Adsorption of Ammonium Ions and Phosphates on Natural and Modified Clinoptilolite: Isotherm and Breakthrough Curve Measurements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of Adsorbents
- (1)
- CL_nat-natural clinoptilolite;
- (2)
- CL_thermo-clinoptilolite calcinated;
- (3)
- CL_Fe-Fe-modified microwaved clinoptilolite;
- (4)
- CL_Cu-Cu-modified microwaved clinoptilolite;
- (5)
- CL_Ca-Ca-modified microwaved clinoptilolite.
BET Surface Area Pore Size Distribution
2.2. Characterization of Solutions and Wastewater Used for Adsorption
2.2.1. Model Solutions (Used in Static Conditions)
2.2.2. Real Solutions (Used in Dynamic Conditions)
2.3. Experiments Methodology
2.3.1. Adsorption Isotherms
2.3.2. Breakthrough Curves
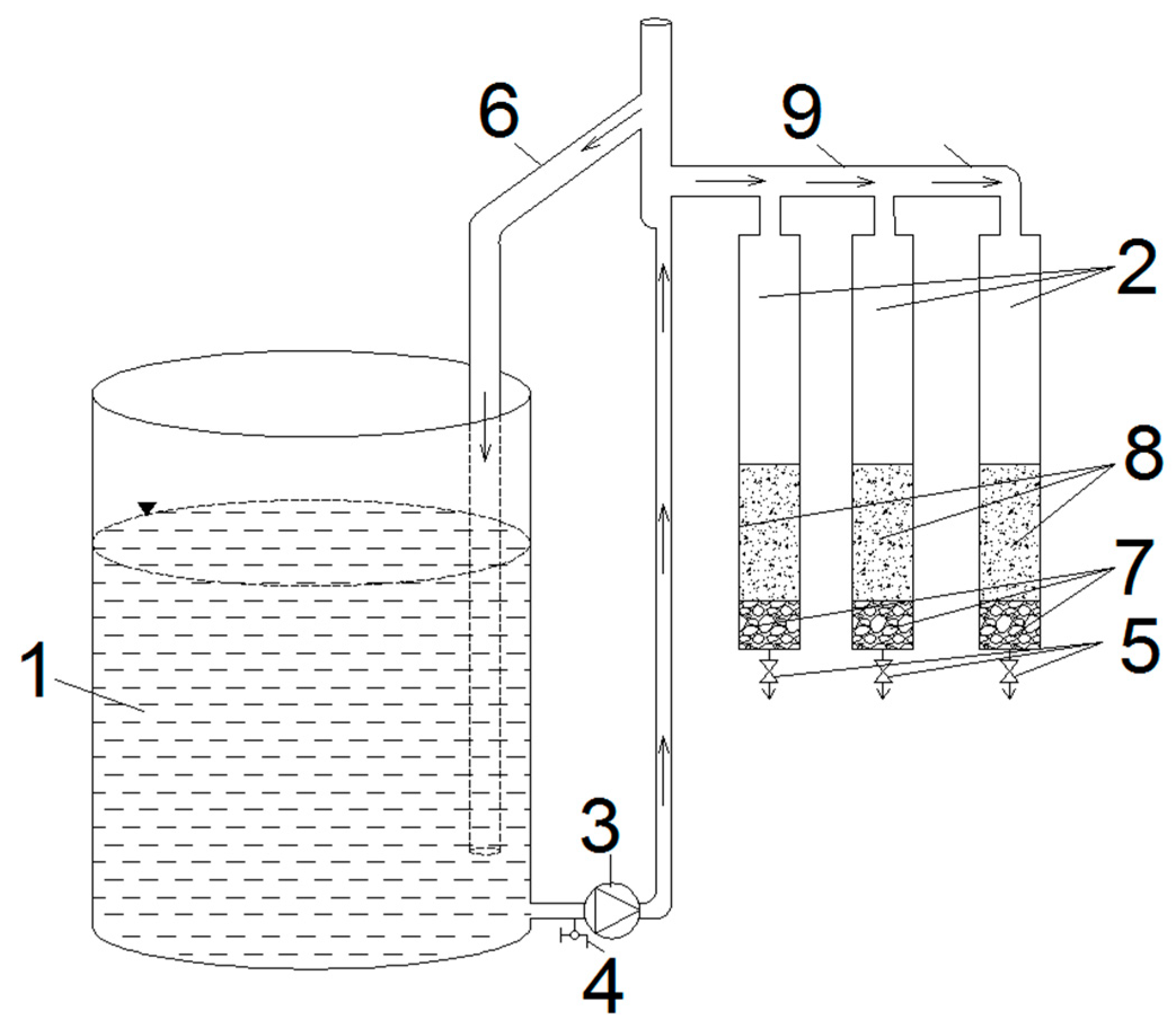
Description of the Experimental Unit
3. Results and Discussion
3.1. Surface Area and Porosity
3.2. Adsorption Isotherms
3.3. Breakthrough Capacity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Feng, Y.; Zhang, Y.; Liang, N.; Wu, H.; Liu, F. Allometric releases of nitrogen and phosphorus from sediments mediated by bacteria determines water eutrophication in coastal river basins of Bohai Bay. Ecotoxicol. Environ. Saf. 2022, 235, 113426. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xu, C.-C.; Ridoutt, B.G.; Wang, X.-C.; Ren, P.-A. Nitrogen and phosphorus losses and eutrophication potential associated with fertilizer application to cropland in China. J. Clean. Prod. 2017, 159, 171–179. [Google Scholar] [CrossRef]
- Baldwin, D.S. Water quality in the Murray–Darling Basin: The potential impacts of climate change. Murray-Darling Basin Aust. Its Future Manag. 2021, 1, 137–159. [Google Scholar] [CrossRef]
- Prokopchuk, O.I.; Hrubinko, V.V. Phosphates in aquatic ecosystems. Sci. Notes Ternopil Natl. Pedagog. Univ. Seriia Biol. 2013, 3, 78–85. [Google Scholar]
- Zhang, L.; Huang, X.; Fu, G.; Zhang, Z. Aerobic electrotrophic denitrification coupled with biologically induced phosphate precipitation for nitrogen and phosphorus removal from high-salinity wastewater: Performance, mechanism, and microbial community. Bioresour. Technol. 2023, 372, 128696. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Liu, Y.; Ge, F.; Xu, Y.; Tao, N.; Peng, F.; Wong, M. Efficiency assessment and pH effect in removing nitrogen and phosphorus by algae-bacteria combined system of Chlorella vulgaris and Bacillus licheniformis. Chemosphere 2013, 92, 1383–1389. [Google Scholar] [CrossRef]
- Lin, Z.; He, L.; Zhou, J.; Shi, S.; He, X.; Fan, X.; Wang, Y.; He, Q. Biologically induced phosphate precipitation in heterotrophic nitrification processes of different microbial aggregates: Influences of nitrogen removal metabolisms and extracellular polymeric substances. Bioresour. Technol. 2022, 356, 127319. [Google Scholar] [CrossRef]
- Prashantha Kumar, T.K.M.; Mandlimath, T.R.; Sangeetha, P.; Revathi, S.K.; Ashok Kumar, S.K. Nanoscale materials as sorbents for nitrate and phosphate removal from water. Environ. Chem. Lett. 2018, 16, 389–400. [Google Scholar] [CrossRef]
- Mitrogiannis, D.; Psychoyou, M.; Baziotis, I.; Inglezakis, V.J.; Koukouzas, N.; Tsoukalas, N.; Palles, D.; Kamitsos, E.; Oikonomou, G.; Markou, G. Removal of phosphate from aqueous solutions by adsorption onto Ca(OH)2 treated natural clinoptilolite. Chem. Eng. J. 2017, 320, 510–522. [Google Scholar] [CrossRef]
- Dai, S.; Wen, Q.; Huang, F.; Bao, Y.; Xi, X.; Liao, Z.; Shi, J.; Ou, C.; Qin, J. Preparation and application of MgO-loaded tobermorite to simultaneously remove nitrogen and phosphorus from wastewater. Chem. Eng. J. 2022, 446, 136809. [Google Scholar] [CrossRef]
- Mažeikienė, A.; Šarko, J. Removal of Nitrogen and Phosphorus from Wastewater Using Layered Filter Media. Sustainability 2022, 14, 10713. [Google Scholar] [CrossRef]
- Li, G.; Dong, G.; Li, B.; Li, Q.; Kronzucker, H.J.; Shi, W. Isolation and characterization of a novel ammonium overly sensitive mutant, amos2, in Arabidopsis thaliana. Planta 2012, 235, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, W.; Chen, H.; Zhan, J.; He, C.; Wang, Q. Ammonium Nitrogen Tolerant Chlorella Strain Screening and Its Damaging Effects on Photosynthesis. Front. Microbiol. 2019, 9, 3250. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Khan, S.A.; Khan, T.A. Remediation of Textile Wastewater by Ozonation. In Sustainable Practices in the Textile Industry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021; pp. 273–284. [Google Scholar] [CrossRef]
- Zapolskyi, A.; Mishkova-Klymenko, N.; Astrelin, I.; Bryk, M. Physical and Chemical Fundamentals of Wastewater Treatment Technology; Libra: Kyiv, Ukraine, 2000; p. 552. ISBN 966-7035-28-X. [Google Scholar]
- Priya, E.; Kumar, S.; Verma, C.; Sarkar, S.; Maji, P.K. A comprehensive review on technological advances of adsorption for removing nitrate and phosphate from waste water. J. Water Process Eng. 2022, 49, 103159. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Lin, F.; Pang, W.-Q. Ammonium exchange in aqueous solution using Chinese natural clinoptilolite and modified zeolite. J. Hazard. Mater. 2007, 142, 160–164. [Google Scholar] [CrossRef]
- Zhou, K.; Wu, B.; Su, L.; Gao, X.; Chai, X.; Dai, X. Development of nano-CaO2-coated clinoptilolite for enhanced phosphorus adsorption and simultaneous removal of COD and nitrogen from sewage. Chem. Eng. J. 2017, 328, 35–43. [Google Scholar] [CrossRef]
- Chen, H.; Hu, X.; Song, W.; Wang, Z.; Li, M.; Liu, H.; Li, J. Effect of pistachio shell as a carbon source to regulate C/N on simultaneous removal of nitrogen and phosphorus from wastewater. Bioresour. Technol. 2023, 367, 128234. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, Y.; Wang, S. Increasing the hydrophyte removal rate of dissolved inorganic phosphorus using a novel Fe-Mg-loaded activated carbon hydroponic substrate with adsorption-release dual functions. J. Environ. Manag. 2022, 313, 114998. [Google Scholar] [CrossRef]
- Du, M.; Zhang, Y.; Wang, Z.; Lv, M.; Xu, Q.; Chen, Z.; Wen, Q.; Li, A. La-doped activated carbon as high-efficiency phosphorus adsorbent: DFT exploration of the adsorption mechanism. Sep. Purif. Technol. 2022, 298, 121585. [Google Scholar] [CrossRef]
- Sonoda, A.; Makita, Y.; Sugiura, Y.; Ogata, A.; Suh, C.; Lee, J.H.; Ooi, K. Influence of coexisting calcium ions during on-column phosphate adsorption and desorption with granular ferric oxide. Sep. Purif. Technol. 2020, 249, 117143. [Google Scholar] [CrossRef]
- Liu, R.; Chi, L.; Wang, X.; Sui, Y.; Wang, Y.; Arandiyan, H. Review of metal (hydr)oxide and other adsorptive materials for phosphate removal from water. J. Environ. Chem. Eng. 2018, 6, 5269–5286. [Google Scholar] [CrossRef]
- Hu, G.; Yang, J.; Duan, X.; Farnood, R.; Yang, C.; Yang, J.; Liu, W.; Liu, Q. Recent developments and challenges in zeolite-based composite photocatalysts for environmental applications. Chem. Eng. J. 2021, 417, 129209. [Google Scholar] [CrossRef]
- Adam, M.R.; Othman, M.H.D.; Hubadillah, S.K.; Abd Aziz, M.H.; Jamalludin, M.R. Application of natural zeolite clinoptilolite for the removal of ammonia in wastewater. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Kannan, A.D.; Parameswaran, P. Ammonia adsorption and recovery from swine wastewater permeate using naturally occurring clinoptilolite. J. Water Process. Eng. 2021, 43, 102234. [Google Scholar] [CrossRef]
- Vocciante, M.; De Folly D’Auris, A.; Finocchi, A.; Tagliabue, M.; Bellettato, M.; Ferrucci, A.; Reverberi, A.P.; Ferro, S. Adsorption of ammonium on clinoptilolite in presence of competing cations: Investigation on groundwater remediation. J. Clean. Prod. 2018, 198, 480–487. [Google Scholar] [CrossRef]
- Uzunova, E.L.; Mikosch, H. Adsorption of phosphates and phosphoric acid in zeolite clinoptilolite: Electronic structure study. Microporous Mesoporous Mater. 2016, 232, 119–125. [Google Scholar] [CrossRef]
- Ahmad, K.; Nazir, M.A.; Qureshi, A.K.; Hussain, E.; Najam, T.; Javed, M.S.; Shah, S.S.A.; Tufail, M.K.; Hussain, S.; Khan, N.A.; et al. Engineering of Zirconium based metal-organic frameworks (Zr-MOFs) as efficient adsorbents. Mater. Sci. Eng. B 2020, 262, 114766. [Google Scholar] [CrossRef]
- Fallatah, A.M.; Shah, H.U.R.; Ahmad, K.; Ashfaq, M.; Rauf, A.; Muneer, M.; Ibrahim, M.M.; El-Bahy, Z.M.; Shahzad, A.; Babras, A. Rational synthesis and characterization of highly water stable MOF@GO composite for efficient removal of mercury (Hg2+) from water. Heliyon 2022, 8, e10936. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lin, H.; Dong, Y.; Li, B.; Wang, L.; Chu, S.; Luo, M.; Liu, J. Zeolite supported Fe/Ni bimetallic nanoparticles for simultaneous removal of nitrate and phosphate: Synergistic effect and mechanism. Chem. Eng. J. 2018, 347, 669–681. [Google Scholar] [CrossRef]
- Yang, W.; Shi, X.; Wang, J.; Chen, W.; Zhang, L.; Zhang, W.; Zhang, X.; Lu, J. Fabrication of a Novel Bifunctional Nanocomposite with Improved Selectivity for Simultaneous Nitrate and Phosphate Removal from Water. ACS Appl. Mater. Interfaces 2019, 11, 35277–35285. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Wu, B.; Dai, X.; Chai, X. Development of polymeric iron/zirconium-pillared clinoptilolite for simultaneous removal of multiple inorganic contaminants from wastewater. Chem. Eng. J. 2018, 347, 819–827. [Google Scholar] [CrossRef]
- Goscianska, J.; Ptaszkowska-Koniarz, M.; Frankowski, M.; Franus, M.; Panek, R.; Franus, W. Removal of phosphate from water by lanthanum-modified zeolites obtained from fly ash. J. Colloid Interface Sci. 2018, 513, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Alshameri, A.; He, H.; Dawood, A.S.; Zhu, J. Simultaneous removal of NH4+ and PO43− from simulated reclaimed waters by modified natural zeolite. Preparation, characterization and thermodynamics. Environ. Prot. Eng. 2017, 43, 73–92. [Google Scholar] [CrossRef]
- Tian, J.; Gaoming, L.; Feng, X.-T.; Li, Y.; Zhang, X. Experimental study on the microwave sensitivity of main rock-forming minerals. Yantu Lixue Rock Soil Mech. 2019, 40, 2066–2074. [Google Scholar] [CrossRef]
- Queiroga, L.N.F.; Pereira, M.B.B.; Silva, L.S.; Silva Filho, E.C.; Santos, I.M.G.; Fonseca, M.G.; Georgelin, T.; Jaber, M. Microwave bentonite silylation for dye removal: Influence of the solvent. Appl. Clay Sci. 2019, 168, 478–487. [Google Scholar] [CrossRef]
- Korichi, S.; Elias, A.; Mefti, A.; Bensmaili, A. The effect of microwave irradiation and conventional acid activation on the textural properties of smectite: Comparative study. Appl. Clay Sci. 2012, 59–60, 76–83. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Kim, D.G.; Shin, H.S. Mechanism study of nitrate reduction by nano zero valent iron. J. Hazard. Mater. 2011, 185, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.I.; Oh, J.S.; Yoo, S.C.; Jho, E.H.; Lee, C.G.; Park, S.J. Removal of phosphorus from water using calcium-rich organic waste and its potential as a fertilizer for rice growth. J. Environ. Chem. Eng. 2022, 10, 107367. [Google Scholar] [CrossRef]
- Huong, P.T.; Lee, B.K.; Kim, J. Improved removal of 2-chlorophenol by a synthesized Cu-nano zeolite. Process Saf. Environ. Prot. 2016, 100, 272–280. [Google Scholar] [CrossRef]
- Mahdavi, S.; Akhzari, D. The removal of phosphate from aqueous solutions using two nano-structures: Copper oxide and carbon tubes. Clean Technol. Environ. Policy 2016, 18, 817–827. [Google Scholar] [CrossRef]
- Hu, Q.; Lan, R.; He, L.; Liu, H.; Pei, X. A critical review of adsorption isotherm models for aqueous contaminants: Curve characteristics, site energy distribution and common controversies. J. Environ. Manage. 2023, 329, 117104. [Google Scholar] [CrossRef] [PubMed]
- Stepova, K.; Sysa, L.; Kontsur, A.; Myakush, O. Adsorption of Copper Ions by Microwave Treated Bentonite. Phys. Chem. Solid State 2020, 21, 537–544. [Google Scholar] [CrossRef]
- Mažeikienė, A.; Vaiškūnaitė, R.; Šarko, J. Sand from groundwater treatment coated with iron and manganese used for phosphorus removal from wastewater. Sci. Total Environ. 2021, 764, 142915. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.N.; Xiao, R.L.; Liu, F.; Wu, J.S. Interception effect of vegetated drainage ditch on nitrogen and phosphorus from drainage ditches. Huanjing Kexue/Environ. Sci. 2015, 36, 4516–4522. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Lambert, S.; Tran, K.Y.; Arrachart, G.; Noville, F.; Henrist, C.; Bied, C.; Moreau, J.J.E.; Wong Chi Man, M.; Heinrichs, B. Tailor-made morphologies for Pd/SiO2 catalysts through sol–gel process with various silylated ligands. Microporous Mesoporous Mater. 2008, 115, 609–617. [Google Scholar] [CrossRef]
- Lin, Y.J.; Chang, Y.H.; Yang, W.D.; Tsai, B.S. Synthesis and characterization of ilmenite NiTiO3 and CoTiO3 prepared by a modified Pechini method. J. Non-Cryst. Solids 2006, 352, 789–794. [Google Scholar] [CrossRef]
- Sydorchuk, O.; Matsuska, O.; Sabadash, V.; Gumnitsky, J. Parallel-serial adsorption of phosphate ions by natural sorbents. East. -Eur. J. Enterp. Technol. 2014, 6, 56–60. [Google Scholar] [CrossRef]
- Guaya, D.; Hermassi, M.; Valderrama, C.; Farran, A.; Cortina, J.L. Recovery of ammonium and phosphate from treated urban wastewater by using potassium clinoptilolite impregnated hydrated metal oxides as N-P-K fertilizer. J. Environ. Chem. Eng. 2016, 4, 3519–3526. [Google Scholar] [CrossRef]
- Sun, S.; Ji, G.; Lv, Y.; Liu, H.; Hu, T.; Chen, Z.; Xu, S. Simultaneous recovery of ammonium and total phosphorus from toilet tail water by modified palygorskite-bentonite clay. Water Environ. Res. 2021, 93, 1077–1086. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, X.; Pan, B.; Zhang, W.; Hua, M.; Lv, L.; Zhang, W. Preferable removal of phosphate from water using hydrous zirconium oxide-based nanocomposite of high stability. J. Hazard. Mater. 2015, 284, 35–42. [Google Scholar] [CrossRef] [PubMed]






| NH4-N (mg/L) | PO4-P (mg/L) | NO3-N (mg/L) | BOD (mg/L) | TSS (mg/L) | pH | T (°C) |
|---|---|---|---|---|---|---|
| 9.7 ± 0.4 | 3.37 ± 0.3 | 1.96 ± 0.2 | 5.5 ± 0.4 | 6.6 ± 0.4 | 7.35 ± 0.2 | 20 ± 0.5 |
| Sample Code | SBET, m2/g | Smic | Sext | Vp, mL/g | dpart | ε |
|---|---|---|---|---|---|---|
| CL_nat | 18.254 | 4.773 | 13.481 | 0.041 | 347.2 | 0.037 |
| CL_thermo | 11.658 | 0.379 | 11.279 | 0.035 | 543.6 | 0.031 |
| CL_Fe | 19.025 | 7.18 | 11.845 | 0.039 | 333.1 | 0.035 |
| CL_Cu | 15.9 | 5.21 | 10.69 | 0.035 | 398.6 | 0.031 |
| CL_Ca | 14.129 | 3.85 | 10.279 | 0.034 | 448.6 | 0.031 |
| Sample Index | |||||
|---|---|---|---|---|---|
| CL_nat | CL_thermo | CL_Fe | CL_Cu | CL_Ca | |
| Langmuir isotherm parameters | |||||
| qm | 1.311 | 4.398 | 0.027 | 83.913 | 7.286 |
| KL | 0.093 | 0.029 | 0.467 | 0.0002 | 0.002 |
| SNE | 3.942 | 3.642 | 3.591 | 3.900 | 3.900 |
| R2 | 0.95 | 0.99 | 0.47 | 0.81 | 0.98 |
| Freundlich isotherm parameters | |||||
| n | 3.158 | 2.456 | 1.197 | 0.757 | 3.962 |
| KF | 0.321 | 0.479 | 0.205 | 0.007 | 0.217 |
| SNE | 3.917 | 3.465 | 3.597 | 3.878 | 3.781 |
| R2 | 0.72 | 1.0 | 0.98 | 1.0 | 0.44 |
| Langmuir–Freundlich isotherm parameters | |||||
| qm | 1.006 | 2.879 | 4.375 | 1.750 | 0.875 |
| KFL | 0.147 | 0.061 | 0.061 | 0.025 | 0.033 |
| nFL | 2.455 | 1.514 | 1.384 | 1.893 | 1.629 |
| SNE | 3.883 | 3.593 | 3.560 | 4.146 | 3.015 |
| R2 | 1.0 | 0.76 | 1.0 | 0.96 | 1.0 |
| Sample Index | |||||
|---|---|---|---|---|---|
| CL_nat | CL_thermo | CL_Fe | CL_Cu | CL_Ca | |
| Langmuir isotherm parameters | |||||
| qm | 200.0 | 1127.41 | 700.00 | 1989.83 | 2745.80 |
| KL | 4.21 × 10−5 | 6.30 × 10−6 | 1.58 × 10−5 | 7.63 × 10−6 | 1.21 × 10−5 |
| SNE | 3.510 | 3.490 | 3.562 | 3.557 | 3.487 |
| R2 | 0.28 | 0.26 | 0.27 | 0.27 | 0.75 |
| Freundlich isotherm parameters | |||||
| n | 0.511 | 0.503 | 0.513 | 0.525 | 0.532 |
| KF | 4.73 × 10−6 | 1.41 × 10−6 | 8.69 × 10−6 | 1.14 × 10−5 | 1.49 × 10−5 |
| SNE | 3.487 | 3.52 | 3.570 | 3.519 | 3.506 |
| R2 | 0.70 | 0.33 | 0.82 | 0.88 | 0.89 |
| Langmuir–Freundlich isotherm parameters | |||||
| qm | 280.86 | 713.568 | 813.14 | 800.62 | 708.33 |
| KFL | 1.8 × 10−4 | 1.18 × 10−4 | 0.12 × 10−3 | 0.12 × 10−3 | 0.12 × 10−3 |
| nFL | 5.704 | 4.270 | 3.835 | 3.40 | 3.254 |
| SNE | 3.465 | 3.476 | 3.559 | 3.516 | 3.947 |
| R2 | 0.99 | 1.0 | 0.96 | 0.98 | 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepova, K.; Fediv, I.; Mažeikienė, A.; Šarko, J.; Mažeika, J. Adsorption of Ammonium Ions and Phosphates on Natural and Modified Clinoptilolite: Isotherm and Breakthrough Curve Measurements. Water 2023, 15, 1933. https://doi.org/10.3390/w15101933
Stepova K, Fediv I, Mažeikienė A, Šarko J, Mažeika J. Adsorption of Ammonium Ions and Phosphates on Natural and Modified Clinoptilolite: Isotherm and Breakthrough Curve Measurements. Water. 2023; 15(10):1933. https://doi.org/10.3390/w15101933
Chicago/Turabian StyleStepova, Kateryna, Iryna Fediv, Aušra Mažeikienė, Julita Šarko, and Jonas Mažeika. 2023. "Adsorption of Ammonium Ions and Phosphates on Natural and Modified Clinoptilolite: Isotherm and Breakthrough Curve Measurements" Water 15, no. 10: 1933. https://doi.org/10.3390/w15101933
APA StyleStepova, K., Fediv, I., Mažeikienė, A., Šarko, J., & Mažeika, J. (2023). Adsorption of Ammonium Ions and Phosphates on Natural and Modified Clinoptilolite: Isotherm and Breakthrough Curve Measurements. Water, 15(10), 1933. https://doi.org/10.3390/w15101933






