Benthic Fish Communities Associated with Posidonia oceanica Beds May Reveal the Fishing Impact and Effectiveness of Marine Protected Areas: Two Case Studies in the Southern Tyrrhenian Sea
Abstract
1. Introduction
2. Materials and Methods
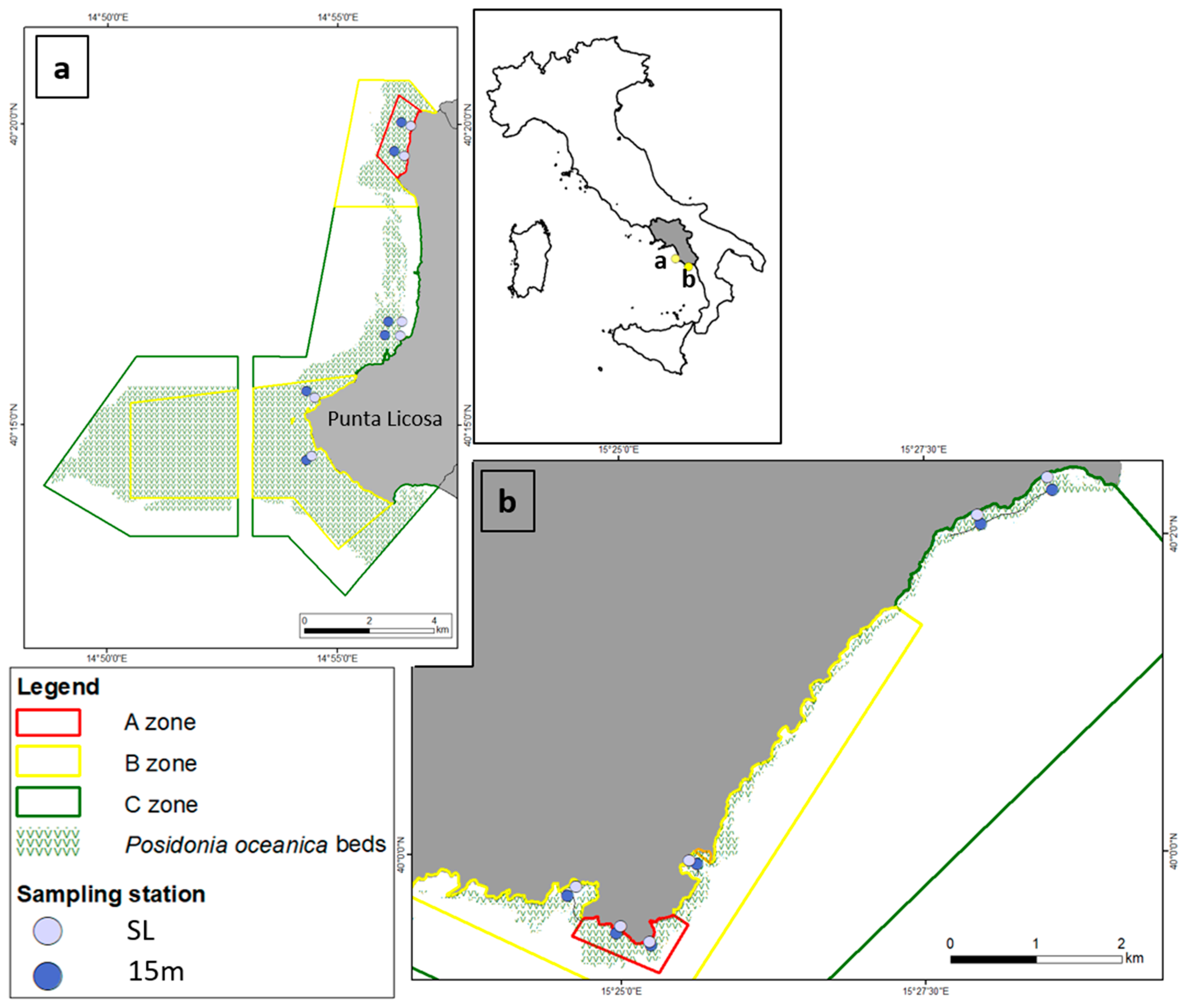
2.1. Study Area
2.2. Sampling Activities and Data Collection
2.3. Data Analyses
3. Results
3.1. Analysis of Posidonia oceanica Beds
3.2. Descriptive Analysis of Total Assemblages
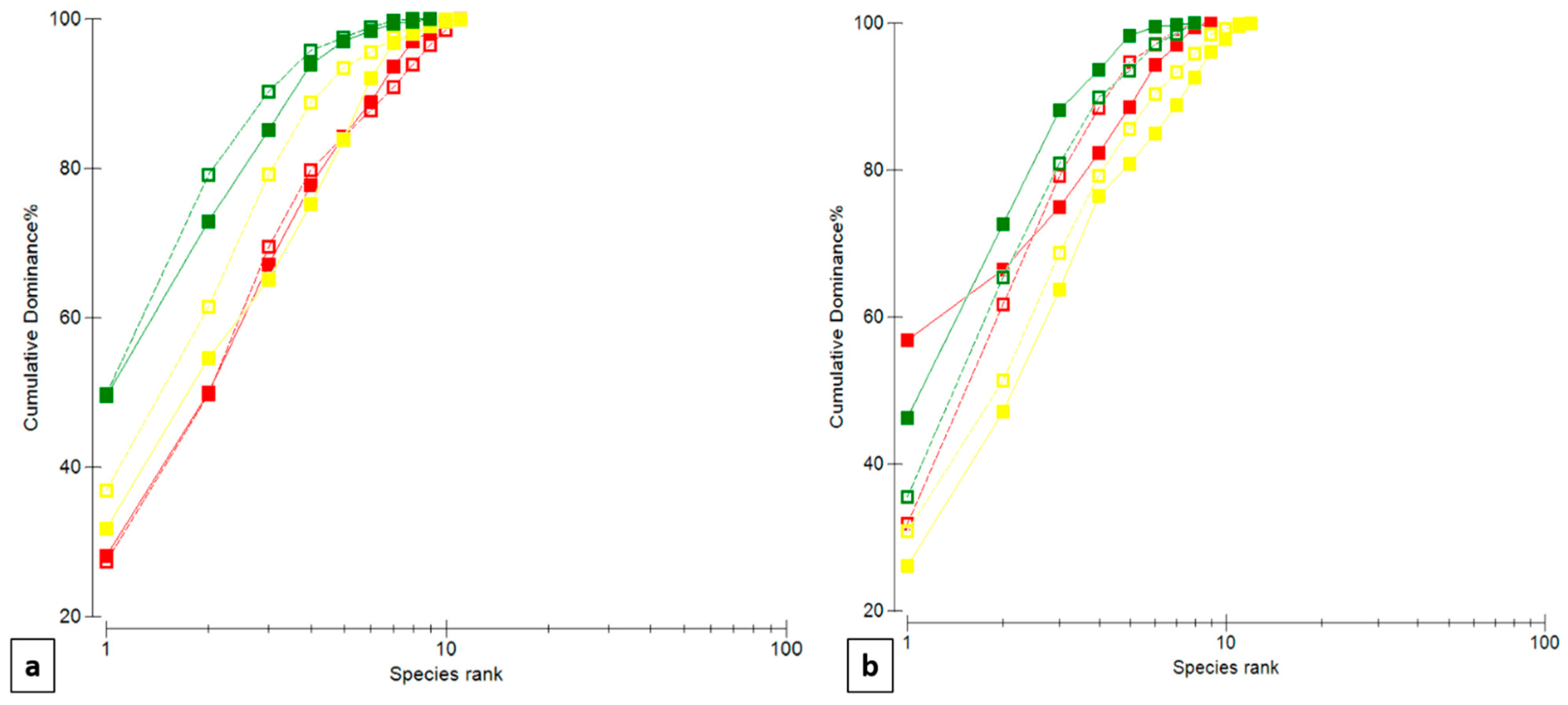
3.3. Multivariate Analyses on Fish Assemblages
3.4. Relations between P. oceanica Bed Characteristics and Fish Communities
4. Discussion
4.1. Posidonia oceanica Beds
4.2. Fish Communities
4.3. Relationship between P. oceanica Beds and Fish Communities
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gall, S.C.; Rodwell, L.D. Evaluating the social acceptability of Marine Protected Areas. Mar. Policy 2016, 65, 30–38. [Google Scholar] [CrossRef]
- Francour, P.; Harmelin, J.G.; Pollard, D.; Sartoretto, S. A review of marine protected areas in the northwestern Mediterranean region: Siting, usage, zonation and management. Aquat. Conserv. Mar. Freshw. Ecosyst. 2001, 11, 155–188. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Stelzenmüller, V.; South, A.; Sørensen, T.K.; Jones, P.J.S.; Kerr, S.; Badalamenti, F.; Anagnostou, C.; Breen, P.; Chust, G.; et al. Ecosystem-based marine spatial management: Review of concepts, policies, tools, and critical issues. Ocean Coast. Manag. 2011, 54, 807–820. [Google Scholar] [CrossRef]
- Grorud-colvert, K.; Sullivan-stack, J.; Roberts, C.; Constant, V.; Horta e Costa, B.; Pike, E.P.; Kingston, N.; Laffoley, D.; Sala, E.; Claudet, J.; et al. The MPA Guide: A framework to achieve global goals for the ocean. Science 2021, 373, eabf0861. [Google Scholar] [CrossRef] [PubMed]
- La Mesa, G.; Vacchi, M. An analysis of the coastal fish assemblage of the Ustica Island marine reserve (Mediterranean Sea). Mar. Ecol. 1999, 20, 147–165. [Google Scholar] [CrossRef]
- Valls, A.; Gascuel, D.; Guénette, S.; Francour, P. Modeling trophic interactions to assess the effects of a marine protected area: Case study in the NW Mediterranean Sea. Mar. Ecol. Prog. Ser. 2012, 456, 201–214. [Google Scholar] [CrossRef]
- Appolloni, L.; Bevilacqua, S.; Sbrescia, L.; Sandulli, R.; Terlizzi, A.; Russo, G.F. Does full protection count for the maintenance of β-diversity patterns in marine communities? Evidence from Mediterranean fish assemblages. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 27, 828–838. [Google Scholar] [CrossRef]
- Giakoumi, S.; Scianna, C.; Plass-Johnson, J.; Micheli, F.; Grorud-Colvert, K.; Thiriet, P.; Claudet, J.; Di Carlo, G.; Di Franco, A.; Gaines, S.D.; et al. Ecological effects of full and partial protection in the crowded Mediterranean Sea: A regional meta-analysis. Sci. Rep. 2017, 7, 8940. [Google Scholar] [CrossRef]
- Harmelin-Vivien, M.; Le Diréach, L.; Bayle-Sempere, J.; Charbonnel, E.; García-Charton, J.A.; Ody, D.; Pérez-Ruzafa, A.; Reñones, O.; Sánchez-Jerez, P.; Valle, C. Gradients of abundance and biomass across reserve boundaries in six Mediterranean marine protected areas: Evidence of fish spillover? Biol. Conserv. 2008, 141, 1829–1839. [Google Scholar] [CrossRef]
- White, J.W.; Scholz, A.J.; Rassweiler, A.; Steinback, C.; Botsford, L.W.; Kruse, S.; Costello, C.; Mitarai, S.; Siegel, D.A.; Drake, P.T.; et al. A comparison of approaches used for economic analysis in marine protected area network planning in California. Ocean Coast. Manag. 2013, 74, 77–89. [Google Scholar] [CrossRef]
- Rossiter, J.S.; Levine, A. What makes a “successful” marine protected area? The unique context of Hawaii’s fish replenishment areas. Mar. Policy 2014, 44, 196–203. [Google Scholar] [CrossRef]
- Frazão Santos, C.; Ehler, C.N.; Agardy, T.; Andrade, F.; Orbach, M.K.; Crowder, L.B. Marine spatial planning. In World Seas: An Environmental Evaluation Volume III: Ecological Issues and Environmental Impacts; Sheppard, C., Ed.; Candice Janco; Academic Press: Cambridge, MA, USA, 2019; pp. 571–592. ISBN 9780128050521. [Google Scholar] [CrossRef]
- Jentoft, S.; Pascual-Fernandez, J.J.; de la Cruz Modino, R.; Gonzalez-Ramallal, M.; Chuenpagdee, R. What Stakeholders Think About Marine Protected Areas: Case Studies from Spain. Hum. Ecol. 2012, 40, 185–197. [Google Scholar] [CrossRef]
- Appolloni, L.; Sandulli, R.; Vetrano, G.; Russo, G.F. A new approach to assess marine opportunity costs and monetary values-in-use for spatial planning and conservation; the case study of Gulf of Naples, Mediterranean Sea, Italy. Ocean Coast. Manag. 2018, 152, 135–144. [Google Scholar] [CrossRef]
- Buonocore, E.; Grande, U.; Franzese, P.P.; Russo, G.F. Trends and evolution in the concept of marine ecosystem services: An overview. Water 2021, 13, 2060. [Google Scholar] [CrossRef]
- Buonocore, E.; Russo, G.F.; Franzese, P.P. Assessing natural capital value in the network of Italian marine protected areas: A comparative approach. Ecol. Quest. 2020, 31, 67–76. [Google Scholar] [CrossRef]
- Appolloni, L.; Buonocore, E.; Russo, G.F.; Franzese, P.P. The use of remote sensing for monitoring Posidonia oceanica and Marine Protected Areas: A systemic review. Ecol. Quest. 2020, 31, 7–17. [Google Scholar] [CrossRef]
- Duarte, C.M.; Chiscano, C.L. Seagrass biomass and production: A reassessment. Aquat. Bot. 1999, 65, 159–174. [Google Scholar] [CrossRef]
- Pergent-Martini, C.; Rico-Raimondino, V.; Pergent, G. Primary production of Posidonia oceanica in the Mediterranean Basin. Mar. Biol. 1994, 120, 9–15. [Google Scholar] [CrossRef]
- Hemminga, M.A.; Duarte, C.M. Seagrass Ecology; Cambridge University Press: Cambridge, UK, 2000; ISBN 0521661846. [Google Scholar]
- Ott, J.A. Growth and Production in Posidonia oceanica (L.) Delile. Mar. Ecol. 1980, 1, 47–64. [Google Scholar] [CrossRef]
- Vacchi, M.; De Falco, G.; Simeone, S.; Montefalcone, M.; Morri, C.; Ferrari, M.; Bianchi, C.N. Biogeomorphology of the Mediterranean Posidonia oceanica seagrass meadows. Earth Surf. Process. Landforms 2017, 42, 42–54. [Google Scholar] [CrossRef]
- Montefalcone, M.; Vacchi, M.; Carbone, C.; Cabella, R.; Schiaffino, C.F.; Elter, F.M.; Morri, C.; Bianchi, C.N.; Ferrari, M. Seagrass on the rocks: Posidonia oceanica settled on shallow-water hard substrata withstands wave stress beyond predictions. Estuar. Coast. Shelf Sci. 2016, 180, 114–122. [Google Scholar] [CrossRef]
- Borum, J.; Duarte, C.M.; Greve, T.M.; Krause-Jensen, D. European Seagrasses: An Introduction to Monitoring and Management; M & MS Project; 2004; ISBN 8789143213. Available online: http://www.seagrasses.org (accessed on 4 May 2023).
- Boudouresque, C.F.; Pergent, G.; Pergent-Martini, C.; Ruitton, S.; Thibaut, T.; Verlaque, M. The necromass of the Posidonia oceanica seagrass meadow: Fate, role, ecosystem services and vulnerability. Hydrobiologia 2015, 781, 25–42. [Google Scholar] [CrossRef]
- Guidetti, P. Differences among fish assemblages associated with nearshore Posidonia oceanica seagrass beds, rocky-algal reefs and unvegetated sand habitats in the adriatic sea. Estuar. Coast. Shelf Sci. 2000, 50, 515–529. [Google Scholar] [CrossRef]
- Giakoumi, S.; Kokkoris, G.D. Effects of habitat and substrate complexity on shallow sublittoral fish assemblages in the Cyclades Archipelago, North-eastern Mediterranean sea. Mediterr. Mar. Sci. 2013, 14, 58–68. [Google Scholar] [CrossRef]
- Di Franco, A.; Bussotti, S.; Navone, A.; Panzalis, P.; Guidetti, P. Evaluating effects of total and partial restrictions to fishing on Mediterranean rocky-reef fish assemblages. Mar. Ecol. Prog. Ser. 2009, 387, 275–285. [Google Scholar] [CrossRef]
- La Mesa, G.; Guidetti, P.; Bussotti, S.; Cattaneo-Vietti, R.; Manganaro, A.; Molinari, A.; Russo, G.F.; Spanò, N.; Vetrano, G.; Tunesi, L. Rocky reef fish assemblages at six Mediterranean marine protected areas: Broad-scale patterns in assemblage structure, species richness and composition. Ital. J. Zool. 2013, 80, 90–103. [Google Scholar] [CrossRef]
- Karachle, P.K.; Stergiou, K.I. Diet and feeding habits of Spicara maena and S. smaris (Pisces, Osteichthyes, Centracanthidae) in the North Aegean Sea. Acta Adriat. 2014, 55, 75–84. [Google Scholar]
- Zubak Čižmek, I.; Stewart, S.T.; Kruschel, C.; Čižmek, H. Seascape Context as a Driver of the Fish Community Structure of Posidonia oceanica Meadows in the Adriatic Sea. Croat. J. Fish. 2021, 79, 89–109. [Google Scholar] [CrossRef]
- Diaz-Gil, C.; Grau, A.; Grau, A.M.; Palmer, M.; Cabrera Castro, R.; Jordà, G.; Catalán, I. Changes in the juvenile fish assemblage of a Mediterranean shallow Posidonia oceanica seagrass nursery area after half a century. Mediterr. Mar. Sci. 2019, 20, 603–615. [Google Scholar] [CrossRef]
- Harmelin-vivien, M.; Harmelin, J.; Chauvet, C.; Duval, C.; Galzin, R.; Lejeune, P.; Barnabe, G.; Blanc, F.; Chevalier, R.; Duclerc, J.; et al. Evaluation visuelle des peuplements et populations de poissons: Méthodes et problèmes. Rev. d’Écologie 1985, 40, 467–539. [Google Scholar] [CrossRef]
- Harmelin, J.G. Structure and Variability of the Ichthyo-fauna in a Mediterranean Protected Rocky Area (National Park of Port-Cros, France). Mar. Ecol. 1987, 8, 263–284. [Google Scholar] [CrossRef]
- Bell, J.D.; Harmelin-Vivien, M.L. Fish fauna of French Mediterranean Posidonia oceanica seagrass meadows. II: Feeding habits. Tethys 1983, 11, 1–14. [Google Scholar]
- Francour, P. Fish assemblages of Posidonia oceanica beds at port-cros (France, NW Mediterranean): Assessment of composition and long-term fluctuations by visual census. Mar. Ecol. 1997, 18, 157–173. [Google Scholar] [CrossRef]
- Moranta, J.; Palmer, M.; Morey, G.; Ruiz, A.; Morales-nin, B. Multi-scale spatial variability in fish assemblages associated with Posidonia oceanica meadows in the Western Mediterranean Sea. Estuar. Coast. Shelf Sci. 2006, 68, 579–592. [Google Scholar] [CrossRef]
- Kalogirou, S.; Corsini-Foka, M.; Sioulas, A.; Wennhage, H.; Pihl, L. Diversity, structure and function of fish assemblages associated with Posidonia oceanica beds in an area of the eastern Mediterranean Sea and the role of non-indigenous species. J. Fish Biol. 2010, 77, 2338–2357. [Google Scholar] [CrossRef] [PubMed]
- Zubak Čižmek, I.; Kruschel, C.; Schultz, S.T. Predators structure fish communities in Posidonia oceanica meadows: Meta-analysis of available data across the Mediterranean basin. Mar. Ecol. Prog. Ser. 2017, 566, 145–157. [Google Scholar] [CrossRef]
- Budillon, F.; Amodio, S.; Alberico, I.; Contestabile, P.; Vacchi, M.; Innangi, S.; Molisso, F. Present-day infralittoral prograding wedges (IPWs) in Central-Eastern Tyrrhenian Sea: Critical issues and challenges to their use as geomorphological indicators of sea level. Mar. Geol. 2022, 450, 106821. [Google Scholar] [CrossRef]
- Budillon, F.; Amodio, S.; Contestabile, P.; Alberico, I.; Innangi, S.; Molisso, F. The present-day nearshore submarine depositional terraces off the Campania coast (South-eastern Tyrrhenian Sea): An analysis of their morpho-bathymetric variability. In Proceedings of the International Workshop on Metrology for the Sea, Naples, Italy, 5–7 October 2020; pp. 132–138. [Google Scholar]
- Rendina, F.; Falace, A.; Alongi, G.; Buia, M.C.; Neiva, J.; Appolloni, L.; Marletta, G.; Russo, G.F. The Lush Fucales Underwater Forests off the Cilento Coast: An Overlooked Mediterranean Biodiversity Hotspot. Plants 2023, 12, 1497. [Google Scholar] [CrossRef]
- Appolloni, L.; Russo, G.F. Nel Regno di Leucosia; Appolloni, L., Russo, G.F., Eds.; Edizioni dell’Ippogrifo: Castellabate, Italy, 2021. [Google Scholar]
- Russo, G.F.; Di Donato, R.; Di Stefano, F. Gli habitat sottomarini delle coste della Campania. Ecodinamica 2008, 6, 37–56. [Google Scholar]
- Bacci, T.; Penna, M.; Sante Rende, F.; Tomasello, A.; Calvo, S. Scheda Metodologica Posidonia oceanica (L.) Delile Descrittore 1 Biodiversità (Dlgs 190/10) Elemento di Qualità Biologica Angiosperme (Dlgs 152/06) 2020, 14. Available online: https://www.researchgate.net/profile/Marina-Penna/publication/349589723_Nuova_scheda_metodologica_EQB_Angiosperme_Posidonia_dic_2020_-/links/60376b074585158939ca6c97/Nuova-scheda-metodologica-EQB-Angiosperme-Posidonia-dic-2020.pdf (accessed on 20 May 2023).
- Froese, R.; Pauly, D. FishBase World Wide Web Electronic Publication, Version (09/2010). 2012. Available online: www.fishbase.org (accessed on 19 May 2023).
- Stergiou, K.; Karpouzi, V.S. Feeding Habits and Trophic Levels of Mediterranean Fish. Rev. Fish Biol. Fish. 2002, 11, 217–254. [Google Scholar] [CrossRef]
- Pauly, D.; Palomares, M. Fishing down marine food web: It is far more pervasive than we thought. Bull. Mar. Sci. 2005, 76, 197–211. [Google Scholar]
- Evagelopoulos, A.; Batjakas, I.E.; Drosos, K. Structure and Diversity of the Demersal Fish Assemblages off Psara Island (Central Aegean Sea) Caught by Experimental Bottom Trawling. Ann. Int. J. Mar. Sci. 2021, 37, 379–391. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutation tests for univariate or multivariate analysis of variance and regression. Can. J. Fish. Aquat. Sci. 2001, 58, 626–639. [Google Scholar] [CrossRef]
- Anderson, M.J.; Ter Braak, C.J.F. Permutation Tests for Multi-Factorial Analysis of Variance. J. Stat. Comput. Simul. 2003, 73, 85–113. [Google Scholar] [CrossRef]
- Anderson, M.J.; Willis, T.J. Canonical Analysis of Principal Coordinates: A Useful Method of Constrained Ordination for Ecology. Ecology 2003, 84, 511–525. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation; Primer-E Ltd: Plymouth, UK, 1994; pp. 1–172. [Google Scholar]
- Anderson, M.J. DISTLM v.5: A FORTRAN computer program to calculate a distance-based multivariate analysis for a linear model. Dep. Stat. Univ. Auckl. N. Z. 2004, 10, 2016. [Google Scholar]
- Härdle, W.K.; Simar, L. Canonical correlation analysis. In Applied Multivariate Statistical Analysis; Springer: Berlin/Heidelberg, Germany, 2015; pp. 443–454. [Google Scholar]
- Terlizzi, A.; Anderson, M.J.; Fraschetti, S.; Benedetti-Cecchi, L. Scales of spatial variation in Mediterranean subtidal sessile assemblages at different depths. Mar. Ecol. Prog. Ser. 2007, 332, 25–39. [Google Scholar] [CrossRef]
- Rojo, I.; Anadon, J.D.; Garcia-Charton, J.A. Exceptionally high but still growing predatory reef fish biomass after 23 years of protection in a Marine Protected Area. PLoS ONE 2021, 16, e0246335. [Google Scholar] [CrossRef]
- Brondizio, E.S.; Settele, J.; Díaz, S.; Ngo, H.T. Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2019. [Google Scholar]
- United Nations, E. The First Global Integrated Marine Assessment: World Ocean Assessment; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Giraud, G. Contribution à la Description et à la Phénologie Quantitative des Herbiers de Posidonia oceanica (L.) Delile; Université d’Aix-Marseille II: Marseille, France, 1977. [Google Scholar]
- Pergent, G.; Pergent-Martini, C.; Boudouresque, C.-F. Utilisation de l’herbier à Posidonia oceanica comme indicateur biologique de la qualité du milieu littoral en Méditerranée: État des connaissances. Mésogée 1995, 54, 3–27. [Google Scholar]
- Pergent-Martini, C.; Pergent, G. Spatio-temporal dynamics of Posidonia oceanica beds near a sewage outfall (Mediterranean, France). In Proceedings of the International Workshop on Seagrass Biology, Rottnest Island, Australia, 25–29 January 1996; pp. 299–306. [Google Scholar]
- Donnarumma, L.; Lombardi, C.; Cocito, S.; Gambi, M.C. Settlement pattern of Posidonia oceanica epibionts along a gradient of ocean acidification: An approach with mimics. Mediterr. Mar. Sci. 2014, 15, 498–509. [Google Scholar] [CrossRef]
- Bedini, R.; Bedini, M.; Bonechi, L.; Piazzi, L. Patterns of spatial variability of mobile macro-invertebrate assemblages within a Posidonia oceanica meadow. J. Nat. Hist. 2015, 49, 2559–2581. [Google Scholar] [CrossRef]
- Peirano, A.; Niccolai, I.; Mauro, R.; Bianchi, C.N. Seasonal grazing and food preference of herbivores in a Posidonia oceanica meadow. Sci. Mar. 2001, 65, 367–374. [Google Scholar] [CrossRef]
- Montefalcone, M.; Chiantore, M.; Lanzone, A.; Morri, C.; Albertelli, G.; Bianchi, C.N. BACI design reveals the decline of the seagrass Posidonia oceanica induced by anchoring. Mar. Pollut. Bull. 2008, 56, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, P.; Baiata, P.; Ballesteros, E.; Di Franco, A.; Hereu, B.; Macpherson, E.; Micheli, F.; Pais, A.; Panzalis, P.; Rosenberg, A.A.; et al. Large-scale assessment of mediterranean marine protected areas effects on fish assemblages. PLoS ONE 2014, 9, e91841. [Google Scholar] [CrossRef]
- Valle, C.; Bayle-Sempere, J.T. Effects of a marine protected area on fish assemblage associated with Posidonia oceanica seagrass beds: Temporal and depth variations. J. Appl. Ichthyol. 2009, 25, 537–544. [Google Scholar] [CrossRef]
- Letourneur, Y.; Ruitton, S.; Sartoretto, S. Environmental and benthic habitat factors structuring the spatial distribution of a summer infralittoral fish assemblage in the north-western Mediterranean Sea. J. Mar. Biol. Assoc. 2003, 83, 193–204. [Google Scholar] [CrossRef]
- Halpern, B.S.; Warner, R.R. Matching marine reserve design to reserve objectives. Proc. R. Soc. B Biol. Sci. 2003, 270, 1871–1878. [Google Scholar] [CrossRef]
- Soler, G.A.; Edgar, G.J.; Thomson, R.J.; Kininmonth, S.; Campbell, J.; Dawson, T.P.; Barrett, N.S.; Bernard, A.T.F.; Galván, E.; Willis, T.J.; et al. Reef Fishes at All Trophic Levels Respond Positively to Effective Marine Protected Areas. PLoS ONE 2015, 10, e0140270. [Google Scholar] [CrossRef]
- Jennings, S.; Kaiser, M.J. The effects of fishing on marine ecosystems. In Advances in Marine Biology; Academic Press: Cambridge, MA, USA, 1998; pp. 201–212. [Google Scholar] [CrossRef]
- Mackay, M.; Hardesty, B.D.; Wilcox, C. The Intersection between Illegal Fishing, Crimes at Sea, and Social Well-Being. Front. Mar. Sci. 2020, 7, 589000. [Google Scholar] [CrossRef]
- Prato, G.; Barrier, C.; Francour, P.; Cappanera, V.; Markantonatou, V.; Guidetti, P.; Mangialajo, L.; Cattaneo-Vietti, R.; Gascuel, D. Assessing interacting impacts of artisanal and recreational fisheries in a small Marine Protected Area (Portofino, NW Mediterranean Sea). Ecosphere 2016, 7, e01601. [Google Scholar] [CrossRef]
- Ravard, D.; Brind’Amour, A.; Trenkel, V.M. Evaluating the potential impact of fishing on demersal species in the Bay of Biscay using simulations and survey data. Fish. Res. 2014, 157, 86–95. [Google Scholar] [CrossRef]
- Stagličić, N.; Matić-Skoko, S.; Pallaoro, A.; Grgičević, R.; Kraljević, M.; Tutman, P.; Dragičević, B.; Dulčić, J. Long-term trends in the structure of eastern Adriatic littoral fish assemblages: Consequences for fisheries management. Estuar. Coast. Shelf Sci. 2011, 94, 263–271. [Google Scholar] [CrossRef]
- Jouffre, D.; Inejih, C.A. Assessing the impact of fisheries on demersal fish assemblages of the Mauritanian continental shelf, 1987-1999, using dominance curves. ICES J. Mar. Sci. 2005, 62, 380–383. [Google Scholar] [CrossRef]
- Dehghan-Madiseh, S.; Nabavi, S.M.B.; Ghofleh-Marammazi, J.; Jahani, N.; Koochaknejad, E. Application of Abundance Biomass Curve in ecological health assessment of Khure-Mussa (northwest of the Persian Gulf). J. Persian Gulf 2012, 3, 1–10. [Google Scholar]
- Appolloni, L.; Ciorciaro, D.; Di Stefano, F.; Donnarumma, L.; Ferrigno, F.; Iacono, C.; Miccio, A.; Rendina, F.; Sandulli, R.; Russo, G.F. Different Metiers Affect Fish Catches Accounting in Marine Protected Areas: A Pilot Investigation Method. J. Environ. Account. Manag. 2022, 10, 237–252. [Google Scholar] [CrossRef]
- Soler, G.A.; Edgar, G.J.; Stuart-Smith, R.D.; Smith, A.D.M.; Thomson, R.J. Moving beyond trophic groups: Evaluating fishing-induced changes to temperate reef food webs. Mar. Ecol. Prog. Ser. 2018, 587, 175–186. [Google Scholar] [CrossRef]
- Zhou, S.; Smith, A.D.M.; Punt, A.E.; Richardson, A.J.; Gibbs, M.; Fulton, E.A.; Pascoe, S.; Bulman, C.; Bayliss, P.; Sainsbury, K. Ecosystem-based fisheries management requires a change to the selective fishing philosophy. Proc. Natl. Acad. Sci. USA 2010, 107, 9485–9489. [Google Scholar] [CrossRef]
- Garcia, S.M.; Kolding, J.; Rice, J.; Rochet, M.J.; Zhou, S.; Arimoto, T.; Beyer, J.E.; Borges, L.; Bundy, A.; Dunn, D.; et al. Reconsidering the consequences of selective fisheries. Science 2012, 335, 1045–1047. [Google Scholar] [CrossRef]
- Ghosh, B.; Kar, T.K. Sustainable use of prey species in a prey-predator system: Jointly determined ecological thresholds and economic trade-offs. Ecol. Modell. 2014, 272, 49–58. [Google Scholar] [CrossRef]
- Walters, C.; Christensen, V.; Fulton, B.; Smith, A.D.M.; Hilborn, R. Predictions from simple predator-prey theory about impacts of harvesting forage fishes. Ecol. Modell. 2016, 337, 272–280. [Google Scholar] [CrossRef]
- Moullec, F.; Barrier, N.; Drira, S.; Guilhaumon, F.; Marsaleix, P.; Somot, S.; Ulses, C.; Velez, L.; Shin, Y.J. An end-to-end model reveals losers and winners in a warming Mediterranean Sea. Front. Mar. Sci. 2019, 6, 345. [Google Scholar] [CrossRef]
- Zhou, S.; Smith, A.D.M. Effect of fishing intensity and selectivity on trophic structure and fishery production. Mar. Ecol. Prog. Ser. 2017, 585, 185–198. [Google Scholar] [CrossRef]
- Gambi, M.C.; Lorenti, M.; Russo, G.F.; Scipione, M.B.; Zupo, V. Depth and Seasonal Distribution of Some Groups of the Vagile Fauna of the Posidonia oceanica Leaf Stratum: Structural and Trophic Analyses. Mar. Ecol. 1992, 13, 17–39. [Google Scholar] [CrossRef]
- Bussotti, S.; Guidetti, P. Fish communities associated with different seagrass systems in the Mediterranean Sea. Nat. Sicil. 1999, 23, 245–259. [Google Scholar]




| D | LL | Bp | LS | Mec | Bio | |||
|---|---|---|---|---|---|---|---|---|
| SMC | SL | A | 550.6 ± 75.0 | 27.9 ± 11.6 | 16.60% | 7.1 ± 3.7 | 16.67% | 4.17% |
| B | 599.5 ± 131.0 | 42.9 ± 18.7 | 23.00% | 12.2 ± 7.1 | 20.25% | 2.53% | ||
| C | 427.3 ± 217.2 | 37.4 ± 17.0 | 11.60% | 7.5 ± 5.3 | 13.58% | 6.17% | ||
| 15 m | A | 599.2 ± 160.7 | 39.5 ± 19.9 | 16.40% | 12.3 ± 9.0 | 13.51% | 0.11% | |
| B | 569.2 ± 93.2 | 44.6 ± 17.8 | 15.80% | 13.8 ± 8.3 | 18.18% | 2.27% | ||
| C | 563.2 ± 97.2 | 45.0 ± 18.1 | 15.90% | 12.6 ± 7.5 | 23.26% | 2.33% | ||
| CIM | SL | A | 461.9 ± 114.6 | 38.4 ± 19.4 | 16.00% | 9.81 ± 6.79 | 20.23% | 3.37% |
| B | 539.4 ± 85.9 | 46.9 ± 19.1 | 17.80% | 13.50 ± 7.78 | 15.91% | 4.55% | ||
| C | 443.2 ± 86.3 | 34.0 ± 22.1 | 20.70% | 7.87 ± 5.95 | 25.93% | 3.71% | ||
| 15 m | A | 345.9 ± 109.6 | 53.9 ± 23.9 | 14.70% | 9.41 ± 6.44 | 20.48% | 4.82% | |
| B | 384.2 ± 94.8 | 55.9 ± 22.9 | 13.00% | 11.73 ± 7.03 | 23.86% | 4.55% | ||
| C | 353.6 ± 69.6 | 44.2 ± 22.2 | 14.20% | 7.93 ± 5.76 | 17.86% | 1.19% |
| Family | Species | CV | TG | SMC | CIM |
|---|---|---|---|---|---|
| Apogonidae | Apogon imberbis (Linnaeus, 1758) | NC | Micro | x | x |
| Carangidae | Seriola dumerili (Risso, 1810) | C | Pisc | - | x |
| Alosidae | Sardina pilchardus (Walbaum, 1792) | LC | Plan | x | - |
| Labridae | Coris julis (Linnaeus, 1758) | NC | Micro | x | x |
| Labrus merula Linnaeus, 1758 | C | Micro | x | x | |
| Labrus viridis Linnaeus, 1758 | LC | Micro | x | x | |
| Symphodus doderleini Jordan, 1890 | NC | Micro | x | - | |
| Centrolabrus melanocercus (Risso, 1810) | NC | Micro | - | x | |
| Symphodus ocellatus (Linnaeus, 1758) | NC | Micro | x | x | |
| Symphodus roissali (Risso, 1810) | NC | Micro | x | - | |
| Symphodus rostratus (Bloch, 1791) | NC | Micro | x | x | |
| Symphodus tinca (Linnaeus, 1758) | LC | Micro | x | x | |
| Thalassoma pavo (Linnaeus, 1758) | NC | Micro | x | x | |
| Moronidae | Dicentrarchus labrax (Linnaeus, 1758) | C | Pisc | x | x |
| Mugilidae | Mugil cephalus Linnaeus, 1758 | C | Detr | - | x |
| Mullidae | Mullus surmuletus Linnaeus, 1758 | C | Detr | x | - |
| Mullus barbatus Linnaeus, 1758 | C | Detr | x | x | |
| Pomacentridae | Chromis chromis (Linnaeus, 1758) | NC | Planc | x | x |
| Serranidae | Serranus cabrilla (Linnaeus, 1758) | C | Macro | x | x |
| Serranus scriba (Linnaeus, 1758) | C | Macro | x | x | |
| Epinephelus marginatus (Lowe, 1834) | C | Pisc | x | - | |
| Sparidae | Boops boops (Linnaeus, 1758) | C | Planc | - | x |
| Dentex dentex (Linnaeus, 1758) | C | Pisc | x | x | |
| Diplodus annularis (Linnaeus, 1758) | C | Omni | x | x | |
| Diplodus puntazzo (Walbaum, 1792) | C | Omni | x | - | |
| Diplodus vulgaris (Geoffroy Saint-Hilaire, 1817) | C | Omni | x | x | |
| Diplodus sargus (Linnaeus, 1758) | C | Omni | x | x | |
| Oblada melanurus (Linnaeus, 1758) | C | Planc | x | x | |
| Sarpa salpa (Linnaeus, 1758) | LC | Herb | x | x | |
| Sparus aurata Linnaeus, 1758 | C | Omni | - | x | |
| Spicara maena (Linnaeus, 1758) | LC | Plan | x | x | |
| Spicara smaris (Linnaeus, 1758) | LC | Plan | x | x | |
| Spondyliosoma cantharus (Linnaeus, 1758) | C | Omni | x | x | |
| Sphyraenidae | Sphyraena viridensis Cuvier, 1829 | C | Pisc | - | x |
| MPA | De | PL | SR | D | H’ | J | B |
|---|---|---|---|---|---|---|---|
| SMC | SL | A | 10.5 ± 1.8 | 106.7 ± 48.1 | 2.3 ± 0.4 | 0.71 ± 0.1 | 1.4 ± 0.9 |
| B | 8.3 ± 2.5 | 127.2 ± 41.7 | 2.2 ± 0.6 | 0.74 ± 0.1 | 1.3 ± 1.1 | ||
| C | 7.2 ± 2.9 | 95.3 ± 56.2 | 1.7 ± 0.6 | 0.59 ± 0.2 | 1.3 ± 0.1 | ||
| 15 m | A | 7.8 ± 2.0 | 92.5 ± 56.0 | 2.0 ± 0.2 | 0.68 ± 0.1 | 0.9 ± 0.4 | |
| B | 7.5 ± 2.5 | 116.3 ± 57.4 | 2.0 ± 0.5 | 0.69 ± 0.1 | 1.7 ± 1.6 | ||
| C | 7.0 ± 0.9 | 122.7 ± 54.8 | 2.0 ± 0.5 | 0.69 ± 0.2 | 2.5 ± 1.4 | ||
| CIM | SL | A | 9.0 ± 2.5 | 141.5 ± 76.2 | 1.7 ± 0.3 | 0.56 ± 0.2 | 0.2 ± 0.2 |
| B | 8.2 ± 1.6 | 111.3 ± 61.2 | 2.3 ± 0.3 | 0.78 ± 0.1 | 0.7 ± 0.6 | ||
| C | 7.2 ± 2.2 | 121.3 ± 49.4 | 1.9 ± 0.5 | 0.68 ± 0.1 | 0.2 ± 0.1 | ||
| 15 m | A | 7.2 ± 1.9 | 66.5 ± 46.0 | 2.1 ± 0.4 | 0.76 ± 0.1 | 0.01 ± 0.0 | |
| B | 7.7 ± 1.0 | 90.7 ± 34.9 | 2.0 ± 0.5 | 0.69 ± 0.1 | 0.9 ± 0.5 | ||
| C | 5.8 ± 0.7 | 99.0 ± 40.7 | 1.8 ± 0.3 | 0.71 ± 0.1 | 0.1 ± 0.1 |
| SMC | CIM | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Density Data | Biomass Data | Density Data | Biomass Data | ||||||
| Factor | Pseudo-F | p | Pseudo-F | p | Factor | Pseudo-F | p | Pseudo-F | p |
| PL | 1.89 | 0.021 | 1.88 | 0.018 | PL | 3.31 | 0.002 | 2.88 | 0.002 |
| De | 2.75 | 0.005 | 2.27 | 0.0182 | De | 2.77 | 0.004 | 2.86 | 0.006 |
| PLxDe | 0.92 | 0.5578 | 1.19 | 0.26 | PLxDe | 2.21 | 0.003 | 1.95 | 0.003 |
| Within PL factor | Within A level | ||||||||
| A-B | 1.15 | 0.23 | 1.29 | 0.11 | SL-15 m | 1.69 | 0.005 | 1.82 | 0.002 |
| A-C | 1.71 | 0.003 | 1.56 | 0.006 | Within B level | ||||
| B-C | 1.24 | 0.16 | 1.28 | 0.11 | SL-15 m | 1.06 | 0.341 | 1.23 | 0.108 |
| Within C level | |||||||||
| SL-15 m | 1.71 | 0.009 | 1.28 | 0.134 | |||||
| Within SL level | |||||||||
| A–B | 1.62 | 0.015 | 1.25 | 0.103 | |||||
| A–C | 1.11 | 0.322 | 0.93 | 0.532 | |||||
| B–C | 1.19 | 0.228 | 1.11 | 0.279 | |||||
| Within 15 m level | |||||||||
| A–B | 1.41 | 0.071 | 2.01 | 0.002 | |||||
| A–C | 2.21 | 0.004 | 2.01 | 0.003 | |||||
| B–C | 1.91 | 0.004 | 1.38 | 0.051 | |||||
| SMC | CIM | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Adj R2 | Pseudo-F | p | Variable | Adj R2 | Pseudo-F | p |
| Densities | Densities | ||||||
| Total length | 2.61 × 10−2 | 1.9393 | 0.0476 | Brown | 1.37 × 10−2 | 1.4875 | 0.0465 |
| Suface | 5.06 × 10−2 | 1.8775 | 0.0638 | Density | 2.58 × 10−2 | 1.4204 | 0.1806 |
| Biomasses | Green part | 2.70 × 10−2 | 1.0397 | 0.0408 | |||
| Total length | 1.83 × 10−2 | 1.6513 | 0.094 | Biomasses | |||
| Suface | 5.11 × 10−2 | 2.1769 | 0.285 | Density | 6.10 × 10−3 | 1.215 | 0.025 |
| Density | 5.20 × 10−2 | 1.0303 | 0.045 | Green part | 1.51 × 10−2 | 1.3104 | 0.0138 |
| Suface | 2.46 × 10−2 | 1.322 | 0.1894 | ||||
| Brown | 3.21 × 10−2 | 1.2478 | 0.2356 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Appolloni, L.; Pagliarani, A.; Cocozza di Montanara, A.; Rendina, F.; Donnarumma, L.; Ciorciaro, D.; Ferrigno, F.; Di Stefano, F.; Sandulli, R.; Russo, G.F. Benthic Fish Communities Associated with Posidonia oceanica Beds May Reveal the Fishing Impact and Effectiveness of Marine Protected Areas: Two Case Studies in the Southern Tyrrhenian Sea. Water 2023, 15, 1967. https://doi.org/10.3390/w15101967
Appolloni L, Pagliarani A, Cocozza di Montanara A, Rendina F, Donnarumma L, Ciorciaro D, Ferrigno F, Di Stefano F, Sandulli R, Russo GF. Benthic Fish Communities Associated with Posidonia oceanica Beds May Reveal the Fishing Impact and Effectiveness of Marine Protected Areas: Two Case Studies in the Southern Tyrrhenian Sea. Water. 2023; 15(10):1967. https://doi.org/10.3390/w15101967
Chicago/Turabian StyleAppolloni, Luca, Alberto Pagliarani, Adele Cocozza di Montanara, Francesco Rendina, Luigia Donnarumma, Domenico Ciorciaro, Federica Ferrigno, Floriana Di Stefano, Roberto Sandulli, and Giovanni Fulvio Russo. 2023. "Benthic Fish Communities Associated with Posidonia oceanica Beds May Reveal the Fishing Impact and Effectiveness of Marine Protected Areas: Two Case Studies in the Southern Tyrrhenian Sea" Water 15, no. 10: 1967. https://doi.org/10.3390/w15101967
APA StyleAppolloni, L., Pagliarani, A., Cocozza di Montanara, A., Rendina, F., Donnarumma, L., Ciorciaro, D., Ferrigno, F., Di Stefano, F., Sandulli, R., & Russo, G. F. (2023). Benthic Fish Communities Associated with Posidonia oceanica Beds May Reveal the Fishing Impact and Effectiveness of Marine Protected Areas: Two Case Studies in the Southern Tyrrhenian Sea. Water, 15(10), 1967. https://doi.org/10.3390/w15101967









