Effects of Warming on Aquatic Snails and Periphyton in Freshwater Ecosystems with and without Predation by Common Carp
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Experiment Set-Up
2.3. Analyses
3. Results
3.1. Treatment Manipulation
3.2. Snail Species Responses
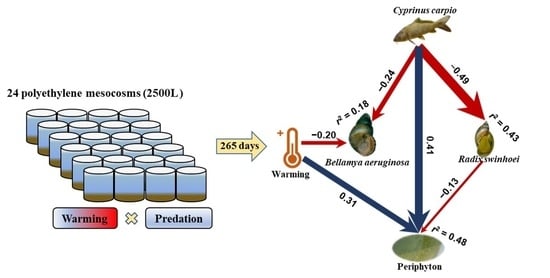
3.3. Structural Equation Model
4. Discussion
4.1. Warming (without Fish) Effects on Snails
4.2. Warming (with Fish) Effects on Snails
4.3. Predation Effects on Snails
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strong, E.E.; Gargominy, O.; Ponder, W.F.; Bouchet, P. Global diversity of gastropods (Gastropoda; Mollusca) in freshwater. Hydrobiologia 2008, 595, 149–166. [Google Scholar] [CrossRef]
- Vaughn, C.C. Ecosystem services provided by freshwater mussels. Hydrobiologia 2018, 810, 15–27. [Google Scholar] [CrossRef]
- Phillips, G.; Willby, N.; Moss, B. Submerged macrophyte decline in shallow lakes: What have we learnt in the last forty years? Aquat. Bot. 2016, 135, 37–45. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, P.; Chen, J.; Xu, J. Freshwater snail and shrimp differentially affect water turbidity and benthic primary producers. Water Biol. Secur. 2021, 1, 100004. [Google Scholar] [CrossRef]
- Covich, A.P.; Palmer, M.A.; Crowl, T.A. The role of benthic invertebrate species in freshwater ecosystems—Zoobenthic species influence energy flows and nutrient cycling. Bioscience 1999, 49, 119–127. [Google Scholar] [CrossRef]
- Zheng, Z.; Lv, J.; Lu, K.; Jin, C.; Zhu, J.; Liu, X. The Impact of Snail (Bellamya aeruginosa) Bioturbation on Sediment Characteristics and Organic Carbon Fluxes in an Eutrophic Pond. Clean-Soil Air Water 2011, 39, 566–571. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Lu, K.H.; Liu, X.S. Can the freshwater snail Bellamya aeruginosa (Mollusca) affect phytoplankton community and water quality? Hydrobiologia 2013, 707, 147–157. [Google Scholar] [CrossRef]
- Lv, T.; Guan, X.; Fan, S.F.; Han, C.; Gao, Z.Y.; Liu, C.H. Snail communities increase submerged macrophyte growth by grazing epiphytic algae and phytoplankton in a mesocosm experiment. Ecol. Evol. 2022, 12, e8615. [Google Scholar] [CrossRef]
- Brönmark, C. Interactions between macrophytes, epiphytes and herbivores: An experimental approach. Oikos 1985, 45, 26–30. [Google Scholar] [CrossRef]
- Roberts, E.; Kroker, J.; Körner, S.; Nicklisch, A. The role of periphyton during the re-colonization of a shallow lake with submerged macrophytes. Hydrobiologia 2003, 506, 525–530. [Google Scholar] [CrossRef]
- Brönmark, C. Effects of tench and perch on interactions in a freshwater, benthic food chain. Ecology 1994, 75, 1818–1828. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, P.; Cheng, H.; Frenken, T.; Xu, J.; Zhang, M. Interactive effects of light and snail herbivory rather than nutrient loading determine early establishment of submerged macrophytes. Ecol. Evol. 2022, 12, e9070. [Google Scholar] [CrossRef] [PubMed]
- Rothmeier, L.M.; Martens, A.; Watermann, B.; Grabow, K.; Bartz, J.; Sahm, R. The Danubian cryptic invader Theodoxus fluviatilis (Gastropoda: Neritidae) in the River Rhine: A potential indicator for metal pollution? Ecotoxicology 2022, 31, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, V.; Pant, D. Environmental biomonitoring by snails. Biomarkers 2021, 26, 221–239. [Google Scholar] [CrossRef]
- Mahmoud, K.M.A.; Abu Taleb, H.M.A. Fresh water snails as bioindicator for some heavy metals in the aquatic environment. Afr. J. Ecol. 2013, 51, 193–198. [Google Scholar] [CrossRef]
- Johnson, P.D.; Bogan, A.E.; Brown, K.M.; Burkhead, N.M.; Cordeiro, J.R.; Garner, J.T.; Hartfield, P.D.; Lepitzki, D.A.W.; Mackie, G.L.; Pip, E.; et al. Conservation Status of Freshwater Gastropods of Canada and the United States. Fisheries 2013, 38, 247–282. [Google Scholar] [CrossRef]
- Bohm, M.; Dewhurst-Richman, N.I.; Seddon, M.; Ledger, S.E.H.; Albrecht, C.; Allen, D.; Bogan, A.E.; Cordeiro, J.; Cummings, K.S.; Cuttelod, A.; et al. The conservation status of the world’s freshwater molluscs. Hydrobiologia 2021, 848, 3231–3254. [Google Scholar] [CrossRef]
- Giannelli, A.; Cantacessi, C.; ColeIla, V.; Dantas-Torres, F.; Otranto, D. Gastropod-Borne Helminths: A Look at the Snail-Parasite Interplay. Trends Parasitol. 2016, 32, 255–264. [Google Scholar] [CrossRef]
- Steffen, W.; Richardson, K.; Rockstrom, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; de Vries, W.; de Wit, C.A.; et al. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef]
- Brönmark, C.; Klosiewski, S.P.; Stein, R.A. Indirect effects of predation in a fresh-water, benthic food-chain. Ecology 1992, 73, 1662–1674. [Google Scholar] [CrossRef]
- Brönmark, C.; Weisner, S.E.B. Decoupling of cascading trophic interactions in a freshwater, benthic food chain. Oecologia 1996, 108, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Vander Zanden, M.J.; Vadeboncoeur, Y. Putting the lake back together 20 years later: What in the benthos have we learned about habitat linkages in lakes? Inland Waters 2020, 10, 305–321. [Google Scholar] [CrossRef]
- Martin, T.H.; Crowder, L.B.; Dumas, C.F.; Burkholder, J.M. Indirect effects of fish on macrophytes in bays mountain lake—Evidence for a littoral trophic cascade. Oecologia 1992, 89, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Paine, R.T. Food web complexity and species diversity. Am. Nat. 1966, 100, 65–75. [Google Scholar] [CrossRef]
- Karakoc, C.; Clark, A.T.; Chatzinotas, A. Diversity and coexistence are influenced by time-dependent species interactions in a predator-prey system. Ecol. Lett. 2020, 23, 983–993. [Google Scholar] [CrossRef]
- Chesson, P.; Kuang, J.J. The interaction between predation and competition. Nature 2008, 456, 235–238. [Google Scholar] [CrossRef]
- Thackeray, S.J.; Henrys, P.A.; Hemming, D.; Bell, J.R.; Botham, M.S.; Burthe, S.; Helaouet, P.; Johns, D.G.; Jones, I.D.; Leech, D.I.; et al. Phenological sensitivity to climate across taxa and trophic levels. Nature 2016, 535, 241–245. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Zhang, P.; van Leeuwen, C.H.; Bogers, D.; Poelman, M.; Xu, J.; Bakker, E.S. Ectothermic omnivores increase herbivory in response to rising temperature. Oikos 2020, 129, 1028–1039. [Google Scholar] [CrossRef]
- Schaum, C.E.; Ffrench-Constant, R.; Lowe, C.; Olafsson, J.S.; Padfield, D.; Yvon-Durocher, G.; Student Res, T. Temperature-driven selection on metabolic traits increases the strength of an algal-grazer interaction in naturally warmed streams. Glob. Chang. Biol. 2018, 24, 1793–1803. [Google Scholar] [CrossRef]
- O’Gorman, E.J.; Petchey, O.L.; Faulkner, K.J.; Gallo, B.; Gordon, T.A.; Neto-Cerejeira, J.; Ólafsson, J.S.; Pichler, D.E.; Thompson, M.S.; Woodward, G. A simple model predicts how warming simplifies wild food webs. Nat. Clim. Chang. 2019, 9, 611–616. [Google Scholar] [CrossRef]
- Sunday, J.M.; Bates, A.E.; Dulvy, N.K. Thermal tolerance and the global redistribution of animals. Nat. Clim. Chang. 2012, 2, 686–690. [Google Scholar] [CrossRef]
- Rohr, J.R.; Civitello, D.J.; Cohen, J.M.; Roznik, E.A.; Sinervo, B.; Dell, A.I. The complex drivers of thermal acclimation and breadth in ectotherms. Ecol. Lett. 2018, 21, 1425–1439. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.; Przeslawski, R. Multistressor impacts of warming and acidification of the ocean on marine invertebrates’ life histories. Integr. Comp. Biol. 2013, 53, 582–596. [Google Scholar] [CrossRef]
- Przeslawski, R.; Ahyong, S.; Byrne, M.; Worheide, G.; Hutchings, P. Beyond corals and fish: The effects of climate change on noncoral benthic invertebrates of tropical reefs. Glob. Chang. Biol. 2008, 14, 2773–2795. [Google Scholar] [CrossRef]
- Meza-Lopez, M.M.; Siemann, E. Warming alone increased exotic snail reproduction and together with eutrophication influenced snail growth in native wetlands but did not impact plants. Sci. Total Environ. 2020, 704, 135271. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, J.A.; Bickford, D. Shrinking body size as an ecological response to climate change. Nat. Clim. Chang. 2011, 1, 401–406. [Google Scholar] [CrossRef]
- Cao, Y.; Li, W.; Jeppesen, E. The response of two submerged macrophytes and periphyton to elevated temperatures in the presence and absence of snails: A microcosm approach. Hydrobiologia 2014, 738, 49–59. [Google Scholar] [CrossRef]
- Hansson, L.A.; Nicolle, A.; Graneli, W.; Hallgren, P.; Kritzberg, E.; Persson, A.; Bjork, J.; Nilsson, P.A.; Bronmark, C. Food-chain length alters community responses to global change in aquatic systems. Nat. Clim. Chang. 2013, 3, 228–233. [Google Scholar] [CrossRef]
- Thakur, M.P.; Kunne, T.; Griffin, J.N.; Eisenhauer, N. Warming magnifies predation and reduces prey coexistence in a model litter arthropod system. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162570. [Google Scholar] [CrossRef]
- Thakur, M.P.; Griffin, J.N.; Kunne, T.; Dunker, S.; Fanesi, A.; Eisenhauer, N. Temperature effects on prey and basal resources exceed that of predators in an experimental community. Ecol. Evol. 2018, 8, 12670–12680. [Google Scholar] [CrossRef] [PubMed]
- Ockendon, N.; Baker, D.J.; Carr, J.A.; White, E.C.; Almond, R.E.A.; Amano, T.; Bertram, E.; Bradbury, R.B.; Bradley, C.; Butchart, S.H.M.; et al. Mechanisms underpinning climatic impacts on natural populations: Altered species interactions are more important than direct effects. Glob. Chang. Biol. 2014, 20, 2221–2229. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, M.; Xu, J.; Heino, J.; Burridge, C. Geographical gradients in the biodiversity of Chinese freshwater molluscs: Implications for conservation. Divers. Distrib. 2018, 24, 485–496. [Google Scholar] [CrossRef]
- Gu, Q.H.; Husemann, M.; Ding, B.; Luo, Z.; Xiong, B.X. Population genetic structure of Bellamya aeruginosa (Mollusca: Gastropoda: Viviparidae) in China: Weak divergence across large geographic distances. Ecol. Evol. 2015, 5, 4906–4919. [Google Scholar] [CrossRef]
- Ma, T.; Gong, S.; Zhou, K.; Zhu, C.; Deng, K.; Luo, Q.; Wang, Z. Laboratory culture of the freshwater benthic gastropod Bellamya aeruginosa (Reeve) and its utility as a test species for sediment toxicity. J. Environ. Sci. 2010, 22, 304–313. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Zhang, W.Z.; Wang, Y.X. Medical Malacology; China Ocean Press: Beijing, China, 1993. [Google Scholar]
- Zhu, T.B.; Zhang, L.H.; Zhang, T.L.; Wang, Y.P.; Hu, W.; Ringo, E.; Zhu, Z.Y. Effects of sustained predation by fast-growing transgenic common carp (Cyprinus carpio Linnaeus, 1758) on gastropods in artificial environments. J. Appl. Ichthyol. 2017, 33, 22–28. [Google Scholar] [CrossRef]
- Chen, Q.; Song, G. A preliminary study on reproduction and growth of the snail, Bellamya aeruginosa (Veeve). Acta Hydrobiol. Sin. 1975, 5, 519–534. [Google Scholar]
- Qi, Z. Economic Mollusca of China; China Agriculture Press: Beijing, China, 1998. [Google Scholar]
- Zhi, Y.; Liu, Y.; Li, W.; Cao, Y. Responses of four submerged macrophytes to freshwater snail density (Radix swinhoei) under clear-water conditions: A mesocosm study. Ecol. Evol. 2020, 10, 7644–7653. [Google Scholar] [CrossRef]
- Pfenninger, M.; Cordellier, M.; Streit, B. Comparing the efficacy of morphologic and DNA-based taxonomy in the freshwater gastropod genus Radix (Basommatophora, Pulmonata). BMC Evol. Biol. 2006, 6, 100. [Google Scholar] [CrossRef]
- Johansson, M.P.; Laurila, A. Maximum thermal tolerance trades off with chronic tolerance of high temperature in contrasting thermal populations of Radix balthica. Ecol. Evol. 2017, 7, 3149–3156. [Google Scholar] [CrossRef]
- Johansson, M.P.; Ermold, F.; Kristjansson, B.K.; Laurila, A. Divergence of gastropod life history in contrasting thermal environments in a geothermal lake. J. Evol. Biol. 2016, 29, 2043–2053. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.K.S. Intraspecific life-history variation in Radix plicatulus (gastropoda, pulmonata, lymnaeidae). J. Zool. 1994, 232, 435–446. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Z.; Jeppesen, E. Fish community assemblages changed but biomass remained similar after lake restoration by biomanipulation in a Chinese tropical eutrophic lake. Hydrobiologia 2014, 724, 127–140. [Google Scholar] [CrossRef]
- Zhang, P.Y.; Zhang, H.; Wang, H.; Hilt, S.; Li, C.; Yu, C.; Zhang, M.; Xu, J. Warming alters juvenile carp effects on macrophytes resulting in a shift to turbid conditions in freshwater mesocosms. J. Appl. Ecol. 2022, 59, 165–175. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report; Cambridge University Press: New York, USA, 2014. [Google Scholar]
- Wang, T.; Xu, J.; Molinos, J.G.; Li, C.; Hu, B.; Pan, M.; Zhang, M. A dynamic temperature difference control recording system in shallow lake mesocosm. MethodsX 2020, 7, 100930. [Google Scholar] [CrossRef]
- He, H.; Han, Y.; Li, Q.; Jeppesen, E.; Li, K.; Yu, J.; Liu, Z. Crucian carp (Carassius carassius) strongly affect C/N/P stoichiometry of suspended particulate matter in shallow warm water eutrophic lakes. Water 2019, 11, 524. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lefcheck, J.S. piecewiseSEM: Piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Velthuis, M.; Domis, L.N.d.S.; Frenken, T.; Stephan, S.; Kazanjian, G.; Aben, R.; Hilt, S.; Kosten, S.; van Donk, E.; Van de Waal, D.B. Warming advances top-down control and reduces producer biomass in a freshwater plankton community. Ecosphere 2017, 8, e01651. [Google Scholar] [CrossRef]
- Tao, J.; He, D.; Kennard, M.J.; Ding, C.; Bunn, S.E.; Liu, C.; Jia, Y.; Che, R.; Chen, Y. Strong evidence for changing fish reproductive phenology under climate warming on the Tibetan Plateau. Glob. Chang. Biol. 2018, 24, 2093–2104. [Google Scholar] [CrossRef]
- Gong, Z.; Li, Y.; Xie, P. Population dynamics and production of Bellamya aeruginosa (Reeve) (Mollusca: Viviparidae) in Lake Donghu, Wuhan. J. Lake Sci. 2009, 21, 401–407. [Google Scholar] [CrossRef]
- Yan, Y.; Liang, Y.; Wang, H. Annual production of Bellamya aeruginosa in Houhu Lake, Wuhan. J. Lake Sci. 2000, 12, 68–72. [Google Scholar] [CrossRef]
- Chen, Q. A preliminary study on the population dynamics and annual production of Bellamya Aeruginosa (Reeve) in Lake Dong Hu, Wuhan. Acta Hydrobiol. Sin. 1987, 11, 117–130. [Google Scholar]
- Twardochleb, L.A.; Treakle, T.C.; Zarnetske, P.L. Foraging strategy mediates ectotherm predator–Prey responses to climate warming. Ecology 2020, 101, e03146. [Google Scholar] [CrossRef]
- Miller, A.I.; Beckman, L.G. First record of predation on white sturgeon eggs by sympatric fishes. Trans. Am. Fish. Soc. 1996, 125, 338–340. [Google Scholar] [CrossRef]
- Zhu, T.; Zhang, L.; Cheng, Q.; Li, W.; Zhang, T. Primary studies on the morphology and shell strength of four species of gastropods in the Liangzi Lake, Hubei. J. Hydroecol. 2013, 34, 91–95. [Google Scholar] [CrossRef]
- Chase, J.M.; Abrams, P.A.; Grover, J.P.; Diehl, S.; Chesson, P.; Holt, R.D.; Richards, S.A.; Nisbet, R.M.; Case, T.J. The interaction between predation and competition: A review and synthesis. Ecol. Lett. 2002, 5, 302–315. [Google Scholar] [CrossRef]
- Bonsall, M.B.; Hassell, M.P. Apparent competition structures ecological assemblages. Nature 1997, 388, 371–373. [Google Scholar] [CrossRef]
- Nagelkerken, I.; Connell, S.D. Global alteration of ocean ecosystem functioning due to increasing human CO2 emissions. Proc. Natl. Acad. Sci. USA 2015, 112, 13272–13277. [Google Scholar] [CrossRef]
- Entrekin, S.A.; Rosi, E.J.; Tank, J.L.; Hoellein, T.J.; Lamberti, G.A. Quantitative Food Webs Indicate Modest Increases in the Transfer of Allochthonous and Autochthonous C to Macroinvertebrates Following a Large Wood Addition to a Temperate Headwater Stream. Front. Ecol. Evol. 2020, 8, 114. [Google Scholar] [CrossRef]
- Nagelkerken, I.; Goldenberg, S.U.; Ferreira, C.M.; Ullah, H.; Connell, S.D. Trophic pyramids reorganize when food web architecture fails to adjust to ocean change. Science 2020, 369, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Ives, A.R.; Cardinale, B.J. Food-web interactions govern the resistance of communities after non-random extinctions. Nature 2004, 429, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Birk, S.; Chapman, D.; Carvalho, L.; Spears, B.M.; Andersen, H.E.; Argillier, C.; Auer, S.; Baattrup-Pedersen, A.; Banin, L.; Beklioğlu, M.; et al. Impacts of multiple stressors on freshwater biota across spatial scales and ecosystems. Nat. Ecol. Evol. 2020, 4, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Janssen, A.B.G.; Hilt, S.; Kosten, S.; Klein, J.J.M.; Paerl, H.W.; Van de Waal, D.B. Shifting states, shifting services: Linking regime shifts to changes in ecosystem services of shallow lakes. Freshw. Biol. 2021, 66, 1–12. [Google Scholar] [CrossRef]
- Vadeboncoeur, Y.; Moore, M.V.; Stewart, S.D.; Chandra, S.; Atkins, K.S.; Baron, J.S.; Bouma-Gregson, K.; Brothers, S.; Francoeur, S.N.; Genzoli, L.; et al. Blue waters, green bottoms: Benthic filamentous algal blooms are an emerging threat to clear lakes worldwide. Bioscience 2021, 71, 1011–1027. [Google Scholar] [CrossRef]



| Without Fish | With Fish | ||||
|---|---|---|---|---|---|
| Parameters | F/χ2 | p | F/χ2 | p | |
| Measured response variables | Log (Turbidity + 1) | 0.195 | 0.668 | 13.119 | 0.005 |
| Log (Chl a + 1) | 1.289 | 0.283 | 4.840 | 0.053 | |
| Log (Periphyton + 1) | 27.593 | <0.001 | 14.597 | 0.003 | |
| B. aeruginosa | Log (Biomass + 0.01) | 2.029 | 0.185 | 3.725 | 0.082 |
| Density | 2.007 | 0.157 | 3.692 | 0.054 | |
| R. swinhoei | Log (Biomass + 0.01) | 5.910 | 0.035 | 0.112 | 0.745 |
| Density | 4.269 | 0.039 | 0.201 | 0.654 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.; Feng, M.; Zhang, P.; Zhang, H.; Wang, H.; Xu, J.; Zhang, M. Effects of Warming on Aquatic Snails and Periphyton in Freshwater Ecosystems with and without Predation by Common Carp. Water 2023, 15, 153. https://doi.org/10.3390/w15010153
Cheng H, Feng M, Zhang P, Zhang H, Wang H, Xu J, Zhang M. Effects of Warming on Aquatic Snails and Periphyton in Freshwater Ecosystems with and without Predation by Common Carp. Water. 2023; 15(1):153. https://doi.org/10.3390/w15010153
Chicago/Turabian StyleCheng, Haowu, Mingjun Feng, Peiyu Zhang, Huan Zhang, Huan Wang, Jun Xu, and Min Zhang. 2023. "Effects of Warming on Aquatic Snails and Periphyton in Freshwater Ecosystems with and without Predation by Common Carp" Water 15, no. 1: 153. https://doi.org/10.3390/w15010153
APA StyleCheng, H., Feng, M., Zhang, P., Zhang, H., Wang, H., Xu, J., & Zhang, M. (2023). Effects of Warming on Aquatic Snails and Periphyton in Freshwater Ecosystems with and without Predation by Common Carp. Water, 15(1), 153. https://doi.org/10.3390/w15010153