SiO2 Nanoparticles Suspension Exposures with Marine Invertebrates: Genotoxicity Response
Abstract
:1. Introduction
2. Material and Methods
2.1. Preparation of Working Suspensions
2.2. Description of the Experiment
2.3. Silicon Concentration Measurement
2.4. Comet Assay
2.5. Statistical Analysis
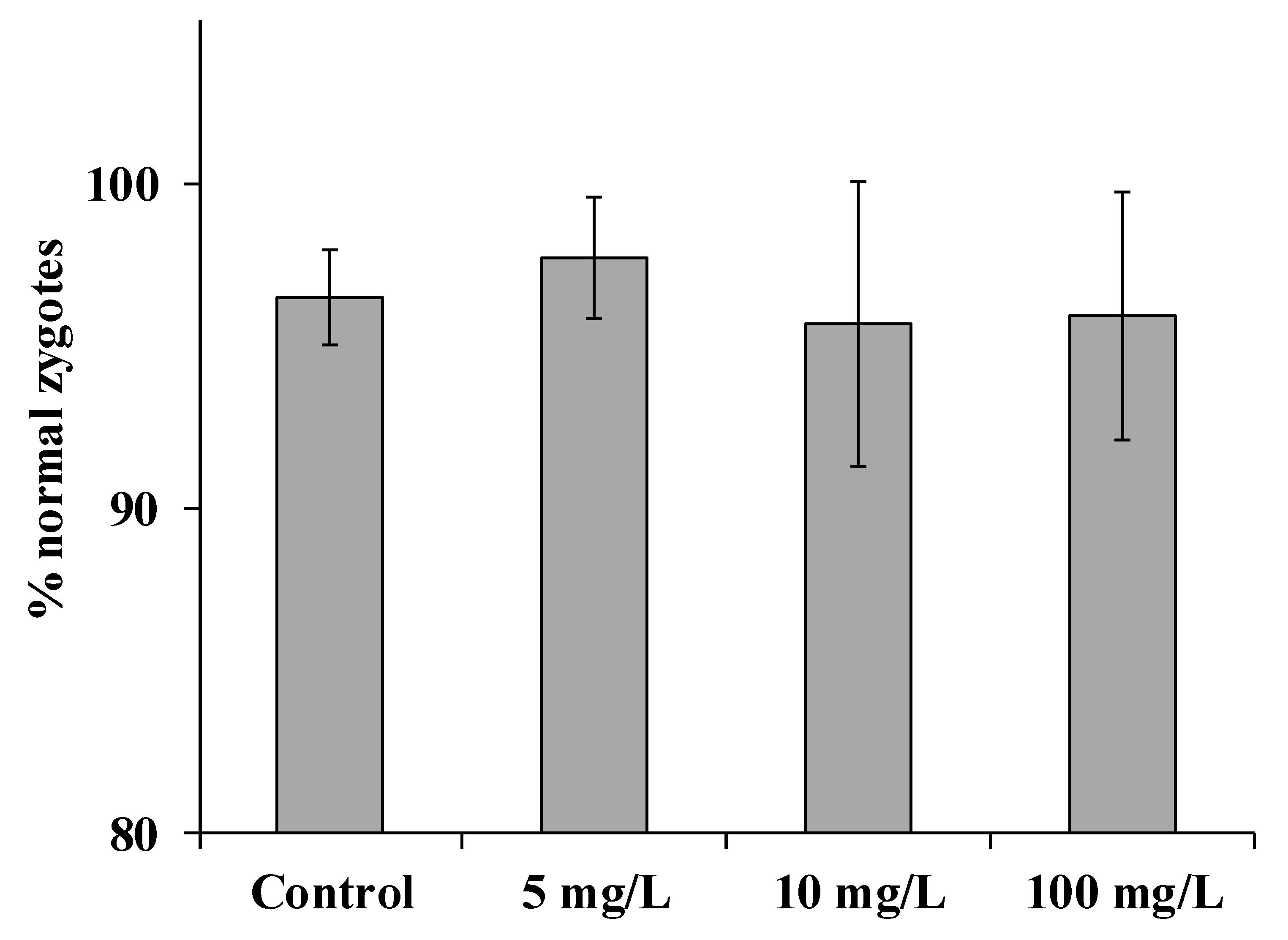
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giese, B.; Klaessig, F.; Park, B.; Kaegi, R.; Steinfeldt, M.; Wigger, H.; von Gleich, A.; Gottschalk, F. Risks, release and concentrations of engineered nanomaterial in the environment. Sci. Rep. 2018, 8, 1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahamed, A.; Liang, L.; Lee, M.Y.; Bobacka, J.; Lisak, G. Too small to matter? Physicochemical transformation and toxicity of engineered nTiO2, nSiO2, nZnO, carbon nanotubes, and nAg. J. Hazard. Mater. 2021, 404 Pt A, 124107. [Google Scholar] [CrossRef]
- D’Mello, S.R.; Cruz, C.N.; Chen, M.L.; Kapoor, M.; Lee, S.L.; Tyne, K.M. The evolving landscape of drug products containing nanomaterials in the United States. Nat. Nanotechnol. 2017, 12, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Fytianos, G.; Rahdar, A.; Kyzas, G.Z. Nanomaterials in cosmetics: Recent updates. Nanomaterials 2020, 10, 979. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, H.; Cui, M.; Huang, R.; Su, R.; Qi, W.; He, Z. Interaction of particles with mucosae and cell membranes. Colloids. Surf. B Biointerfaces 2020, 186, 110657. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, K.; Czajka, M.; Matysiak-Kucharek, M.; Fal, B.; Drop, B.; Meczy’nska-Wielgosz, S.; Sikorska, K.; Kruszewski, M.; Kapka-Skrzypczak, L. Toxicity of metallic nanoparticles in the central nervous system. Nanotechnol. Rev. 2019, 8, 175–200. [Google Scholar] [CrossRef] [Green Version]
- Bongaerts, E.; Nawrot, T.S.; van Pee, T.; Ameloot, M.; Bové, H. Translocation of (ultra) fine particles and nanoparticles across the placenta; a systematic review on the evidence of in vitro, ex vivo, and in vivo studies. Part. Fibre Toxicol. 2020, 17, 56. [Google Scholar] [CrossRef]
- Kaegi, R.; Ulrich, A.; Sinnet, B.; Vonbank, R.; Wichser, A.; Zuleeg, S.; Simmler, H.; Brunner, S.; Vonmont, H.; Burkhardt, M.; et al. Synthetic TiO2 nanoparticle emission from exterior facades into the aquatic environment. Environ. Pollut. 2008, 156, 233–239. [Google Scholar] [CrossRef]
- Gondikas, A.P.; von der Kammer, F.; Reed, R.B.; Wagner, S.; Ranville, J.F.; Hofmann, T. Release of TiO2 nanoparticles from sunscreens into surface waters: A one-year survey at the old Danube recreational Lake. Environ. Sci. Technol. 2014, 48, 5415–5422. [Google Scholar] [CrossRef]
- Markus, A.A.; Krystek, P.; Tromp, P.C.; Parsons, J.R.; Roex, E.W.M.; de Voogt, P.; Laane, R.W.P.M. Determination of metal-based nanoparticles in the river Dommel in the Netherlands via ultrafiltration, HR-ICP-MS and SEM. Sci. Total Environ. 2018, 631–632, 485–495. [Google Scholar] [CrossRef]
- Souza, I.C.; Mendes, V.A.S.; Duarte, I.D.; Rocha, L.D.; Azevedo, V.C.; Matsumoto, S.T.; Elliott, M.; Wunderlin, D.A.; Monferrán, M.V.; Fernandes, M.N. Nanoparticle transport and sequestration: Intracellular titanium dioxide nanoparticles in a neotropical fish. Sci. Total Environ. 2019, 658, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.J.; Tyler, C.R.; Galloway, T.S. Impacts of metal and metal oxide nanoparticles on marine organisms. Environ. Pollut. 2014, 186, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Wei, Y.; Wang, X.; Liu, S.; Zhang, H.; Yuan, S. Mechanistic study of the adsorption and penetration of modified SiO2 nanoparticles on cellular membrane. Chemosphere 2022, 294, 133793. [Google Scholar] [CrossRef]
- Ale, A.; Gutierrez, M.F.; Rossi, A.S.; Bacchetta, C.; Desimone, M.F.; Cazenave, J. Ecotoxicity of silica nanoparticles in aquatic organisms: An updated review. Environ. Toxicol. Pharmacol. 2021, 87, 103689. [Google Scholar] [CrossRef] [PubMed]
- Bundschuh, M.; Filser, J.; Luderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the environment: Where do we come from, where do we go to? Environ. Sci. Eur. 2018, 30, 6. [Google Scholar] [CrossRef] [Green Version]
- Breznan, D.; Das, D.D.; O’Brien, J.S.; MacKinnon-Roy, C.; Nimesh, S.; Vuong, N.Q.; Bernatchez, S.; DeSilva, N.; Hill, M.; Kumarathasan, P.; et al. Differential cytotoxic and inflammatory potency of amorphous silicon dioxide nanoparticles of similar size in multiple cell lines. Nanotoxicology 2017, 11, 223–235. [Google Scholar] [CrossRef] [Green Version]
- Dinnel, P.A.; Stober, Q.J.; Crumley, S.C.; Nakatani, R.E. Development of a sperm cell toxicity test for marine water. Aquat. Toxicol. Haz. Asses. 1982, 1, 82–98. [Google Scholar] [CrossRef]
- Canesi, L.; Fabbri, R.; Gallo, G.; Vallotto, D.; Marcomini, A.; Pojana, G. Biomarkers in Mytilus galloprovincialis exposed to suspensions of selected nanoparticles (Nano carbon black, C60 fullerene, Nano-TiO2, Nano-SiO2). Aquat. Toxicol. 2010, 100, 168–177. [Google Scholar] [CrossRef]
- Browne, M.A.; Dissanayake, A.; Galloway, T.S.; Lowe, D.M.; Thompson, R.C. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ. Sci. Technol. 2008, 42, 5026–5031. [Google Scholar] [CrossRef]
- Al-Sid-Cheikh, M.; Rouleau, C.; Pelletier, E. Tissue distribution and kinetics of dissolved and nanoparticulate silver in Iceland scallop (Chlamys islandica). Mar. Environ. Res. 2013, 86, 21–28. [Google Scholar] [CrossRef]
- Chelomin, V.P.; Slobodskova, V.V.; Zakhartsev, M.K.; Kukla, S.P. Genotoxic potential of copper oxide nanoparticles in the bivalve mollusk Mytilus trossulus. J. Ocean Univ. China 2017, 16, 339–345. [Google Scholar] [CrossRef]
- Kukla, S.; Slobodskova, V.; Mazur, A.; Chelomin, V.; Kamenev, Y. Genotoxic testing of titanium dioxide nanoparticles in Far Eastern mussels, Mytilus trossulus. Pollution 2021, 7, 129–140. [Google Scholar] [CrossRef]
- Yang, S.; Ye, R.; Han, B.; Wei, C.; Yang, X. Ecotoxicological effect of nano-silicon dioxide particles on Daphnia magna. Integr. Ferroelectr. 2014, 154, 64–72. [Google Scholar] [CrossRef]
- Pham, D.H.; Roo, B.D.; Nguyen, X.B.; Vervaele, M.; Kecskés, A.; Ny, A.; Copmans, D.; Vriens, H.; Locquet, J.P.; Hoet, P.; et al. Use of zebrafish larvae as a multi-endpoint platform to characterize the toxicity profile of silica nanoparticles. Sci. Rep. 2016, 6, 37145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eom, H.J.; Choi, J. Clathrin-mediated endocytosis is involved in uptake and toxicity of silica nanoparticles in Caenohabditis elegans. Chem. Biol. Interact. 2019, 311, 108774. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.N. Ecotoxicological applications and significance of the comet assay. Mutagenesis 2008, 23, 207–221. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.A.; Fernandez-Triana, J.; Roughley, R.; Hebert, D.N. DNA barcode accumulation curves for understudied taxa and areas. Mol. Ecol. Resour. 2009, 9, 208–216. [Google Scholar] [CrossRef]
- Pikula, K.; Chaika, V.; Zakharenko, A.; Savelyeva, A.; Kirsanova, I.; Anisimova, A.; Golokhvast, K. Toxicity of carbon, silicon, and metal-based nanoparticles to the hemocytes of three marine bivalves. Animals 2020, 10, 827. [Google Scholar] [CrossRef]
- Canesi, L.; Ciacci, C.; Vallotto, D.; Gallo, G.; Marcomini, A.; Pojana, G. In Vitro effects of suspensions of selected nanoparticles (C60 fullerene, TiO2, SiO2) on Mytilus hemocytes. Aquat. Toxicol. 2010, 96, 151–158. [Google Scholar] [CrossRef]
- Gambardella, C.; Ferrando, S.; Gatti, A.M.; Cataldi, E.; Ramoino, P.; Aluigi, M.G.; Faimali, M.; Diaspro, A.; Falugi, C. Review: Morphofunctional and biochemical markers of stress in sea urchin life stages exposed to engineered nanoparticles. Environ. Toxicol. 2016, 11, 1552–1562. [Google Scholar] [CrossRef]
- Mahaye, N.; Thwala, M.; Cowan, D.A.; Musee, N. Genotoxicity of metal based engineered nanoparticles in aquatic organisms: A review. Mutat. Res. 2017, 773, 134–160. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Yuvaraju, P.; Beegam, S.; Yasin, J.; Kazzam, E.E.; Ali, B.H. Oxidative stress, inflammation, and DNA damage in multiple organs of mice acutely exposed to amorphous silica nanoparticles. Int. J. Nanomed. 2016, 11, 919–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maser, E.; Schulz, M.; Sauer, U.G.; Wiemann, M.; Ma-Hock, L.; Wohlleben, W.; Hartwig, A.; Landsiedel, R. In Vitro and in vivo genotoxicity investigations of differently sized amorphous SiO2 nanomaterials. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015, 794, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Li, Q.; Jiang, L.; Zou, Y.; Duan, J.; Sun, Z. Genome-Wide transcriptional analysis of silica nanoparticle-induced toxicity in zebrafish embryos. Toxicol. Res. 2016, 5, 609–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrick, A.; Mouneyrac, C.; Manier, N.; De Lantivy, L.; Jrad, N.; Châtel, A. Towards the development of a high throughput screening approach for Mytilus edulis hemocytes: A case study on silicon-based nanomaterials. Mar. Environ. Res. 2018, 142, 306–318. [Google Scholar] [CrossRef]
- Tacconi, S.; Augello, S.; Persano, F.; Sbarigia, C.; Carata, E.; Leporatti, S.; Fidaleo, M.; Dini, L. Amino-Functionalized Mesoporous silica nanoparticles (NH 2-MSiNPs) impair the embryonic development of the sea urchin Paracentrotus lividus. Environ. Toxicol. Pharmacol. 2022, 95, 103956. [Google Scholar] [CrossRef] [PubMed]
- Magdolenova, Z.; Collins, A.; Kumar, A.; Dhawan, A.; Stone, V.; Dusinska, M. Mechanisms of genotoxicity. A review of in vitro and in vivo studies with engineered nanoparticles. Nanotoxicology 2014, 8, 233–278. [Google Scholar] [CrossRef]
- Chang, Y.N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef] [Green Version]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed. Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; von Mikecz, A. Formation of nucleoplasmic protein aggregates impairs nuclear function in response to SiO2 nanoparticles. Exp. Cell Res. 2005, 305, 51–62. [Google Scholar] [CrossRef]
- Nabeshi, H.; Yoshikawa, T.; Matsuyama, K.; Nakazato, Y.; Tochigi, S.; Kondoh, S.; Hirai, T.; Akase, T.; Nagano, K.; Abe, Y.; et al. Amorphous nanosilica induce endocytosis-dependent ROS generation and DNA damage in human keratinocytes. Part. Fibre Toxicol. 2011, 8, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, C.; Galloway, T.S. Genotoxic damage in Polychaetes: A study of species and cell-type sensitivities. Mutat. Res. Genet. Toxicol. Environ. Mutat. 2008, 654, 69–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devaux, A.; Fiat, L.; Gillet, C.; Bony, S. Reproduction impairment following paternal genotoxin exposure in brown trout (Salmo trutta) and Arctic charr (Salvelinus alpinus). Aquat. Toxicol. 2011, 101, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Kazama, M.; Hino, A. Sea urchin spermatozoa generate at least two reactive oxygen species; the type of reactive oxygen species changes under different conditions. Mol. Reprod. Dev. 2012, 79, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Manfra, L.; Boni, R.; Rotini, A.; Migliore, L.; Tosti, E. Cytotoxicity and genotoxicity of CuO nanoparticles in sea urchin spermatozoa through oxidative stress. Environ. Int. 2018, 118, 325–333. [Google Scholar] [CrossRef]
- Kukla, S.P.; Chelomin, V.P.; Mazur, A.A.; Slobodskova, V.V. Zinc oxide nanoparticles induce DNA damage in sand dollar Scaphechinus mirabilis sperm. Toxics 2022, 10, 348. [Google Scholar] [CrossRef]
- Mazur, A.A.; Chelomin, V.P.; Zhuravel, E.V.; Kukla, S.P.; Slobodskova, V.V.; Dovzhenko, N.V. Genotoxicity of polystyrene (PS) microspheres in short-term exposure to gametes of the sand dollar Scaphechinus mirabilis (Agassiz, 1864) (Echinodermata, Echinoidea). J. Mar. Sci. Eng. 2021, 9, 1088. [Google Scholar] [CrossRef]
- Gambardella, C.; Morgana, S.; Bari, G.D.; Ramoino, P.; Bramini, M.; Diaspro, A.; Falugi, C.; Faimali, M. Multidisciplinary screening of toxicity induced by silica nanoparticles during sea urchin development. Chemosphere 2015, 139, 486–495. [Google Scholar] [CrossRef]
- Burić, P.; Jakšić, Ž.; Štajner, L.; Dutour Sikirić, M.; Jurašin, D.; Cascio, C.; Calzolai, L.; Lyons, D.M. Effect of silver nanoparticles on Mediterranean sea urchin embryonal development is species specific and depends on moment of first exposure. Mar. Environ. Res. 2015, 111, 50–59. [Google Scholar] [CrossRef] [Green Version]
- Mazur, A.A.; Zhuravel, E.V.; Slobodskova, V.V.; Mazur, M.A.; Kukla, S.P.; Chelomin, V.P. Waterborne exposure of adult sand dollar, Scaphechinus mirabilis (Agassiz, 1864), to zinc ions and zinc oxide nanoparticles affects early development of its offspring. Water Air Soil Pollut. 2020, 231, 115. [Google Scholar] [CrossRef]
- Kukla, S.P.; Slobodskova, V.V.; Zhuravel, E.V.; Mazur, A.A.; Chelomin, V.P. Exposure of adult sand dollars (Scaphechinus mirabilis) (Agassiz, 1864) to copper oxide nanoparticles induces gamete DNA damage. Environ. Sci. Pollut. Res. Int. 2022, 26, 39451–39460. [Google Scholar] [CrossRef] [PubMed]

| Size, nm | Hydrodynamic Size, nm | Purity, % | Total Surface Area, m2/g | Zetta Potential, mV |
|---|---|---|---|---|
| 20 ± 4.6 | 576 ± 104 | 95.5 | 613 ± 47 | −11.5 ± 0.7 |
| Parameter | Control Group | Experimental Group |
|---|---|---|
| Silicon concentration, µg/gdw | n.d. | 11.04 ± 0.3 * |
| DNA damage, %DNA in tail | 7.41 ± 0.33 | 19.28 ± 0.86 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kukla, S.P.; Chelomin, V.P.; Mazur, A.A.; Slobodskova, V.V.; Mazur, M.A. SiO2 Nanoparticles Suspension Exposures with Marine Invertebrates: Genotoxicity Response. Water 2023, 15, 162. https://doi.org/10.3390/w15010162
Kukla SP, Chelomin VP, Mazur AA, Slobodskova VV, Mazur MA. SiO2 Nanoparticles Suspension Exposures with Marine Invertebrates: Genotoxicity Response. Water. 2023; 15(1):162. https://doi.org/10.3390/w15010162
Chicago/Turabian StyleKukla, Sergey Petrovich, Victor Pavlovich Chelomin, Andrey Alexandrovich Mazur, Valentina Vladimirovna Slobodskova, and Marina Alexandrovna Mazur. 2023. "SiO2 Nanoparticles Suspension Exposures with Marine Invertebrates: Genotoxicity Response" Water 15, no. 1: 162. https://doi.org/10.3390/w15010162
APA StyleKukla, S. P., Chelomin, V. P., Mazur, A. A., Slobodskova, V. V., & Mazur, M. A. (2023). SiO2 Nanoparticles Suspension Exposures with Marine Invertebrates: Genotoxicity Response. Water, 15(1), 162. https://doi.org/10.3390/w15010162





