phenol biodegradation and aerobic granulation by two coaggregating bacterial strains. Environ. Sci. Technol. 2006, 40, 6137–6142. [Google Scholar] [CrossRef]
- Liang, J.; Li, W.; Zhang, H.; Jiang, X.; Wang, L.; Liu, X.; Shen, J. Coaggregation mechanism of pyridine-degrading strains for the acceleration of the aerobic granulation process. Chem. Eng. J. 2018, 338, 176–183. [Google Scholar] [CrossRef]
- Jiang, H.L.; Tay, J.H.; Liu, Y.; Tay, S.T.L. Ca2+ augmentation for enhancement of aerobically grown microbial granules in sludge blanket reactors. Biotechnol. Lett. 2003, 25, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gao, D.W.; Zhang, M.; Fu, Y. Comparison of Ca2+ and Mg2+ enhancing aerobic granulation in SBR. J. Hazard. Mater. 2010, 181, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Su, J.F.; Shi, J.X.; Ma, F. Aerobic denitrification and biomineralization by a novel heterotrophic bacterium, Acinetobacter sp. H36. Mar. Pollut Bull. 2017, 116, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Buswell, C.M.; Herlihy, Y.M.; Marsh, P.D.; Keevil, C.W.; Leach, S.A. Coaggregation amongst aquatic biofilm bacteria. J. Appl. Microbiol. 1997, 83, 477–484. [Google Scholar] [CrossRef]
- Shuai, J.; Hu, X.; Wang, B.; Lyu, W.; Chen, R.; Guo, W.; Wang, H.; Zhou, D. Response of aerobic sludge to AHL-mediated QS: Granulation, simultaneous nitrogen and phosphorus removal performance. Chin. Chem. Lett. 2021, 32, 3402–3409. [Google Scholar] [CrossRef]
- Maddela, N.R.; Sheng, B.; Yuan, S.; Zhou, Z.; Villamar-Torres, R.; Meng, F. Roles of quorum sensing in biological wastewater treatment: A critical review. Chemosphere 2019, 221, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Szabó, E.; Hermansson, M.; Modin, O.; Persson, F.; Wilén, B.-M. Effects of Wash-Out Dynamics on Nitrifying Bacteria in Aerobic Granular Sludge During Start-Up at Gradually Decreased Settling Time. Water 2016, 8, 172. [Google Scholar] [CrossRef]
- He, Q.; Zhang, S.; Zou, Z.; Zheng, L.A.; Wang, H. Unraveling characteristics of simultaneous nitrification, denitrification and phosphorus removal (SNDPR) in an aerobic granular sequencing batch reactor. Bioresour. Technol. 2016, 220, 651–655. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; APHA: Washington, DC, USA, 2005. [Google Scholar]
- Li, X.Y.; Yang, S.F. Influence of loosely bound extracellular polymeric substances (EPS) on the flocculation, sedimentation and dewaterability of activated sludge. Water Res. 2007, 41, 1022–1030. [Google Scholar] [CrossRef]
- He, Q.; Song, Q.; Zhang, S.; Zhang, W.; Wang, H. Simultaneous nitrification, denitrification and phosphorus removal in an aerobic granular sequencing batch reactor with mixed carbon sources: Reactor performance, extracellular polymeric substances and microbial successions. Chem. Eng. J. 2018, 331, 841–849. [Google Scholar] [CrossRef]
- He, Q.; Yuan, Z.; Zhang, J.; Zhang, S.; Zhang, W.; Zou, Z.; Wang, H. Insight into the impact of ZnO nanoparticles on aerobic granular sludge under shock loading. Chemosphere 2017, 173, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, A.; Cui, C.; Ma, F.; Cui, D.; Zhao, H.; Wang, Q.; Ni, B.; Yang, J. AHL-mediated quorum sensing regulates the variations of microbial community and sludge properties of aerobic granular sludge under low organic loading. Environ. Int. 2019, 130, 104946. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, Q.; Chen, D.; Wei, L.; Zou, Z.; Zhou, J.; Yang, K.; Zhang, H. Microbial community in a hydrogenotrophic denitrification reactor based on pyrosequencing. Appl. Microbiol. Biotechnol. 2015, 99, 10829–10837. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhang, J.; Gao, S.; Chen, L.; Lyu, W.; Zhang, W.; Song, J.; Hu, X.; Chen, R.; Wang, H.; et al. A comprehensive comparison between non-bulking and bulking aerobic granular sludge in microbial communities. Bioresour. Technol. 2019, 294, 122151. [Google Scholar] [CrossRef]
- Zhang, D.; Li, W.; Hou, C.; Shen, J.; Jiang, X.; Sun, X.; Li, J.; Han, W.; Wang, L.; Liu, X. Aerobic granulation accelerated by biochar for the treatment of refractory wastewater. Chem. Eng. J. 2017, 314, 88–97. [Google Scholar] [CrossRef]
- Tay, J.H.; Liu, Q.S.; Liu, Y. The role of cellular polysaccharides in the formation and stability of aerobic granules. Lett. Appl. Microbiol. 2001, 33, 222–226. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.Y.; Lee, D.J.; Tay, J.H. Distribution of extracellular polymeric substances in aerobic granules. Appl. Microbiol. Biotechnol. 2007, 73, 1463–1469. [Google Scholar] [CrossRef]
- Adav, S.S.; Lee, D.J.; Tay, J.H. Extracellular polymeric substances and structural stability of aerobic granule. Water Res. 2008, 42, 1644–1650. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Deng, D.; Li, R.; Guo, C.; Ma, J.; Chen, M. Investigation of extracellular polymeric substances (EPS) in four types of sludge: Factors influencing EPS properties and sludge granulation. J. Water Process. Eng. 2021, 40, 101924. [Google Scholar] [CrossRef]
- Higgins, M.J.; Novak, J.T. Characterization of exocellular protein and its role in bioflocculation. J. Environ. Eng.-Asce 1997, 123, 479–485. [Google Scholar] [CrossRef]
- Morgan, J.W.; Forster, C.F.; Evison, L. A comparative study of the nature of biopolymers extracted from anaerobic and activated sludges. Water Res. 1990, 24, 743–750. [Google Scholar] [CrossRef]
- Yan, L.; Liu, Y.; Wen, Y.; Ren, Y.; Hao, G.; Zhang, Y. Role and significance of extracellular polymeric substances from granular sludge for simultaneous removal of organic matter and ammonia nitrogen. Bioresour. Technol. 2015, 179, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.T.; Yu, H.Q.; Li, X.Y. The quorum-sensing effect of aerobic granules on bacterial adhesion, biofilm formation, and sludge granulation. Appl. Microbiol. Biotechnol. 2010, 88, 789–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Y.; Yu, H.; Cao, R.; Xu, X.; Zhu, L. Promoting the granulation process of aerobic sludge via a sustainable strategy of effluent reflux in view of AHLs-mediated quorum sensing. J. Environ. Manag. 2021, 303, 114091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cao, R.; Jin, L.; Zhu, W.; Ji, Y.; Xu, X.; Zhu, L. The regulation of N-acyl-homoserine lactones (AHLs)-based quorum sensing on EPS secretion via ATP synthetic for the stability of aerobic granular sludge. Sci. Total Environ. 2019, 673, 83–91. [Google Scholar] [CrossRef]
- Liu, X.; Wu, S.; Zhang, D.; Shen, J.; Han, W.; Sun, X.; Li, J.; Wang, L. Simultaneous pyridine biodegradation and nitrogen removal in an aerobic granular system. J. Environ. Sci. (China) 2018, 67, 318–329. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, Z.; Yu, Z.; Dai, X.; Xu, X.; Alvarez, P.J.J.; Zhu, L. Evolution and functional analysis of extracellular polymeric substances during the granulation of aerobic sludge used to treat p-chloroaniline wastewater. Chem. Eng. J. 2017, 330, 596–604. [Google Scholar] [CrossRef]
- Xiong, H.; Dong, S.; Zhang, J.; Zhou, D.; Rittmann, B.E. Roles of an easily biodegradable co-substrate in enhancing tetracycline treatment in an intimately coupled photocatalytic-biological reactor. Water Res. 2018, 136, 75–83. [Google Scholar] [CrossRef]
- Tang, P.; Yu, D.; Chen, G.; Zhang, P.; Wang, X.; Liu, C.; Huang, S. Novel aerobic granular sludge culture strategy: Using granular sludge Anammox process effluent as a biocatalyst. Bioresour. Technol. 2019, 294, 122156. [Google Scholar] [CrossRef]
- Cai, F.; Lei, L.; Li, Y.; Chen, Y. A review of aerobic granular sludge (AGS) treating recalcitrant wastewater: Refractory organics removal mechanism, application and prospect. Sci. Total Environ. 2021, 782, 146852. [Google Scholar] [CrossRef]
- He, Q.; Chen, L.; Zhang, S.; Chen, R.; Wang, H.; Zhang, W.; Song, J. Natural sunlight induced rapid formation of water-born algal-bacterial granules in an aerobic bacterial granular photo-sequencing batch reactor. J. Hazard. Mater. 2018, 359, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Zhou, S.; Wang, X.; Chen, Y.; Lin, X.; Yan, Y.; Ma, X.; Wu, M.; Han, H. 16S rRNA gene high-throughput sequencing reveals shift in nitrogen conversion related microorganisms in a CANON system in response to salt stress. Chem. Eng. J. 2017, 317, 512–521. [Google Scholar] [CrossRef]
- Ding, H.H.H.; Chang, S.; Liu, Y. Biological hydrolysis pretreatment on secondary sludge: Enhancement of anaerobic digestion and mechanism study. Bioresour. Technol. 2017, 244, 989–995. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhou, J.; Wang, H.; Zhang, J.; Wei, L. Microbial population dynamics during sludge granulation in an A/O/A sequencing batch reactor. Bioresour. Technol. 2016, 214, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gong, B.; Huang, W.; Wang, Y.; Zhou, J. Bacterial community structure in simultaneous nitrification, denitrification and organic matter removal process treating saline mustard tuber wastewater as revealed by 16S rRNA sequencing. Bioresour. Technol. 2017, 228, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Watanabe, Y.; Okabe, S. Significance of Chloroflexi in performance of submerged membrane Bioreactors (MBR) treating municipal wastewater. Environ. Sci. Technol. 2007, 41, 7787–7794. [Google Scholar] [CrossRef]
- Cheng, C.; Zhou, Z.; Niu, T.; An, Y.; Shen, X.; Pan, W.; Chen, Z.; Liu, J. Effects of side-stream ratio on sludge reduction and microbial structures of anaerobic side-stream reactor coupled membrane bioreactors. Bioresour. Technol. 2017, 234, 380–388. [Google Scholar] [CrossRef]
- Kindaichi, T.; Yamaoka, S.; Uehara, R.; Ozaki, N.; Ohashi, A.; Albertsen, M.; Nielsen, P.H.; Nielsen, J.L. Phylogenetic diversity and ecophysiology of Candidate phylum Saccharibacteria in activated sludge. Fems Microbiol. Ecol. 2016, 92, fiw078. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Chandran, K.; Stensel, D. Microbial ecology of denitrification in biological wastewater treatment. Water Res. 2014, 64, 237–254. [Google Scholar] [CrossRef]
- Baldwin, S.A.; Khoshnoodi, M.; Rezadehbashi, M.; Taupp, M.; Hallam, S.; Mattes, A.; Sanei, H. The microbial community of a passive biochemical reactor treating arsenic, zinc, and sulfate-rich seepage. Front. Bioeng. Biotechnol. 2015, 3, 27. [Google Scholar] [CrossRef]
- Hou, Y.-N.; Yang, C.; Zhou, A.; Liu, W.; Liu, C.; Cai, W.-W.; Zhou, J.; Wang, A.-J. Microbial community response and SDS-PAGE reveal possible mechanism of waste activated sludge acidification enhanced by microaeration coupled thermophilic pretreatment. Process. Biochem. 2018, 64, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Seviour, T.W.; Lambert, L.K.; Pijuan, M.; Yuan, Z.G. Selectively inducing the synthesis of a key structural exopolysaccharide in aerobic granules by enriching for Candidatus “Competibacter phosphatis”. Appl. Microbiol. Biotechnol. 2011, 92, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Yu, D.; Zhao, J.; Wang, X.; Bi, C.; Zhen, J.; Yuan, M. Achieving deep-level nutrient removal via combined denitrifying phosphorus removal and simultaneous partial nitrification-endogenous denitrification process in a single-sludge sequencing batch reactor. Bioresour. Technol. 2019, 289, 121690. [Google Scholar] [CrossRef] [PubMed]



| Sample | OTU | Shannon | Simpson | Ace | Chao | Coverage |
|---|---|---|---|---|---|---|
| Seed | 1042 | 5.114 | 0.015 | 1281.6 | 1280.1 | 0.994 |
| R1 | 1027 | 4.815 | 0.030 | 1357.1 | 1330.1 | 0.991 |
| R2 | 969 | 4.650 | 0.042 | 1209.0 | 1258.8 | 0.993 |
| R3 | 938 | 4.263 | 0.064 | 1246.0 | 1268.6 | 0.992 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Wang, C.; Xie, Y.; Chen, R.; Huang, M.; Hu, X.; Wang, B.; Guo, W.; Huang, H.; Wang, R.; et al. Self-Aggregation and Denitrifying Strains Accelerate Granulation and Enhance Denitrification. Water 2022, 14, 1482. https://doi.org/10.3390/w14091482
Zhang S, Wang C, Xie Y, Chen R, Huang M, Hu X, Wang B, Guo W, Huang H, Wang R, et al. Self-Aggregation and Denitrifying Strains Accelerate Granulation and Enhance Denitrification. Water. 2022; 14(9):1482. https://doi.org/10.3390/w14091482
Chicago/Turabian StyleZhang, Shujia, Chunyan Wang, Yijia Xie, Rongfan Chen, Mengyuan Huang, Xiaoling Hu, Bin Wang, Wenbin Guo, Haiyun Huang, Rongrong Wang, and et al. 2022. "Self-Aggregation and Denitrifying Strains Accelerate Granulation and Enhance Denitrification" Water 14, no. 9: 1482. https://doi.org/10.3390/w14091482