Microplastics in the Ecosystem: An Overview on Detection, Removal, Toxicity Assessment, and Control Release
Abstract
:1. Introduction
1.1. Properties of MPs
1.2. Primary and Secondary MPs
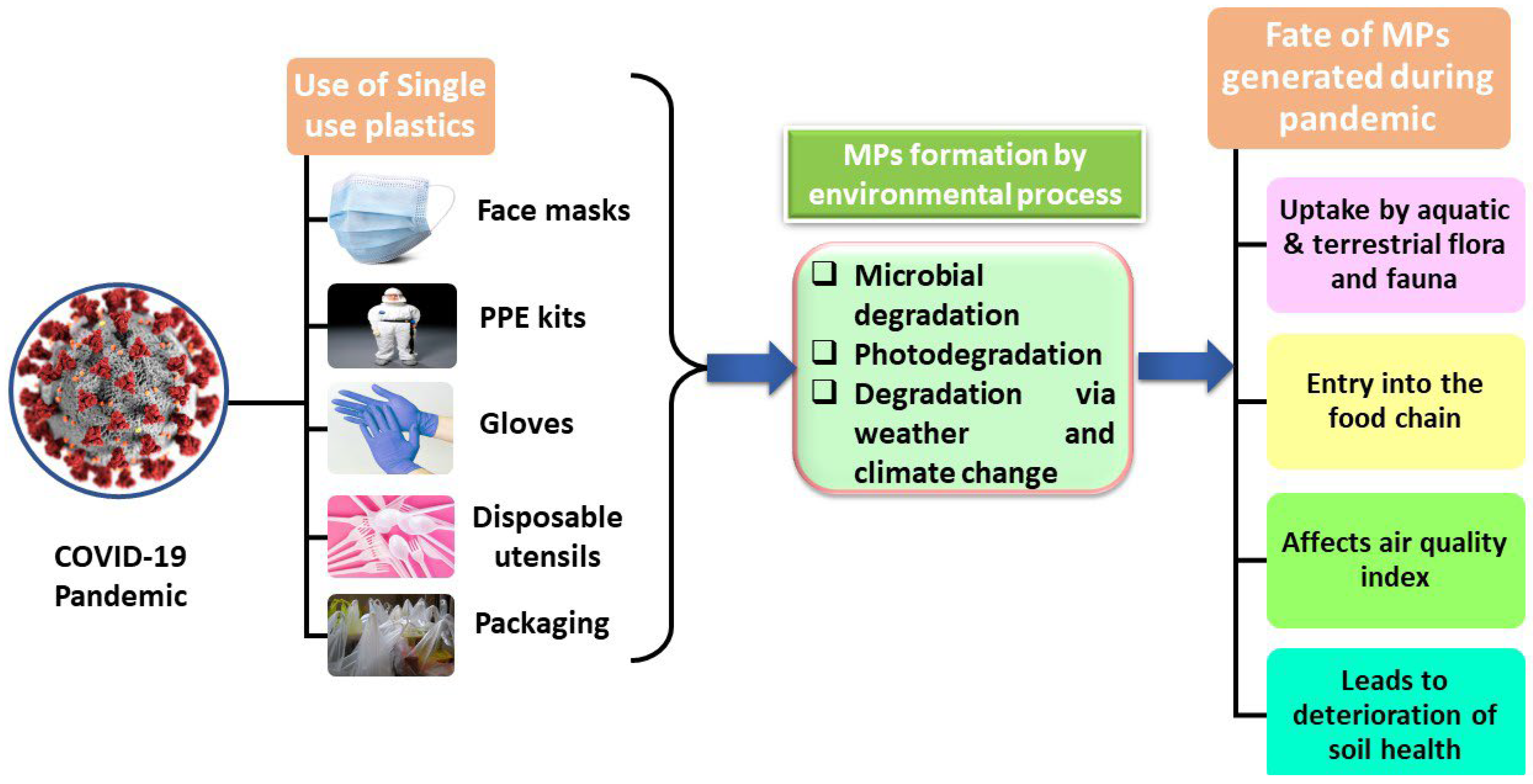
1.3. Impacts of COVID-19 on Release of MPs in the Environment
2. Detection and Identification of MPs
2.1. Identification of Morphology
2.2. Identification of Chemical Structure and Composition
2.3. Identification of Thermal Properties and Chemical Bonding
3. Removal Methods
- (i)
- Filtration and segregation
- (ii)
- Surface adhesion and growth
- (iii)
- Deterioration
- Filtration and segregation methods: These methods involve the separation of MPs from contaminated water by physical barriers such as membranes and filter mechanisms. These physical barriers only allow the passage of liquids, thereby separating microplastics from aqueous media. However, these methods are often found to be ineffective in the removal of microplastics from sludge waste with higher viscosities. In addition, filtration methods require intensive manpower and require the movement of enormous quantities of water for the separation of micro- and nano-sized microplastics present in minimal concentrations. By using these methods, we only obtain information about the quantification of separated microplastics and do not gather any information about microplastic pollutant type and structure. To obtain detailed information about the type and structure of MPs, we need to adopt other characterisation techniques [47].
- Surface adhesion and growth methods: This method involves the capture and attachment of MPs onto the surface of the added materials (e.g., coagulants, disinfectants, oxidants, surfactants, etc.), causing them to form macrostructures such as aggregates, facilitating their easy removal. This methodology utilizes techniques such as coagulation, flocculation and sedimentation (CFS), adsorption, and ion exchange. Unlike the filtration and segregation methods, these methods are efficient, easy to handle and monitor, and are even helpful in the removal of other pollutants. However, due to a lack of information, they are still only performed at the pilot scale instead of large-scale operations. However, these methods possess certain limitations, i.e., they are often time-intensive and ineffective for the uptake of smooth, small-sized microplastics due to a lack of sufficient surface area to either adhere to the surface of the added materials or form flocs [48].
- Deterioration methods: Another method used for the separation of microplastics is the deterioration method which makes use of the action of external factors such as radiation, heat, and microorganisms to bring about changes in the physiological structure of MPs and break them down into simpler molecules such as CO2, H2O, H2S, methane, etc. Photocatalytic, thermal and microbial degradation fall under this category. Degradation methods are one of the most efficient methods for combating MP waste but these methods are not much explored and still need further in-depth studies for understanding the detailed mechanisms involved in degradation to fully exploit their potential. The breakdown capacities efficiencies can also be enhanced which can ultimately lead to a reduced degradation time span [49]. Table 2 presents the advantages and disadvantages of the above-mentioned removal methods.
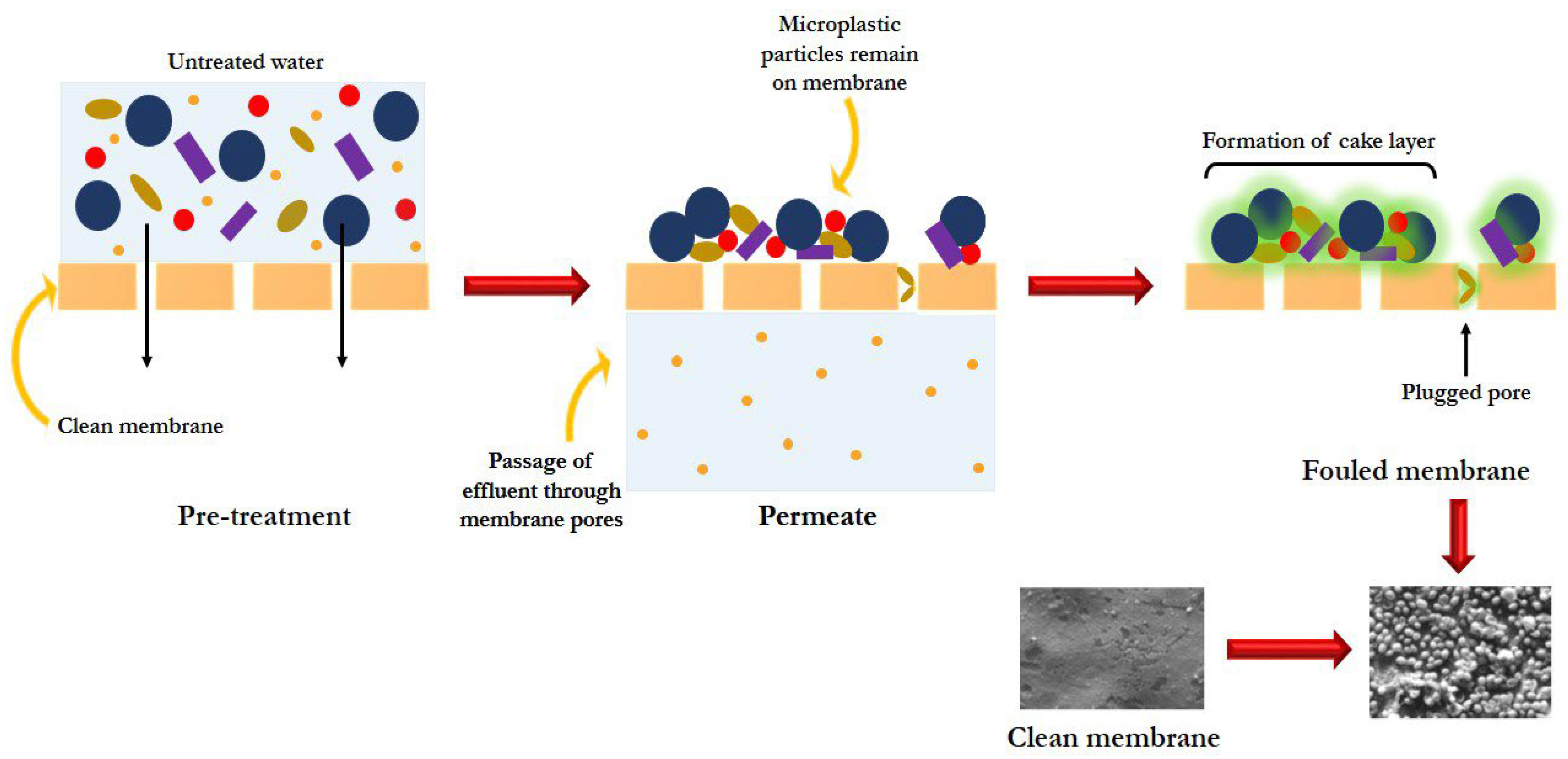
3.1. Membrane Filtration
3.2. Adsorption
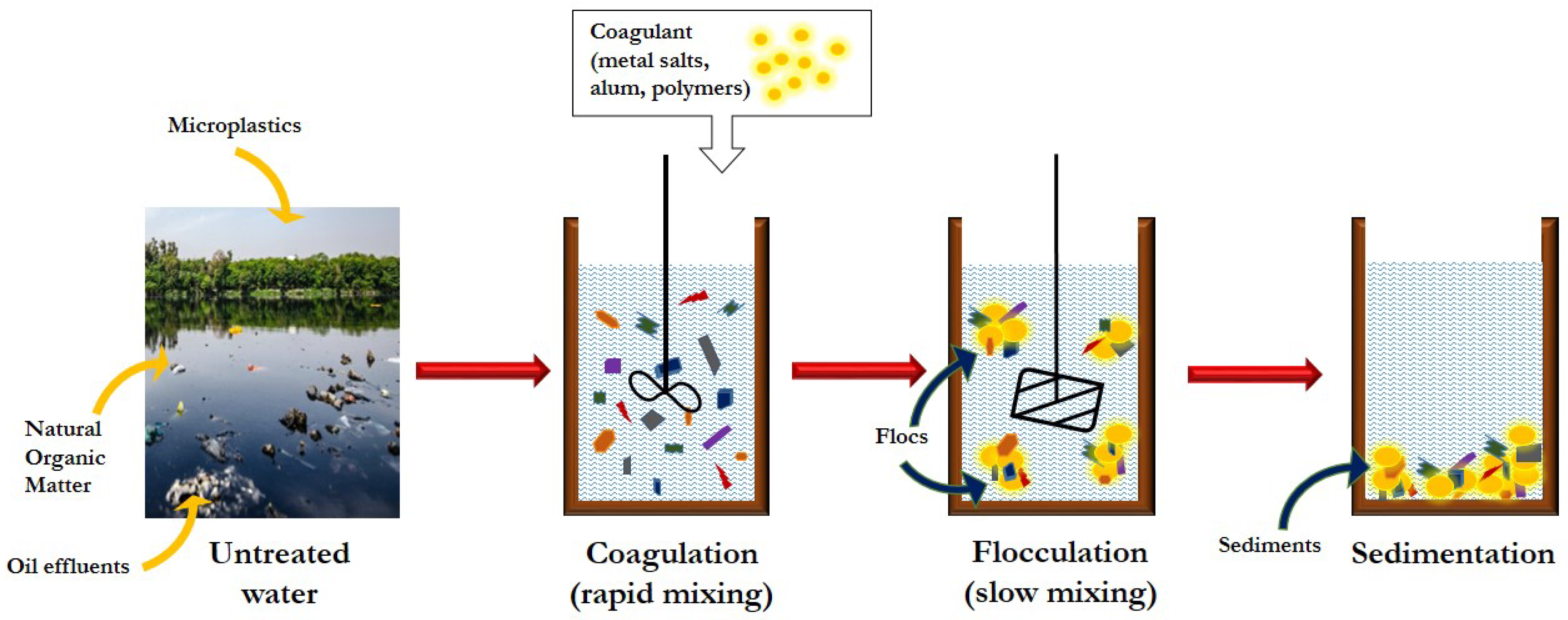
3.3. Coagulation, Flocculation and Sedimentation (CFS)
3.4. Biological Degradation
3.5. MP Shape, Size, and Polymer Type and Their Impact on Efficiency of Removal Methods
3.5.1. Impact of Shape of MP
3.5.2. Impact of Size of MP
3.5.3. Impact of Polymer Type of MP
4. Accumulation of MPs in the Ecosystem and Their Toxicity Assessment
4.1. Impacts of Microplastics on Human Health
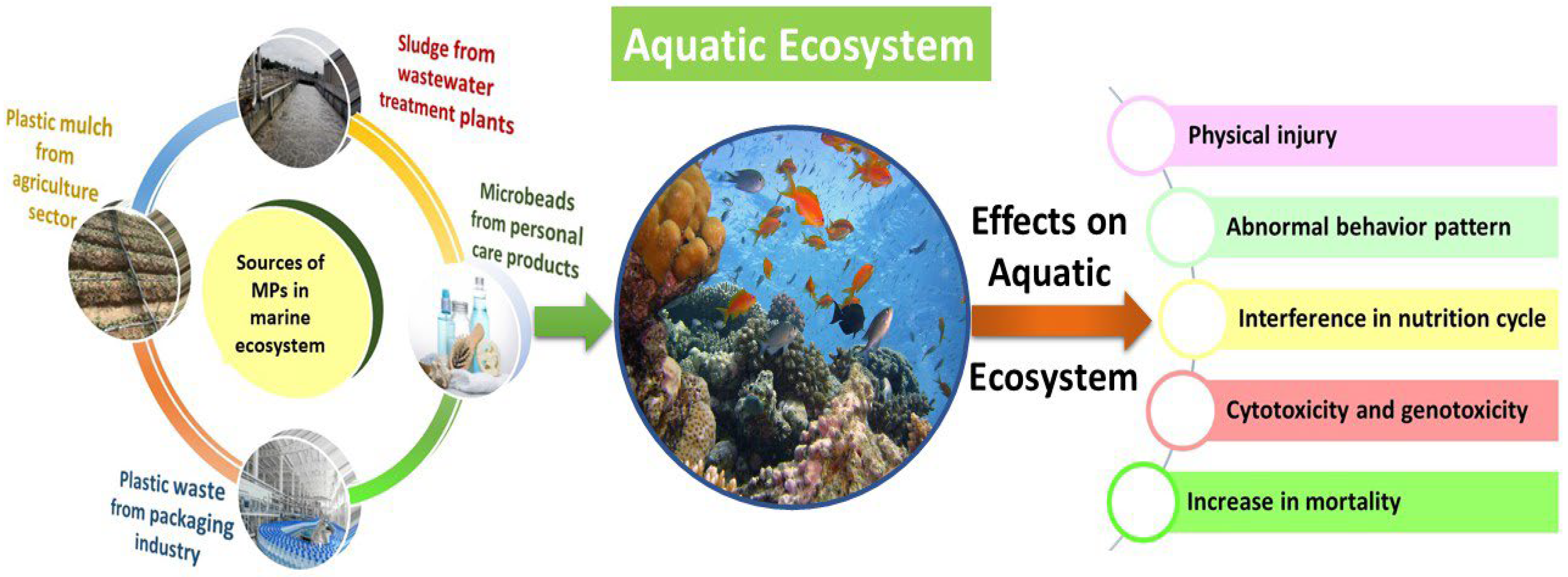
4.2. Impacts of MPs on Aquatic Environments
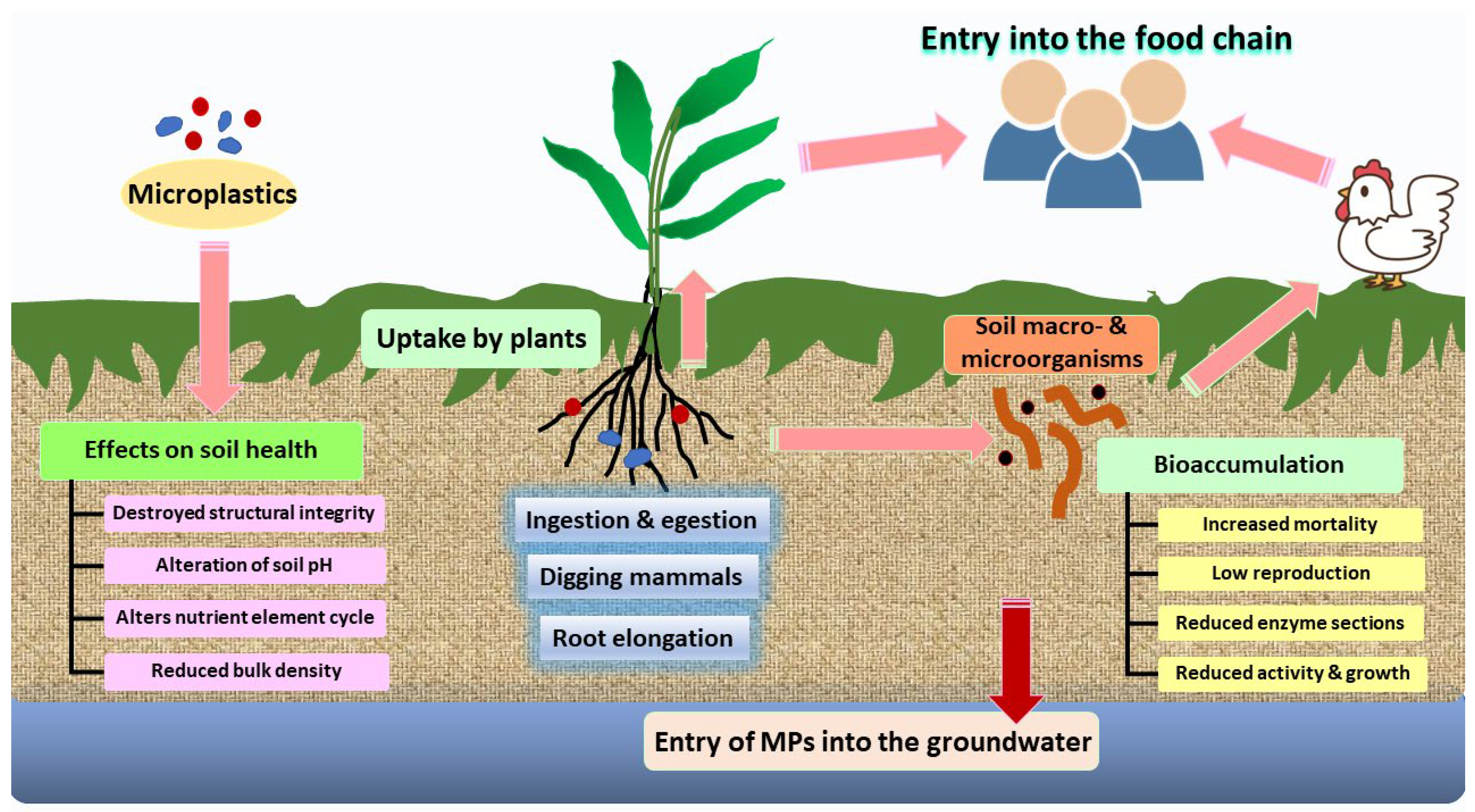
4.3. Impacts of MPs on Soil
5. Protocols and Existing Infrastructure in Place for Controlling MP Release
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, S.; Ma, J.; Ji, R.; Pan, K.; Miao, A.J. Microplastics in Aquatic Environments: Occurrence, Accumulation, and Biological Effects. Sci. Total Environ. 2020, 703, 134699. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Browne, M.A.; Halpern, B.S.; Hentschel, B.T.; Hoh, E.; Karapanagioti, H.K.; Rios-Mendoza, L.M.; Takada, H.; Teh, S.; Thompson, R.C. Classify Plastic Waste as Hazardous. Nature 2013, 494, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Faunce, T.; Kolodziejczyk, B. Nanowaste: Need for Disposal and Recycling Standards. G20 Insights 2017, 148, 202–213. [Google Scholar]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gall, S.C.; Thompson, R.C. The Impact of Debris on Marine Life. Mar. Pollut. Bull. 2015, 92, 170–179. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Alsabri, A.; Tahir, F.; Al-Ghamdi, S.G. Environmental Impacts of Polypropylene (PP) Production and Prospects of Its Recycling in the GCC Region. Mater. Today Proc. 2022, 56, 2245–2251. [Google Scholar] [CrossRef]
- Anderson, J.C.; Park, B.J.; Palace, V.P. Microplastics in Aquatic Environments: Implications for Canadian Ecosystems. Environ. Pollut. 2016, 218, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Mangaraj, S.; Goswami, T.K.; Mahajan, P.V. Applications of Plastic Films for Modified Atmosphere Packaging of Fruits and Vegetables: A Review. Food Eng. Rev. 2009, 1, 133–158. [Google Scholar] [CrossRef]
- Bach, C.; Dauchy, X.; Severin, I.; Munoz, J.F.; Etienne, S.; Chagnon, M.C. Effect of Temperature on the Release of Intentionally and Non-Intentionally Added Substances from Polyethylene Terephthalate (PET) Bottles into Water: Chemical Analysis and Potential Toxicity. Food Chem. 2013, 139, 672–680. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, D.; Han, S.; Choi, J.; Hong, J. An Assessment of the Toxicity of Polypropylene Microplastics in Human Derived Cells. Sci. Total Environ. 2019, 684, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, M.S.; Huyghebaert, A. Polystyrene Cups and Containers: Styrene Migration. Food Addit. Contam. 1998, 15, 592–599. [Google Scholar] [CrossRef]
- Nakamura, S.; Nakajima, K.; Yoshizawa, Y.; Matsubae-Yokoyama, K.; Nagasaka, T. Analyzing Polyvinyl Chloride in Japan with The Waste Input-Output Material Flow Analysis Model. J. Ind. Ecol. 2009, 13, 706–717. [Google Scholar] [CrossRef]
- Das, A.; Mahanwar, P. A Brief Discussion on Advances in Polyurethane Applications. Adv. Ind. Eng. Polym. Res. 2020, 3, 93–101. [Google Scholar] [CrossRef]
- Hearle, J.W.S. Textile Fibers: A Comparative Overview. Encycl. Mater. Sci. Technol. 2001, 2001, 9100–9116. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and Fragmentation of Plastic Debris in Global Environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [Green Version]
- Andrady, A.L. The Plastic in Microplastics: A Review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef]
- Lee, K.W.; Shim, W.J.; Kwon, O.Y.; Kang, J.H. Size-Dependent Effects of Micro Polystyrene Particles in the Marine Copepod Tigriopus Japonicus. Environ. Sci. Technol. 2013, 47, 11278–11283. [Google Scholar] [CrossRef]
- Au, S.Y.; Bruce, T.F.; Bridges, W.C.; Klaine, S.J. Responses of Hyalella Azteca to Acute and Chronic Microplastic Exposures. Environ. Toxicol. Chem. 2015, 34, 2564–2572. [Google Scholar] [CrossRef]
- Llorca, M.; Schirinzi, G.; Martínez, M.; Barceló, D.; Farré, M. Adsorption of Perfluoroalkyl Substances on Microplastics under Environmental Conditions. Environ. Pollut. 2018, 235, 680–691. [Google Scholar] [CrossRef]
- Chen, D.R.; Bei, J.Z.; Wang, S.G. Polycaprolactone Microparticles and Their Biodegradation. Polym. Degrad. Stab. 2000, 67, 455–459. [Google Scholar] [CrossRef]
- Stolte, A.; Forster, S.; Gerdts, G.; Schubert, H. Microplastic Concentrations in Beach Sediments along the German Baltic Coast. Mar. Pollut. Bull. 2015, 99, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Friot, D.; Boucher, J. Primary Microplastics in the Oceans: Aglobal Evaluation of Sources; IUCN: Gland, Switzerland, 2017; ISBN 0231137079. [Google Scholar]
- An, L.; Liu, Q.; Deng, Y.; Wu, W.; Gao, Y.; Ling, W. Sources of Microplastic in the Environment. Handb. Environ. Chem. 2020, 95, 143–159. [Google Scholar] [CrossRef]
- Karlsson, T.M.; Arneborg, L.; Broström, G.; Almroth, B.C.; Gipperth, L.; Hassellöv, M. The Unaccountability Case of Plastic Pellet Pollution. Mar. Pollut. Bull. 2018, 129, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Napper, I.E.; Bakir, A.; Rowland, S.J.; Thompson, R.C. Characterisation, Quantity and Sorptive Properties of Microplastics Extracted from Cosmetics. Mar. Pollut. Bull. 2015, 99, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Atugoda, T.; Vithanage, M.; Wijesekara, H.; Bolan, N.; Sarmah, A.K.; Bank, M.S.; You, S.; Ok, Y.S. Interactions between Microplastics, Pharmaceuticals and Personal Care Products: Implications for Vector Transport. Environ. Int. 2021, 149, 106367. [Google Scholar] [CrossRef]
- Gouin, T.; Avalos, J.; Brunning, I.; Brzuska, K.; De Graaf, J.; Kaumanns, J.; Koning, T.; Meyberg, M.; Rettinger, K.; Schlatter, H.; et al. Use of Micro-Plastic Beads in Cosmetic Products in Europe and Their Estimated Emissions to the North Sea Environment. SOFW J. 2015, 141, 40–46. [Google Scholar]
- Nagaraj, V.; Skillman, L.; Li, D.; Ho, G. Review—Bacteria and Their Extracellular Polymeric Substances Causing Biofouling on Seawater Reverse Osmosis Desalination Membranes. J. Environ. Manag. 2018, 223, 586–599. [Google Scholar] [CrossRef]
- Montarsolo, A.; Mossotti, R.; Patrucco, A.; Caringella, R.; Zoccola, M.; Pozzo, P.D.; Tonin, C. Study on the Microplastics Release from Fishing Nets. Eur. Phys. J. Plus 2018, 133, 494. [Google Scholar] [CrossRef]
- Napper, I.E.; Thompson, R.C. Release of Synthetic Microplastic Plastic Fibres from Domestic Washing Machines: Effects of Fabric Type and Washing Conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Jan Kole, P.; Löhr, A.J.; Van Belleghem, F.G.A.J.; Ragas, A.M.J. Wear and Tear of Tyres: A Stealthy Source of Microplastics in the Environment. Int. J. Environ. Res. Public Health 2017, 14, 1265. [Google Scholar] [CrossRef] [PubMed]
- Piret, J.; Boivin, G. Pandemics Throughout History. Front. Microbiol. 2021, 11, 631736. [Google Scholar] [CrossRef] [PubMed]
- Shams, M.; Alam, I.; Mahbub, M.S. Plastic Pollution During COVID-19: Plastic Waste Directives and Its Long-Term Impact on The Environment. Environ. Adv. 2021, 5, 100119. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Silva, A.L.P.; Walker, T.R.; Duarte, A.C.; Rocha-Santos, T. COVID-19 Pandemic Repercussions on the Use and Management of Plastics. Environ. Sci. Technol. 2020, 54, 7760–7765. [Google Scholar] [CrossRef] [PubMed]
- Benson, N.U.; Bassey, D.E.; Palanisami, T. COVID Pollution: Impact of COVID-19 Pandemic on Global Plastic Waste Footprint. Heliyon 2021, 7, e06343. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, P.; Schartup, A.T.; Zhang, Y. Plastic Waste Release Caused by COVID-19 and Its Fate in the Global Ocean. Proc. Natl. Acad. Sci. USA 2021, 118, e2111530118. [Google Scholar] [CrossRef]
- Morgana, S.; Casentini, B.; Amalfitano, S. Uncovering the Release of Micro/Nanoplastics from Disposable Face Masks at Times of COVID-19. J. Hazard. Mater. 2021, 419, 126507. [Google Scholar] [CrossRef]
- Okuku, E.; Kiteresi, L.; Owato, G.; Otieno, K.; Mwalugha, C.; Mbuche, M.; Gwada, B.; Nelson, A.; Chepkemboi, P.; Achieng, Q.; et al. The Impacts of COVID-19 Pandemic on Marine Litter Pollution along the Kenyan Coast: A Synthesis after 100 Days Following the First Reported Case in Kenya. Mar. Pollut. Bull. 2021, 162, 111840. [Google Scholar] [CrossRef]
- Haque, F.; Fan, C. Prospect of Microplastic Pollution Control under the “New Normal” Concept beyond COVID-19 Pandemic. J. Clean. Prod. 2022, 367, 133027. [Google Scholar] [CrossRef]
- Poerio, T.; Piacentini, E.; Mazzei, R. Membrane Processes for Microplastic Removal. Molecules 2019, 24, 4148. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Hernández-Crespo, C.; Du, B.; Van Hulle, S.W.H.; Rousseau, D.P.L. Fate and Removal of Microplastics in Unplanted Lab-Scale Vertical Flow Constructed Wetlands. Sci. Total Environ. 2021, 778, 146152. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, M.; Farner, J.M.; Hernandez, L.M.; Tufenkji, N. Understanding and Improving Microplastic Removal during Water Treatment: Impact of Coagulation and Flocculation. Environ. Sci. Technol. 2020, 54, 8719–8727. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.H.; Liang, Y.; Kim, M.; Byun, J.; Choi, H. Microplastics with Adsorbed Contaminants: Mechanisms and Treatment. Environ. Chall. 2021, 3, 100042. [Google Scholar] [CrossRef]
- Lee, Q.Y.; Li, H. Photocatalytic Degradation of Plastic Waste: A Mini Review. Micromachines 2021, 12, 907. [Google Scholar] [CrossRef] [PubMed]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and Enzymatic Degradation of Synthetic Plastics. Front. Microbiol. 2020, 11, 580709. [Google Scholar] [CrossRef]
- Dey, T.K.; Uddin, M.E.; Jamal, M. Detection and Removal of Microplastics in Wastewater: Evolution and Impact. Environ. Sci. Pollut. Res. 2021, 28, 16925–16947. [Google Scholar] [CrossRef]
- Kim, K.T.; Park, S. Enhancing Microplastics Removal from Wastewater Using Electro-Coagulation and Granule-Activated Carbon with Thermal Regeneration. Processes 2021, 9, 617. [Google Scholar] [CrossRef]
- Arpia, A.A.; Chen, W.H.; Ubando, A.T.; Naqvi, S.R.; Culaba, A.B. Microplastic Degradation as a Sustainable Concurrent Approach for Producing Biofuel and Obliterating Hazardous Environmental Effects: A State-of-the-Art Review. J. Hazard. Mater. 2021, 418, 126381. [Google Scholar] [CrossRef]
- Ma, B.; Xue, W.; Hu, C.; Liu, H.; Qu, J.; Li, L. Characteristics of Microplastic Removal via Coagulation and Ultrafiltration during Drinking Water Treatment. Chem. Eng. J. 2019, 359, 159–167. [Google Scholar] [CrossRef]
- Barbier, J.S.; Dris, R.; Lecarpentier, C.; Raymond, V.; Delabre, K.; Thibert, S.; Tassin, B.; Gasperi, J. Microplastic Occurrence after Conventional and Nanofiltration Processes at Drinking Water Treatment Plants: Preliminary Results. Front. Water 2022, 2022, 4. [Google Scholar] [CrossRef]
- Yaranal, N.A.; Subbiah, S.; Mohanty, K. Identification, Extraction of Microplastics from Edible Salts and Its Removal from Contaminated Seawater. Environ. Technol. Innov. 2021, 21, 101253. [Google Scholar] [CrossRef]
- Hidayaturrahman, H.; Lee, T.G. A Study on Characteristics of Microplastic in Wastewater of South Korea: Identification, Quantification, and Fate of Microplastics during Treatment Process. Mar. Pollut. Bull. 2019, 146, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Tadsuwan, K.; Babel, S. Microplastic Abundance and Removal via an Ultrafiltration System Coupled to a Conventional Municipal Wastewater Treatment Plant in Thailand. J. Environ. Chem. Eng. 2022, 10, 107142. [Google Scholar] [CrossRef]
- Li, L.; Xu, G.; Yu, H.; Xing, J. Dynamic Membrane for Micro-Particle Removal in Wastewater Treatment: Performance and Influencing Factors. Sci. Total Environ. 2018, 627, 332–340. [Google Scholar] [CrossRef]
- Xing, J.; Wang, H.; Cheng, X.; Tang, X.; Luo, X.; Wang, J.; Wang, T.; Li, G.; Liang, H. Application of Low-Dosage UV/Chlorine Pre-Oxidation for Mitigating Ultrafiltration (UF) Membrane Fouling in Natural Surface Water Treatment. Chem. Eng. J. 2018, 344, 62–70. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D.L. Wastewater Treatment Plants as a Pathway for Microplastics: Development of a New Approach to Sample Wastewater-Based Microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef]
- Ben Hassan, I.; Ennouri, M.; Lafforgue, C.; Schmitz, P.; Ayadi, A. Experimental Study of Membrane Fouling during Crossflow Microfiltration of Yeast and Bacteria Suspensions: Towards an Analysis at the Microscopic Level. Membranes 2013, 3, 44–68. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Zhang, S.; Su, Y.; Wu, D.; Zhao, Y.; Xie, B. Removal of Microplastics from Aqueous Solutions by Magnetic Carbon Nanotubes. Chem. Eng. J. 2021, 406, 126804. [Google Scholar] [CrossRef]
- Siipola, V.; Pflugmacher, S.; Romar, H.; Wendling, L.; Koukkari, P. Low-Cost Biochar Adsorbents for Water Purification Including Microplastics Removal. Appl. Sci. 2020, 10, 788. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Wang, Z.; Chen, L.; Li, F. Fabrication of Robust and Compressive Chitin and Graphene Oxide Sponges for Removal of Microplastics with Different Functional Groups. Chem. Eng. J. 2020, 393, 124796. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Liu, Z.; Tian, S.; Lu, J.; Mu, R.; Yuan, H. Coagulation Removal of Microplastics from Wastewater by Magnetic Magnesium Hydroxide and PAM. J. Water Process Eng. 2021, 43, 102250. [Google Scholar] [CrossRef]
- Kumar, A.; Upadhyay, P.; Prajapati, S.K. Impact of microplastics on riverine greenhouse gas emissions: A view point. Environ. Sci. Pollut. Res. 2022, 2022, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Danso, D.; Chow, J.; Streita, W.R. Plastics: Environmental and Biotechnological Perspectives on Microbial Degradation. Appl. Environ. Microbiol. 2019, 85, AEM-01095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, B.Y.; Chen, Z.; Chen, J.; Yu, H.; Zhou, X.; Criddle, C.S.; Wu, W.M.; Zhang, Y. Biodegradation of Polyvinyl Chloride (PVC) in Tenebrio Molitor (Coleoptera: Tenebrionidae) Larvae. Environ. Int. 2020, 145, 106106. [Google Scholar] [CrossRef]
- Huang, S.; Peng, C.; Wang, Z.; Xiong, X.; Bi, Y.; Liu, Y.; Li, D. Spatiotemporal Distribution of Microplastics in Surface Water, Biofilms, and Sediments in the World’s Largest Drinking Water Diversion Project. Sci. Total Environ. 2021, 789, 148001. [Google Scholar] [CrossRef]
- Jimenez-Cárdenas, V.; Luna-Acosta, A.; Gómez-Méndez, L.D. Differential Presence of Microplastics and Mesoplastics in Coral Reef and Mangrove Fishes in Isla Grande, Colombia. Microplastics 2022, 1, 477–493. [Google Scholar] [CrossRef]
- Prokić, M.D.; Radovanović, T.B.; Gavrić, J.P.; Faggio, C. Ecotoxicological Effects of Microplastics: Examination of Biomarkers, Current State and Future Perspectives. TrAC Trends Anal. Chem. 2019, 111, 37–46. [Google Scholar] [CrossRef]
- Microplastics in Drinking-Water; World Health Organization: Geneva, Switzerland, 2019.
- Cho, Y.; Shim, W.J.; Jang, M.; Han, G.M.; Hong, S.H. Abundance and Characteristics of Microplastics in Market Bivalves from South Korea. Environ. Pollut. 2019, 245, 1107–1116. [Google Scholar] [CrossRef]
- Karami, A.; Golieskardi, A.; Choo, C.K.; Larat, V.; Karbalaei, S.; Salamatinia, B. Microplastic and Mesoplastic Contamination in Canned Sardines and Sprats. Sci. Total Environ. 2018, 612, 1380–1386. [Google Scholar] [CrossRef]
- Ghosh, G.C.; Akter, S.M.; Islam, R.M.; Habib, A.; Chakraborty, T.K.; Zaman, S.; Kabir, A.H.M.E.; Shipin, O.V.; Wahid, M.A. Microplastics Contamination in Commercial Marine Fish from the Bay of Bengal. Reg. Stud. Mar. Sci. 2021, 44, 101728. [Google Scholar] [CrossRef]
- Halstead, J.E.; Smith, J.A.; Carter, E.A.; Lay, P.A.; Johnston, E.L. Assessment Tools for Microplastics and Natural Fibres Ingested by Fish in an Urbanised Estuary. Environ. Pollut. 2018, 234, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; McHugh, M.; Thompson, R.C. Occurrence of Microplastics in the Gastrointestinal Tract of Pelagic and Demersal Fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Takada, H. Microplastic Fragments and Microbeads in Digestive Tracts of Planktivorous Fish from Urban Coastal Waters. Sci. Rep. 2016, 6, 34351. [Google Scholar] [CrossRef] [PubMed]
- Daniel, D.B.; Ashraf, P.M.; Thomas, S.N. Abundance, Characteristics and Seasonal Variation of Microplastics in Indian White Shrimps (Fenneropenaeus Indicus) from Coastal Waters off Cochin, Kerala, India. Sci. Total Environ. 2020, 737, 139839. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qu, X.; Su, L.; Zhang, W.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in Mussels along the Coastal Waters of China. Environ. Pollut. 2016, 214, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.T.; Cai, Y.F.; Chen, Y.X.; Yang, Y.W.; Xing, S.C.; Liao, X. Di Occurrence of Microplastic in Livestock and Poultry Manure in South China. Environ. Pollut. 2021, 277, 116790. [Google Scholar] [CrossRef]
- Huerta Lwanga, E.; Mendoza Vega, J.; Ku Quej, V.; de los Chi, J.A.; del Cid Sanchez, L.; Chi, C.; Escalona Segura, G.; Gertsen, H.; Salánki, T.; van der Ploeg, M.; et al. Field Evidence for Transfer of Plastic Debris along a Terrestrial Food Chain. Sci. Rep. 2017, 7, 14071. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Shi, H.; Li, L.; Li, J.; Jabeen, K.; Kolandhasamy, P. Microplastic Pollution in Table Salts from China. Environ. Sci. Technol. 2015, 49, 13622–13627. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, H.J.; Kim, S.K.; Kim, H.J. Global Pattern of Microplastics (MPs) in Commercial Food-Grade Salts: Sea Salt as an Indicator of Seawater MP Pollution. Environ. Sci. Technol. 2018, 52, 12819–12828. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Xu, E.G.; Larsson, H.C.E.; Tahara, R.; Maisuria, V.B.; Tufenkji, N. Plastic Teabags Release Billions of Microparticles and Nanoparticles into Tea. Environ. Sci. Technol. 2019, 53, 12300–12310. [Google Scholar] [CrossRef]
- Schymanski, D.; Goldbeck, C.; Humpf, H.U.; Fürst, P. Analysis of Microplastics in Water by Micro-Raman Spectroscopy: Release of Plastic Particles from Different Packaging into Mineral Water. Water Res. 2018, 129, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Kutralam-Muniasamy, G.; Pérez-Guevara, F.; Elizalde-Martínez, I.; Shruti, V.C. Branded Milks—Are They Immune from Microplastics Contamination? Sci. Total Environ. 2020, 714, 136823. [Google Scholar] [CrossRef] [PubMed]
- Mintenig, S.M.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Low Numbers of Microplastics Detected in Drinking Water from Ground Water Sources. Sci. Total Environ. 2019, 648, 631–635. [Google Scholar] [CrossRef]
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic Contamination of Tap Water, Beer, and Sea Salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Basantes, M.F.; Conesa, J.A.; Fullana, A. Microplastics in Honey, Beer, Milk and Refreshments in Ecuador as Emerging Contaminants. Sustainability 2020, 12, 5514. [Google Scholar] [CrossRef]
- Liebezeit, G.; Liebezeit, E. Non-Pollen Particulates in Honey and Sugar. Food Addit. Contam. Part A 2013, 30, 2136–2140. [Google Scholar] [CrossRef]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; dos Santos Galvão, L.; Ando, R.A.; Mauad, T. Presence of Airborne Microplastics in Human Lung Tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental Exposure to Microplastics: An Overview on Possible Human Health Effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Pironti, C.; Ricciardi, M.; Motta, O.; Miele, Y.; Proto, A.; Montano, L. Microplastics in the Environment: Intake through the Food Web, Human Exposure and Toxicological Effects. Toxics 2021, 9, 224. [Google Scholar] [CrossRef]
- Prata, J.C. Airborne Microplastics: Consequences to Human Health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef]
- Li, L.; Zhao, X.; Li, Z.; Song, K. COVID-19: Performance Study of Microplastic Inhalation Risk Posed by Wearing Masks. J. Hazard. Mater. 2021, 411, 124955. [Google Scholar] [CrossRef] [PubMed]
- Salvioni, L.; Morelli, L.; Ochoa, E.; Labra, M.; Fiandra, L.; Palugan, L.; Prosperi, D.; Colombo, M. The Emerging Role of Nanotechnology in Skincare. Adv. Colloid Interface Sci. 2021, 293, 102437. [Google Scholar] [CrossRef] [PubMed]
- Revel, M.; Châtel, A.; Mouneyrac, C. Micro(Nano)Plastics: A Threat to Human Health? Curr. Opin. Environ. Sci. Health 2018, 1, 17–23. [Google Scholar] [CrossRef]
- Schirinzi, G.F.; Pérez-Pomeda, I.; Sanchís, J.; Rossini, C.; Farré, M.; Barceló, D. Cytotoxic Effects of Commonly Used Nanomaterials and Microplastics on Cerebral and Epithelial Human Cells. Environ. Res. 2017, 159, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wu, X.; Liu, S.; Wang, Z.; Chen, L. Size-Dependent Effects of Polystyrene Microplastics on Cytotoxicity and Efflux Pump Inhibition in Human Caco-2 cells. Chemosphere 2019, 221, 333–341. [Google Scholar] [CrossRef]
- Stock, V.; Böhmert, L.; Lisicki, E.; Block, R.; Cara-Carmona, J.; Pack, L.K.; Selb, R.; Lichtenstein, D.; Voss, L.; Henderson, C.J.; et al. Uptake and Effects of Orally Ingested Polystyrene Microplastic Particles in Vitro and in Vivo. Arch. Toxicol. 2019, 93, 1817–1833. [Google Scholar] [CrossRef]
- Hesler, M.; Aengenheister, L.; Ellinger, B.; Drexel, R.; Straskraba, S.; Jost, C.; Wagner, S.; Meier, F.; von Briesen, H.; Büchel, C.; et al. Multi-Endpoint Toxicological Assessment of Polystyrene Nano- and Microparticles in Different Biological Models in Vitro. Toxicol. In Vitro 2019, 61, 104610. [Google Scholar] [CrossRef]
- Xu, H.; Hoet, P.H.M.; Nemery, B. In Vitro Toxicity Assessment of Polyvinyl Chloride Particles and Comparison of Six Cellular Systems. J. Toxicol. Environ. Health Part A 2002, 65, 1141–1159. [Google Scholar] [CrossRef]
- Guzzetti, E.; Sureda, A.; Tejada, S.; Faggio, C. Microplastic in Marine Organism: Environmental and Toxicological Effects. Environ. Toxicol. Pharmacol. 2018, 64, 164–171. [Google Scholar] [CrossRef]
- Allsopp, M.; Walters, A.; Santillo, D.; Johnston, P. Plastic Debris in the World ’ s Oceans, Greenspace; UN Environment Programme, UNEP: Nairobi, Kenya, 2006. [Google Scholar]
- Carbery, M.; O’Connor, W.; Palanisami, T. Trophic Transfer of Microplastics and Mixed Contaminants in the Marine Food Web and Implications for Human Health. Environ. Int. 2018, 115, 400–409. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.; Chen, B.; Xia, B.; Shi, X.; Qu, K. Polystyrene Microplastics Alter the Behavior, Energy Reserve and Nutritional Composition of Marine Jacopever (Sebastes schlegelii). J. Hazard. Mater. 2018, 360, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gundlach, M.; Yang, S.; Jiang, J.; Velki, M.; Yin, D.; Hollert, H. Quantitative Investigation of the Mechanisms of Microplastics and Nanoplastics toward Zebrafish Larvae Locomotor Activity. Sci. Total Environ. 2017, 584–585, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Sussarellu, R.; Suquet, M.; Thomas, Y.; Lambert, C.; Fabioux, C.; Pernet, M.E.J.; Le Goïc, N.; Quillien, V.; Mingant, C.; Epelboin, Y.; et al. Oyster Reproduction Is Affected by Exposure to Polystyrene Microplastics. Proc. Natl. Acad. Sci. USA 2016, 113, 2430–2435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardon, T.; Reisser, C.; Soyez, C.; Quillien, V.; Le Moullac, G. Microplastics Affect Energy Balance and Gametogenesis in the Pearl Oyster Pinctada Margaritifera. Environ. Sci. Technol. 2018, 52, 5277–5286. [Google Scholar] [CrossRef] [Green Version]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Galloway, T.S. The Impact of Polystyrene Microplastics on Feeding, Function and Fecundity in the Marine Copepod Calanus Helgolandicus. Environ. Sci. Technol. 2015, 49, 1130–1137. [Google Scholar] [CrossRef]
- Banaee, M.; Gholamhosseini, A.; Sureda, A.; Soltanian, S.; Fereidouni, M.S.; Ibrahim, A.T.A. Effects of Microplastic Exposure on the Blood Biochemical Parameters in the Pond Turtle (Emys orbicularis). Environ. Sci. Pollut. Res. 2021, 28, 9221–9234. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Kaposi, K.L.; Mos, B.; Kelaher, B.P.; Dworjanyn, S.A. Ingestion of Microplastic Has Limited Impact on a Marine Larva. Environ. Sci. Technol. 2014, 48, 1638–1645. [Google Scholar] [CrossRef] [Green Version]
- Weber, A.; Scherer, C.; Brennholt, N.; Reifferscheid, G.; Wagner, M. PET Microplastics Do Not Negatively Affect the Survival, Development, Metabolism and Feeding Activity of the Freshwater Invertebrate Gammarus Pulex. Environ. Pollut. 2018, 234, 181–189. [Google Scholar] [CrossRef]
- Rist, S.E.; Assidqi, K.; Zamani, N.P.; Appel, D.; Perschke, M.; Huhn, M.; Lenz, M. Suspended Micro-Sized PVC Particles Impair the Performance and Decrease Survival in the Asian Green Mussel Perna Viridis. Mar. Pollut. Bull. 2016, 111, 213–220. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested Plastic Transfers Hazardous Chemicals to Fish and Induces Hepatic Stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Browne, M.A.; Niven, S.J.; Galloway, T.S.; Rowland, S.J.; Thompson, R.C. Microplastic Moves Pollutants and Additives to Worms, Reducing Functions Linked to Health and Biodiversity. Curr. Biol. 2013, 23, 2388–2392. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Wu, S.; Lu, S.; Liu, M.; Song, Y.; Fu, Z.; Shi, H.; Raley-Susman, K.M.; He, D. Microplastic Particles Cause Intestinal Damage and Other Adverse Effects in Zebrafish Danio Rerio and Nematode Caenorhabditis Elegans. Sci. Total Environ. 2018, 619–620, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, X.; Wang, J.; Tan, L. Toxic Effects of Microplastic on Marine Microalgae Skeletonema Costatum: Interactions between Microplastic and Algae. Environ. Pollut. 2016, 220, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.; Garcia, A.R.; Pereira, B.P.; Fonseca, M.; Mestre, N.C.; Fonseca, T.G.; Ilharco, L.M.; Bebianno, M.J. Microplastics Effects in Scrobicularia Plana. Mar. Pollut. Bull. 2017, 122, 379–391. [Google Scholar] [CrossRef]
- Dawson, A.; Huston, W.; Kawaguchi, S.; King, C.; Cropp, R.; Wild, S.; Eisenmann, P.; Townsend, K.; Bengtson Nash, S.M. Uptake and Depuration Kinetics Influence Microplastic Bioaccumulation and Toxicity in Antarctic Krill (Euphausia superba). Environ. Sci. Technol. 2018, 52, 3195–3201. [Google Scholar] [CrossRef]
- Chisada, S.; Yoshida, M.; Karita, K. Ingestion of Polyethylene Microbeads Affects the Growth and Reproduction of Medaka, Oryzias Latipes. Environ. Pollut. 2019, 254, 113094. [Google Scholar] [CrossRef]
- Green, D.S. Effects of Microplastics on European Flat Oysters, Ostrea Edulis and Their Associated Benthic Communities. Environ. Pollut. 2016, 216, 95–103. [Google Scholar] [CrossRef]
- Devriese, L.I.; van der Meulen, M.D.; Maes, T.; Bekaert, K.; Paul-Pont, I.; Frère, L.; Robbens, J.; Vethaak, A.D. Microplastic Contamination in Brown Shrimp (Crangon Crangon, Linnaeus 1758) from Coastal Waters of the Southern North Sea and Channel Area. Mar. Pollut. Bull. 2015, 98, 179–187. [Google Scholar] [CrossRef]
- Messinetti, S.; Mercurio, S.; Scarì, G.; Pennati, A.; Pennati, R. Ingested Microscopic Plastics Translocate from the Gut Cavity of Juveniles of the Ascidian Ciona Intestinalis. Eur. Zool. J. 2019, 86, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Lo, H.K.A.; Chan, K.Y.K. Negative Effects of Microplastic Exposure on Growth and Development of Crepidula Onyx. Environ. Pollut. 2018, 233, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Nizzetto, L.; Futter, M.; Langaas, S. Are Agricultural Soils Dumps for Microplastics of Urban Origin? Environ. Sci. Technol. 2016, 50, 10777–10779. [Google Scholar] [CrossRef] [PubMed]
- Möller, J.N.; Löder, M.G.J.; Laforsch, C. Finding Microplastics in Soils: A Review of Analytical Methods. Environ. Sci. Technol. 2020, 54, 2078–2090. [Google Scholar] [CrossRef] [PubMed]
- Gabet, E.J.; Reichman, O.J.; Seabloom, E.W. The Effects of Bioturbation on Soil Processes and Sediment Transport. Annu. Rev. Earth Planet. Sci. 2003, 31, 249–273. [Google Scholar] [CrossRef]
- Hurley, R.R.; Nizzetto, L. Fate and Occurrence of Micro(Nano)Plastics in Soils: Knowledge Gaps and Possible Risks. Curr. Opin. Environ. Sci. Health 2018, 1, 6–11. [Google Scholar] [CrossRef]
- Gutiérrez-López, M.; Salmon, S.; Trigo, D. Movement Response of Collembola to the Excreta of Two Earthworm Species: Importance of Ammonium Content and Nitrogen Forms. Soil Biol. Biochem. 2011, 43, 55–62. [Google Scholar] [CrossRef]
- Cao, D.; Wang, X.; Luo, X.; Liu, G.; Zheng, H. Effects of Polystyrene Microplastics on the Fitness of Earthworms in an Agricultural Soil. IOP Conf. Ser. Earth Environ. Sci. 2017, 61, 012148. [Google Scholar] [CrossRef]
- Huerta Lwanga, E.; Gertsen, H.; Gooren, H.; Peters, P.; Salánki, T.; van der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Incorporation of Microplastics from Litter into Burrows of Lumbricus Terrestris. Environ. Pollut. 2017, 220, 523–531. [Google Scholar] [CrossRef]
- Lahive, E.; Walton, A.; Horton, A.A.; Spurgeon, D.J.; Svendsen, C. Microplastic Particles Reduce Reproduction in the Terrestrial Worm Enchytraeus Crypticus in a Soil Exposure. Environ. Pollut. 2019, 255, 113174. [Google Scholar] [CrossRef]
- Rillig, M.C.; Ziersch, L.; Hempel, S. Microplastic Transport in Soil by Earthworms. Sci. Rep. 2017, 7, 1362. [Google Scholar] [CrossRef] [Green Version]
- Lei, L.; Liu, M.; Song, Y.; Lu, S.; Hu, J.; Cao, C.; Xie, B.; Shi, H.; He, D. Polystyrene (Nano)Microplastics Cause Size-Dependent Neurotoxicity, Oxidative Damage and Other Adverse Effects in Caenorhabditis Elegans. Environ. Sci. Nano 2018, 5, 2009–2020. [Google Scholar] [CrossRef]
- Zhu, D.; Chen, Q.L.; An, X.L.; Yang, X.R.; Christie, P.; Ke, X.; Wu, L.H.; Zhu, Y.G. Exposure of Soil Collembolans to Microplastics Perturbs Their Gut Microbiota and Alters Their Isotopic Composition. Soil Biol. Biochem. 2018, 116, 302–310. [Google Scholar] [CrossRef]
- Song, Y.; Cao, C.; Qiu, R.; Hu, J.; Liu, M.; Lu, S.; Shi, H.; Raley-Susman, K.M.; He, D. Uptake and Adverse Effects of Polyethylene Terephthalate Microplastics Fibers on Terrestrial Snails (Achatina fulica) after Soil Exposure. Environ. Pollut. 2019, 250, 447–455. [Google Scholar] [CrossRef]
- Kim, S.W.; An, Y.J. Soil Microplastics Inhibit the Movement of Springtail Species. Environ. Int. 2019, 126, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.; Zhu, D.; Qiao, M. Effects of Polyethylene Microplastics on the Gut Microbial Community, Reproduction and Avoidance Behaviors of the Soil Springtail, Folsomia Candida. Environ. Pollut. 2019, 247, 890–897. [Google Scholar] [CrossRef]
- Yi, M.; Zhou, S.; Zhang, L.; Ding, S. The Effects of Three Different Microplastics on Enzyme Activities and Microbial Communities in Soil. Water Environ. Res. 2020, 93, 24–32. [Google Scholar] [CrossRef]
- Wan, Y.; Wu, C.; Xue, Q.; Hui, X. Effects of Plastic Contamination on Water Evaporation and Desiccation Cracking in Soil. Sci. Total Environ. 2019, 654, 576–582. [Google Scholar] [CrossRef]
- Prendergast-Miller, M.T.; Katsiamides, A.; Abbass, M.; Sturzenbaum, S.R.; Thorpe, K.L.; Hodson, M.E. Polyester-Derived Microfibre Impacts on the Soil-Dwelling Earthworm Lumbricus Terrestris. Environ. Pollut. 2019, 251, 453–459. [Google Scholar] [CrossRef]
- Rodríguez-Seijo, A.; da Costa, J.P.; Rocha-Santos, T.; Duarte, A.C.; Pereira, R. Oxidative Stress, Energy Metabolism and Molecular Responses of Earthworms (Eisenia fetida) Exposed to Low-Density Polyethylene Microplastics. Environ. Sci. Pollut. Res. 2018, 25, 33599–33610. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, H.; Hua, X.; Dang, Y.; Han, Y.; Yu, Z.; Chen, X.; Ding, P.; Li, H. Polystyrene Microplastics (PS-MPs) Toxicity Induced Oxidative Stress and Intestinal Injury in Nematode Caenorhabditis Elegans. Sci. Total Environ. 2020, 726, 138679. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, X.; Pelaez, A.M.; Huerta Lwanga, E.; Beriot, N.; Gertsen, H.; Garbeva, P.; Geissen, V. Macro- and Micro- Plastics in Soil-Plant System: Effects of Plastic Mulch Film Residues on Wheat (Triticum aestivum) Growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Bosker, T.; Bouwman, L.J.; Brun, N.R.; Behrens, P.; Vijver, M.G. Microplastics Accumulate on Pores in Seed Capsule and Delay Germination and Root Growth of the Terrestrial Vascular Plant Lepidium Sativum. Chemosphere 2019, 226, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Chen, H.; Liao, Y.; Ye, Z.; Li, M.; Klobučar, G. Ecotoxicity and Genotoxicity of Polystyrene Microplastics on Higher Plant Vicia Faba. Environ. Pollut. 2019, 250, 831–838. [Google Scholar] [CrossRef] [PubMed]
- De Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics Can Change Soil Properties and Affect Plant Performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Liu, Y.; Song, Z. Effects of Polyethylene Microplastic on the Phytotoxicity of Di-n-Butyl Phthalate in Lettuce (Lactuca sativa L. Var. Ramosa Hort). Chemosphere 2019, 237, 124482. [Google Scholar] [CrossRef]
- Hernández-Arenas, R.; Beltrán-Sanahuja, A.; Navarro-Quirant, P.; Sanz-Lazaro, C. The Effect of Sewage Sludge Containing Microplastics on Growth and Fruit Development of Tomato Plants. Environ. Pollut. 2021, 268, 115779. [Google Scholar] [CrossRef]
- Kumar, A.; Mishra, S.; Pandey, R.; Yu, Z.G.; Kumar, M. Microplastics in terrestrial ecosystems: Un-ignorable impacts on soil characterises, nutrient storage and its cycling. TrAC Trends Anal. Chem. 2023, 158, 116869. [Google Scholar] [CrossRef]
- Ministry of Foreign Affairs of Japan Japan’s “MARINE Initiative” toward Realization of the Osaka Blue Ocean Vision; Ministry of Foreign Affairs of Japan: Tokyo, Japan, 2019; p. 2050.
- Marine Debris Program, N. Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Synthetic Particles in Waters and Sediments; NOAA Marine Debris Division: Silver Spring, MD, USA, 2015.
- Wu, J.F.; Yao, H.X.; Yuan, X.; Lin, B.Q. Dissolved organic carbon response to hydrological drought characteristics: Based on long-term measurements of headwater streams. Water Res. 2022, 215, 115252. [Google Scholar] [CrossRef]
- Pasquier, G.; Doyen, P.; Kazour, M.; Dehaut, A.; Diop, M.; Duflos, G.; Amara, R. Manta Net: The Golden Method for Sampling Surface Water Microplastics in Aquatic Environments. Front. Environ. Sci. 2022, 10, 811112. [Google Scholar] [CrossRef]
- Zobkov, M.B.; Esiukova, E.E.; Zyubin, A.Y.; Samusev, I.G. Microplastic Content Variation in Water Column: The Observations Employing a Novel Sampling Tool in Stratified Baltic Sea. Mar. Pollut. Bull. 2019, 138, 193–205. [Google Scholar] [CrossRef]








| Polymer | Structure | Applications | Toxic Effects | References |
|---|---|---|---|---|
| Polyethylene (PE) |  | Packaging | Detrimental to environment | [9] |
| Polyethylene terephthalate (PET) | 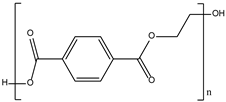 | Packaging | Disruption of endocrine system | [10] |
| Polypropylene (PP) |  | Automotives and furniture | Carcinogenic and cytotoxic | [11] |
| Polystyrene (PS) | 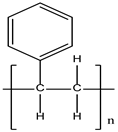 | Food packaging | Inhibition of growth and mortality | [12] |
| Polyvinyl chloride (PVC) |  | Constructions and buildings | Damage to immune system and causes infertility | [13] |
| Polyurethane (PU) |  | Constructions and buildings | Cause neurological impairment | [14] |
| Polyamide (PA) | 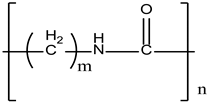 | Textiles and automotives | Liver damage | [15] |
| Removal Method | Advantages | Disadvantages |
|---|---|---|
| Filtration [41] |
|
|
| Constructed wetlands [42] |
|
|
| Coagulation-Flocculation-Sedimentation [43] |
|
|
| Adsorption and ion exchange [44] |
|
|
| Photocatalytic degradation [45] |
|
|
| Microbial degradation [46] |
|
|
| Consumable Products | Polymer Types | Size | MPs Concentration | References |
|---|---|---|---|---|
| Seafood | ||||
| Bivalve (oyster, mussel, Manila clam, and scallop) | PE, PP, PS, PES, PEVA, PET, PUR | 0.1–0.2 mm | 0.97 (0–2.8) particles/individual 0.15 (0–1.8) particles/g | [70] |
| Canned Sardines | PE, PET, PVC, PP | 190–3800 µm | 6 MPs per item | [71] |
| Fish | PET, PP, PUR, PES | <500 µm | 2.2 ± 0.89 MPs/individual | [72] |
| Acanthopagrus australis (Yellowfin bream) | PET, RY, PES | - | Mean 0.6 MPs/fish | [73] |
| Pelagic and demersal fish | Cellulose, PA, RY | 0.13–14.3 mm | 1.90 particles/individual | [74] |
| Engraulis japonicus (Japanese anchovy) | PE, PP, PS | 150–1000 µm | Mean 2.3 MPs/individual | [75] |
| Fenneropenaeus indicus (Indian white shrimp) | PA, PES, PE, PP | 0.157–2.785 mm | 0.39 ± 0.6 items/shrimp 0.04 ± 0.07 items/g | [76] |
| Mytilus edulis (Mussels) | CPH, PET, PES PE, | 0.033–4.7 mm | 0.9–4.6 particles/individual 1.5–7.6 particles/g | [77] |
| Meat | ||||
| Poultry, cows, and pigs | PP, PE, PET | <5 mm | Poultry manure: 667 ± 990 particles/kg Cow manure: 74 ± 129 particles/kg Pig manure: 902 ± 1290 particles/kg | [78] |
| Chicken gizzards | PS | 0.1–5 mm | 10.2 ± 13.8 particles/g | [79] |
| Salts | ||||
| Salt | CPH, PE, PET | <200 µm | Lake salt: 43–364 particles/kg Rock salt: 7–204 particles/kg Sea salts: 550–681 particles/kg | [80] |
| Sea/lake/rock salt | PE, PET, PP | <500 µm | Lake salt: 28–462 particles/kg Rock salt: 0–148 particles/kg Sea salts: 0–1674 particles/kg | [81] |
| Drinks | ||||
| Tea | PA, PET | 25 µm | ~11.6 microplastics/cup of the beverage | [82] |
| Drinking water | PET, PE, PA, PP | 0.005–0.1 mm | 1 ± 8 particles/L (beverage cartons) 118 ± 88 particles/L (returnable plastic bottles) | [83] |
| Milk | Polysulfone | 0.1–5 mm | 6500 particles/m3 | [84] |
| Drinking water | PES, PVC, PE, PA, EP | 0.05–0.105 mm | 0–7000 particles/L | [85] |
| Beer | - | 0.1–5 mm | 0–14.3 particles/L | [86] |
| Sugar and honey | ||||
| Honey | PP, PE, PAAm | 0.013–0.25 mm | 54 particles/L (industrial honey) 67 particles/L (craft honey) | [87] |
| Honey | - | 0.01–9 mm | 166 ± 147 particles/kg (fibers) 9 ± 9 particles/kg (fragments) | [88] |
| Sugar | 217 ± 123 particles kg−1 (fibres) 32 ± 7 particles kg−1 (fragments) |
| Aquatic Organisms | Polymer Types | Size | Effects | References |
|---|---|---|---|---|
| Zebrafish Larvae | PS | 45 µm | Suppressed catalytic performance of AchE | [105] |
| Oyster | PS | 2 and 6 µm | Reduced sperm count and speed | [106] |
| Pinctada margaritifera (Oyster) | PS | 6 and 10 µm | Reduced assimilation efficiency and reproduction | [107] |
| Calanus helgolandicus (Copepods) | PS | 20 µm | Reduction in carbon biomass | [108] |
| Emys orbicularis (Pond turtle) | PE | - | Adverse impact on the liver and kidney functioning | [109] |
| Danio rerio (Zebrafish) | PS | 5 and 20 µm | Inhibited liver functions and metabolism of fish | [110] |
| Danio rerio (Zebrafish) | PA, PE, PVC, PP | 70 µm | Damage to intestine | [116] |
| Tripneustes gratilla (Sea urchin) | PE | 10–45 µm | Decreased larval width and survival affected by 50% | [111] |
| Gammarus pulex (Amphipoda) | PET | 10–150 µm | Metabolic rate, behavior, and growth were not affected | [112] |
| Skeletonema costatum (Microalgae) | PVC | 1 µm and mm | Inhibition in growth and affected photosynthesis | [117] |
| Perna viridis (Asian green mussel) | PVC | 1–50 µm | Negative impacts on physiological functions of mussels | [113] |
| Scrobicularia plana (Bivalve mollusc) | PS | 20 µm | MPs inhibited antioxidant activity, damaged DNA, and caused neurotoxicity and oxidative stress. | [118] |
| Euphausia superba (Antarctic Kill) | PE | 27–32 µm | Loss in weight | [119] |
| Oryzias lapites (Japanese medaka fish) | LDPE | - | Resulted in formation of tumours, liver damage, and accumulation of toxic chemicals | [114] |
| Oryzias lapites (Japanese medaka fish) | PE | <1 mm | Adverse effects on reproduction and growth | [120] |
| Arenicola marina L. (Lugworms) | PVC, PS | <10 µm | Mortality and dysfunction of immune system | [115] |
| Ostrea edulis (Flat Oysters) | HDPE and PLA | Varying sizes | Increase in respiration rate | [121] |
| Crangon crangon L. (Brown shrimp) | - | 200–1000 µm | No adverse impact on the shrimp’s nutritional condition | [122] |
| Ciona intestinalis (Sea squirt) | PS | 1 µm | Negative effects on growth and food intake | [123] |
| Crepidula onyx (Mollusca) | PS | 2 µm | Growth inhibition | [124] |
| Soil Biota and Properties | Polymer Types | Size | MPs Effects | References |
|---|---|---|---|---|
| Eisenia Foetida (Earthworm) | PS | 58 µm | Inhibition in growth and increased mortality | [130] |
| Lumbricus Terrestris (earthworm) | PE | ≥50 µm | Growth inhibition and mortality | [131] |
| Lumbricus terrestris (Earthworm) | PE | 40.7 ± 3.8 µm | Cellular stress | [141] |
| Eisenia fetida (Earthworm) | PE | 250–1000 µm | Gut damage | [142] |
| Enchytraeus crypticus (Soil worm) | PA | 20 and 160 µm | Rate of reproduction was affected | [132] |
| Lumbricus terrestris (Anecic earthworm) | PE | Varying sizes | Earthworms transported MPs deeper into the soil | [133] |
| Caenorhabditis Elegans (Roundworm) | PS | 1–5 µm | MPs caused reduction in body growth and low survival rate | [134] |
| Caenorhabditis elegans (Nematode) | PS | 1 µm | Oxidative stress and intestinal damage | [143] |
| Folsomia candida (Collembolans) | PVC | 80–250 mm | Inhibition of reproduction and growth | [135] |
| Achatina Fulica (snail) | PET | 76.3 µm | Reduction in food intake and damage to digestive tract | [136] |
| Lobella sokamensis (Soil springtail) | PE and PS | 0.47~1155 μm | Movement inhibition | [137] |
| Folsomia candida (Soil springtail) | PE | 281 µm | Decreased survival and reproduction rate | [138] |
| Soil enzyme (urease and phosphatase) | Membranous PE, PP microsphere and fibrous PP | - | Inhibition of enzymatic activity | [139] |
| Soil property | PE | 2, 5 and 10 mm | Increased water evaporation of soil leading to soil drying | [140] |
| Triticum aestivum (wheat plant) | PE | - | Inhibited the vegetative and reproductive growth | [144] |
| Lepidium sativum (cress seed) | - | <5 mm | Delayed germination rate and growth of its root | [145] |
| Vicia faba (Broad bean) | PS | 5 µm | Oxidative damage, Inhibition of plant growth, and induced genotoxicity and ecotoxicity | [146] |
| Allium fistulosum (Spring onions) | PEHD, PA, PES, PET, PP, and PS | Varying sizes | Affected plant performance | [147] |
| Lactuca sativa L. var. ramose Hort (Lettuce) | PS | 23 µm | Lettuce’s growth rate, photosynthesis, and chlorophyll content were significantly reduced by MPs | [148] |
| Lycopersicon esculentum Mill (Tomato) | PET, PP, PE | 0.4–2.6 mm | MP sludge stimulated tomato plant growth but delayed the production and yield | [149] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, B.; Pathak, J.; Singh, P.; Kumar, R.; Kumar, A.; Kaushik, S.; Thakur, T.K. Microplastics in the Ecosystem: An Overview on Detection, Removal, Toxicity Assessment, and Control Release. Water 2023, 15, 51. https://doi.org/10.3390/w15010051
Pandey B, Pathak J, Singh P, Kumar R, Kumar A, Kaushik S, Thakur TK. Microplastics in the Ecosystem: An Overview on Detection, Removal, Toxicity Assessment, and Control Release. Water. 2023; 15(1):51. https://doi.org/10.3390/w15010051
Chicago/Turabian StylePandey, Bhamini, Jigyasa Pathak, Poonam Singh, Ravinder Kumar, Amit Kumar, Sandeep Kaushik, and Tarun Kumar Thakur. 2023. "Microplastics in the Ecosystem: An Overview on Detection, Removal, Toxicity Assessment, and Control Release" Water 15, no. 1: 51. https://doi.org/10.3390/w15010051
APA StylePandey, B., Pathak, J., Singh, P., Kumar, R., Kumar, A., Kaushik, S., & Thakur, T. K. (2023). Microplastics in the Ecosystem: An Overview on Detection, Removal, Toxicity Assessment, and Control Release. Water, 15(1), 51. https://doi.org/10.3390/w15010051







