Enhanced Removal of As(V) and Pb(II) from Drinking and Irrigating Water Effluents Using Hydrothermally Synthesized Zeolite 5A
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
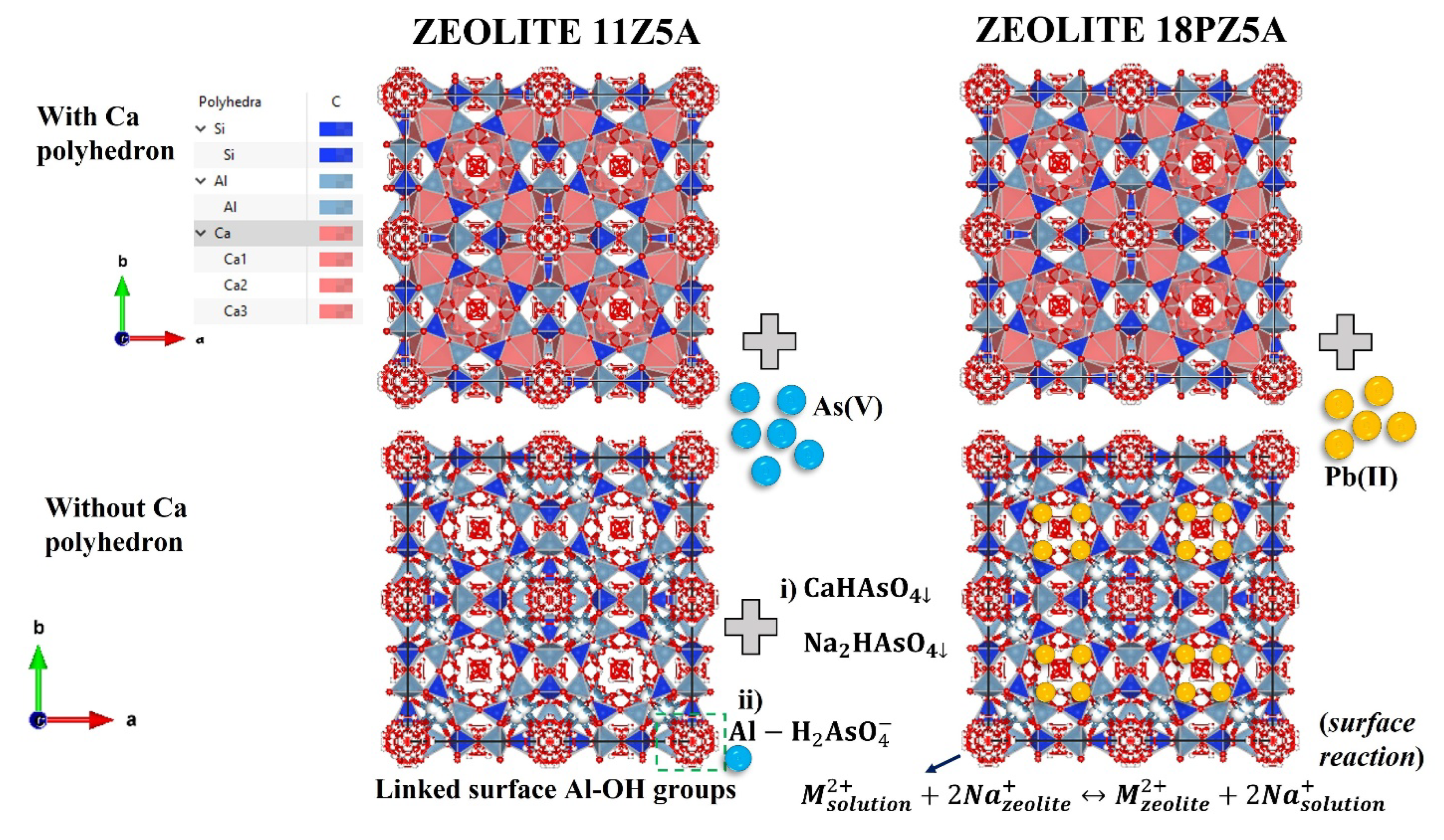
2.2. Synthesis of Adsorbents
2.3. Characterization of the Adsorbents
2.4. Adsorption Experiments
2.4.1. Arsenic Removal
- Adsorption kinetics
- pH effect
- Adsorbent dose
- Adsorption isotherms
- Removal of As from drinking water using 11Z5A
2.4.2. Lead Removal
- Adsorption kinetics
- pH effect
- Adsorption isotherms
- Removal of Pb from irrigation water with 18PZ5A
2.4.3. Theorical Background of Adsorption Models
- Adsorption kinetics models
- Adsorption isotherm models
2.4.4. Error Analysis
- Residual sum of squares (RSS):
- Bayesian information criterion (BIC):
3. Results and Discussion
3.1. X-ray Diffraction and Rietveld Analysis of Zeolite 11Z5A (before and after As Adsorption Experiments)
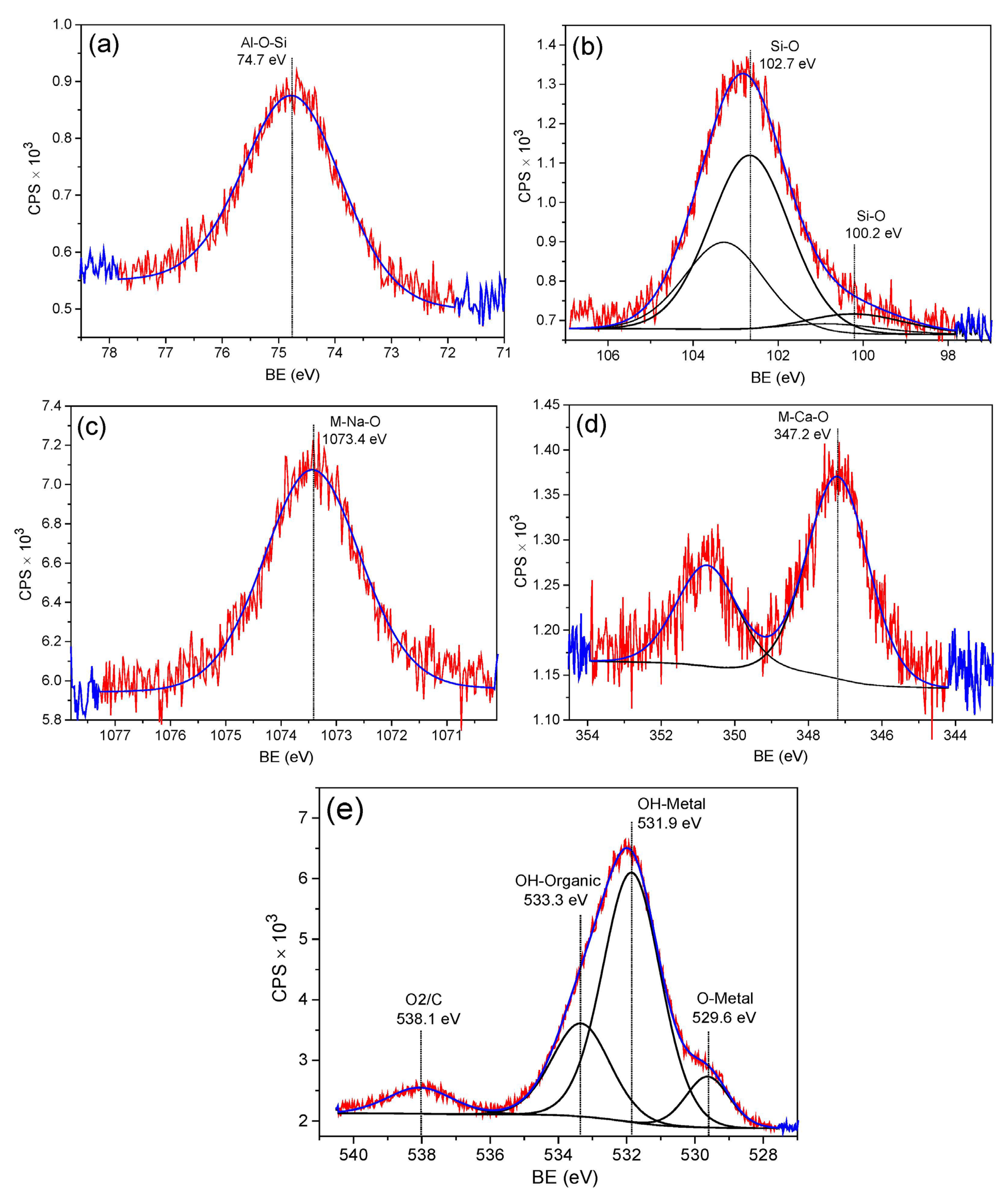
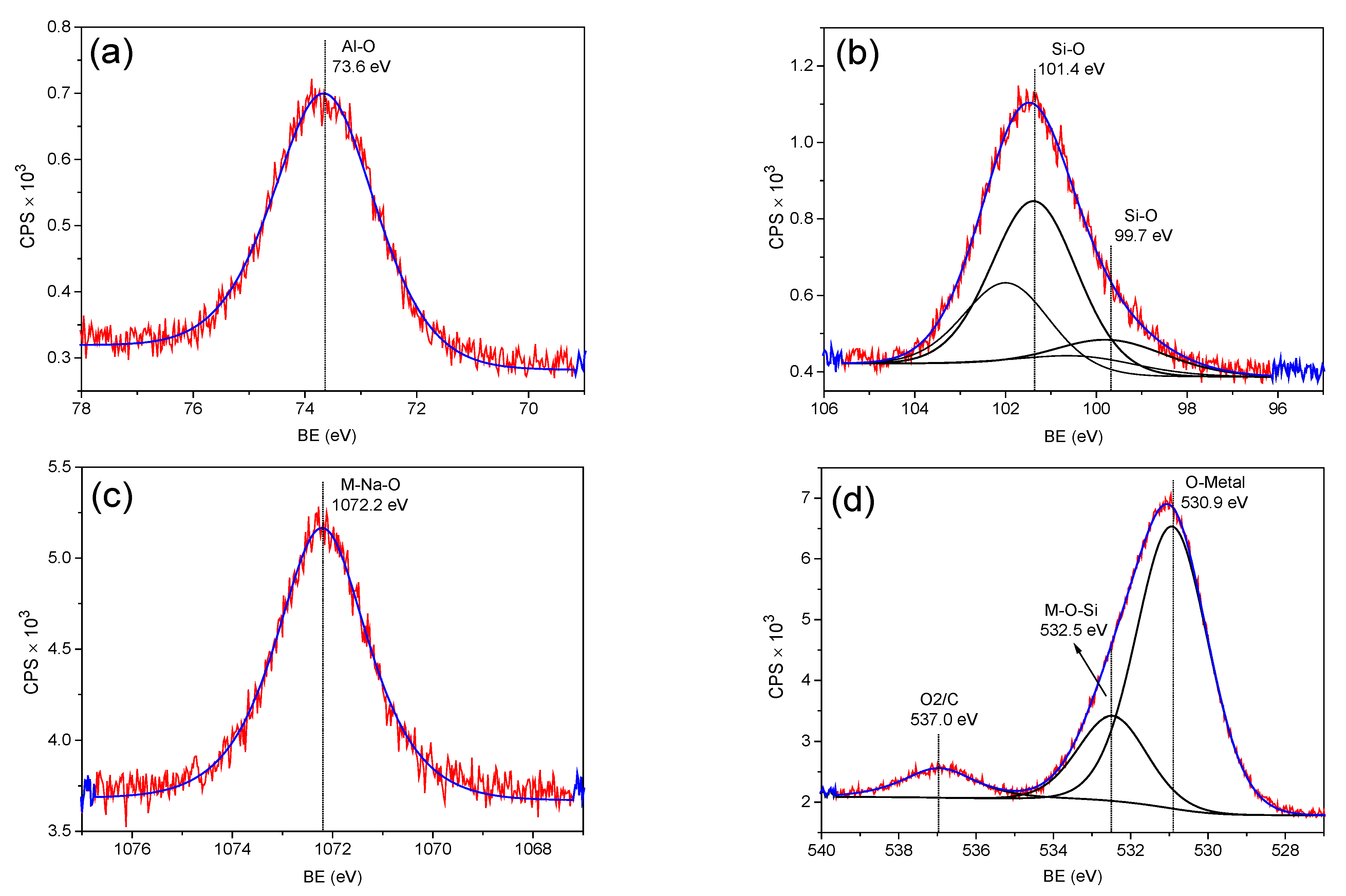
3.2. XPS Analysis of Zeolite 11Z5A (before and after As Adsorption Experiments)
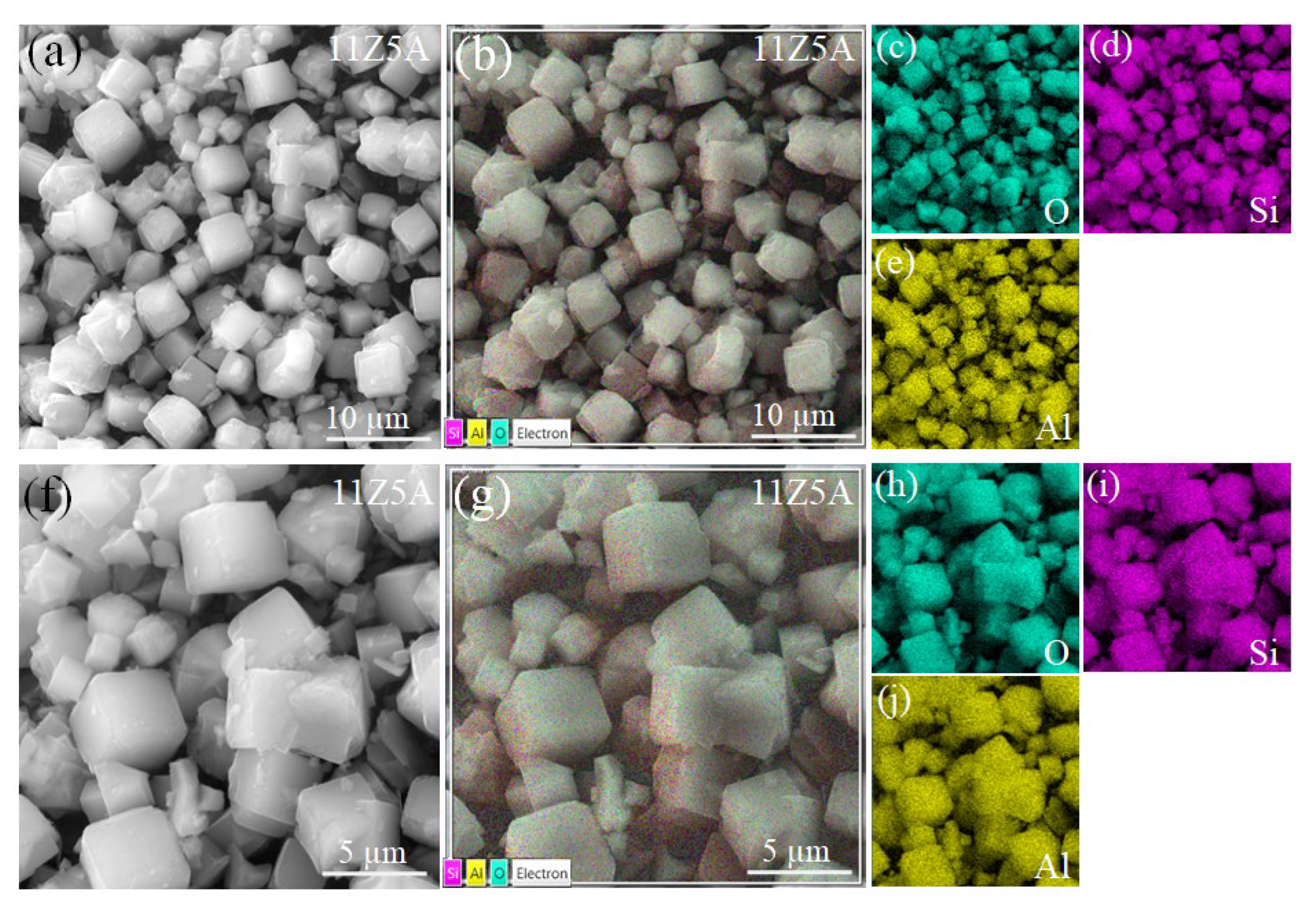
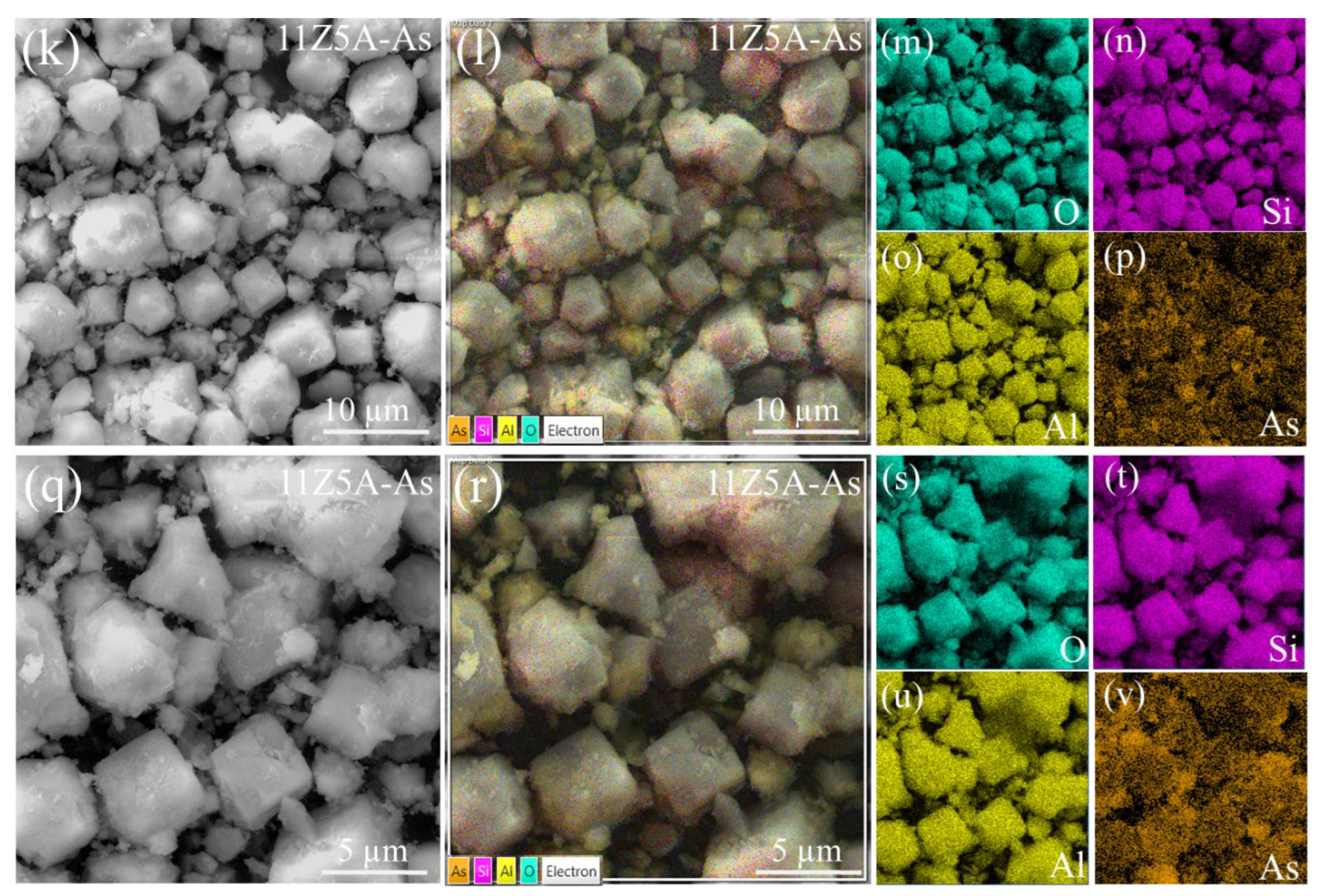
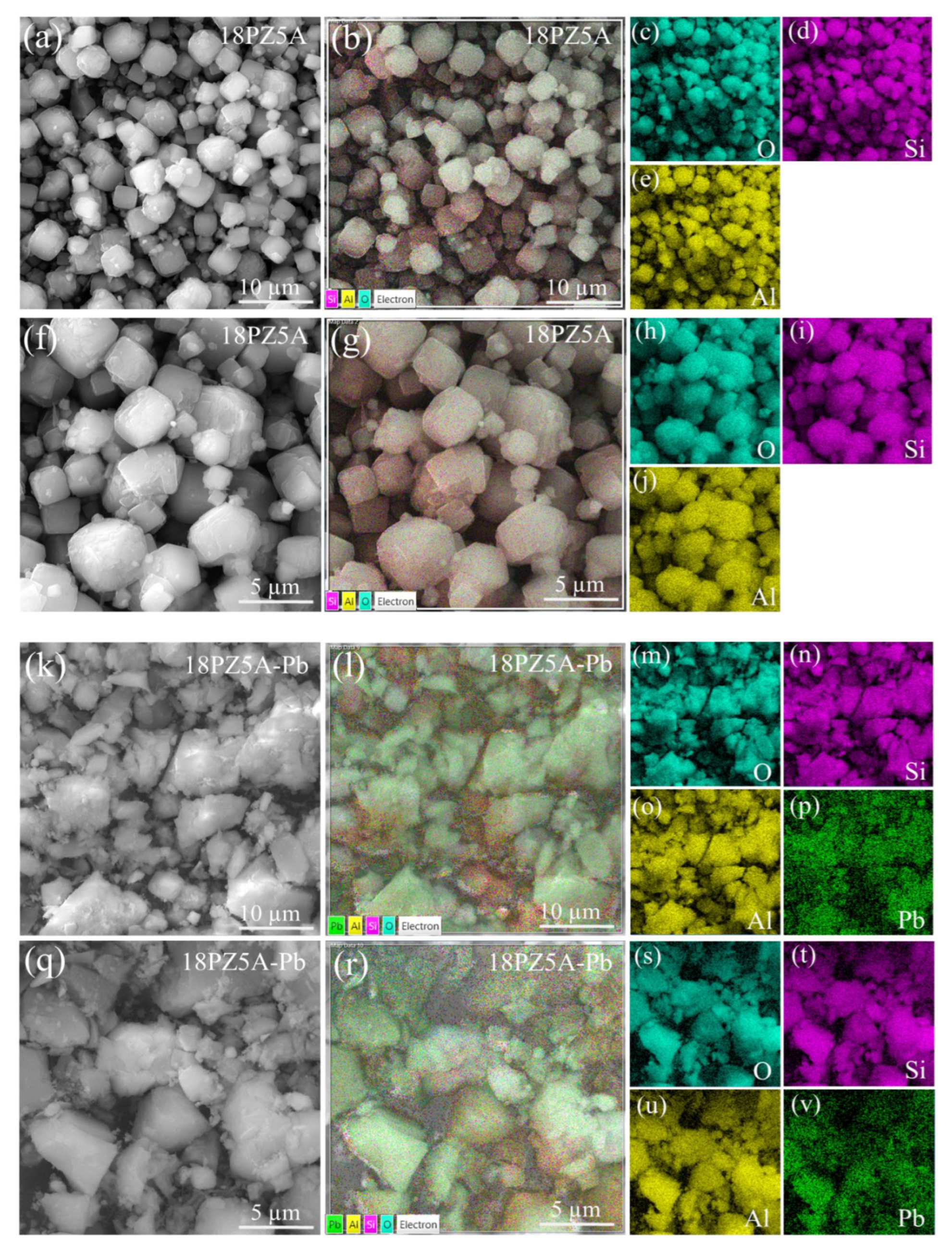
3.3. SEM Analysis of Zeolite 11Z5A (before and after As Adsorption Experiments)
3.4. X-ray Diffraction and Rietveld Analysis of Zeolite 18PZ5A (before and after Pb Adsorption)
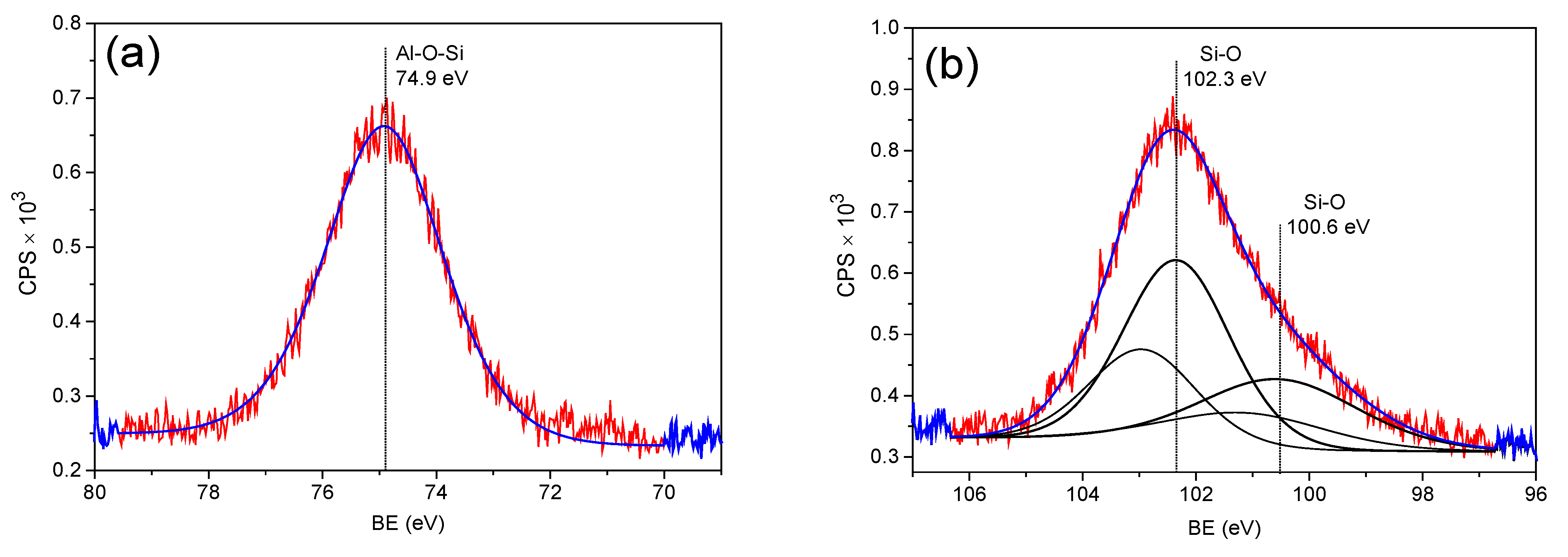
3.5. XPS Analysis of Zeolite 18PZ5A (before and after Pb Adsorption)
3.6. SEM Analysis of Zeolite 18PZ5A (before and after Pb Adsorption)
3.7. Removal Experiments
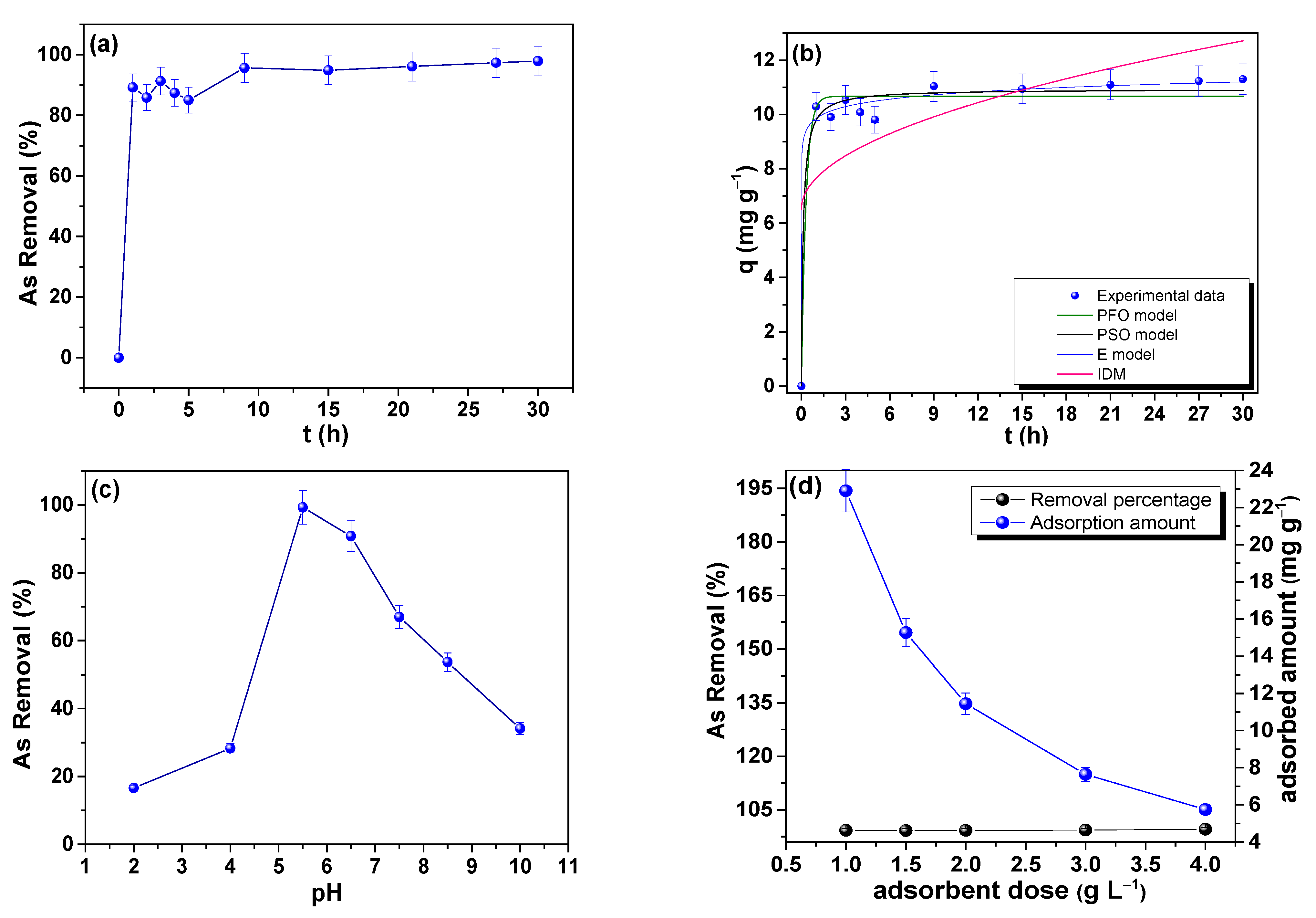
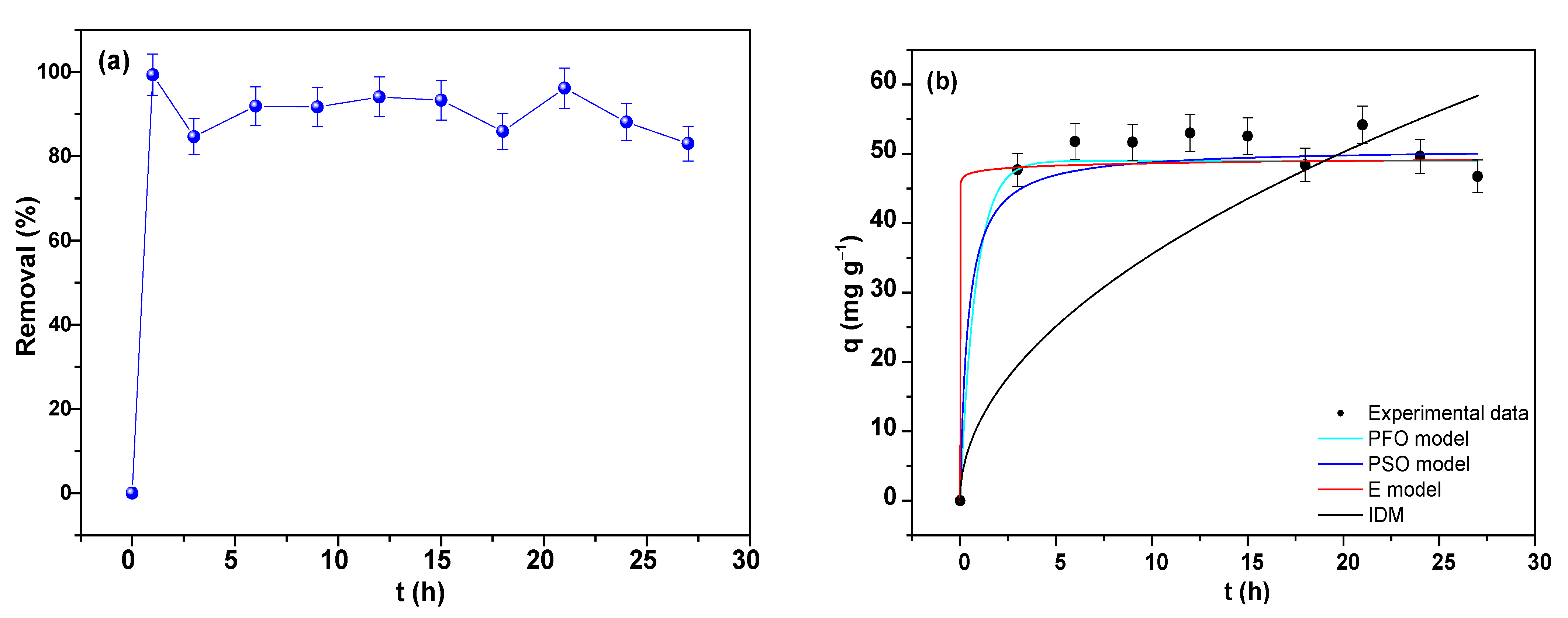
3.7.1. Adsorption Kinetics of As
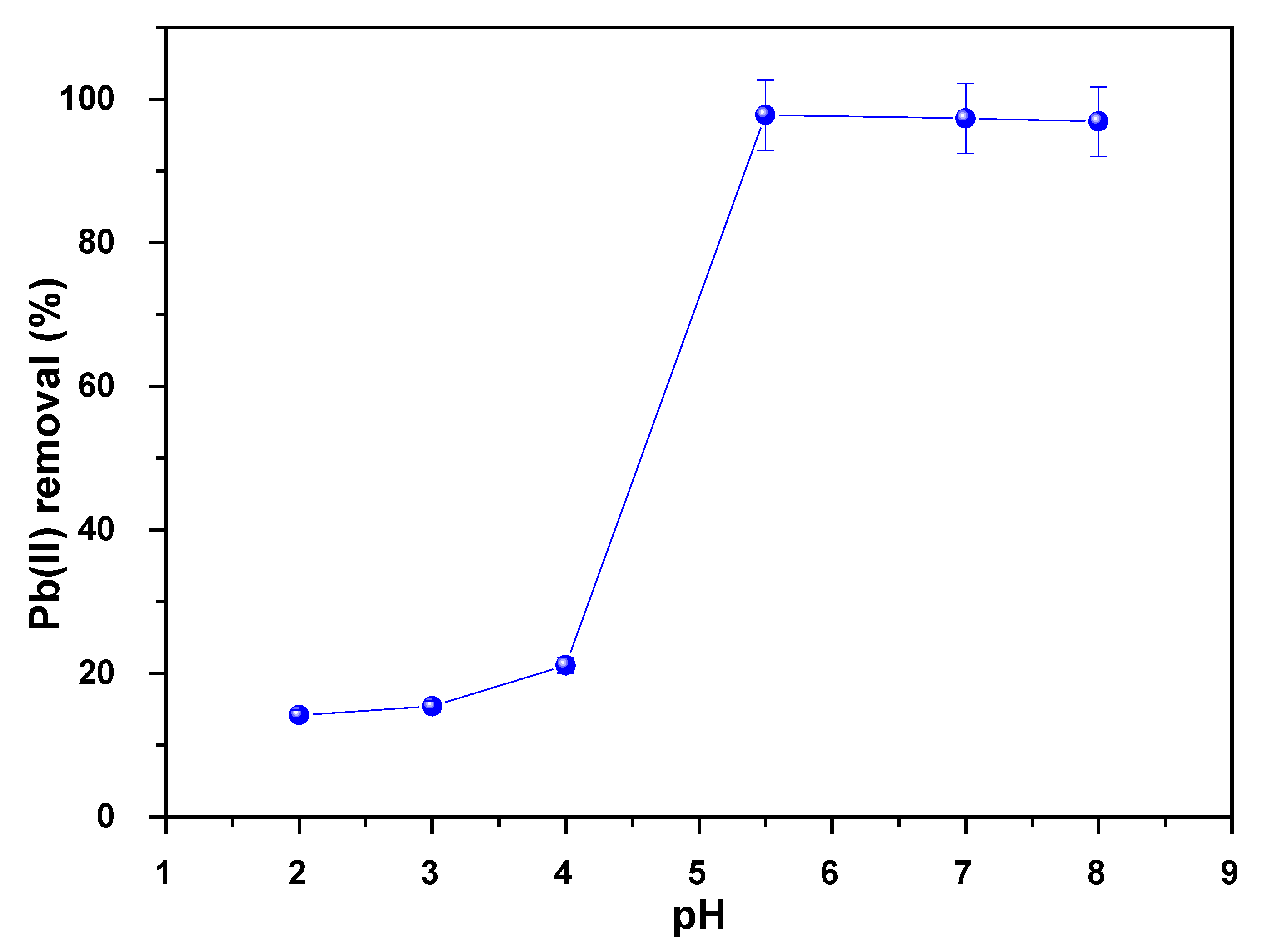
3.7.2. Effect of pH on the As Adsorption
3.7.3. Effect of Adsorbent Dose on As Adsorption
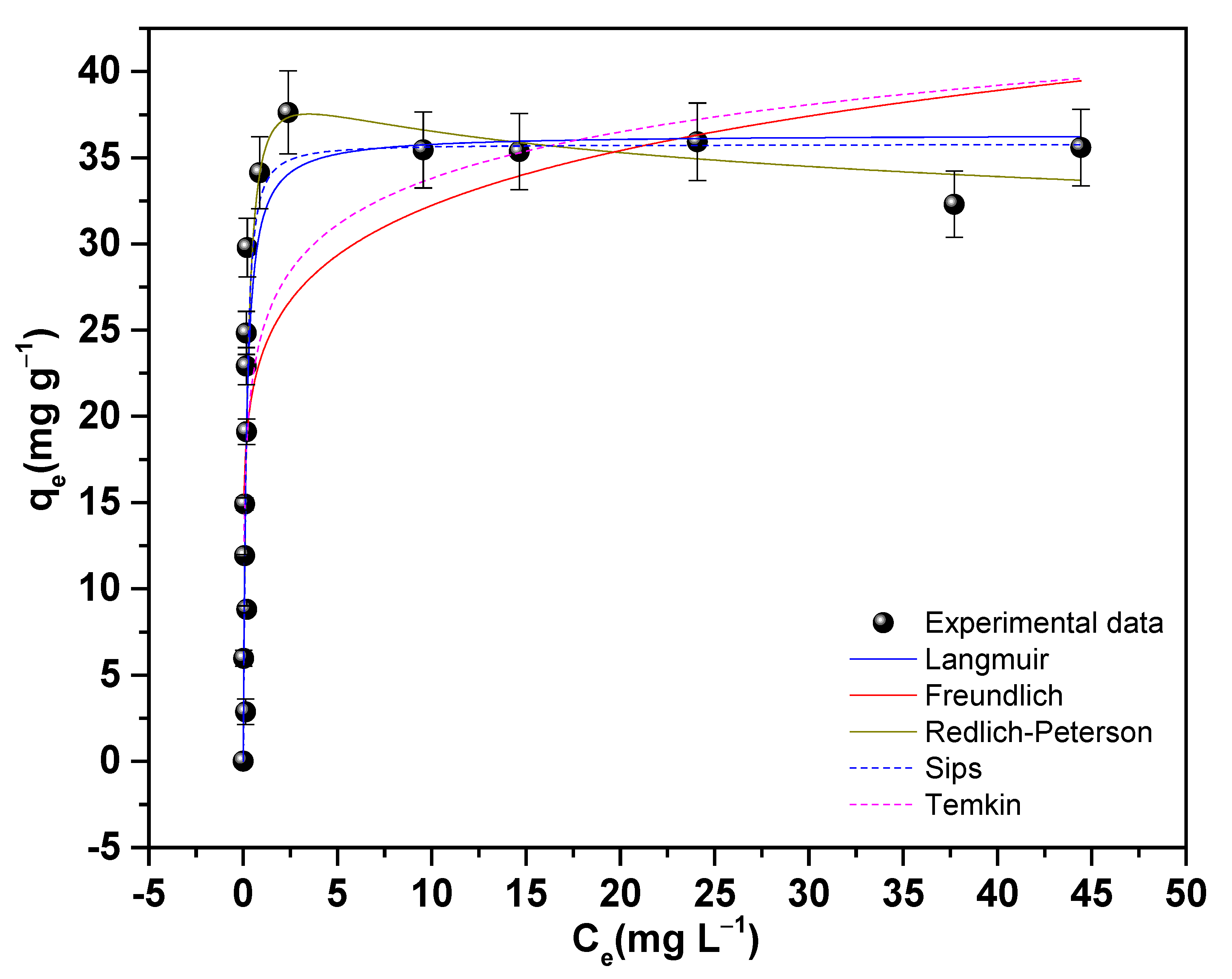
3.7.4. Adsorption Isotherms of As(V) on 11Z5A
3.7.5. Removal of As from Drinking Water with 11Z5A
| Adsorbent | pH | Time (h) | Dose | qmax. (mg g−1) | Removal % | Kinetic Model | Adsorption Model | Ref. |
|---|---|---|---|---|---|---|---|---|
| NZVI-5A zeolite | Not affected | - | 0.2 g | 72.09 | >80 | PSO | Langmuir R2 = 0.99 | [23] |
| Zeolite 5A | 4 | 4 | 0.2 g | 31.27 | >75 | Linear-PSO | Freundlich R2 = 0.99 | [23] |
| Zeolite/Fe3O4 | 4 | 0.67 | - | 47.4 | 96.8 | Linear-PSO | Langmuir | [28] |
| Copper exchanged zeolite A (CEZ) | 7 | - | - | 1.48 | 98 | Linear-PSO | Langmuir R2 = 0.90 | [58] |
| Fe3O4-NaA zeolite | 7 | 1.5 | 0.05 g L−1 | - | 87 | - | - | [59] |
| Zeolite 5A | 6 | 24 | 4.1 | - | - | - | [57] | |
| γ-Fe2O3-coated zeolite (MNCZ) | 2.5 | 0.25 | 0.5 g L−1 | 44 | 95.6 | - | Freundlich R2 = 0.89 | [60] |
| Zeolite 5A | 12 | 5 g L−1 | - | - | - | - | [50] | |
| This work (zeolite 11Z5A) | 5.5 | 9 | 1 g L−1 | 36.35 | 99.3 | Nor-linear PSO | Langmuir R2 = 0.81 |
3.7.6. Adsorption Kinetics of Pb
3.7.7. Effect of pH on the Pb Adsorption
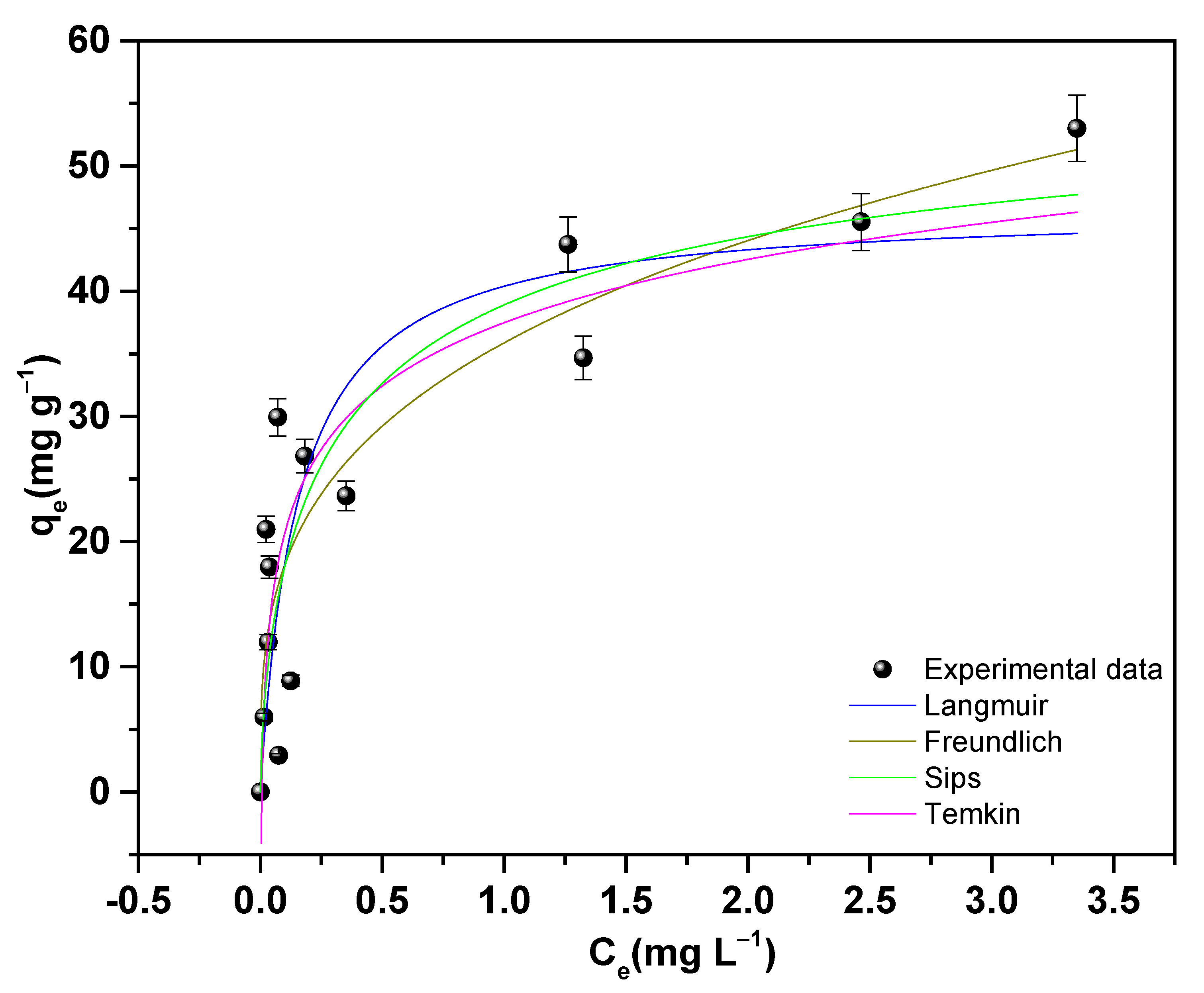
3.7.8. Adsorption Isotherms of Pb(II) on 18PZ5A
3.7.9. Removal of Pb from Irrigation Water with 18PZ5A
| Adsorbent | pH | Time (h) | Dose (g L−1) | qmax. (mg g−1) | Removal % | Kinetic Model | Adsorption Model | Ref. |
|---|---|---|---|---|---|---|---|---|
| H-clinoptilolite | 5.4 | 1 | 10.0 | 9.98 | 75 | Nonlinear-PFO | Freundlich R2 = 0.92 | [62] |
| ZSM-5 | 0.75 | 2 | - | 74.1 | - | - | Langmuir R2 = 0.99 | [77] |
| zeolite/Fe3O4 | 4 | 0.67 | 3.0 | 33.9 | 81.3 | Linear-PSO | Langmuir R2 = 0.99 | [28] |
| Zeolite/CuO | 4 | 0.67 | 3.0 | 47.4 | 96.8 | Linear-PSO | Langmuir R2 = 0.99 | [28] |
| K-Type Zeolite | 5 | 6 | 0.2 | 102.0 | 75.7 | Nonlinear-PSO | Freundlich R2 = 0.99 | [19] |
| Clay-zeolite | 4.97 | 24 | 1.0 | 131.6 | Linear-PSO | Langmuir R2 = 0.99 | [32] | |
| Natural zeolite | 3-6 | 2 | 2 | 100 | 90 | Nonlinear-Double Exponential model | Langmuir R2 = 0.99 | [74] |
| New zeolite-type | 7.3 | 1 | 2.5 | 25.88 | 99.6 | Nonlinear-Pseudo second order | Langmuir R2 = 0.98 | [78] |
| Fe3O4@PDA@L-Cys | 5.5 | 14 | 1.5 | 46.95 | >90 | Linear Pseudo second order | Langmuir R2 = 0.9903–0.9970 | [79] |
| This work (zeolite 18PZ5A) | 6.5 | 12 | 1 | 46.67 | 97.8 | Nonlinear Pseudo first order | Freundlich R2 = 0.75 |
3.7.10. Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aguilar-Ibarra, A. Calidad Del Agua: Un Enfoque Multidisciplinario, 1st ed.; Instituto de Investigaciones Económicas, UNAM: Ciudad de Mexico, Mexico, 2010. [Google Scholar]
- OMS. Arsenic in Drinking-Water; WHO: Geneva, Switzerland, 2011; Volume 27. [Google Scholar] [CrossRef]
- Payne, K.B.; Abdel-Fattah, T.M. Adsorption of Arsenate and Arsenite by Iron-Treated Activated Carbon and Zeolites: Effects of PH, Temperature, and Ionic Strength. J. Environ. Sci. Health Part A 2005, 40, 723–749. [Google Scholar] [CrossRef] [PubMed]
- Henke, K.R. Arsenic: Environmental Chemistry, Health Threats and Waste Treatment, 1st ed.; John Wiley & Sons: West Sussex, UK, 2009. [Google Scholar] [CrossRef]
- Anthony, J.W.; Bideaux, R.A.; Bladh, K.W.; Nichols, M.C. (Eds.) “Galena”. Handbook of Mineralogy; Mineralogical Society of America: Chantilly, VA, USA, 1990; Volume 1, ISBN 0962209708. [Google Scholar]
- Mostafa, N.G.; Yunnus, A.F.; Elawwad, A. Adsorption of Pb(II) from Water onto ZnO, TiO2, and Al2O3: Process Study, Adsorption Behaviour, and Thermodynamics. Adsorpt. Sci. Technol. 2022, 1, 1–13. [Google Scholar] [CrossRef]
- Elawwad, A.; Naguib, A.; Abdel-Halim, H. Modeling of Phenol and Cyanide Removal in a Full-Scale Coke-Oven Wastewater Treatment Plant. Desalin. Water Treat. 2016, 57, 25181–25193. [Google Scholar] [CrossRef]
- Ghorbani, M.; Seyedin, O.; Aghamohammadhassan, M. Adsorptive Removal of Lead (II) Ion from Water and Wastewater Media Using Carbon-Based Nanomaterials as Unique Sorbents: A Review. J. Environ. Manag. 2020, 254, 109814. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lu, M.; Chen, D.; Chen, R.; Li, L.; Han, W. Efficient and Rapid Removal of Pb2+ from Water by Magnetic Fe3O4@MnO2 Core-Shell Nanoflower Attached to Carbon Microtube: Adsorption Behavior and Process Study. J. Colloid Interface Sci. 2020, 563, 218–228. [Google Scholar] [CrossRef]
- Karapinar, H.S.; Kilicel, F.; Ozel, F.; Sarilmaz, A. Fast and Effective Removal of Pb(II), Cu(II) and Ni(II) Ions from Aqueous Solutions with TiO2 Nanofibers: Synthesis, Adsorption-Desorption Process and Kinetic Studies. Int. J. Environ. Anal. Chem. 2021, 1, 1–21. [Google Scholar] [CrossRef]
- Shu, Y.; Huang, R.; Wei, X.; Liu, L.; Jia, Z. Pb(II) Removal Using TiO2-Embedded Monolith Composite Cryogel as an Alternative Wastewater Treatment Method. Water. Air. Soil Pollut. 2017, 228, 1–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, X.; Zhang, P.; Gao, H.; Ou, C.; Kong, X. Production of Activated Carbons from Four Wastes via One-Step Activation and Their Applications in Pb2+ Adsorption: Insight of Ash Content. Chemosphere 2020, 245, 125587. [Google Scholar] [CrossRef]
- Vu, K.B.; Kaminski, M.D.; Nunez, L. Review of Arsenic Removal Technologies for Contaminated Groundwaters; U.S. Department of Energy, Office of Scientific and Technical Information: IL, USA, 2003. [Google Scholar]
- Kurian, M. Cerium Oxide Based Materials for Water Treatment-A Review. J. Environ. Chem. Eng. 2020, 8, 104439. [Google Scholar] [CrossRef]
- Rudi, N.N.; Muhamad, M.S.; Te Chuan, L.; Alipal, J.; Omar, S.; Hamidon, N.; Hamid, N.H.A.; Sunar, N.M.; Ali, R.; Harun, H. Evolution of adsorption process for manganese removal in water via agricultural waste adsorbents. Heliyon 2020, 6, e05049. [Google Scholar] [CrossRef]
- Taamneh, Y.; Sharadqah, S. The Removal of Heavy Metals from Aqueous Solution Using Natural Jordanian Zeolite. Appl. Water Sci. 2017, 7, 2021–2028. [Google Scholar] [CrossRef]
- Wingenfelder, U.; Hansen, C.; Furrer, G.; Schulin, R. Removal of Heavy Metals from Mine Waters by Natural Zeolites. Environ. Sci. Technol. 2005, 39, 4606–4613. [Google Scholar] [CrossRef] [PubMed]
- Bilici Baskan, M.; Pala, A. Removal of Arsenic from Drinking Water Using Modified Natural Zeolite. Desalination 2011, 281, 396–403. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ogata, F.; Saenjum, C. Removal of Pb2+ from Aqueous Solutions Using K-Type Zeoite Synthesized from Coal Fly Ash. Water 2020, 12, 2375. [Google Scholar] [CrossRef]
- Anis, S.F.; Hashaikeh, R.; Hilal, N. Functional materials in desalination: A review. Desalination 2019, 468, 114077. [Google Scholar] [CrossRef]
- Shahmirzadi, M.A.A.; Hosseini, S.S.; Luo, J.; Ortiz, I. Significance, evolution and recent advances in adsorption technology, materials and processes for desalination, water softening and salt removal. J. Environ. Manag. 2018, 215, 324–344. [Google Scholar] [CrossRef]
- Busca, G. Zeolites and Other Structurally Microporous Solids as Acid–Base Materials. In Heterogeneous Catalytic Materials; Elsevier: Amsterdam, The Netherlands, 2014; pp. 197–249. [Google Scholar] [CrossRef]
- Yang, L.; Gao, M.; Wei, T.; Nagasaka, T. Synergistic Removal of As(V) from Aqueous Solution by Nanozero Valent Iron Loaded with Zeolite 5A Synthesized from Fly Ash. J. Hazard. Mater. 2021, 424, 127428. [Google Scholar] [CrossRef] [PubMed]
- Payne, K.B.; Abdel-Fattah, T.M. Adsorption of Divalent Lead Ions by Zeolites and Activated Carbon: Effects of PH, Temperature, and Ionic Strength. J. Environ. Sci. Health Part A 2012, 39, 2275–2291. [Google Scholar] [CrossRef]
- 000519-2022/DIN; Método Para La Obtención De Nanozeolita 5A Mediante Intercambio Iónico De Una Zeolita 4A Sintetizada Por El Método Hidrotermal a Partir De Caolín. INDECOPI: Lima, Perú, 2022.
- Canchanya-Huaman, Y.; Mayta-Armas, A.F.; Pomalaya-Velasco, J.; Bendezú-Roca, Y.; Guerra, J.A.; Ramos-Guivar, J.A. Strain and Grain Size Determination of CeO2 and TiO2 Nanoparticles: Comparing Integral Breadth Methods versus Rietveld, μ-Raman, and TEM. Nanomaterials 2021, 11, 2311. [Google Scholar] [CrossRef]
- Rueda-Vellasmin, R.; Checca-Huaman, N.R.; Passamani, E.C.; Litterst, F.J.; Ramos-Guivar, J.A. Mössbauer Studies of Core-Single-Shell and Core-Double-Shell Polymer Functionalized Magnetic Nanoparticles. Hyperfine Interact. 2022, 243, 1–19. [Google Scholar] [CrossRef]
- Alswat, A.A.; Ahmad, M.B.; Saleh, T.A. Zeolite Modified with Copper Oxide and Iron Oxide for Lead and Arsenic Adsorption from Aqueous Solutions. J. Water Supply Res. Technol.-AQUA 2016, 65, 465–479. [Google Scholar] [CrossRef]
- Pinzón-Bedoya, M.L.; Villamizar, L.E.V. Modelamiento de La Cinética de Bioadsorción de Cr (III) Usando Cáscara de Naranja. DYNA 2009, 76, 95–106. [Google Scholar]
- Imessaoudene, A.; Cheikh, S.; Bollinger, J.C.; Belkhiri, L.; Tiri, A.; Bouzaza, A.; El Jery, A.; Assadi, A.; Amrane, A.; Mouni, L. Zeolite Waste Characterization and Use as Low-Cost, Ecofriendly, and Sustainable Material for Malachite Green and Methylene Blue Dyes Removal: Box–Behnken Design, Kinetics, and Thermodynamics. Appl. Sci. 2022, 12, 7587. [Google Scholar] [CrossRef]
- Choy, K.K.H.; Ko, D.C.K.; Cheung, C.W.; Porter, J.F.; McKay, G. Film and Intraparticle Mass Transfer during the Adsorption of Metal Ions onto Bone Char. J. Colloid Interface Sci. 2004, 271, 284–295. [Google Scholar] [CrossRef]
- Medina-Rodríguez, D.F.; Martin-Torres, D.M.S.; García, C.M.L.; De Luis, V.; Aguilar-ramírez, S.D.; Jaramillo-Fierro, X.V.; Daniel, J.; Garcia-López, A.L. Removal of Pb (II) in Aqueous Solutions Using Synthesized Zeolite X from Ecuadorian Clay. Ing. Investig. 2021, 41, e89671. [Google Scholar] [CrossRef]
- Redlich, O.; Peterson, D.L. A Useful Adsorption Isotherm. J. Phys. Chem. 1959, 63, 1024. [Google Scholar] [CrossRef]
- Tor, A.; Danaoglu, N.; Arslan, G.; Cengeloglu, Y. Removal of Fluoride from Water by Using Granular Red Mud: Batch and Column Studies Removal of Fluoride from Water by Using Granular Red Mud: Batch and Column Studies. J. Hazard. Mater. 2009, 164, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.Y.; Hameed, B.H. Insights into the Modeling of Adsorption Isotherm Systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Ramos Guivar, J.A.; Bustamante, D.A.; Gonzalez, J.C.; Sanches, E.A.; Morales, M.A.; Raez, J.M.; López-Muñoz, M.J.; Arencibia, A. Adsorption of Arsenite and Arsenate on Binary and Ternary Magnetic Nanocomposites with High Iron Oxide Content. Appl. Surf. Sci. 2018, 454, 87–100. [Google Scholar] [CrossRef]
- Muthuselvi, R.; Vashantha, R. Kinetic and Isotherm Studies on Adsorption of Nickel (II) Ions by Brassera Oleracea as a Bioadsorbent. Int. J. Sci. Res. 2016, 5, 2413–2420. [Google Scholar] [CrossRef]
- Lima, E.C.; Sher, F.; Guleria, A.; Reza, M.; Anastopoulos, I.; Nguyen, H.; Hosseini-bandegharaei, A. Is One Performing the Treatment Data of Adsorption Kinetics Correctly? J. Environ. Chem. Eng. 2021, 9, 104813. [Google Scholar] [CrossRef]
- Cao, Y.; Dong, S.; Dai, Z.; Zhu, L.; Xiao, T.; Zhang, X. Adsorption Model Identification for Chromium (VI) Transport in Unconsolidated Sediments. J. Hydrol. 2021, 598, 126228. [Google Scholar] [CrossRef]
- Nguyen, T.T.Q.; Loganathan, P.; Nguyen, T.V.; Vigneswaran, S. Removing Arsenic from Water with an Original and Modified Natural Manganese Oxide Ore: Batch Kinetic and Equilibrium Adsorption Studies. Environ. Sci. Pollut. Res. 2019, 27, 5490–5502. [Google Scholar] [CrossRef]
- Barr, T.L. The Nature of the Relative Bonding Chemistry in Zeolites: An XPS Study. Zeolites 1990, 10, 760–765. [Google Scholar] [CrossRef]
- Mcintyre, N.S.; Cook, M.G. X-Ray Photoelectron Studies on Some Oxides and Hydroxides of Cobalt, Nickel, and Copper. Anal. Chem. 1975, 47, 2208–2213. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Domben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1995. [Google Scholar]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. The Scienta ESCA300 Database; Wiley: Chichester, UK, 1992; Volume 5, p. 2205. [Google Scholar]
- Nilsson, A.; Palmer, R.E.; Tillborg, H.; Hernnäs, B.; Guest, R.J.; Mårtensson, N. Orientation-Dependent Final-State Effects in Photoelectron Spectra of Physisorbed Molecules. Phys. Rev. Lett. 1992, 68, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.G.C.; Curtis, M.T.; Hooper, A.J.; Tucker, P.M.; Generat-, C.E.; Laboratories, B.N.; Gloucestershire, G.L.; Parkinson, R.F.L.D.; Mallett, S.; Dalton, J.C.S. X-Ray Photoelectron Spectroscopy of Iron-Oxygen Systems. J. Chem. Soc. Dalt. Trans. 1974, 1, 1525–1530. [Google Scholar] [CrossRef]
- Stec, W.J.; Morgan, W.E.; Albridge, R.G.; Van Wazer, J.R. Measured Binding Energy Shifts of the “3p” and “3d” Electrons in Arsenic Compounds. Inorg. Chem. 1972, 11, 219–225. [Google Scholar] [CrossRef]
- Kazak, O.; Tor, A. In Situ Preparation of Magnetic Hydrochar by Co-Hydrothermal Treatment of Waste Vinasse with Red Mud and Its Adsorption Property for Pb(II) in Aqueous Solution. J. Hazard. Mater. 2020, 393, 122391. [Google Scholar] [CrossRef]
- Melo, C.R.; Riella, H.G.; Kuhnen, N.C.; Angioletto, E.; Melo, A.R.; Bernardin, A.M.; Da Rocha, M.R.; Da Silva, L. Synthesis of 4A Zeolites from Kaolin for Obtaining 5A Zeolites through Ionic Exchange for Adsorption of Arsenic. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2012, 177, 345–349. [Google Scholar] [CrossRef]
- Munthali, M.W.; Elsheikh, M.A.; Johan, E.; Matsue, N. Proton Adsorption Selectivity of Zeolites in Aqueous Media: Effect of Si/Al Ratio of Zeolites. Molecules 2014, 19, 20468–20481. [Google Scholar] [CrossRef] [PubMed]
- Gorzin, F.; Bahri Rasht Abadi, M.M. Adsorption of Cr(VI) from Aqueous Solution by Adsorbent Prepared from Paper Mill Sludge: Kinetics and Thermodynamics Studies. Adsorpt. Sci. Technol. 2018, 36, 149–169. [Google Scholar] [CrossRef]
- Katenta, J.; Nakiguli, C.; Mukasa, P.; Ntambi, E. Removal of Chromium (VI) from Tannery Effluent Using Bio-Char of Phoenix Reclinata Seeds. Green Sustain. Chem. 2020, 10, 91–107. [Google Scholar] [CrossRef]
- Tahir, H. Comparative Trace Metal Contents in Sediments and Liquid Wastes from Tanneries and the Removal of Chromium Using Zeolite-5A. Electron. J. Environ. Agric. Food Chem. 2005, 4, 1021–1032. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Keren, Y.; Borisover, M.; Bukhanovsky, N. Chemosphere Sorption Interactions of Organic Compounds with Soils Affected by Agricultural Olive Mill Wastewater. Chemosphere 2015, 138, 462–468. [Google Scholar] [CrossRef]
- Qiu, W.; Zheng, Y. Arsenate Removal from Water by an Alumina-Modified Zeolite Recovered from Fly Ash. J. Hazard. Mater. 2007, 148, 721–726. [Google Scholar] [CrossRef]
- Pillewan, P.; Mukherjee, S.; Meher, A.K.; Rayalu, S.; Bansiwal, A. Removal of Arsenic (III) and Arsenic (V) Using Copper Exchange Zeolite-A. Environ. Prog. Sustain. Energy 2014, 33, 1274–1282. [Google Scholar] [CrossRef]
- Sanaei, L.; Tahmasebpour, M.; Khatamian, M.; Divband, B. Arsenic Removal from Aqueous Solutions Using Iron Oxide-Modified Zeolite: Experimental and Modeling Investigations. AUT J. Mech. Eng. 2021, 5, 141–152. [Google Scholar] [CrossRef]
- Salem Attia, T.M.; Hu, X.L.; Yin, D.Q. Synthesised Magnetic Nanoparticles Coated Zeolite (MNCZ) for the Removal of Arsenic (As) from Aqueous Solution. J. Exp. Nanosci. 2012, 9, 551–560. [Google Scholar] [CrossRef]
- Revellame, E.D.; Lord, D.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption Kinetic Modeling Using Pseudo- Fi Rst Order and Pseudo-Second Order Rate Laws: A Review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Tsvetanova, L.; Barbov, B.; Rusew, R. Equilibrium Isotherms and Kinetic Effects during the Adsorption of Pb (II) on Titanosilicates Compared with Natural Zeolite Clinoptilolite. Water 2022, 14, 2152. [Google Scholar] [CrossRef]
- Figueroa, D.; Moreno, A.; Hormaza, A. Equilibrium, Thermodynamic and Kinetic Models for the Adsorption of Red 40 onto Corn Cob. Ing. Univ. Medellín 2014, 14, 105–120. [Google Scholar] [CrossRef]
- Simonin, J. On the Comparison of Pseudo-First Order and Pseudo-Second Order Rate Laws in the Modeling of Adsorption Kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Mamun, O.; Winther, K.T.; Boes, J.R.; Bligaard, T. A Bayesian Framework for Adsorption Energy Prediction on Bimetallic Alloy Catalysts. NPJ Comput. Mater. 2020, 6, 177. [Google Scholar] [CrossRef]
- He, K.; Chen, Y.; Tang, Z.; Hu, Y. Removal of Heavy Metal Ions from Aqueous Solution by Zeolite Synthesized from Fly Ash. Environ. Sci. Pollut. Res. 2015, 23, 2778–2788. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Wang, M.; Chen, B.; Sun, Y.; Chen, K.; Du, Q. Adsorption Capacity of Tetracycline in Solution by Cu-BTC@Carboxyl-Functionalized Carbon Nanotubes@Copper Alginate Composite Aerogel Beads. Coat. Artic. 2022, 12, 1298. [Google Scholar] [CrossRef]
- Joseph, I.V.; Tosheva, L.; Doyle, A.M. Simultaneous Removal of Cd (II), Co (II), Cu (II), Pb (II), and Zn (II) Ions from Aqueous Solutions via Adsorption on FAU-Type Zeolites Prepared from Coal Fl y Ash. J. Environ. Chem. Eng. 2020, 8, 103895. [Google Scholar] [CrossRef]
- Zamzow, M.J.; Eichbaum, B.R.; Sandgren, K.R.; Shanks, D.E. Removal of Heavy Metals and Other Cations from Wastewater Using Zeolites. Sep. Sci. Technol. 1990, 25, 1555–1569. [Google Scholar] [CrossRef]
- Belova, T.P. Heliyon Adsorption of Heavy Metal Ions (Cu2+, Ni2+, Co2+ and Fe2+) from Aqueous Solutions by Natural Zeolite. Heliyon 2019, 5, e02320. [Google Scholar] [CrossRef]
- Senila, M.; Neag, E.; Cadar, O.; Kovacs, E.D.; Aschilean, I.; Kovacs, M.H. Simultaneous Removal of Heavy Metals (Cu, Cd, Cr, Ni, Zn and Pb) from Aqueous Solutions Using Thermally Treated Romanian Zeolitic Volcanic Tuff. Molecules 2022, 27, 3938. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Yu, L.; Wang, Y.; Zhang, J.; Chen, Z.; Dong, L.; Zan, Q.; Li, R. Heavy Metal Adsorption with Zeolites: The Role of Hierarchical Pore Architecture. Chem. Eng. J. 2019, 359, 363–372. [Google Scholar] [CrossRef]
- Yuna, Z. Review of the Natural, Modified, and Synthetic Zeolites for Heavy Metals Removal from Wastewater. Environ. Eng. Sci. 2016, 33, 443–454. [Google Scholar] [CrossRef]
- Yousefi, T.; Mohsen, M.A.; Mahmudian, H.R.; Torab-mostaedi, M.; Moosavian, M.A.; Aghayan, H. Removal of Pb(II) by Modified Natural Adsorbent; Thermodynamics and Kinetics Studies. J. Water Environ. Nanotechnol. 2018, 3, 265–272. [Google Scholar] [CrossRef]
- Irannajad, M.; Haghighi, H.K. Removal of Heavy Metals from Polluted Solutions by Zeolitic Adsorbents: A Review. Environ. Process. 2021, 8, 7–35. [Google Scholar] [CrossRef]
- Jha, V.K.; Matsuda, M.; Miyake, M. Sorption Properties of the Activated Carbon-Zeolite Composite Prepared from Coal Fly Ash for Ni2+, Cu2+, Cd2+ and Pb2+. J. Hazard. Mater. 2008, 160, 148–153. [Google Scholar] [CrossRef]
- Priyadi; Iskandar; Suwardi; Mukti, R.R. Characteristics of Heavy Metals Adsorption Cu, Pb and Cd Using Synthetics Zeolite Zsm-5. J. Tanah Trop. Kualitas Lingkung. 2015, 20, 77–83. [Google Scholar] [CrossRef]
- Xing, P.; Wang, C.; Ma, B.; Chen, Y. Removal of Pb (II) from Aqueous Solution Using a New Zeolite-Type Absorbent: Potassium Ore Leaching Residue. J. Environ. Chem. Eng. 2018, 6, 7138–7143. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Y.; Wang, Q.; Wu, J.; Duan, G.; Xu, W.; Jian, S. Magnetically separable and recyclable Fe3O4@ PDA covalent grafted by l-cysteine core-shell nanoparticles toward efficient removal of Pb2+. Vacuum 2021, 189, 110229. [Google Scholar] [CrossRef]
- Shevade, S.; Ford, R.G. Use of Synthetic Zeolites for Arsenate Removal from Pollutant Water. Water Res. 2004, 38, 3197–3204. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.H.; Sankaran, R.; Show, P.L.; Ooi, C.W.; Liu, B.L.; Chai, W.S.; Chang, Y.K. Removal of Protein Wastes by Cylinder-Shaped NaY Zeolite Adsorbents Decorated with Heavy Metal Wastes. Int. J. Biol. Macromol. 2021, 185, 761–772. [Google Scholar] [CrossRef] [PubMed]
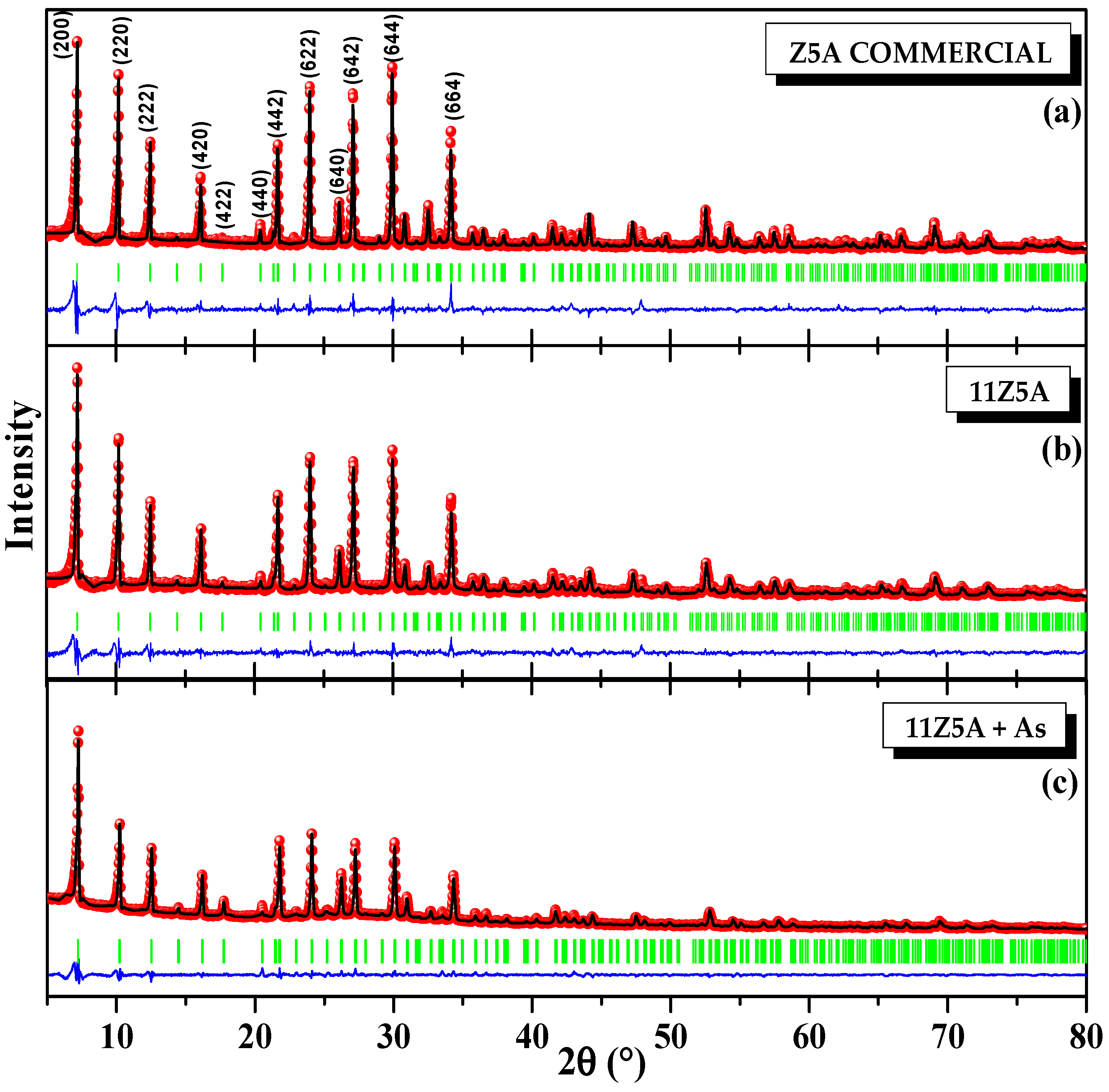



| Refinement Parameters | Z5A Commercial | 11Z5A Before the Adsorption | 11Z5A After the Adsorption |
|---|---|---|---|
| a (Å) | 24.6050 (3) | 24.5883 (3) | 24.5002 (2) |
| b (Å) | 24.6050 (3) | 24.5883 (3) | 24.5002 (2) |
| c (Å) | 24.6050 (3) | 24.5883 (3) | 24.5002 (2) |
| α (°) | 90 | 90 | 90 |
| β (°) | 90 | 90 | 90 |
| γ (°) | 90 | 90 | 90 |
| V (Å3) | 14896.05 (3) | 14865.68 (4) | 14706.39 (2) |
| Average max strain | 6.42 (8) | 9.98 (2) | 10.91 (3) |
| Average size (nm) | 163.79 (8) | 99.21 (9) | 99.76 (2) |
| 15.35 | 7.43 | 7.40 | |
| 27.5 | 8.53 | 19.1 | |
| 27 | 11.3 | 16.5 | |
| χ2 | 3.10 | 2.31 | 4.99 |
| Sample | Level | BE (eV) | at.% | Bond Type | Reference |
|---|---|---|---|---|---|
| 11Z5A | O 1s | 529.6 | 5.1 | O-Metal | [42,43] |
| O 1s | 531.9 | 33.2 | OH-Metal | [41,44] | |
| O 1s | 533.3 | 12.4 | OH-Organic | [45] | |
| O 1s | 538.1 | 3.8 | O=C=O | [46] | |
| Al 2p | 74.7 | 15.9 | Al-O-Si | [41] | |
| Ca 2p3/2 | 347.2 | 1.5 | M-Ca-O | [43] | |
| Si 2p3/2 | 102.7 | 21.7 | Si-O | [41] | |
| Si 2p3/2 | 100.2 | 3.2 | Si-O | [41] | |
| Na 1s | 1073.4 | 3.2 | M-Na-O | [47] | |
| 11Z5A-As | O 1s | 529.3 | 3.5 | O-Metal | [42,43] |
| O 1s | 531.1 | 18.9 | OH-Metal | [44] | |
| O 1s | 532.2 | 31.4 | M-O-Si | [41] | |
| Al 2p | 74.4 | 22.6 | Al-O-Si | [41] | |
| Si 2p3/2 | 99.9 | 7.1 | Si-O | [41] | |
| Si 2p3/2 | 101.9 | 13.4 | Si-O | [41] | |
| As 3p3/2 | 143.9 | 3.1 | As(V) | [48] | |
| 18PZ5A | O 1s | 530.9 | 37.7 | O-Metal | [41] |
| O 1s | 532.5 | 10.8 | M-O-Si | [41] | |
| O 1s | 537.0 | 4.2 | O=C=O | [46] | |
| Al 2p | 73.6 | 17.8 | Al-O | [41] | |
| Si 2p3/2 | 99.7 | 5.6 | Si-O | [41] | |
| Si 2p3/2 | 101.4 | 19.7 | Si-O | [41] | |
| Na 1s | 1072.2 | 4.3 | M-Na-O | [41] | |
| 18PZ5A-Pb | O 1s | 532.1 | 53.7 | M-O-Si | [41] |
| Al 2p | 74.9 | 20.9 | Al-O-Si | [41] | |
| Si 2p3/2 | 100.6 | 7.5 | Si-O | [41] | |
| Si 2p3/2 | 102.3 | 13.1 | Si-O | [41] | |
| F 1s | 685.2 | 1.0 | F-M | [43] | |
| F 1s | 689.3 | 3.1 | F-Organic | [45] | |
| Pb 4f7/2 | 139.7 | 0.7 | Pb(II) | [43,49] |
| PFO Model | PSO Model | E Model | IDM | ||||
|---|---|---|---|---|---|---|---|
| qe (mg g−1) | 10.670 (2) | qe (mg g−1) | 10.935(2) | β (g mg−1) | 2.528 (6) | kp (mg g−1 h−0.5) | 1.129 (5) |
| k1 (h−1) | 3.196 (1) | k2 (g mg−1 h−1) | 0.7779 (4) | α (mg h−1) | 2.627 × 1010(2) | C1 (mg g−1) | 6.525 (2) |
| R2 | 0.9709 | R2 | 0.9801 | R2 | 0.9896 | R2 | 0.324 |
| RSS | 2.7646 | 2.7646 | 0.9855 | 64.2019 | |||
| BIC | −8.2516 | −12.0385 | −18.5665 | 23.1997 | |||
| Freundlich | Langmuir | Redlich–Peterson | |||
|---|---|---|---|---|---|
| kF ((mg g−1)/(mg L−1)1/n) | 23.5937 (2) | qmax (mg g−1) | 36.3525 (2) | A (L g−1) | 175.3144 (4) |
| 1/n | 0.1356 (3) | kL (L mg−1) | 6.1698 (1) | B (L mg−1) | 4.0396 (1) |
| n | 7.14 (2) | R2 | 0.81 | β | 1.0656 (6) |
| R2 | 0.55 | R2 | 0.81 | ||
| RSS | 920.1 | 496.4 | 460.2 | ||
| BIC | 70.38 | 60.50 | 62.06 | ||
| Sips | Temkin | ||||
| qms (mg g−1) | 35.7619 (2) | KT (L g−1) | 604.2939 (7) | ||
| ks (L mg−1)ms | 11.7959 (1) | bT (J mol−1) | 638.089 (1) | ||
| ms | 1.3342 (5) | R2 | 0.69 | ||
| R2 | 0.79 | ||||
| RSS | 479.1 | 777.1 | |||
| BIC | 62.7 | 67.67 | |||
| PFO Model | PSO Model | E Model | IDM | ||||
|---|---|---|---|---|---|---|---|
| qe (mg g−1) | 48.991 (4) | qe (mg g−1) | 50.7794 (6) | β (g mg−1) | 2.0203 (2) | kp (mg g−1 h−0.5) | 11.2344 (8) |
| k1 (h−1) | 1.2188 (3) | k2 (g mg−1 h−1) | 0.04813 (8) | α (mg h−1) | 2.045 × 1041 (3) | C1 (mg g−1) | 2.7044 × 10−8 (4) |
| R2 | 0.99 | R2 | 0.94 | R2 | 0.99 | R2 | 0.95 |
| RSS | 536.0106 | 536.0106 | 157.6133 | 4160.0438 | |||
| BIC | 44.4209 | 43.5098 | 32.1808 | 64.9121 | |||
| Freundlich | Langmuir | Sips | Temkin | ||||
|---|---|---|---|---|---|---|---|
| kF ((mg g−1)/(mg L−1)1/n) | 35.8754 (2) | qmax (mg g−1) | 46.6739 (6) | qms (mg g−1) | 59.1141 (3) | KT (L g−1) | 170.4298 (1) |
| 1/n | 0.2959 (5) | kL (L mg−1) | 6.4304 (3) | ks (L mg−1)ms | 1.9252 (3) | bT (J mol−1) | 339.626 (6) |
| n | 3.45 (6) | R2 | 0.69 | ms | 0.6421 (4) | R2 | 0.69 |
| R2 | 0.75 | R2 | 0.73 | ||||
| RSS | 688.39 | 1048.69 | 819.04 | 832.37 | |||
| BIC | 56.73 | 62.2 | 61.56 | 59.20 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayta-Armas, A.F.; Canchanya-Huaman, Y.; Pomalaya-Velasco, J.; Bendezú-Roca, Y.; Checca-Huaman, N.-R.; Ramos-Guivar, J.A. Enhanced Removal of As(V) and Pb(II) from Drinking and Irrigating Water Effluents Using Hydrothermally Synthesized Zeolite 5A. Water 2023, 15, 1892. https://doi.org/10.3390/w15101892
Mayta-Armas AF, Canchanya-Huaman Y, Pomalaya-Velasco J, Bendezú-Roca Y, Checca-Huaman N-R, Ramos-Guivar JA. Enhanced Removal of As(V) and Pb(II) from Drinking and Irrigating Water Effluents Using Hydrothermally Synthesized Zeolite 5A. Water. 2023; 15(10):1892. https://doi.org/10.3390/w15101892
Chicago/Turabian StyleMayta-Armas, Angie F., Yamerson Canchanya-Huaman, Jemina Pomalaya-Velasco, Yéssica Bendezú-Roca, Noemi-Raquel Checca-Huaman, and Juan A. Ramos-Guivar. 2023. "Enhanced Removal of As(V) and Pb(II) from Drinking and Irrigating Water Effluents Using Hydrothermally Synthesized Zeolite 5A" Water 15, no. 10: 1892. https://doi.org/10.3390/w15101892
APA StyleMayta-Armas, A. F., Canchanya-Huaman, Y., Pomalaya-Velasco, J., Bendezú-Roca, Y., Checca-Huaman, N.-R., & Ramos-Guivar, J. A. (2023). Enhanced Removal of As(V) and Pb(II) from Drinking and Irrigating Water Effluents Using Hydrothermally Synthesized Zeolite 5A. Water, 15(10), 1892. https://doi.org/10.3390/w15101892








