Removal and Reclamation of Trace Metals from Copper and Gold Mine Tailing Leachates Using an Alkali Suspension Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Trace Metal Concentrations
2.3. Synthetic Leachate Experiment
2.4. Authentic Leachate Experiment
2.5. Life Cycle Impact Assessment
3. Results and Discussion
3.1. Effect of pH
3.2. The Appropriate HRT and Amount of Soil Additive
3.3. Trace Metal Removal Efficiency from Authentic Leachates
3.4. Effect of Temperature
3.5. Effect of Soil Composition
3.6. Interactions between the Soil and Leachate
3.7. Estimation of the Consumption of Ca(OH)2 in an Alkali–Soil Suspension System
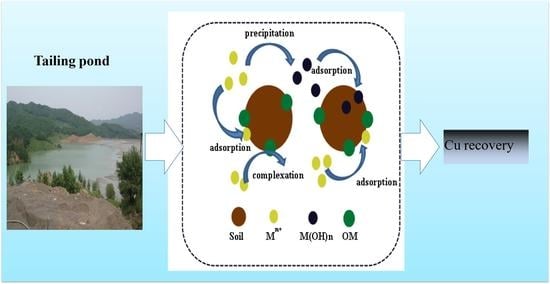
3.8. Reclaiming of Trace Metals
3.9. LCA Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fetisov, V.V.; Menshikova, E.A. Evaluation and Prediction of Seepage Discharge through Tailings Dams When Their Height Increases. In Applied Geology; Springer: Cham, Switzerland, 2020; pp. 21–41. [Google Scholar]
- Komljenovic, D.; Stojanovic, L.; Malbasic, V.; Lukic, A. A resilience-based approach in managing the closure and abandonment of large mine tailing ponds. Int. J. Min. Sci. Technol. 2020, 30, 737. [Google Scholar] [CrossRef]
- Xu, D.M.; Zhan, C.L.; Liu, H.X.; Lin, H.Z. A critical review on environmental implications, recycling strategies, and ecological remediation for mine tailings. Environ. Sci. Pollut. Res. 2019, 26, 35657–35669. [Google Scholar] [CrossRef] [PubMed]
- Davila, R.B.; Fontes, M.P.F.; Pacheco, A.A.; da Silva Ferreira, M. Trace metals in iron ore tailings and floodplain soils affected by the Samarco dam collapse in Brazil. Sci. Total Environ. 2020, 709, 136151. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liu, W.; Xu, H.; Cui, X.; Li, J.; Chen, J.; Zheng, B. Characterizations of trace metal contamination, microbial community, and resistance genes in a tailing of the largest copper mine in China. Environ. Pollut. 2021, 280, 116947. [Google Scholar] [CrossRef]
- Kan, X.; Dong, Y.; Feng, L.; Zhou, M.; Hou, H. Contamination and health risk assessment of trace metals in China’s lead–zinc mine tailings: A meta–analysis. Chemosphere 2021, 267, 128909. [Google Scholar] [CrossRef] [PubMed]
- Tao, E.; Ma, D.; Yang, S.; Sun, Y.; Xu, J.; Kim, E.J. Zirconium dioxide loaded montmorillonite composites as high-efficient adsorbents for the removal of Cr3+ ions from tanning wastewater. J. Solid State Chem. 2019, 277, 502. [Google Scholar]
- Tang, B.; Xu, H.; Song, F.; Ge, H.; Yue, S. Effects of trace metals on microorganisms and enzymes in soils of lead–zinc tailing ponds. Environ. Res. 2022, 207, 112174. [Google Scholar] [CrossRef]
- Young, G.; Chen, Y.; Yang, M. Concentrations, distribution, and risk assessment of trace metals in the iron tailings of Yeshan National Mine Park in Nanjing, China. Chemosphere 2021, 271, 129546. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.S.; Song, H.; Yim, G.J.; Ji, S.W.; Kim, J.G. An engineered cover system for mine tailings using a hardpan layer: A solidification/stabilization method for layer and field performance evaluation. J. Hazard. Mater. 2011, 197, 153–160. [Google Scholar] [CrossRef]
- Shen, L.; Luo, S.; Zeng, X.; Wang, H. Review on Anti-Seepage Technology Development of Tailings Pond in China. Procedia Eng. 2011, 26, 1803. [Google Scholar] [CrossRef]
- Sánchez-Andrea, I.; Sanz, J.L.; Bijmans, M.F.M.; Stams, A.J.M. Sulfate reduction at low pH to remediate acid mine drainage. J. Hazard. Mater. 2014, 269, 98. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.M.; Rodriguez, R.P.; Sancinetti, G.P. Removal sulfate and metals Fe+2, Cu+2, and Zn+2 from acid mine drainage in an anaerobic sequential batch reactor. J. Environ. Chem. Eng. 2017, 5, 1985–1989. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, L.; Zhang, Z.; Chen, G.H.; Jiang, F. Authenticizing high-rate sulfur reduction under sulfate-rich conditions in a biological sulfide production system to treat metal-laden wastewater deficient in organic matter. Water Res. 2018, 131, 239. [Google Scholar] [CrossRef] [PubMed]
- Vargas, F.; Lopez, M.; Rigamonti, L. Environmental impacts evaluation of treated copper tailings as supplementary cementitious materials. Resour. Conserv. Recy. 2020, 160, 104890. [Google Scholar] [CrossRef]
- Yang, L.; Yi, G.; Wang, B.; Shao, P.; Feng, Y.; Liu, Y.; Yu, B.; Liu, F.; Liu, L.; Luo, X.; et al. Atomic H* enhanced electrochemical recovery towards high-value-added metallic Sb from complex mine flotation wastewater. Resour. Conserv. Recy. 2022, 178, 106020. [Google Scholar] [CrossRef]
- He, Y.; Li, B.B.; Zhang, K.N.; Li, Z.; Chen, Y.G.; Ye, W.M. Experimental and numerical study on trace metal contaminant migration and retention behavior of engineered barrier in tailings pond. Environ. Pollut. 2019, 252, 1010–1018. [Google Scholar] [CrossRef]
- Moraci, N.; Calabrò, P.S. Trace metals removal and hydraulic performance in zero-valent iron/pumice permeable reactive barriers. J. Environ. Manag. 2010, 91, 2336–2341. [Google Scholar] [CrossRef]
- Park, M.; Park, J.; Kang, J.; Han, Y.S.; Jeong, H.Y. Removal of hexavalent chromium using mackinawite (FeS)-coated sand. J. Hazard. Mater. 2018, 360, 17. [Google Scholar] [CrossRef]
- Pal, P.; Pal, A.; Nakashima, K.; Yadav, B.K. Applications of chitosan in environmental remediation: A review. Chemosphere 2021, 266, 128934. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, S.; Yang, G.; Wang, G.; Zhang, L.; Xia, D.; Huang, H.; Cheng, Z.; Xu, J.; Sun, C.; et al. Z-scheme Au decorated carbon nitride/cobalt tetroxide plasmonic heterojunction photocatalyst for catalytic reduction of hexavalent chromium and oxidation of Bisphenol A. J. Hazard. Mater. 2021, 410, 124539. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Zhang, T.; Zhao, Y.; Wen, T.; Zhang, Q.; Bao, S.; Song, S. Removal of Cu(II) from wastewater by using mechanochemically activated carbonate-based tailings through chemical precipitation. Environ. Sci. Pollut. Res. 2019, 26, 35198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, B.; Leiviskä, T. Vanadium recovery from spent iron sorbent used for the treatment of mining-influenced water. Resour. Conserv. Recy. 2019, 182, 106291. [Google Scholar] [CrossRef]
- Ye, M.; Li, G.; Yan, P.; Ren, J.; Zheng, L.; Han, D.; Sun, S.; Huang, S.; Zhong, Y. Removal of metals from lead-zinc mine tailings using bioleaching and followed by sulfide precipitation. Chemosphere 2017, 185, 1189. [Google Scholar] [CrossRef]
- Zeng, C.; Hu, H.; Feng, X.; Wang, K.; Zhang, Q. Activating CaCO3 to enhance lead removal from lead-zinc solution to serve as green technology for the purification of mine tailings. Chemosphere 2020, 249, 126227. [Google Scholar] [CrossRef]
- Huang, H.; Liu, J.; Zhang, P.; Zhang, D.; Gao, F. Investigation on the simultaneous removal of fluoride, ammonia nitrogen and phosphate from semiconductor wastewater using chemical precipitation. Chem. Eng. J. 2017, 307, 696–706. [Google Scholar] [CrossRef]
- Ge, J.C.; Yoon, S.K.; Choi, N.J. Application of fly ash as an adsorbent for removal of air and water pollutants. Appl. Sci. 2018, 8, 1116. [Google Scholar] [CrossRef]
- Karanac, M.; Đolić, M.; Veljović, Đ.; Rajaković-Ognjanović, V.; Veličković, Z.; Pavićević, V.; Marinković, A. The removal of Zn2+, Pb2+, and As (V) ions by lime activated fly ash and valorization of the exhausted adsorbent. Waste Manag. 2018, 78, 366. [Google Scholar] [CrossRef]
- Qi, L.; Teng, F.; Deng, X.; Zhang, Y.; Zhong, X. Experimental study on adsorption of Hg(II) with microwave-assisted alkali-modified fly ash. Powder Technol. 2019, 351, 153. [Google Scholar] [CrossRef]
- Hong, X.Q.; Li, R.Z.; Liu, W.J.; Zhang, X.S.; Ding, H.S.; Jiang, H. An investigation on reuse of Cr-contaminated sediment: Cr removal and interaction between Cr and organic matter. Chem. Eng. J. 2012, 189, 222–228. [Google Scholar] [CrossRef]
- Saha, N.; Saba, A.; Reza, M.T. Effect of hydrothermal carbonization temperature on pH, dissociation constants, and acidic functional groups on hydrochar from cellulose and wood. J. Anal. Appl. Pyrol. 2019, 137, 138. [Google Scholar] [CrossRef]
- Hengen, T.J.; Squillace, M.K.; O’Sullivan, A.D.; Stone, J.J. Life cycle assessment analysis of active and passive acid mine drainage treatment technologies. Resour. Conserv. Recy. 2014, 86, 160–167. [Google Scholar] [CrossRef]
- Joseph, L.; Jun, B.M.; Flora, J.R.; Park, C.M.; Yoon, Y. Removal of trace metals from water sources in the developing world using low-cost materials: A review. Chemosphere 2019, 229, 142–159. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhou, G.; Chu, L.; Liu, Y.; Liu, C.; Luo, S.; Wei, Y. Efficient removal of trace metal ions with an EDTA functionalized chitosan/polyacrylamide double network hydrogel. ACS Sustain. Chem. Eng. 2017, 5, 843–851. [Google Scholar] [CrossRef]
- Abd Ali, Z.T.; Naji, L.A.; Almuktar, S.A.; Faisal, A.A.; Abed, S.N.; Scholz, M.; Naushad, M.; Ahamad, T. Predominant mechanisms for the removal of nickel metal ion from aqueous solution using cement kiln dust. J. Water Process Eng. 2020, 33, 101033. [Google Scholar] [CrossRef]
- Qian, T.T.; Zhang, X.S.; Hu, J.Y.; Jiang, H. Effects of environmental conditions on the release of phosphorus from biochar. Chemosphere 2013, 93, 2069. [Google Scholar] [CrossRef]
- Lin, K.Y.A.; Liu, Y.T.; Chen, S.Y. Adsorption of fluoride to UiO-66-NH2 in water: Stability, kinetic, isotherm and thermodynamic studies. J. Colloid Interf. Sci. 2016, 461, 79–87. [Google Scholar] [CrossRef]
- Liu, W.J.; Zeng, F.X.; Jiang, H.; Zhang, X.S. Adsorption of lead (Pb) from aqueous solution with Typha angustifolia biomass modified by SOCl2 activated EDTA. Chem. Eng. J. 2011, 170, 21–28. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.H.; Show, P.L. A review on conventional and novel materials towards trace metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Lai, D.; Deng, L.; Guo, Q.; Fu, Y. Hydrolysis of biomass by magnetic solid acid. Energy Environ. Sci. 2011, 4, 3552–3557. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, M.; Hao, Y. High efficient removal of Pb (II) by amino-functionalized Fe3O4 magnetic nano-particles. Chem. Eng. J. 2012, 191, 104. [Google Scholar] [CrossRef]
- Yong, L.L.; Namal Jayasanka Perera, S.V.A.D.; Syamsir, A.; Emmanuel, E.; Paul, S.C.; Anggraini, V. Stabilization of a residual soil using calcium and magnesium hydroxide nanoparticles: A quick precipitation method. Appl. Sci. 2019, 9, 4325. [Google Scholar] [CrossRef]
- Visa, M. Synthesis and characterization of new zeolite materials obtained from fly ash for trace metals removal in advanced wastewater treatment. Powder Technol. 2016, 294, 338. [Google Scholar] [CrossRef]
- Visa, M.; Isac, L.; Duta, A. Fly ash adsorbents for multi-cation wastewater treatment. Appl. Surf. Sci. 2012, 258, 6345. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A.R. Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 2012, 89, 1467. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Rizwan, M.; Ali, S.; Hassan, M.J.; Brestic, M.; Zhang, X.; Huang, L. Effects of silicon on trace metal uptake at the soil-plant interphase: A review. Ecotox. Environ. Safe 2021, 222, 112510. [Google Scholar] [CrossRef]
- Kusaka, R.; Nihonyanagi, S.; Tahara, T. The photochemical reaction of phenol becomes ultrafast at the air–water interface. Nat. Chem. 2021, 13, 306. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, K.; Fang, Y.; Guo, Z.; Wei, Y.; Sheng, K. Conversion from bamboo waste derived biochar to cleaner composites: Synergistic effects of aramid fiber and silica. J. Clean. Prod. 2022, 347, 131336. [Google Scholar] [CrossRef]
- Kani, A.N.; Dovi, E.; Aryee, A.A.; Han, R.; Qu, L. Efficient removal of 2, 4-D from solution using a novel antibacterial adsorbent based on tiger nut residues: Adsorption and antibacterial study. Environ. Sci. Pollut. Res. 2022, 29, 64177–64191. [Google Scholar] [CrossRef]
- Martin-Gullon, I.; Perez, J.M.; Domene, D.; Salgado-Casanova, A.J.; Radovic, L.R. New insights into oxygen surface coverage and the resulting two-component structure of graphene oxide. Carbon 2020, 158, 406–417. [Google Scholar] [CrossRef]
- Oliver, D.P.; Li, Y.; Orr, R.; Nelson, P.; Barnes, M.; McLaughlin, M.J.; Kookana, R.S. The role of surface charge and pH changes in tropical soils on sorption behaviour of per-and polyfluoroalkyl substances (PFASs). Sci. Total Environ. 2019, 673, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Presura, E.; Robescu, L.D. Energy use and carbon footprint for potable water and wastewater treatment. Proc. Int. Conf. Bus. Excell. 2017, 11, 191–198. [Google Scholar] [CrossRef]
- Martínez, N.M.; Basallote, M.D.; Meyer, A.; Cánovas, C.R.; Macías, F.; Schneider, P. Life cycle assessment of a passive remediation system for acid mine drainage: Towards more sustainable mining activity. J. Clean. Prod. 2019, 211, 1100–1111. [Google Scholar] [CrossRef]








| Trace Metal | Temp. (°C) | Pseudo-First Order Kinetics | Pseudo-Second Order Kinetics | ||||
|---|---|---|---|---|---|---|---|
| qe | k1 | R2 | qe | k2 | R2 | ||
| Cu2+ | 10 | 2.46 | 0.92 | 0.9999 | 2.47 | 8.79 | 0.9999 |
| 20 | 2.44 | 1.02 | 0.9999 | 2.45 | 10.52 | 0.9999 | |
| 30 | 2.43 | 1.07 | 0.9999 | 2.44 | 7.46 | 0.9999 | |
| Zn2+ | 10 | 2.46 | 0.92 | 0.9999 | 2.47 | 8.79 | 0.9999 |
| 20 | 2.44 | 0.83 | 0.9998 | 2.46 | 3.31 | 0.9999 | |
| 30 | 2.46 | 0.92 | 0.9999 | 2.46 | 8.78 | 0.9999 | |
| Cd2+ | 10 | 2.12 | 0.57 | 0.9998 | 2.14 | 2.26 | 0.9999 |
| 20 | 2.13 | 0.72 | 0.9994 | 2.16 | 2.74 | 0.9999 | |
| 30 | 2.16 | 0.63 | 0.9998 | 2.15 | 12.56 | 0.9999 | |
| Cr3+ | 10 | 2.35 | 0.47 | 0.9996 | 2.38 | 1.18 | 0.9999 |
| 20 | 1.87 | 0.29 | 0.9687 | 2.08 | 0.22 | 0.9918 | |
| 30 | 2.01 | 0.69 | 0.9975 | 2.07 | 1.10 | 0.9998 | |
| Substrate | Trace Metal | qe (mg/g) | k2 (min/(mg/g)) | R2 |
|---|---|---|---|---|
| Raw soil | Cu2+ | 2.47 | 8.80 | 0.9999 |
| Zn2+ | 2.47 | 8.79 | 0.9999 | |
| Cd2+ | 2.13 | 2.26 | 0.9999 | |
| Cr3+ | 2.38 | 1.18 | 0.9998 | |
| Organic-free soil | Cu2+ | 2.34 | 9.30 | 0.9988 |
| Zn2+ | 2.31 | 3.25 | 0.9988 | |
| Cd2+ | 2.11 | 1.74 | 0.9997 | |
| Cr3+ | 2.29 | 0.96 | 0.9984 | |
| Sandy soil | Cu2+ | 1.50 | 8.17 | 0.9887 |
| Zn2+ | 1.98 | 1.17 | 0.9995 | |
| Cd2+ | 1.53 | 1.53 | 0.9977 | |
| Cr3+ | 1.87 | 2.96 | 0.9998 | |
| Without soil | Cu2+ | 0.39 | 1.06 | 0.6347 |
| Zn2+ | 0.37 | 1.39 | 0.6796 | |
| Cd2+ | 0.23 | 1.10 | 0.4712 | |
| Cr3+ | 0.30 | 0.55 | 0.8699 |
| Impact Category | Unit | Chemical Reagents | Waste Handling | Transportation Energy | Separation Energy | Operation Energy | Total |
|---|---|---|---|---|---|---|---|
| Abiotic depletion | kg Sb eq | 0.00064 | 1.67 ×10−5 | 4.8 × 10−5 | 0.00017 | 0.00973 | 0.010605 |
| Acidification | kg SO2 eq | 0.001256 | 0 | 2.63 × 10−5 | 9.09 × 10−5 | 0.006928 | 0.008301 |
| Eutrophication | kg PO4 eq | 7.74 × 10−5 | 0 | 3.3 × 10−6 | 7.23 × 10−6 | 0.000445 | 0.000533 |
| Global warming | kg CO2 eq | 0.094831 | 0 | 0.007982 | 0.02362 | 1.226799 | 1.353231 |
| Ozone layer depletion | kg CFC-11 eq | 1.74 × 10−8 | 0 | 6.35 × 10−9 | 1.5 × 10−9 | 2.76 × 10−8 | 5.28 × 10−8 |
| Human toxicity | kg 1,4-DB eq | 0.007139 | 8.41 × 10−5 | 0.001576 | 0.006908 | 0.247261 | 0.262968 |
| Fresh water aquatic ecotoxicity | kg 1,4-DB eq | 0.001418 | 0.006579 | 0.000114 | 0.001515 | 0.049796 | 0.059422 |
| Marine aquatic ecotoxicity | kg 1,4-DB eq | 2.9444 | 1.017661 | 0.270359 | 4.455857 | 124.7827 | 133.471 |
| Terrestrial ecotoxicity | kg 1,4-DB eq | 5.7 × 10−5 | 2.83 × 10−25 | 3.28 × 10−6 | 4.08 × 10−5 | 0.005705 | 0.005806 |
| Photochemical oxidation | kg C2H4 | 4.13 × 10−5 | 0 | 4.48 × 10−6 | 3.69 × 10−6 | 0.000252 | 0.000302 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Chen, Y.; Chen, S.; Hu, Z. Removal and Reclamation of Trace Metals from Copper and Gold Mine Tailing Leachates Using an Alkali Suspension Method. Water 2023, 15, 1902. https://doi.org/10.3390/w15101902
Jiang S, Chen Y, Chen S, Hu Z. Removal and Reclamation of Trace Metals from Copper and Gold Mine Tailing Leachates Using an Alkali Suspension Method. Water. 2023; 15(10):1902. https://doi.org/10.3390/w15101902
Chicago/Turabian StyleJiang, Shunfeng, Yali Chen, Siqin Chen, and Ziying Hu. 2023. "Removal and Reclamation of Trace Metals from Copper and Gold Mine Tailing Leachates Using an Alkali Suspension Method" Water 15, no. 10: 1902. https://doi.org/10.3390/w15101902
APA StyleJiang, S., Chen, Y., Chen, S., & Hu, Z. (2023). Removal and Reclamation of Trace Metals from Copper and Gold Mine Tailing Leachates Using an Alkali Suspension Method. Water, 15(10), 1902. https://doi.org/10.3390/w15101902