Formation of Biochar Nanocomposite Materials Based on CoFe2O4 for Purification of Aqueous Solutions from Chromium Compounds (VI)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pyrolysis of Biochars
2.3. Synthesis of Composite Materials
2.4. Characteristic
2.5. Study of Adsorption Activity
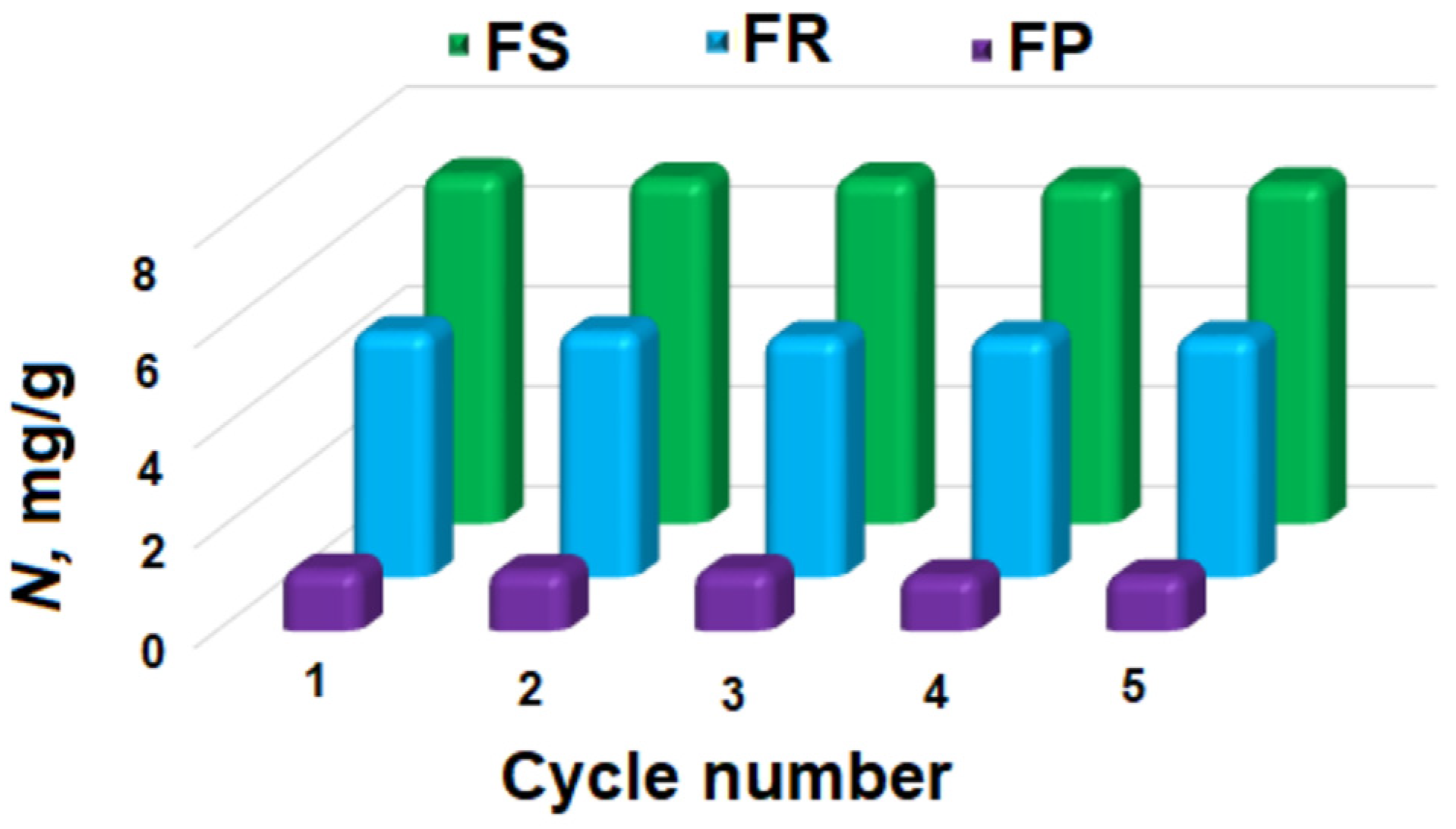
2.6. Recycling
3. Results
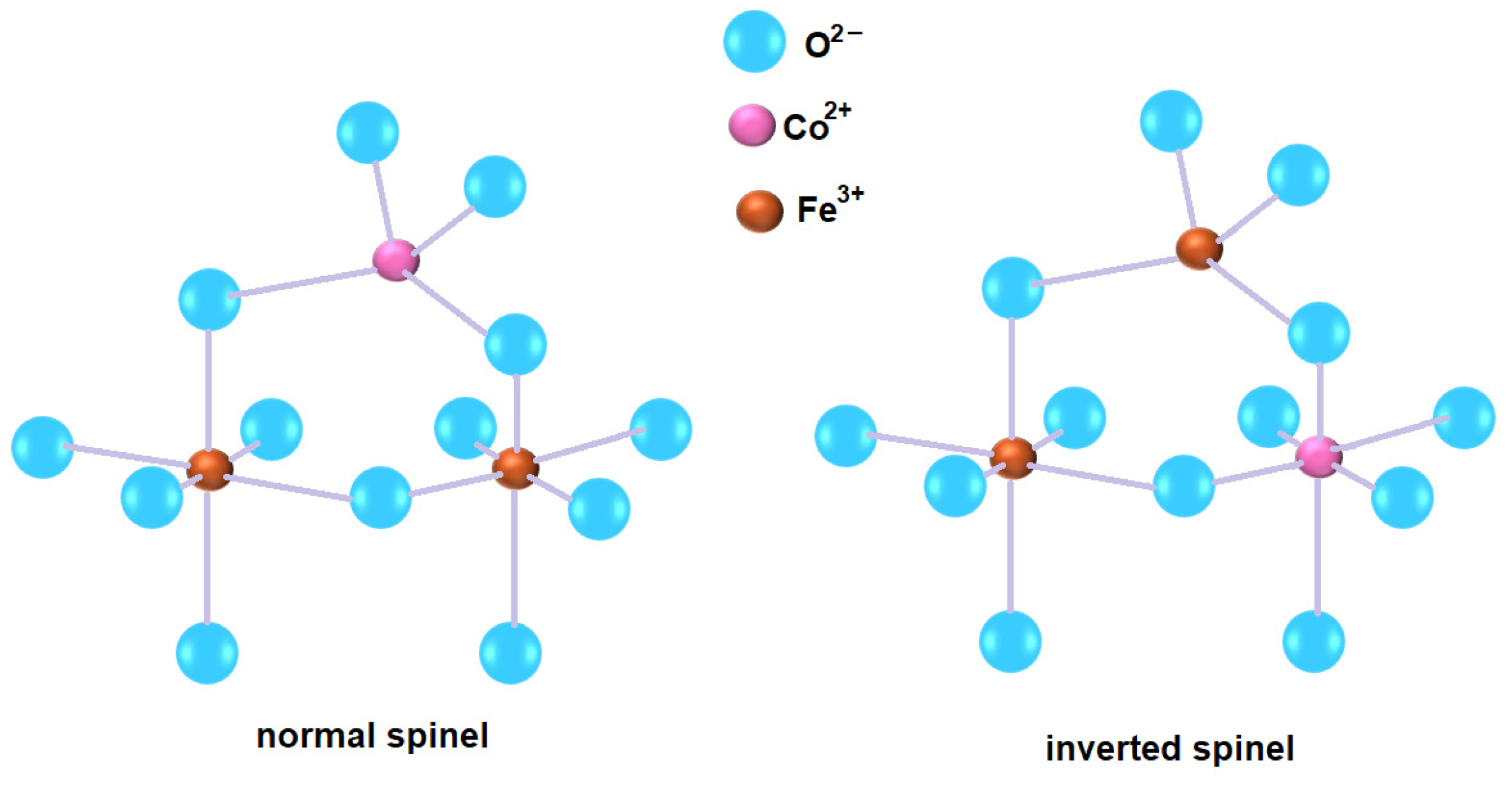
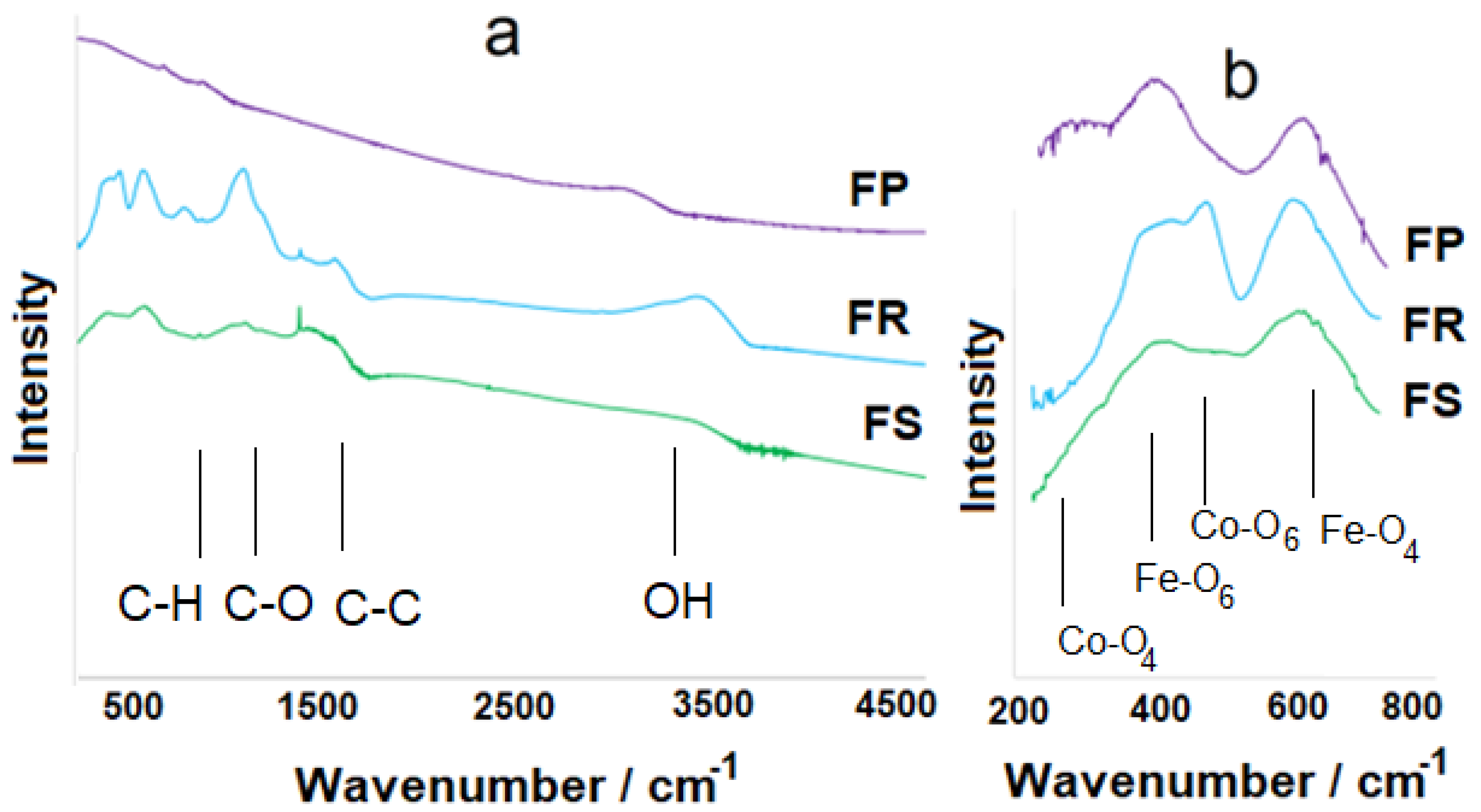
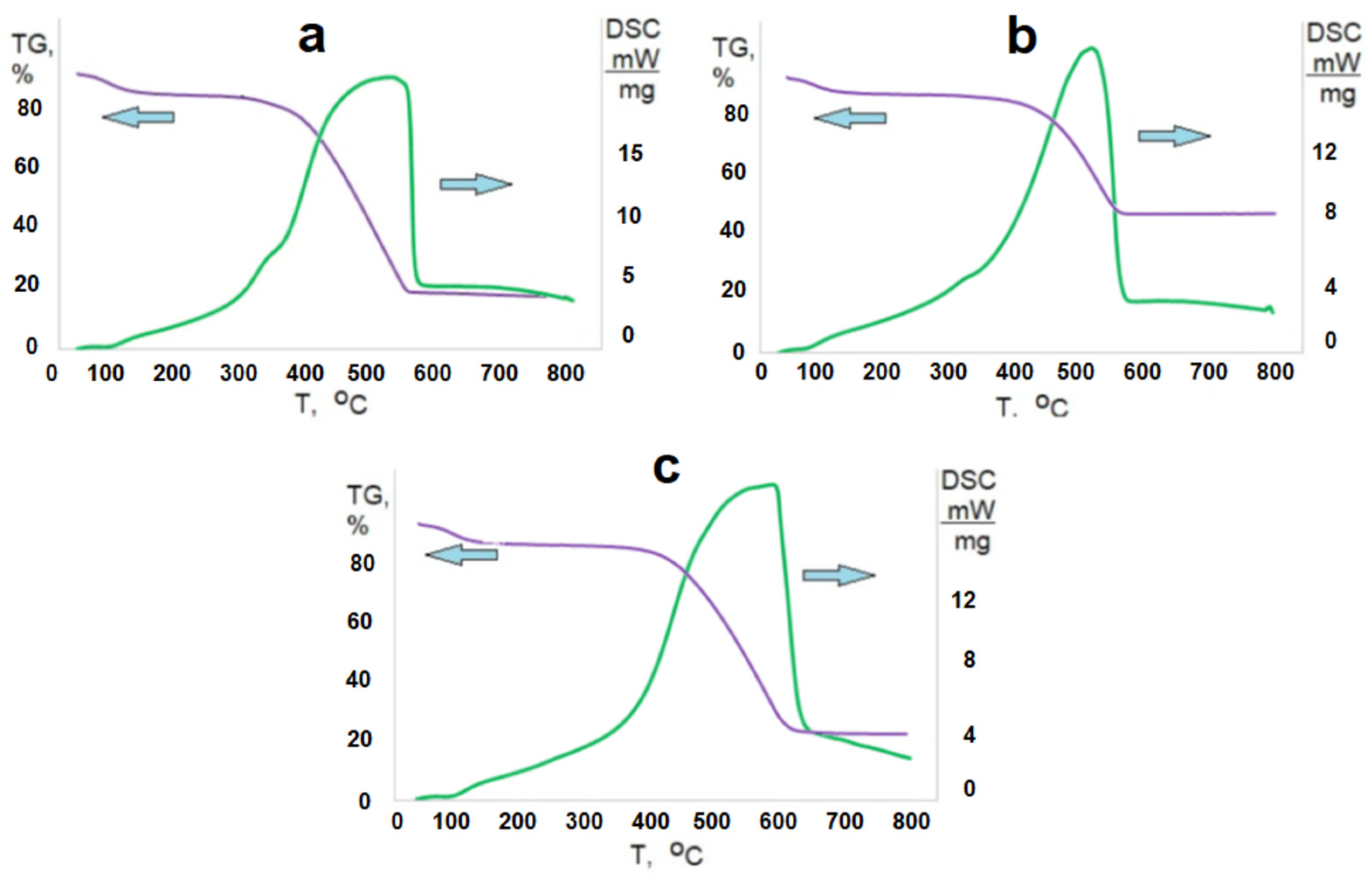
3.1. Study of the Structure and Morphological Features of Samples
3.2. Study of Adsorption Activity
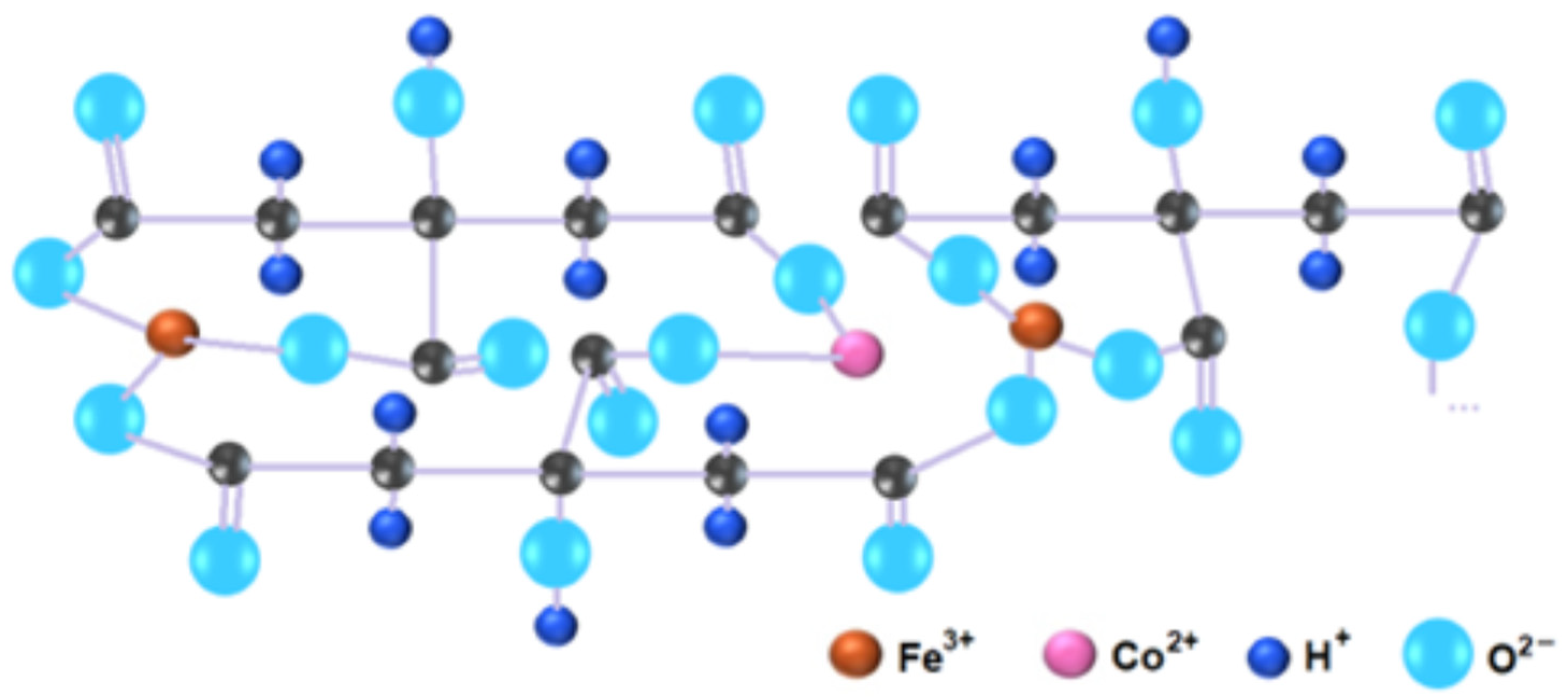
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, C.; Zhang, A.; Chang, T.; Chen, Y.; Zhang, Y.; Tian, F.; Zuo, W.; Ren, Y.; Song, X.; Yang, S. The Phase Diagram and Exotic Magnetostrictive Behaviors in Spinel Oxide Co(Fe1−xAlx)2O4 System. Materials 2019, 12, 1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, S.; Irfan, M.; Naz, M.Y.; Shukrullah, S.; Munir, M.A.; Ayyaz, M.; Alwadie, A.S.; Legutko, S.; Petrů, J.; Rahman, S. Investigating the Impact of Cu2+ Doping on the Morphological, Structural, Optical, and Electrical Properties of CoFe2O4 Nanoparticles for Use in Electrical Devices. Materials 2022, 15, 3502. [Google Scholar] [CrossRef] [PubMed]
- Efimova, N.V.; Krasnopyorova, A.P.; Yuhno, G.D.; Sofronov, D.S.; Rucki, M. Uptake of Radionuclides 60Co, 137Cs, and 90Sr with α-Fe2O3 and Fe3O4 Particles from Aqueous Environment. Materials 2021, 14, 2899. [Google Scholar] [CrossRef] [PubMed]
- Dippong, T.; Levei, E.A.; Cadar, O. Formation, Structure and Magnetic Properties of MFe2O4@SiO2 (M = Co, Mn, Zn, Ni, Cu) Nanocomposites. Materials 2021, 14, 1139. [Google Scholar] [CrossRef]
- Kostuch, A.; Grybo’s, J.; Wierzbicki, S.; Sojka, Z.; Kruczała, K. Selectivity of Mixed Iron-Cobalt Spinels Deposited on a N,S-Doped Mesoporous Carbon Support in the Oxygen Reduction Reaction in Alkaline Media. Materials 2021, 14, 820. [Google Scholar] [CrossRef]
- Barkat, F.; Afzal, M.; Khan, B.S.; Saeed, A.; Bashir, M.; Mukhtar, A.; Mehmood, T.; Wu, K. Formation Mechanism and Lattice Parameter Investigation for Copper-Substituted Cobalt Ferrites from Zingiber officinale and Elettaria cardamom Seed Extracts Using Biogenic Route. Materials 2022, 15, 4374. [Google Scholar] [CrossRef]
- Klekotka, U.; Satuła, D.; Spassov, S.; Kalska-Szostko, B. Influence of Atomic Doping on Thermal Stability of Ferrite Nanoparticles-Structural and Magnetic Studies. Materials 2021, 14, 100. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Abouelanwar, M.E.; Mahmoud, S.E.M.E.; Salam, M.A. Doping starch-gelatin mixed hydrogels with magnetic spinel ferrite@biochar@molybdenum oxide as a highly efficient nanocomposite for removal of lead (II) ions. J. Environ. Chem. Eng. 2021, 9, 106682. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, L.; Li, P.; Yang, L.; He, L.; Chen, S.; Yang, Y.; Gao, F.; Qi, X.; Zhang, Z. A novel, efficient and sustainable magnetic sludge biochar modified by graphene oxide for environmental concentration imidacloprid removal. J. Hazard. Mater. 2021, 4075, 124777. [Google Scholar] [CrossRef]
- Li, S.; Wu, Y.; Zheng, Y.; Jing, T.; Tian, J.; Zheng, H.; Wang, N.; Nan, J.; Ma, J. Free-radical and surface electron transfer dominated bisphenol A degradation in system of ozone and peroxydisulfate co-activated by CoFe2O4-biochar. Appl. Surf. Sci. 2021, 5411, 147887. [Google Scholar] [CrossRef]
- Gan, L.; Zhong, Q.; Geng, A.; Wang, L.; Song, C.; Han, S.; Cui, J.; Xu, L. Cellulose derived carbon nanofiber: A promising biochar support to enhance the catalytic performance of CoFe2O4 in activating peroxymonosulfate for recycled dimethyl phthalate degradation. Sci. Total Environ. 2019, 6941, 133705. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xiang, Y.; Zhou, H.; Yang, J.; He, Y.; Zhu, Z.; Zhou, Y. Manganese ferrite modified biochar from vinasse for enhanced adsorption of levofloxacin: Effects and mechanisms. Environ. Pollut. 2021, 2721, 115968. [Google Scholar] [CrossRef] [PubMed]
- Tony, M.A.; Eltabey, M.M. End-of-life waste criteria: Synthesis and utilization of Mn–Zn ferrite nanoparticles as a superparamagnetic photocatalyst for synergistic wastewater remediation. Appl. Water Sci. 2022, 12, 21. [Google Scholar] [CrossRef]
- Tony, M.A. Central composite design optimization of Bismarck Dye oxidation from textile effluent with Fenton’s reagent. Appl. Water Sci. 2020, 10, 108. [Google Scholar] [CrossRef] [Green Version]
- Mittova, I.Y.; Sladkopevtsev, B.V.; Mittova, V.O. Nanoscale semiconductor and dielectric films and magnetic nanocrystals—New directions of development of the scientific school of Ya. A. Ugai “Solid state chemistry and semiconductors”. Cond. Mat. Interph. 2021, 23, 309–336. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, X.; Yao, J.; Zhan, S.; Li, H.; Zhang, J.; Qiu, Z. Synthesis of polyethyleneimine modified CoFe2O4-loaded porous biochar for selective adsorption properties towards dyes and exploration of interaction mechanisms. Sep. Purif. Technol. 2021, 27715, 119474. [Google Scholar] [CrossRef]
- Ozdes, D.; Duran, C. Preparation of melon peel biochar/CoFe2O4 as a new adsorbent for the separation and preconcentration of Cu(II), Cd(II), and Pb(II) ions by solid-phase extraction in water and vegetable samples. Environ. Monit. Assessm. 2021, 193, 642. [Google Scholar] [CrossRef]
- Harikishore, K.R.D.; Lee, S.-M. Magnetic biochar composite: Facile synthesis, characterization, and application for heavy metal removal. Coll. Surf. A Physicochem. Eng. Asp. 2014, 454, 96–10320. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, G.; Al-Muhtaseb, A.; Naushad, M.; Ghfar, A.; Guo, C.; Stadler, F.J. Biochar-templated g-C3N4/Bi2O2CO3/CoFe2O4 nano-assembly for visible and solar assisted photo-degradation of paraquat, nitrophenol reduction and CO2 conversion. Chem. Eng. J. 2018, 339, 393–4101. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; Ding, D.; Cai, T. From rice straw to magnetically recoverable nitrogen doped biochar: Efficient activation of peroxymonosulfate for the degradation of metolachlor. Appl. Catal. B Environ. 2019, 254, 312–3205. [Google Scholar] [CrossRef]
- Chakhtouna, H.; Benzeid, H.; Zari, N.; Qaiss, A.; Bouhfid, R. Functional CoFe2O4-modified biochar derived from banana pseudostem as an efficient adsorbent for the removal of amoxicillin from water. Sep. Purif. Techn. 2021, 2661, 118592. [Google Scholar] [CrossRef]
- Mahmoud, M.; Abouelanwar, M.; Mahmoud, S.E.M.E.; Abdel Salam, M. Adsorption behavior of silver quantum dots by a novel super magnetic CoFe2O4-biochar-polymeric nanocomposite. J. Coll. Interf. Sci. 2022, 606, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Shi, Z.; Li, Y.; Zhao, Z.; He, B.; Cheng, X. Magnetic cobalt ferrite biochar composite as peroxymonosulfate activator for removal of lomefloxacin hydrochloride. Sep. Purif. Techn. 2021, 2721, 118889. [Google Scholar] [CrossRef]
- Omar, N.; Abdullah, E.C.; Petrus, A.A.; Mubarak, N.M.; Khalid, M.; Agudosi, E.S.; Numan, A.; Aid, S.R. Single-route synthesis of binary metal oxide loaded coconut shell and watermelon rind biochar: Characterizations and cyclic voltammetry analysis. Biomass Conv. Bioref. 2021, 13, 51–62. [Google Scholar] [CrossRef]
- Zhai, Y.; Dai, Y.; Guo, J.; Zhou, L.; Chen, M.; Yang, H.; Peng, L. Novel biochar@CoFe2O4/Ag3PO4 photocatalysts for highly efficient degradation of bisphenol a under visible-light irradiation. J. Coll. Interf. Sci. 2020, 560, 111–121. [Google Scholar] [CrossRef]
- Shabelskaya, N.P.; Egorova, M.A.; Vasileva, E.V.; Polozhentsev, O.E. Photocatalytic properties of nanosized zinc ferrite and zinc chromite. Adv. Nat. Sci. Nanoscien. Nanotechnol. 2021, 12, 015004. [Google Scholar] [CrossRef]
- Egorova, M.A.; Shabelskaya, N.P.; Arzumanova, A.V.; Yakovenko, E.A.; Semchenko, V.V. Synthesis of materials of composition CoM2O4 (M=Al, Fe) for purification of aqueous solutions. IOP Conf. Ser. Earth Environ. Sci. 2021, 723, 042029. [Google Scholar] [CrossRef]
- Lim, D.J.; Marks, N.A.; Rowles, M.R. Universal Scherrer equation for graphene fragments. Carbon 2020, 162, 475–480. [Google Scholar] [CrossRef]
- Denisova, K.O.; Il’in, A.A.; Il’in, A.P.; Sakharova, Y.N. Effect of catalyst composition and process conditions on the catalytic efficiency of cobalt ferrite in the decomposition of nitrogen(I) oxide. Russ. J. Phys. Chem. A 2021, 95, 2014–2019. [Google Scholar] [CrossRef]
- Briceño, S.; Reinoso, C. CoFe2O4-chitosan-graphene nanocomposite for glyphosate removal. Environ. Res. 2022, 212, 113470. [Google Scholar] [CrossRef]
- Oliveira, R.V.M.; Santos, A.F.; Santos, M.D.L.; Cunha, G.C.; Romão, L.P.C. Magnetic solid-phase extraction of bisphenol A from water samples using nanostructured material based on graphene with few layers and cobalt ferrite. Microchem. J. 2022, 181, 107741. [Google Scholar] [CrossRef]
- Gore, S.K.; Jadhav, S.S.; Jadhav, V.V.; Patange, S.M.; Naushad, M.; Mane, R.; Kim, K.H. The structural and magnetic properties of dual phase cobalt ferrite. Scient. Rep. 2017, 7, 2524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krasil’nikov, V.N.; Gyrdasova, O.L.; Bazuev, G.V. Ethylene glycol-modified cobalt and iron oxalates as precursors for the synthesis of oxides as extended microsized and nanosized objects. Russ. J. Inorg. Chem. 2008, 53, 1854–1861. [Google Scholar] [CrossRef]
- Verma, S.; Das, T.; Pandey, V.K.; Verma, B. Nanoarchitectonics of GO/PANI/CoFe2O4 (Graphene Oxide/polyaniline/Cobalt Ferrite) based hybrid composite and its use in fabricating symmetric supercapacitor devices. J. Mol. Struct. 2022, 126615, 133515. [Google Scholar] [CrossRef]
- Kamy, S.T.B.; Esmaeili, M.H. Ultrasound-assisted biodiesel generation from waste edible oil using CoFeO@GO as a superior and reclaimable nanocatalyst: Optimization of two-step transesterification by RSM24. Fuel 2022, 327, 125170. [Google Scholar] [CrossRef]
- Granone, L.I.; Ulpe, A.C.; Robben, L.; Klimke, S.; Jahns, M.; Renz, F.; Gesing, T.M.; Bredow, T.; Dillert, R.; Bahnemann, D.W. Effect of the degree of inversion on optical properties of spinel ZnFe2O4. Phys. Chem. Chem. Phys. 2018, 20, 28267–28278. [Google Scholar] [CrossRef] [Green Version]
- Arimi, A.; Megatif, L.; Granone, L.I.; Dillert, R.; Bahnemann, D.W. Visible-light photocatalytic activity of zinc ferrites. J. photochem. Photobiol. A Chem. 2018, 366, 118–126. [Google Scholar] [CrossRef]
- Zuo, J.; Han, G.; Wang, W.; Huang, Y.; Liu, B.; Su, S. Study on the Application of Modified MOFs to the Treatment of Simulated Metallurgical Wastewater. In Proceedings of the TMS 2022 151st Annual Meeting & Exhibition Supplemental Proceedings, Anaheim, CA, USA, 27 February–3 March 2022; pp. 863–871. [Google Scholar] [CrossRef]
- Anions, K.V.; Ryabchenko, E.S.; Yanovska, V.A.; Tertykh, O.Y.; Kichkiruk; Sternik, D. Adsorption Properties of Vermiculite with In Situ–Immobilized Polyaniline with Respect to Cr(VI), Mo(VI), W(VI), V(V) and P(V). Adsorpt. Sci. Technol. 2014, 32, 89–99. [Google Scholar] [CrossRef]


| Sample | a, nm | Reversibility Parameter λ | Spinel Formula | D, nm | SBET, m2·g−1 |
|---|---|---|---|---|---|
| FS | 0.8376 | 0.9 | (Co0.1Fe0.9)[Co0.9Fe1.1]O4 | 121 | 83.2 |
| FR | 0.8370 | 0.85 | (Co0.15Fe0.85)[Co0.85Fe1.15]O4 | 104 | 87.1 |
| FP | 0.8297 | 0.1 | (Co0.9Fe0.1)[Co0.1Fe1.9]O4 | 208 | 115.9 |
| Sample | Sample Weight, g | n, µmol | N, mg·g−1 |
|---|---|---|---|
| FS | 0.263 | 8.50 | 6.98 |
| FR | 0.430 | 9.75 | 4.90 |
| FP | 0.588 | 3.32 | 1.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabelskaya, N.; Egorova, M.; Radjabov, A.; Burachevskaya, M.; Lobzenko, I.; Minkina, T.; Sushkova, S. Formation of Biochar Nanocomposite Materials Based on CoFe2O4 for Purification of Aqueous Solutions from Chromium Compounds (VI). Water 2023, 15, 93. https://doi.org/10.3390/w15010093
Shabelskaya N, Egorova M, Radjabov A, Burachevskaya M, Lobzenko I, Minkina T, Sushkova S. Formation of Biochar Nanocomposite Materials Based on CoFe2O4 for Purification of Aqueous Solutions from Chromium Compounds (VI). Water. 2023; 15(1):93. https://doi.org/10.3390/w15010093
Chicago/Turabian StyleShabelskaya, Nina, Marina Egorova, Asatullo Radjabov, Marina Burachevskaya, Ilya Lobzenko, Tatiana Minkina, and Svetlana Sushkova. 2023. "Formation of Biochar Nanocomposite Materials Based on CoFe2O4 for Purification of Aqueous Solutions from Chromium Compounds (VI)" Water 15, no. 1: 93. https://doi.org/10.3390/w15010093
APA StyleShabelskaya, N., Egorova, M., Radjabov, A., Burachevskaya, M., Lobzenko, I., Minkina, T., & Sushkova, S. (2023). Formation of Biochar Nanocomposite Materials Based on CoFe2O4 for Purification of Aqueous Solutions from Chromium Compounds (VI). Water, 15(1), 93. https://doi.org/10.3390/w15010093









