The Surface-to-Atmosphere GHG Fluxes in Rewetted and Permanently Flooded Former Peat Extraction Areas Compared to Pristine Peatland in Hemiboreal Latvia
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Sites
2.2. GHG Flux Sampling Design in Rewetted Former Peat Extraction Areas and Undisturbed Peatland
2.3. GHG Flux Sampling Design in Permanently Flooded Former Peat Extraction Areas
2.4. GHG Flux Sampling and Analysis
2.5. Measurements of Environmental Parameters
2.6. Statistical Analysis
3. Results
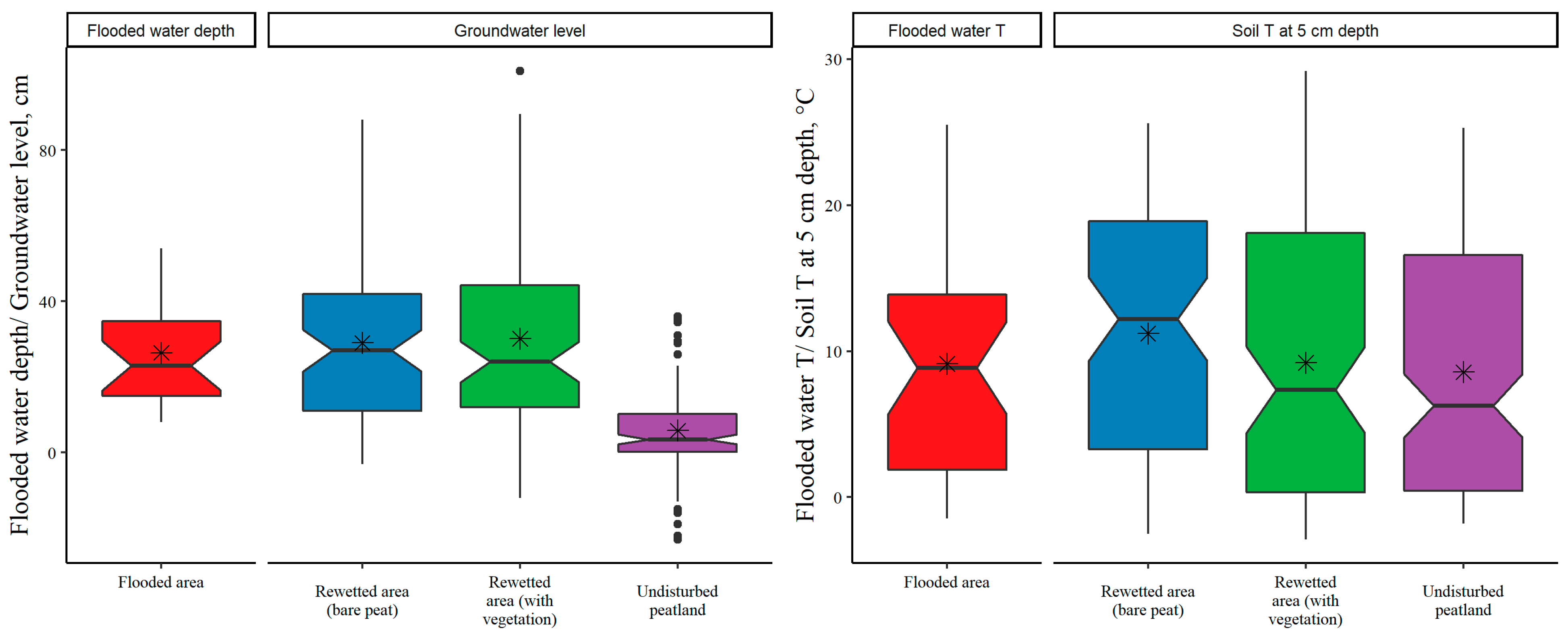
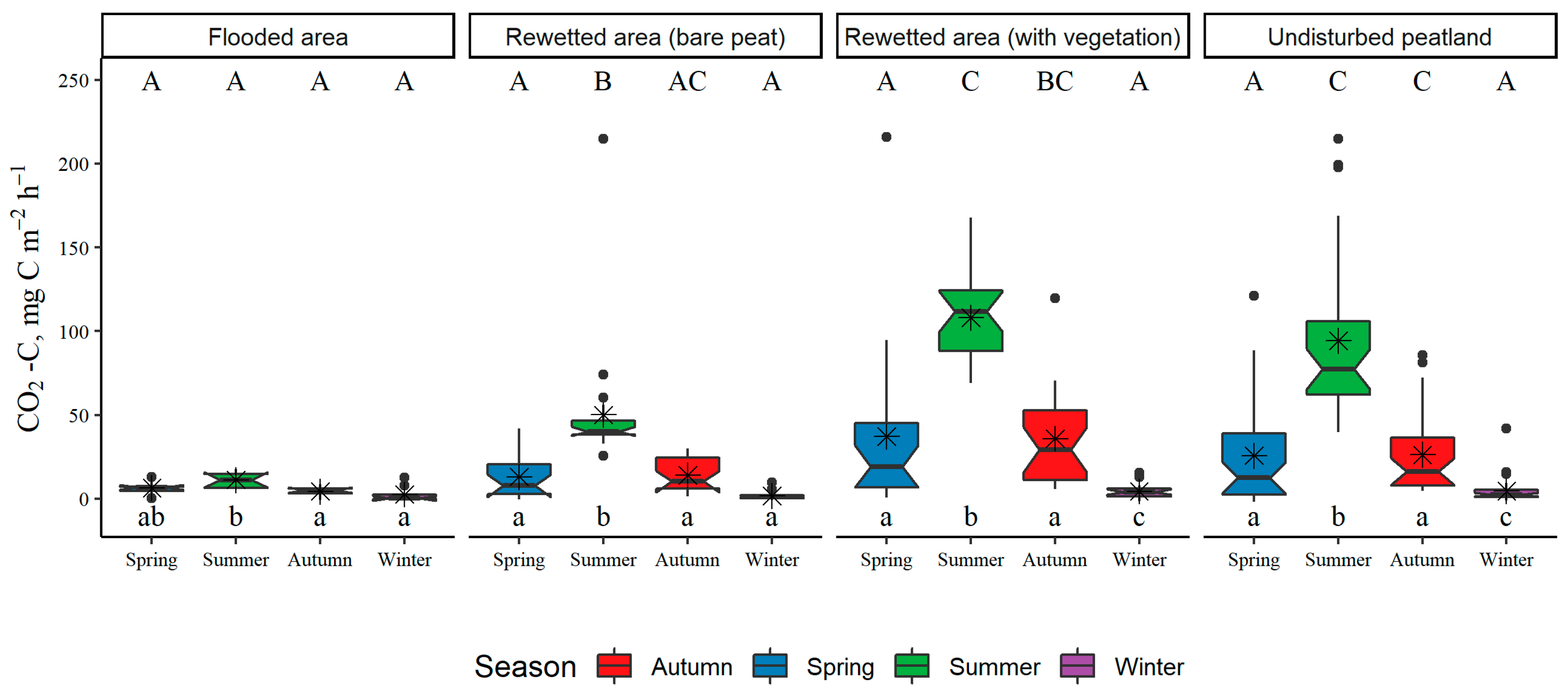
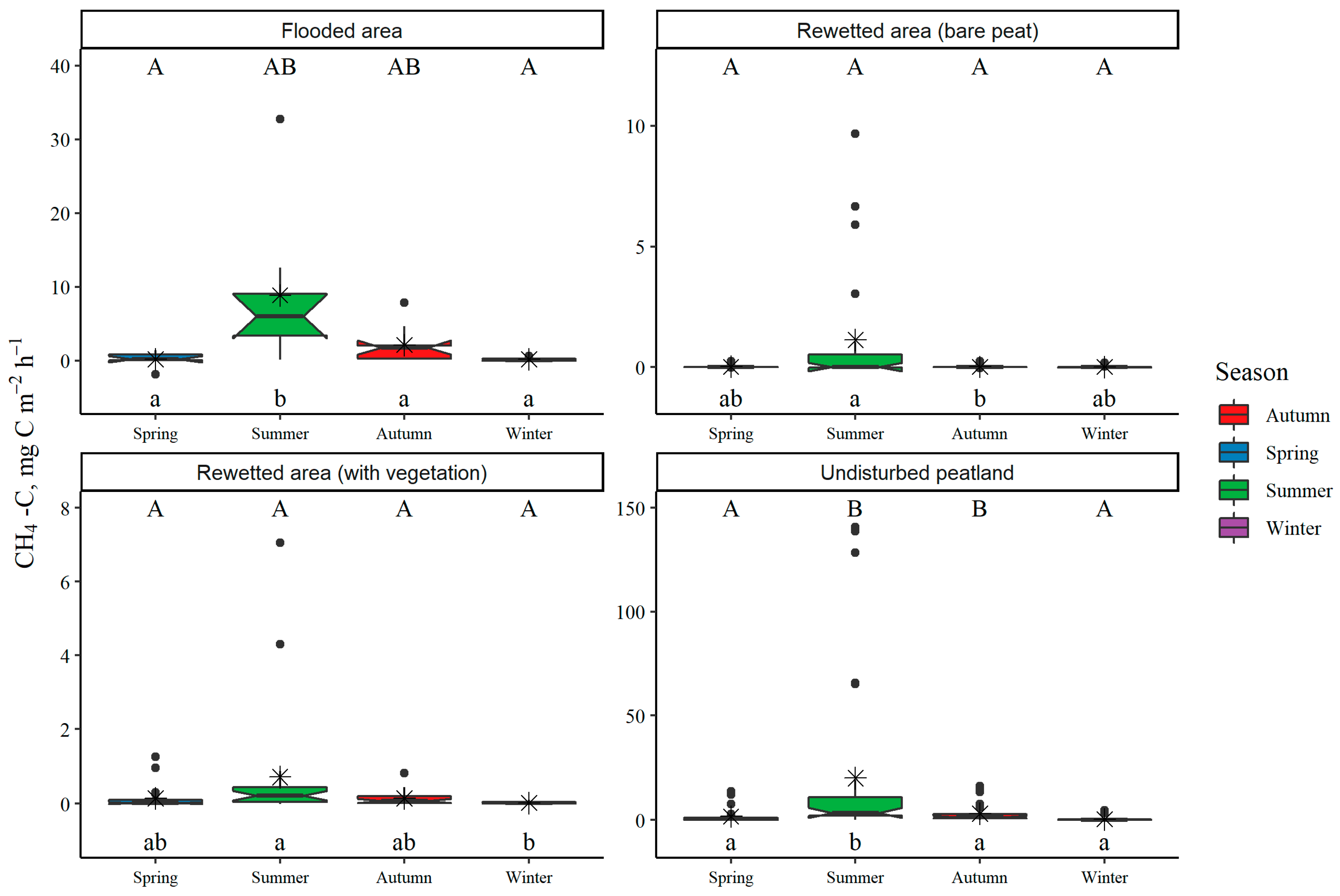
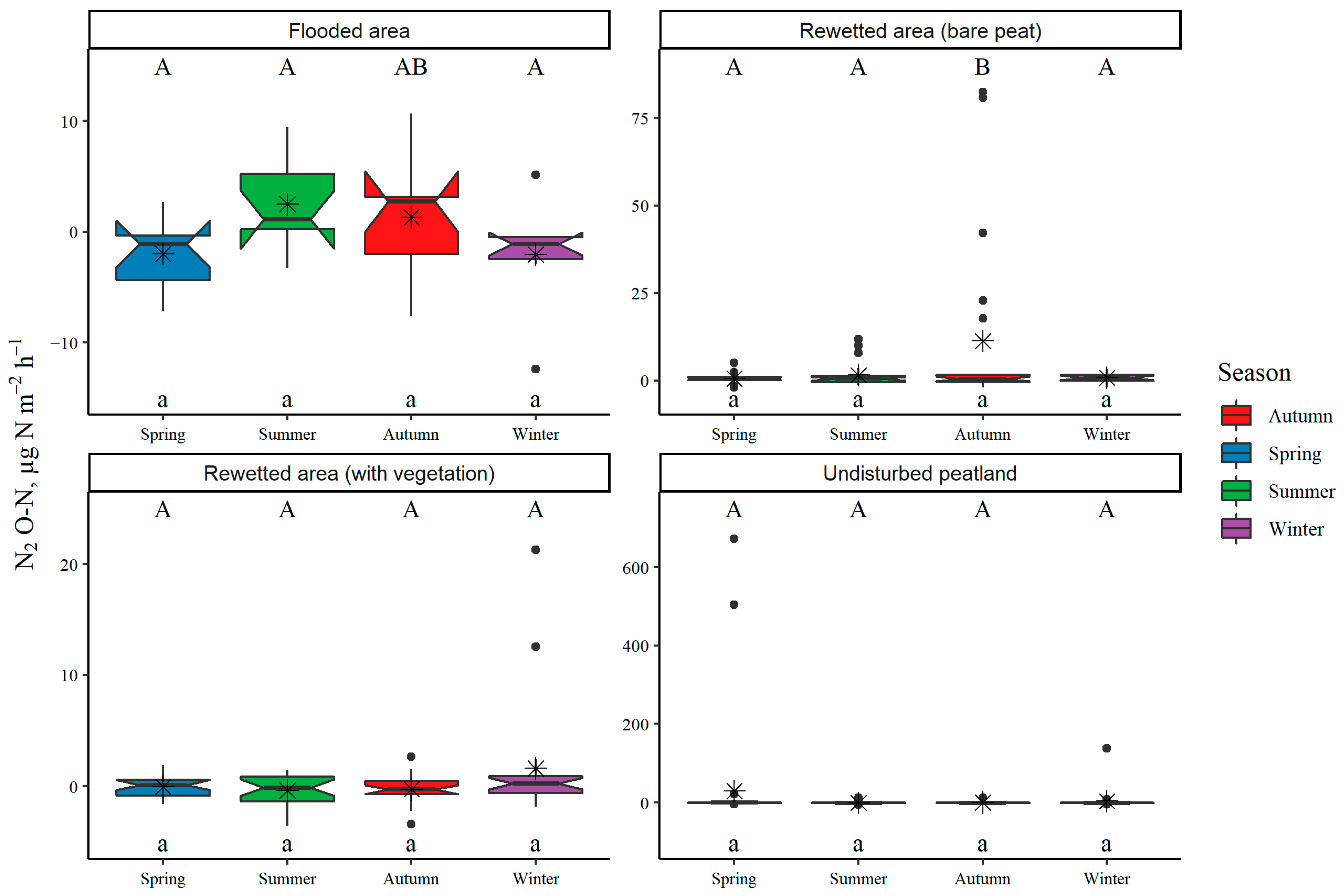
3.1. Land-Use Type and Seasonal Variation in GHG Fluxes from Soil or Water Surface
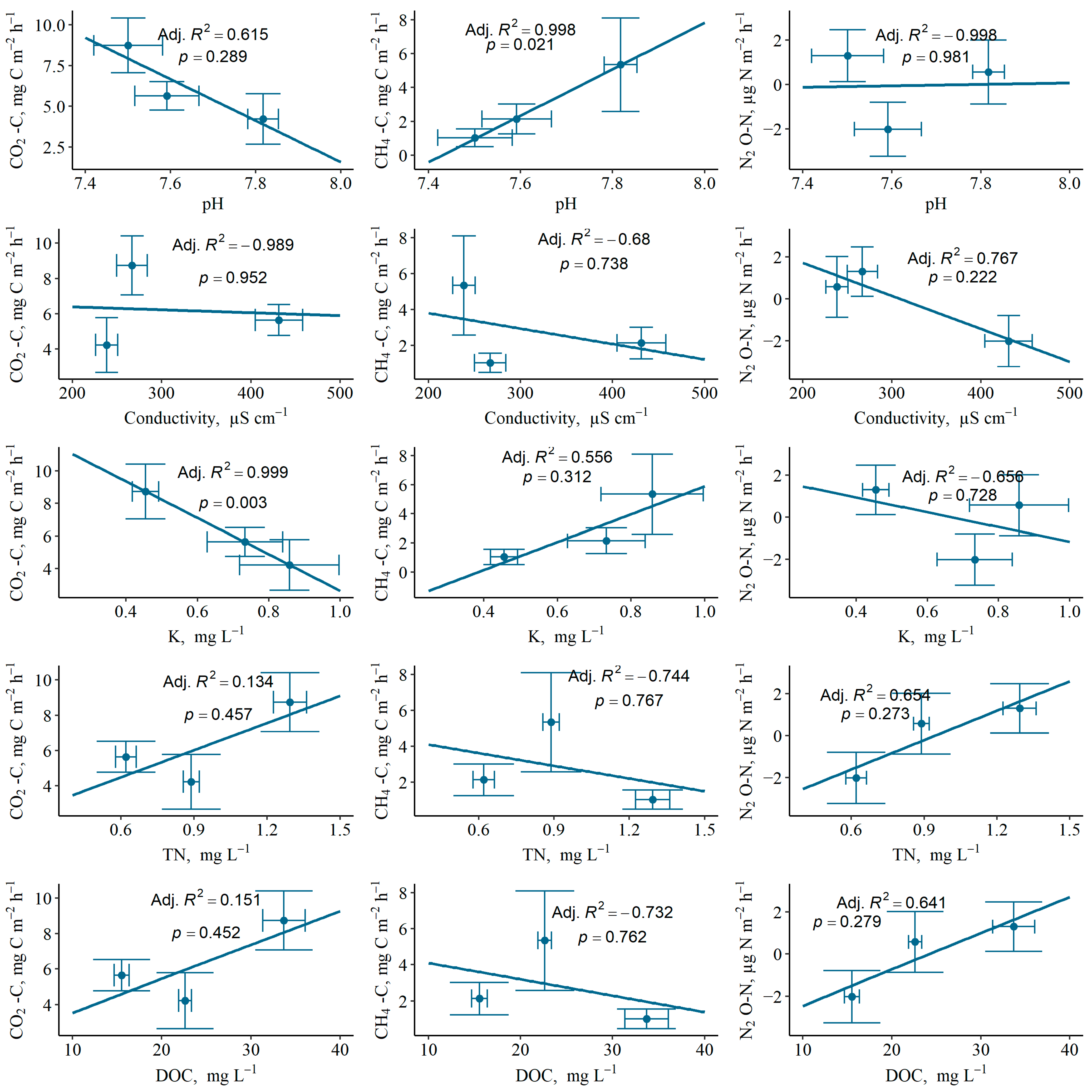
3.2. Impact of Environmental Parameters on Magnitude of GHG Fluxes from Soil or Water Surfaces
3.3. Annual GHG Fluxes from Soil or Water Surfaces
4. Discussion
4.1. CO2 Fluxes
4.2. CH4 Fluxes
4.3. N2O Fluxes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter | Studied Land-Use Types with Organic Soils | ||
|---|---|---|---|
| Rewetted Area (Bare Peat) | Rewetted Area (with Vegetation) | Undisturbed Peatland | |
| Soil at 0–20 cm depth | |||
| OC, g kg−1 | 544.6 ± 3.8 | 539.5 ± 9.5 | 524.1 ± 6.5 |
| pH (CaCl2) | 2.56 ± 0.02 | 3.22 ± 0.33 | 2.87 ± 0.05 |
| TN, g kg−1 | 6.57 ± 0.59 | 11.42 ± 1.05 | 9.83 ± 1.15 |
| P, g kg−1 | 0.12 ± 0.02 | 0.20 ± 0.03 | 0.24 ± 0.03 |
| K, g kg−1 | 0.12 ± 0.03 | 0.88 ± 0.31 | 0.51 ± 0.07 |
| Ca, g kg−1 | 1.35 ± 0.13 | 11.23 ± 5.20 | 2.09 ± 0.52 |
| Mg, g kg−1 | 0.63 ± 0.09 | 1.01 ± 0.31 | 0.71 ± 0.10 |
| C/N ratio | 84.0 ± 6.6 | 50.4 ± 5.2 | 63.6 ± 6.2 |
| Soil at 20–40 cm depth | |||
| OC, g kg−1 | 554.1 ± 3.8 | 553.6 ± 14.6 | 534.2 ± 4.5 |
| pH (CaCl2) | 2.54 ± 0.01 | 3.25 ± 0.34 | 2.93 ± 0.05 |
| TN, g kg−1 | 6.69 ± 0.19 | 10.04 ± 0.96 | 9.64 ± 0.85 |
| P, g kg−1 | 0.10 ± 0.01 | 0.18 ± 0.02 | 0.20 ± 0.02 |
| K, g kg−1 | 0.06 ± 0.01 | 0.23 ± 0.06 | 0.40 ± 0.08 |
| Ca, g kg−1 | 1.06 ± 0.04 | 12.37 ± 5.60 | 1.83 ± 0.32 |
| Mg, g kg−1 | 0.38 ± 0.06 | 0.97 ± 0.34 | 0.54 ± 0.05 |
| C/N ratio | 83.2 ± 2.6 | 58.5 ± 0.9 | 63.4 ± 6.0 |
References
- Waddington, J.M.; Warner, K.D.; Kennedy, G.W. Cutover peatlands: A consistent source of CO2. Glob. Biogeochem. Cycles 2002, 16, 1–7. [Google Scholar] [CrossRef]
- Leifeld, J.; Wüst-Galley, C.; Page, S. Intact and managed peatland soils as a source and sink of GHGs from 1850 to 2100. Nat. Clim. Chang. 2019, 9, 945–947. [Google Scholar] [CrossRef]
- Conchedda, G.; Tubiello, F.N. Drainage of organic soils and GHG emissions: Validation with country data. Earth Syst. Sci. Data 2020, 12, 3113–3137. [Google Scholar] [CrossRef]
- FAO. Drained Organic Soils 1990–2019. Global, Regional and Country Trends; FAOSTAT Analytical Brief Series No 4; FAO: Rome, Italy, 2020; pp. 1–9. [Google Scholar]
- Joosten, H.; Tapio-Biström, M.-L.; Tol, S. Peatlands-Guidance for Climate Change Mitigation through Conservation, Rehabilitation and Sustainable Use, 2nd ed.; FAO and Wetlands International: Rome, Italy, 2012; pp. 1–114. [Google Scholar]
- Gebremichael, A.W.; Osborne, B.; Orr, P. Flooding-related increases in CO2 and N2O emissions from a temperate coastal grassland ecosystem. Biogeosciences 2017, 14, 2611–2626. [Google Scholar] [CrossRef]
- Evans, C.D.; Peacock, M.; Baird, A.J.; Artz, R.R.E.; Burden, A.; Callaghan, N.; Chapman, P.J.; Cooper, H.M.; Coyle, M.; Craig, E.; et al. Overriding water table control on managed peatland greenhouse gas emissions. Nature 2021, 593, 548–552. [Google Scholar] [CrossRef]
- Kreyling, J.; Tanneberger, F.; Jansen, F.; van der Linden, S.; Aggenbach, C.; Blüml, V.; Couwenberg, J.; Emsens, W.-J.; Joosten, H.; Klimkowska, A.; et al. Rewetting does not return drained fen peatlands to their old selves. Nat. Commun. 2021, 12, 5693. [Google Scholar] [CrossRef] [PubMed]
- IPCC. 2013 Supplement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories: Wetlands; Hiraishi, T., Krug, T., Tanabe, K., Srivastava, N., Baasansuren, J., Fukuda, M., Troxler, T.G., Eds.; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- IPCC. 2006 IPCC Guidelines for National Greenhouse Gas Inventories, Prepared by the National Greenhouse Gas Inventories Programme; Eggleston, H.S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; IGES: Hayama, Japan, 2006. [Google Scholar]
- Kim, D.-G.; Vargas, R.; Bond-Lamberty, B.; Turetsky, M.R. Effects of soil rewetting and thawing on soil gas fluxes: A review of current literature and suggestions for future research. Biogeosciences 2012, 9, 2459–2483. [Google Scholar] [CrossRef]
- Waddington, J.M.; Day, S.M. Methane emissions from a peatland following restoration. JGR Biogeosci. 2007, 112, G03018. [Google Scholar] [CrossRef]
- Waddington, J.M.; Warner, K. Atmospheric CO2 sequestration in restored mined peatlands. Ecoscience 2001, 8, 359–368. [Google Scholar] [CrossRef]
- Günther, A.; Barthelmes, A.; Huth, V.; Joosten, H.; Jurasinski, G.; Koebsch, F.; Couwenberg, J. Prompt rewetting of drained peatlands reduces climate warming despite methane emissions. Nat. Commun. 2020, 11, 1644. [Google Scholar] [CrossRef]
- Liu, H.; Wrage-Mönnig, N.; Lennartz, B. Rewetting strategies to reduce nitrous oxide emissions from European peatlands. Commun. Earth Environ. 2020, 1, 17. [Google Scholar] [CrossRef]
- Zou, J.; Ziegler, A.D.; Chen, D.; McNicol, G.; Ciais, P.; Jiang, X.; Zheng, C.; Wu, J.; Wu, J.; Lin, Z.; et al. Rewetting global wetlands effectively reduces major greenhouse gas emissions. Nat. Geosci. 2022, 15, 627–632. [Google Scholar] [CrossRef]
- Aitova, E.; Morley, T.; Wilson, D.; Renou-Wilson, F. A review of greenhouse gas emissions and removals from Irish peatlands. Mires Peat 2023, 29, 1–17. [Google Scholar]
- Wilson, D.; Farrell, C.; Mueller, C.; Hepp, S.; Renou-Wilson, F. Rewetted industrial cutaway peatlands in western Ireland: A prime location for climate change mitigation? Mires Peat 2013, 11, 1–22. [Google Scholar]
- MacDonald, S.M. Carbon Gas Exchange at a Recently Restored Peatland in Alberta, Canada. Master’s Thesis, McGill University, Montreal, QC, Canada, December 2017. [Google Scholar]
- Wilson, D.; Blain, D.; Couwenberg, J.; Evans, C.D.; Murdiyarso, D.; Page, S.E.; Renou-Wilson, F.; Rieley, J.O.; Sirin, A.; Strack, M.; et al. Greenhouse gas emission factors associated with rewetting of organic soils. Mires Peat 2016, 17, 1–28. [Google Scholar]
- Martens, M.; Karlsson, N.P.E.; Ehde, P.M.; Mattsson, M.; Weisner, S.E.B. The greenhouse gas emission effects of rewetting drained peatlands and growing wetland plants for biogas fuel production. J. Environ. Manag. 2021, 277, 111391. [Google Scholar] [CrossRef]
- Butlers, A.; Bārdule, A.; Spalva, G.; Muižnieks, E. N2O and CH4 emissions from naturally wet and drained nutrient-rich organic forest soils. In Proceedings of the 10th International Scientific Conference Rural Development, Kaunas, Lithuania, 21–23 September 2021; pp. 195–200. [Google Scholar] [CrossRef]
- Latvia National Inventory Report. Available online: https://unfccc.int/documents/461908 (accessed on 12 April 2023).
- Butlers, A.; Ivanovs, J. Improved activity data for accounting greenhouse gas emissions due to management of wetlands. Res. Rural Dev. 2018, 1, 27–33. [Google Scholar] [CrossRef]
- Samariks, V.; Lazdiņš, A.; Bārdule, A.; Kalēja, S.; Butlers, A.; Spalva, G.; Jansons, Ā. Impact of former peat extraction field afforestation on soil greenhouse gas emissions in hemiboreal region. Forests 2023, 14, 184. [Google Scholar] [CrossRef]
- Kaleja, S.; Bardule, A. Review of climate change mitigation measures applicable in degraded peatlands in Latvia. Res. Rural Dev. 2022, 37, 56–62. [Google Scholar] [CrossRef]
- EU Biodiversity Strategy for 2030. Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:a3c806a6-9ab3-11ea-9d2d-01aa75ed71a1.0001.02/DOC_1&format=PDF (accessed on 12 April 2023).
- Proposal for a Regulation of the European Parliament and of the Council on Nature Restoration. Available online: https://environment.ec.europa.eu/system/files/2022-06/Proposal%20for%20a%20Regulation%20on%20nature%20restoration.pdf (accessed on 12 April 2023).
- Latvian Environment, Geology and Meteorology Centre. Meteorological Network. Available online: https://klimats.meteo.lv/klimats/latvijas_klimats/ (accessed on 12 April 2023).
- Pavelka, M.; Acosta, M.; Kiese, R.; Altimir, N.; Brümmer, C.; Crill, P.; Darenova, E.; Fuß, R.; Gielen, B.; Graf, A.; et al. Standardisation of chamber technique for CO2, N2O and CH4 fluxes measurements from terrestrial ecosystems. Int. Agrophysics 2018, 32, 569–587. [Google Scholar] [CrossRef]
- Butlers, A.; Lazdiņš, A. Case study on greenhouse gas (GHG) fluxes from flooded former peat extraction fields in central part of Latvia. Res. Rural Dev. 2022, 37, 44–49. [Google Scholar]
- Darenova, E.; Pavelka, M.; Acosta, M. Diurnal deviations in the relationship between CO2 efflux and temperature: A case study. Catena 2014, 123, 263–269. [Google Scholar] [CrossRef]
- Loftfield, N.; Flessa, H.; Augustin, J.; Beese, F. Automated gas chromatographic system for rapid analysis of the atmospheric trace gases methane, carbon dioxide, and nitrous oxide. J. Environ. Qual. 1997, 26, 560–564. [Google Scholar] [CrossRef]
- R Core Team. The R Project for Statistical Computing. Available online: https://www.R-project.org (accessed on 12 April 2023).
- Kuzyakov, Y. Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol. Biochem. 2006, 38, 425–448. [Google Scholar] [CrossRef]
- Tang, X.; Pei, X.; Lei, N.; Luo, X.; Liu, L.; Shi, L.; Chen, G.; Liang, J. Global patterns of soil autotrophic respiration and its relation to climate, soil and vegetation characteristics. Geoderma 2020, 369, 114339. [Google Scholar] [CrossRef]
- Hu, J.; VanZomeren, C.M.; Inglett, K.S.; Wright, A.L.; Clark, M.W.; Reddy, K.R. Greenhouse gas emissions under different drainage and flooding regimes of cultivated peatlands. JGR Biogeosci. 2017, 122, 3047–3062. [Google Scholar] [CrossRef]
- Koebsch, F.; Gottschalk, P.; Beyer, F.; Wille, C.; Jurasinski, G.; Sachs, T. The impact of occasional drought periods on vegetation spread and greenhouse gas exchange in rewetted fens. Philos. Trans. R. Soc. B 2020, 375, 20190685. [Google Scholar] [CrossRef] [PubMed]
- Tiemeyer, B.; Freibauer, A.; Borraz, E.A.; Augustin, J.; Bechtold, M.; Beetz, S.; Beyer, C.; Ebli, M.; Eickenscheidt, T.; Fiedler, S.; et al. A new methodology for organic soils in national greenhouse gas inventories: Data synthesis, derivation and application. Ecol. Indic. 2020, 109, 105838. [Google Scholar] [CrossRef]
- Bārdule, A.; Gerra-Inohosa, L.; Kļaviņš, I.; Kļaviņa, Z.; Bitenieks, K.; Butlers, A.; Lazdiņš, A.; Lībiete, Z. Variation in the mercury concentrations and greenhouse gas emissions of pristine and managed hemiboreal peatlands. Land 2022, 11, 1414. [Google Scholar] [CrossRef]
- Berglund, Ö.; Berglund, K.; Klemedtsson, L. Plant-derived CO2 flux from cultivated peat soils. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2011, 61, 508–513. [Google Scholar]
- Tuittila, E.-S.; Komulainen, V.-M.; Vasander, H.; Nykänen, H.; Martikainen, P.J.; Laine, J. Methane dynamics of a restored cut-away peatland. Glob. Chang. Biol. 2000, 6, 569–581. [Google Scholar] [CrossRef]
- Glatzel, S.; Forbrich, I.; Krüger, C.; Lemke, S.; Gerold, G. Small scale controls of greenhouse gas release under elevated Ndeposition rates in a restoring peat bog in NW Germany. Biogeosciences 2008, 5, 925–935. [Google Scholar] [CrossRef]
- Carmichael, M.J.; Bernhardt, E.S.; Bräuer, S.L.; Smith, W.K. The role of vegetation in methane flux to the atmosphere: Should vegetation be included as a distinct category in the global methane budget? Biogeochemistry 2014, 119, 1–24. [Google Scholar] [CrossRef]
- Akhtar, H.; Lupascu, M.; Sukri, R.S.; Smith, T.E.L.; Cobb, A.R.; Swarup, S. Significant sedge-mediated methane emissions from degraded tropical peatlands. Environ. Res. Lett. 2020, 16, 014002. [Google Scholar] [CrossRef]
- Larmola, T.; Tuittila, E.-S.; Tiirola, M.; Nykänen, H.; Martikainen, P.J.; Yrjälä, K.; Tuomivirta, T.; Fritze, H. The role of Sphagnum mosses in the methane cycling of a boreal mire. Ecology 2010, 91, 2356–2365. [Google Scholar] [CrossRef]
- Klemedtsson, L.; Von Arnold, K.; Weslien, P.; Gundersen, P. Soil CN ratio as a scalar parameter to predict nitrous oxide emissions. Glob. Chang. Biol. 2005, 11, 1142–1147. [Google Scholar] [CrossRef]
- Liimatainen, M.; Voigt, C.; Martikainen, P.J.; Hytönen, J.; Regina, K.; Óskarsson, H.; Maljanen, M. Factors controlling nitrous oxide emissions from managed northern peat soils with low carbon to nitrogen ratio. Soil Biol. Biochem. 2018, 122, 186–195. [Google Scholar] [CrossRef]
- Hendriks, D.M.D.; van Huissteden, J.; Dolman, A.J.; van der Molen, M.K. The full greenhouse gas balance of an abandoned peat meadow. Biogeosciences 2007, 4, 411–424. [Google Scholar] [CrossRef]
- Wrage-Mönnig, N.; Horn, M.A.; Well, R.; Müller, C.; Velthof, G.; Oenema, O. The role of nitrifier denitrification in the production of nitrous oxide revisited. Soil Biol. Biochem. 2018, 123, A3–A16. [Google Scholar] [CrossRef]
- Bārdule, A.; Bārdulis, A.; Polmanis, K.; Krumšteds, L.L.; Butlers, A.; Stola, J.; Skranda, I.; Purviņa, D.; Zvaigzne, Z.A.; Muižnieks, E.; et al. Trends of Scots pine forest health and element flow changes in the ICP Forests monitoring sites in Latvia. Balt. For. 2021, 27, 536. [Google Scholar] [CrossRef]
- Michel, A.; Kirchner, T.; Prescher, A.-K.; Schwärzel, K. Forest Condition in Europe: The 2022 Assessment. ICP Forests Technical Report under the UNECE Convention on Long-Range Transboundary Air Pollution (Air Convention); Thünen Institute: Eberswalde, Germany, 2022. [Google Scholar] [CrossRef]
- Myhre, G.; Shindell, D.; Bréon, F.-M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.-F.; Lee, D.; Mendoza, B.; et al. Anthropogenic and Natural Radiative Forcing. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the In-tergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]



| Type of Research Sites, Study Period (GHG Flux Sampling) | Name of Research Sites | Short Description, Water Level Position during the Study Period | Coordinates (WGS-84 Coordinate System) |
|---|---|---|---|
| Undisturbed peatland (steady stage), December 2016–2018 | Lauga Mire | Raised bog, groundwater level in range from 0 to 35 cm | X: 24.67638; Y: 57.27659 |
| Ķemeri Mire | Raised bog, groundwater level in range from −23 to 20 cm | X: 23.53489; Y: 56.88007 | |
| Lielsala Mire | Raised bog, groundwater level in range from −2 to 15 cm | X: 22.31902; Y: 57.34393 | |
| Igerīšu Mire | Transitional mire, groundwater level in range from −8 to 7 cm | X: 24.61425; Y: 57.10805 | |
| Kalnezera Mire | Transitional mire, groundwater level in range from −16 to 35 cm | X: 24.73494; Y: 56.68246 | |
| Kazu Mire | Transitional mire, groundwater level in range from −10 to 36 cm | X: 24.82005; Y: 57.27762 | |
| Rewetted former peat extraction areas with bare peat, December 2016–2018 | Cenas Mire | Groundwater level in range from 2 to 88 cm | X: 23.98021; Y: 56.82464 |
| Medema Mire | Groundwater level in range from 2 to 74 cm | X: 24.10859; Y: 56.84534 | |
| Ķemeri Mire | Groundwater level in range from −3 to 30 cm | X: 23.52458; Y: 56.89753 | |
| Ķemeri Mire | Groundwater level in range from 0 to 43 cm | X: 23.52478; Y: 56.89730 | |
| Rewetted former peat extraction areas with vegetation, December 2016–2018 | Cenas Mire | Herbaceous plant and shrub vegetation, groundwater level in range from 0 to 101 cm | X: 23.97731; Y: 56.82599 |
| Cepļa Mire | Herbaceous plant and shrub vegetation, groundwater level in range from 9 to 53 cm | X: 26.47581; Y: 57.22005 | |
| Silguldas Mire | Herbaceous plant and shrub vegetation, groundwater level in range from 8 to 79 cm | X: 27.39121; Y: 57.31268 | |
| Briģu Mire | Reed vegetation, groundwater level in range from −12 to 90 cm | X: 24.40517; Y: 56.44364 | |
| Permanently flooded former peat extraction areas, January 2021–December 2022 | Tēvgāršu Swamp | Depth of flooded water level in range from 1.0 to 1.5 m, decrease during summer (by max 0.6 m) | X: 24.95375; Y: 57.67010 |
| Zilākalna Swamp | Depth of flooded water level in range from 0.8 to 1.8 m, relatively stable during summer (decrease by max 0.3 m) | X: 25.16912; Y: 57.59400 | |
| Zilākalna swamp | Depth of flooded water level in range from 0.7 to 1.2 m, decrease during summer (by max 0.5 m) | X: 25.16439; Y: 57.59056 |
| Parameter | Flooded Area | Rewetted Area (Bare Peat) | Rewetted Area (with Vegetation) | Undisturbed Peatland |
|---|---|---|---|---|
| Surface-to-atmosphere CO2 fluxes (sum of soil heterotrophic and autotrophic respiration) | ||||
| Air temperature | 0.52 *** | 0.83 *** | 0.80 *** | 0.80 *** |
| Soil temperature, 5 cm depth | 0.92 *** | 0.92 *** | 0.85 *** | |
| Soil temperature, 10 cm depth | 0.92 *** | 0.92 *** | 0.84 *** | |
| Soil temperature, 15 cm depth | 0.88 *** | 0.92 *** | 0.82 *** | |
| Soil temperature, 30 cm depth | 0.88 *** | 0.92 *** | 0.81 *** | |
| GW level | 0.60 *** | 0.52 *** | 0.29 ** | |
| Water level in flooded area during frost-free period | −0.16 | |||
| Water temperature in flooded area | 0.56 *** | |||
| Surface-to-atmosphere CH4 fluxes | ||||
| Air temperature | 0.52 *** | 0.32 ** | 0.26 * | 0.32 *** |
| Soil temperature, 5 cm depth | 0.34 ** | 0.27 * | 0.31 *** | |
| Soil temperature, 10 cm depth | 0.32 ** | 0.30 * | 0.32 *** | |
| Soil temperature, 15 cm depth | 0.30 * | 0.28 ** | 0.30 *** | |
| Soil temperature, 30 cm depth | 0.30 * | 0.28 ** | 0.28 ** | |
| GW level | −0.02 | −0.08 | −0.01 | |
| Water level in flooded area during frost-free period | −0.04 | |||
| Water temperature in flooded area | 0.62 *** | |||
| Surface-to-atmosphere N2O fluxes | ||||
| Air temperature | 0.32 | −0.04 | −0.19 | −0.05 |
| Soil temperature, 5 cm depth | −0.04 | −0.20 | −0.14 | |
| Soil temperature, 10 cm depth | 0.05 | −0.18 | −0.15 | |
| Soil temperature, 15 cm depth | 0.01 | −0.21 * | −0.16 | |
| Soil temperature, 30 cm depth | 0.01 | −0.21 * | −0.15 | |
| GW level | −0.05 | 0.01 | 0.01 | |
| Water level in flooded area during frost-free period | −0.21 | |||
| Water temperature in flooded area | 0.26 | |||
| Surface-to-Atmosphere GHG Fluxes | Unit | Studied Land-Use Types with Organic Soils | |||
|---|---|---|---|---|---|
| Flooded Area | Rewetted Area (Bare Peat) | Rewetted Area (with Vegetation) | Undisturbed Peatland | ||
| Estimated annual CO2 fluxes (sum of soil heterotrophic and autotrophic respiration) | t CO2-C ha−1 yr−1 | 0.55 ± 0.05 | 1.73 ± 0.17 | 4.10 ± 0.21 | 3.45 ± 0.21 |
| IPCC CO2 emission factor taken from Table 3.1 in the 2013 IPCC Wetlands Supplement [9] | t CO2-C ha−1 yr−1 | - | 0.50 (95% range: from −0.71 to +1.71) | - | |
| Estimated annual CH4 fluxes | kg CH4-C ha−1 yr−1 | 251.1 ± 77.2 | 26.4 ± 12.0 | 22.6 ± 7.6 | 562.4 ± 155.8 |
| IPCC CH4 emission factor taken from Table 3.3 in the 2013 IPCC Wetlands Supplement [9] | kg CH4-C ha−1 yr−1 | - | 216 (95% range: from 0 to 856) | - | |
| Estimated annual N2O fluxes | kg N2O-N ha−1 yr−1 | 0.00 ± 0.06 | 0.32 ± 0.12 | 0.02 ± 0.03 | 0.66 ± 0.41 |
| IPCC N2O emission factor according to the 2013 IPCC Wetlands Supplement [9] | kg N2O-N ha−1 yr−1 | - | negligible | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bārdule, A.; Butlers, A.; Spalva, G.; Ivanovs, J.; Meļņiks, R.N.; Līcīte, I.; Lazdiņš, A. The Surface-to-Atmosphere GHG Fluxes in Rewetted and Permanently Flooded Former Peat Extraction Areas Compared to Pristine Peatland in Hemiboreal Latvia. Water 2023, 15, 1954. https://doi.org/10.3390/w15101954
Bārdule A, Butlers A, Spalva G, Ivanovs J, Meļņiks RN, Līcīte I, Lazdiņš A. The Surface-to-Atmosphere GHG Fluxes in Rewetted and Permanently Flooded Former Peat Extraction Areas Compared to Pristine Peatland in Hemiboreal Latvia. Water. 2023; 15(10):1954. https://doi.org/10.3390/w15101954
Chicago/Turabian StyleBārdule, Arta, Aldis Butlers, Gints Spalva, Jānis Ivanovs, Raitis Normunds Meļņiks, Ieva Līcīte, and Andis Lazdiņš. 2023. "The Surface-to-Atmosphere GHG Fluxes in Rewetted and Permanently Flooded Former Peat Extraction Areas Compared to Pristine Peatland in Hemiboreal Latvia" Water 15, no. 10: 1954. https://doi.org/10.3390/w15101954
APA StyleBārdule, A., Butlers, A., Spalva, G., Ivanovs, J., Meļņiks, R. N., Līcīte, I., & Lazdiņš, A. (2023). The Surface-to-Atmosphere GHG Fluxes in Rewetted and Permanently Flooded Former Peat Extraction Areas Compared to Pristine Peatland in Hemiboreal Latvia. Water, 15(10), 1954. https://doi.org/10.3390/w15101954









