Hydrochemistry of the Geothermal in Gonghe Basin, Northeastern Tibetan Plateau: Implications for Hydro-Circulation and the Geothermal System
Abstract
:1. Introduction
2. Geological Setting
2.1. Geology

2.2. Geothermal and Geological Conditions

| Code | Spring Name | Location | Temperature (°C) | |
|---|---|---|---|---|
| Longitude | Latitude | |||
| 1 | Bayingou | 98.931969 | 36.438813 | 43 |
| 2 | Wahong | 98.821297 | 36.305936 | 46 |
| 3 | Qinggenhe | 99.124605 | 35.070330 | 68 |
| 4 | Sangchi 1 | 99.198644 | 36.051766 | 62 |
| 5 | Sangchi 2 | 99.233341 | 36.016961 | 30 |
| 6 | Wenquan | 99.430527 | 35.405138 | 61 |
| 7 | Ayihai | 100.701491 | 36.159602 | 32 |
| 8 | Lagan | 100.558969 | 35.766052 | 38 |
| 9 | Qunaihai | 101.040638 | 36.141444 | 96 |
| 10 | Zhacang | 102.634475 | 35.968611 | 93 |
| 11 | Xinjie | 101.389222 | 35.958500 | 64 |
| 12 | Reshui | 101.591113 | 36.401655 | 39 |
| 13 | Xiema | 101.678497 | 36.200894 | 32 |
| 14 | Lancai | 101.804258 | 35.587875 | 69 |
| 15 | Qukuhu | 101.971769 | 35.377741 | 49 |
3. Sampling and Analysis
3.1. Sampling
3.2. Methods
4. Results
4.1. Water Chemistry
4.2. Fluids and Gas Isotopes
4.2.1. Isotope Compositions of Fluids Samples
4.2.2. Isotope Compositions of Gas Samples
5. Discussion
5.1. Origin and Recharge Source of Geothermal Fluids
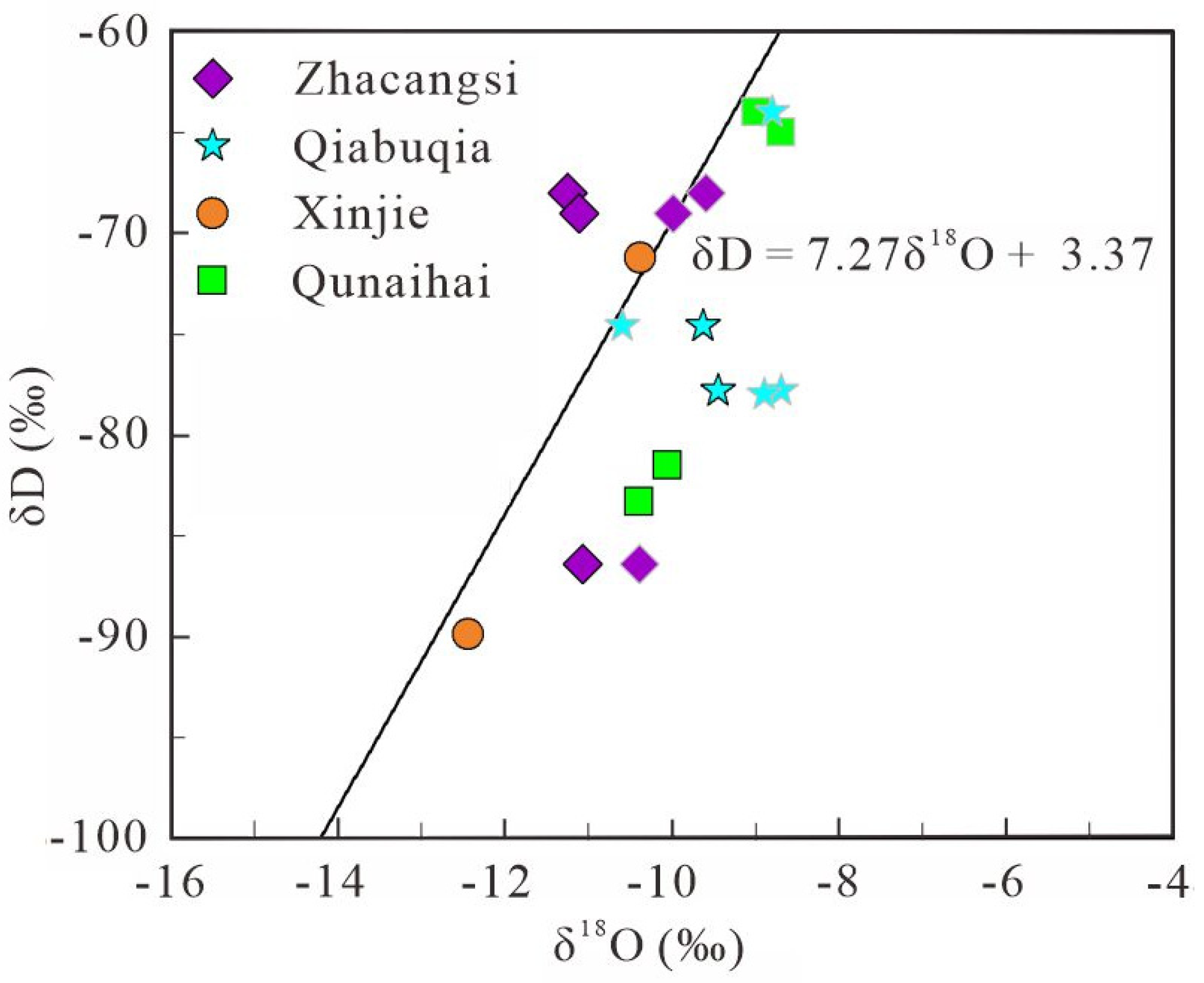
5.1.1. Deuterium and Oxygen Stable Isotopes Characteristics
5.1.2. Recharge Elevation
5.2. Hydrogeochemical Processes
5.2.1. General Hydrogeochemistry
5.2.2. Hydrochemical Facies Variation
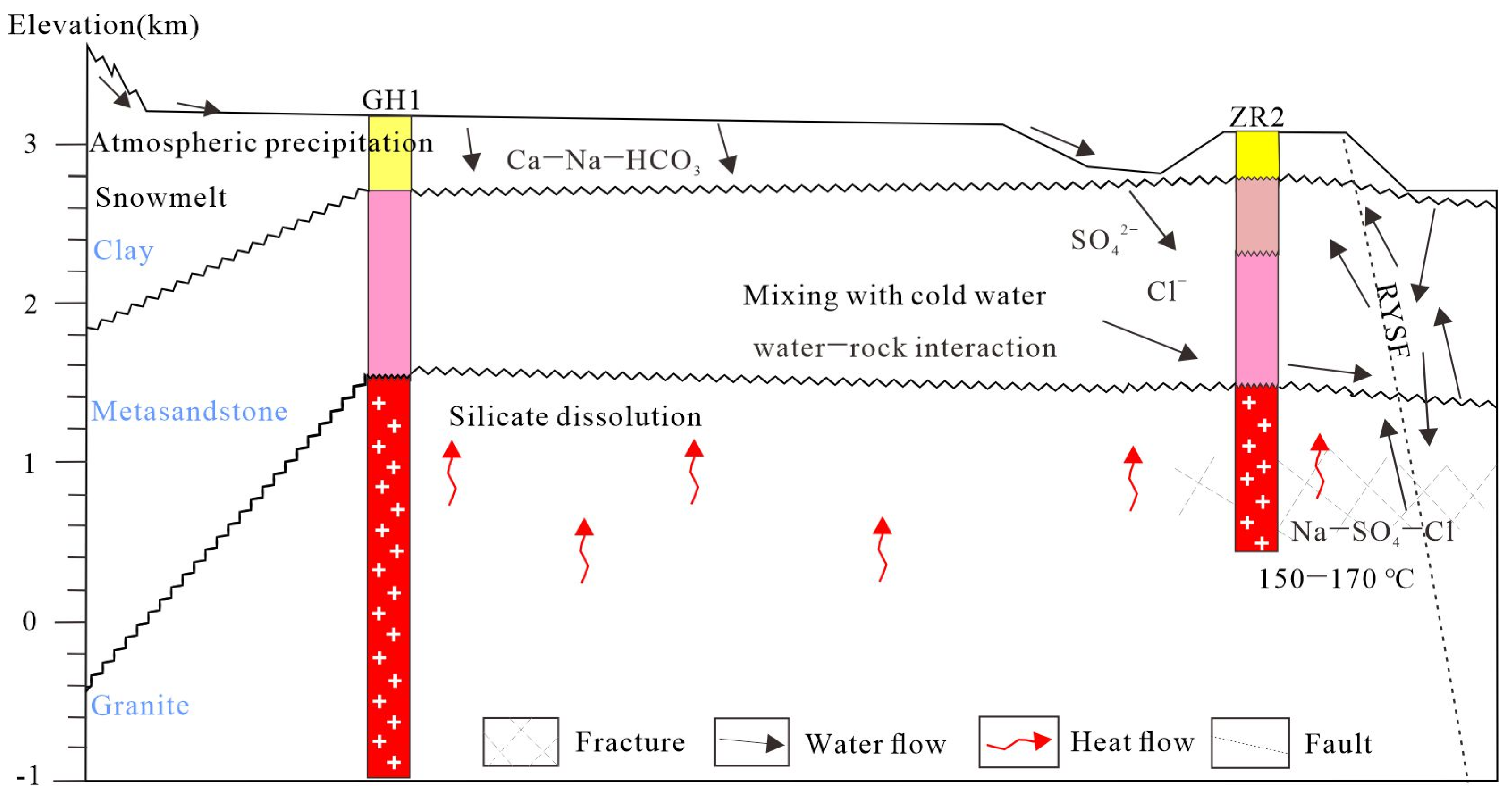
5.3. Deep Circulation of Geothermal Fluid
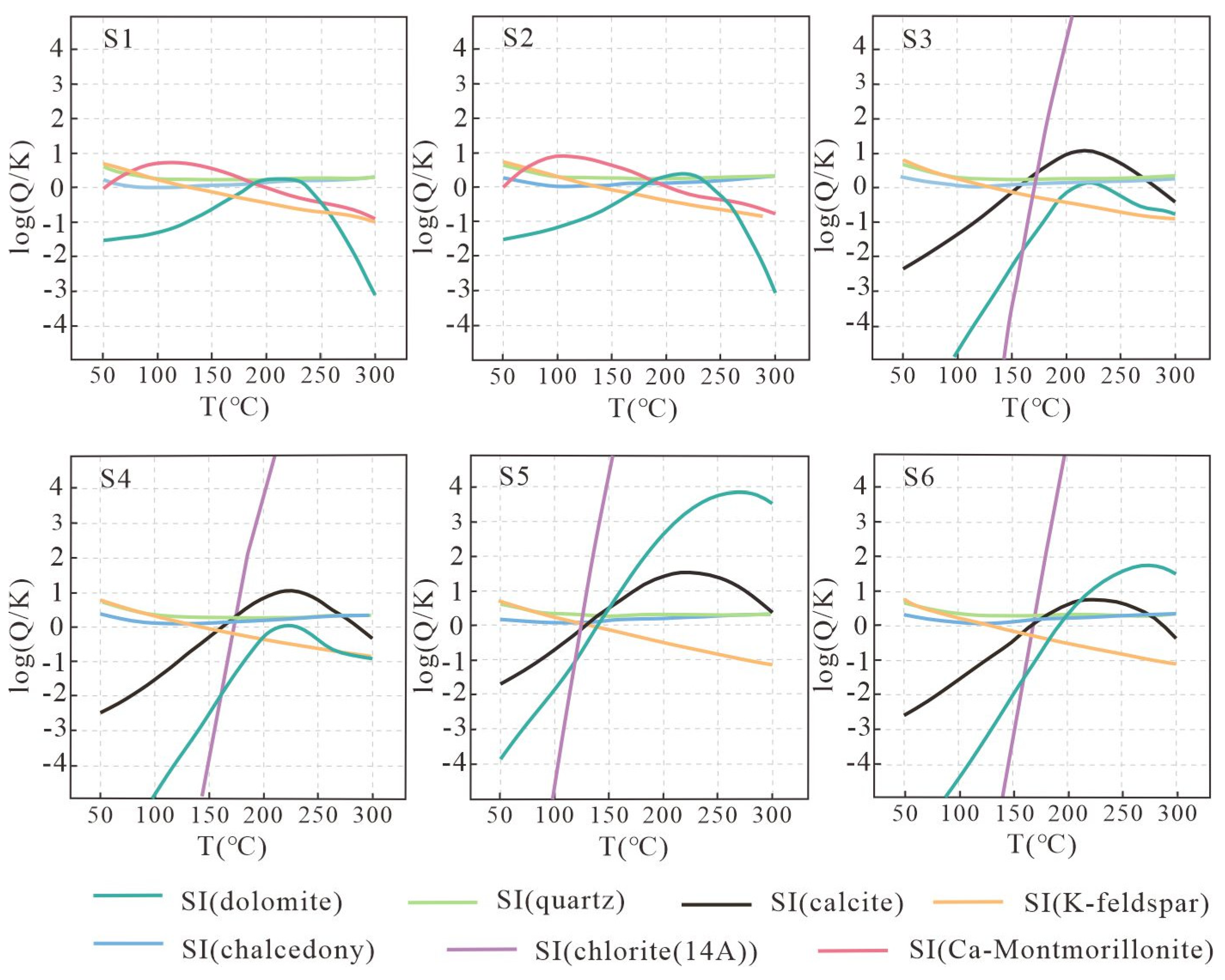
5.4. Heat Source and Reservoir Temperature
5.5. Conceptual Model of the Gonghe Geothermal System
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, X.; Liu, S.; Zhang, D.; Wang, G.; Luo, Y.; Hu, S.; Xu, Q. Geothermal Accumulation Constrained by the Tectonic Transformation in the Gonghe Basin, Northeastern Tibetan Plateau. Lithosphere 2022, 2022, 3936881. [Google Scholar] [CrossRef]
- Hu, S.; He, L.; Wang, J. Compilation of heat flow data in the China continental area(3rd edition). Chin. J. Geophys. 2001, 44, 611–626. [Google Scholar] [CrossRef]
- Xue, J.; Gan, B.; Li, B.; Wang, Z. Geological-geophysical characteristics of enhanced geothermal systems (hot dry rocks) in Gonghe-guide Basin. Geophys. Geochem. Explor. 2013, 37, 35–41. [Google Scholar]
- Zhang, S.; Yan, W.; Li, D.; Jia, X.; Zhang, S.; Li, S.; Fu, L.; Wu, H.; Zeng, Z.; Li, Z.; et al. Characteristics of geothermal geology of the Qiabuqia HDR in Gonghe Basin, Qinghai Province. Geol. China 2018, 45, 1087–1102. [Google Scholar]
- Tang, X.; Wang, G.; Zhang, D.; Ma, Y. Coupling Mechanism of Geothermal Accumulation and the Cenozoic Active Tectonics Evolution in Gonghe Basin, Northeastern Margin of the Tibetan Plateau. Acta Geosci. Sin. 2023, 44, 7–20. [Google Scholar]
- Sun, Z.; Li, B.; Wang, Z. Explortion of the possibility of hot dry rock occurring in the Qinghai Gonghe Basin. Hydrogeol. Eng. Geol. 2011, 38, 119–124+129. [Google Scholar] [CrossRef]
- Yan, W.; Wang, Y.; Gao, X.; Zhang, S.; Ma, Y.; Shang, X.; Guo, S. Distribution and Aggregration Mechanism of Geothermal Energy in Gonghe Basin. Northwest. Geol. 2013, 46, 223–230. [Google Scholar]
- Zhang, C.; Jiang, G.; Shi, Y.; Wang, Z.; Wang, Y.; Li, S.; Jia, X.; Hu, S. Terrestrial heat flow and crustal thermal structure of the Gonghe-Guide area, northeastern Qinghai-Tibetan plateau. Geothermics 2018, 72, 182–192. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, H.; Zhang, S.; Chen, X.; Cheng, Z.; Jia, X.; Li, S.; Fu, L.; Gao, L.; Xin, H. Three-dimensional magnetotelluric imaging of the geothermal system beneath the Gonghe Basin, Northeast Tibetan Plateau. Geothermics 2018, 76, 15–25. [Google Scholar] [CrossRef]
- Tang, X.; Wang, G.; Ma, Y.; Zhang, D.; Liu, Z.; Zhao, X.; Cheng, T. Geological model of heat source and accumulation for geothermal anomalies in the Gonghe basin, northeastern Tibetan Plateau. Acta Geol. Sin. 2020, 94, 2052–2065. [Google Scholar] [CrossRef]
- Feng, Y.-f.; Zhang, X.-x.; Zhang, B.; Liu, J.-t.; Wang, Y.-g.; Jia, D.-l.; Hao, L.-r.; Kong, Z.-y. The geothermal formation mechanism in the Gonghe Basin: Discussion and analysis from the geological background. China Geol. 2018, 1, 331–345. [Google Scholar] [CrossRef]
- Lang, X.; Lin, W.; Liu, Z.; Xing, L.; Wang, G. Hydrochemical Characteristics of Geothermal Water in Guide Basin. Earth Sci. 2016, 41, 1723–1734. [Google Scholar]
- Li, X.; Wu, G.; Lei, Y.; Li, C.; Zhao, J.; Bai, Y.; Zeng, Z.; Zhao, Z.; Zhang, S.; Zhao, A. Suggestions for Geothermal Genetic Mechanismand Exploiation of Zhangcang Temple Geothermal Energy in Guide County, Qinghai Province. J. Jilin Univ. 2016, 46, 220–229. [Google Scholar] [CrossRef]
- Jiang, Z.; Xu, T.; Mallants, D.; Tian, H.; Owen, D.D.R. Numerical modelling of stable isotope (2H and 18O) transport in a hydrogeothermal system: Model development and implementation to the Guide Basin, China. J. Hydrol. 2019, 569, 93–105. [Google Scholar] [CrossRef]
- Zhang, G.; Guo, A.; Yao, A. Western Qinling—Songpan continental tectonic node in China’s continental tectonics. Earth Sci. Front. 2004, 11, 23–32. [Google Scholar]
- Zeng, L.; Zhang, K.-J.; Tang, X.-C.; Zhang, Y.-X.; Li, Z.-W. Mid-Permian rifting in Central China: Record of geochronology, geochemistry and Sr-Nd-Hf isotopes of bimodal magmatism on NE Qinghai-Tibetan Plateau. Gondwana Res. 2018, 57, 77–89. [Google Scholar] [CrossRef]
- Zhang, K.J. Escape hypothesis for North and South China collision and tectonic evolution of the Qinling orogen, eastern Asia. Eclogae Geol. Helv. 2002, 95, 237–247. [Google Scholar]
- Jiang, G.; Hu, S.; Shi, Y.; Zhang, C.; Wang, Z.; Hu, D. Terrestrial heat flow of continental China: Updated dataset and tectonic implications. Tectonophysics 2019, 753, 36–48. [Google Scholar] [CrossRef]
- Hu, S.B.; He, L.J.; Wang, J.Y. Heat flow in the continental area of China: A new data set. Earth Planet. Sci. Lett. 2000, 179, 407–419. [Google Scholar] [CrossRef]
- Klyuev, R.V.; Golik, V.I.; Bosikov, I.I. Comprehensive assessment of hydrogeological conditions for the formation of mineral water resources of the Nizhne-Karmadon deposit. Bull. Tomsk. Polytech. Univ.-Geo Assets Eng. 2021, 332, 206–218. [Google Scholar] [CrossRef]
- Yan, W. Characteristics of Gonghe Basin hot dry rock and its utilization prospects. Sci. Technol. Rev. 2015, 33, 54–57. [Google Scholar]
- Chandrajith, R.; Barth, J.A.C.; Subasinghe, N.D.; Merten, D.; Dissanayake, C.B. Geochemical and isotope characterization of geothermal spring waters in Sri Lanka: Evidence for steeper than expected geothermal gradients. J. Hydrol. 2013, 476, 360–369. [Google Scholar] [CrossRef]
- Cinti, D.; Tassi, F.; Procesi, M.; Brusca, L.; Cabassi, J.; Capecchiacci, F.; Delgado Huertas, A.; Galli, G.; Grassa, F.; Vaselli, O.; et al. Geochemistry of hydrothermal fluids from the eastern sector of the Sabatini Volcanic District (central Italy). Appl. Geochem. 2017, 84, 187–201. [Google Scholar] [CrossRef]
- Fusari, A.; Carroll, M.R.; Ferraro, S.; Giovannetti, R.; Giudetti, G.; Invernizzi, C.; Mussi, M.; Pennisi, M. Circulation path of thermal waters within the Laga foredeep basin inferred from chemical and isotopic (delta 18O, delta D, 3H, 87Sr/86Sr) data. Appl. Geochem. 2017, 78, 23–34. [Google Scholar] [CrossRef]
- Elderfield, H. Strontium isotope stratigraphy. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1986, 57, 71–90. [Google Scholar] [CrossRef]
- Palmer, M.R.; Edmond, J.M. Controls over the strontium isotope composition of river water. Geochim. Cosmochim. Acta 1992, 56, 2099–2111. [Google Scholar] [CrossRef]
- Bottomley, D.J.; Gregoire, D.C.; Raven, K.G. Saline groundwaters and brines in the canadian shield—Geochemical and isotopic evidence for a residual evaporite brine component. Geochim. Cosmochim. Acta 1994, 58, 1483–1498. [Google Scholar] [CrossRef]
- Bullen, T.D.; Krabbenhoft, D.P.; Kendall, C. Kinetic and mineralogic controls on the evolution of groundwater chemistry and Sr-87/Sr-86 in a sandy silicate aquifer, northern Wisconsin, USA. Geochim. Cosmochim. Acta 1996, 60, 1807–1821. [Google Scholar] [CrossRef]
- Hou, Z.Y.; Xu, T.F.; Li, S.T.; Jiang, Z.J.; Feng, B.; Cao, Y.Q.; Feng, G.H.; Yuan, Y.L.; Hu, Z.X. Reconstruction of different original water chemical compositions and estimation of reservoir temperature from mixed geothermal water using the method of integrated multicomponent geothermometry: A case study of the Gonghe Basin, northeastern Tibetan Plateau, China. Appl. Geochem. 2019, 108, 104389. [Google Scholar] [CrossRef]
- Li, Y. Hydrogeochemical Characteristics and Its Origin Analysis of Geothermal Water in the Qia Bu-Qia Area, GongHe Basin. Master’s Thesis, East China University of Technology, Shanghai, China, 2016. [Google Scholar]
- Pan, S.; Kong, Y.; Wang, K.; Ren, Y.; Pang, Z.; Zhang, C.; Wen, D.; Zhang, L.; Feng, Q.; Zhu, G.; et al. Magmatic origin of geothermal fluids constrained by geochemical evidence: Implications for the heat source in the northeastern Tibetan Plateau. J. Hydrol. 2021, 603, 126985. [Google Scholar] [CrossRef]
- Liu, M.; Guo, Q.; Zhang, X.; Luo, J.; Li, J.; Zhou, C.; Guo, W.; Zhang, C.; Zhu, M. Geochemistry of geothermal waters from the Gonghe region, Northwestern China: Implications for identification of the heat source. Environ. Earth Sci. 2016, 75, 682. [Google Scholar] [CrossRef]
- Oxburgh, E.R.; Onions, R.K. Helium loss, tectonics, and the terrestrial heat-budget. Science 1987, 237, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- Taran, Y.A. Geochemistry of volcanic and hydrothermal fluids and volatile budget of the Kamchatka-Kuril subduction zone. Geochim. Et Cosmochim. Acta 2009, 73, 1067–1094. [Google Scholar] [CrossRef]
- Klemperer, S.L.; Zhao, P.; Whyte, C.J.; Darrah, T.H.; Crossey, L.J.; Karlstrom, K.E.; Liu, T.; Winn, C.; Hilton, D.R.; Ding, L. Limited underthrusting of India below Tibet: 3He/4He analysis of thermal springs locates the mantle suture in continental collision. Proc. Natl. Acad. Sci. USA 2022, 119, e2113877119. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, J.; Pang, Z.; Hu, S.; Tian, J.; Bao, S. The eastern Tibetan Plateau geothermal belt, western China: Geology, geophysics, genesis, and hydrothermal system. Tectonophysics 2017, 717, 433–448. [Google Scholar] [CrossRef]
- Gautheron, C.; Moreira, M.; Allegre, C. He, Ne and Ar composition of the European lithospheric mantle. Chem. Geol. 2005, 217, 97–112. [Google Scholar] [CrossRef]
- Tardani, D.; Reich, M.; Roulleau, E.; Takahata, N.; Sano, Y.; Perez-Flores, P.; Sanchez-Alfaro, P.; Cembrano, J.; Arancibia, G. Exploring the structural controls on helium, nitrogen and carbon isotope signatures in hydrothermal fluids along an intra-arc fault system. Geochim. Et Cosmochim. Acta 2016, 184, 193–211. [Google Scholar] [CrossRef]
- Karakus, H. Helium and carbon isotope composition of gas discharges in the Simav Geothermal Field, Turkey: Implications for the heat source. Geothermics 2015, 57, 213–223. [Google Scholar] [CrossRef]
- LIU, J. Research on Water Vapor Sources of Four Provinces in Northwest China Based on Precipitation Stable Isotopes. Master’s Thesis, Shanxi Normal University, Taiyuan, China, 2019. [Google Scholar]
- Siegenthaler, U.; Oeschger, H. Correlation of 18O in precipitation with temperature and altitude. Nature 1980, 285, 314–317. [Google Scholar] [CrossRef]
- Chamberlain, C.P.; Poage, M.A. Reconstructing the paleotopography of mountain belts from the isotopic composition of authigenic minerals. Geology 2000, 28, 115–118. [Google Scholar] [CrossRef]
- Li, J.; Pang, Z. The elevation gradient of stable isotopes in precipitation in the eastern margin of Tibetan Plateau. Sci. China Earth Sci. 2022, 65, 1972–1984. [Google Scholar] [CrossRef]
- Xiao, J.; Jin, Z.; Zhang, F. Geochemical and isotopic characteristics of shallow groundwater within the Lake Qinghai catchment, NE Tibetan Plateau. Quat. Int. 2013, 313, 62–73. [Google Scholar] [CrossRef]
- Dansgaard, W. Stable isotopes in precipitation. Tellus 1964, 16, 436–468. [Google Scholar] [CrossRef]
- Dotsika, E.; Leontiadis, I.; Poutoukis, D.; Cioni, R.; Raco, B. Fluid geochemistry of the Chios geothermal area, Chios Island, Greece. J. Volcanol. Geotherm. Res. 2006, 154, 237–250. [Google Scholar] [CrossRef]
- Tarcan, G.; Gemici, U. Water geochemistry of the Seferihisar geothermal area, Izmir, Turkey. J. Volcanol. Geotherm. Res. 2003, 126, 225–242. [Google Scholar] [CrossRef]
- Michard, G. Behavior of major elements and some trace-elements (Li, Rb, Cs, Sr, Fe, Mn, W, F) in deep hot waters from granitic areas. Chem. Geol. 1990, 89, 117–134. [Google Scholar] [CrossRef]
- Krabbenhoeft, A.; Eisenhauer, A.; Boehm, F.; Vollstaedt, H.; Fietzke, J.; Liebetrau, V.; Augustin, N.; Peucker-Ehrenbrink, B.; Mueller, M.N.; Horn, C.; et al. Constraining the marine strontium budget with natural strontium isotope fractionations (87Sr/86Sr*, delta 88/86Sr) of carbonates, hydrothermal solutions and river waters. Geochim. Cosmochim. Acta 2010, 74, 4097–4109. [Google Scholar] [CrossRef]
- Peucker-Ehrenbrink, B.; Fiske, G.J. A continental perspective of the seawater 87Sr/86Sr record: A review. Chem. Geol. 2019, 510, 140–165. [Google Scholar] [CrossRef]
- Capo, R.C.; Stewart, B.W.; Chadwick, O.A. Strontium isotopes as tracers of ecosystem processes: Theory and methods. Geoderma 1998, 82, 197–225. [Google Scholar] [CrossRef]
- Han, G.L.; Liu, C.Q. Strontium isotope and major ion chemistry of the rainwaters from Guiyang, Guizhou Province, China. Sci. Total Environ. 2006, 364, 165–174. [Google Scholar] [CrossRef]
- Cook, P.G.; Solomon, D.K. Recent advances in dating young groundwater: Chlorofluorocarbons, 3H/3He and 85Kr. J. Hydrol. 1997, 191, 245–265. [Google Scholar] [CrossRef]
- Edmunds, W.M.; Ma, J.; Aeschbach-Hertig, W.; Kipfer, R.; Darbyshire, D.P.F. Groundwater recharge history and hydrogeochemical evolution in the Minqin Basin, North West China. Appl. Geochem. 2006, 21, 2148–2170. [Google Scholar] [CrossRef]
- Jasechko, S. Global Isotope Hydrogeology?Review. Rev. Geophys. 2019, 57, 835–965. [Google Scholar] [CrossRef]
- Munnich, K.O. Heidelberg natural radiocarbon measurements-I. Science 1957, 126, 194–199. [Google Scholar] [CrossRef]
- Chacha, N.; Njau, K.N.; Lugomela, G.V.; Muzuka, A.N.N. Groundwater age dating and recharge mechanism of Arusha aquifer, northern Tanzania: Application of radioisotope and stable isotope techniques. Hydrogeol. J. 2018, 26, 2693–2706. [Google Scholar] [CrossRef]
- Hoke, L.; Hilton, D.R.; Lamb, S.H.; Hammerschmidt, K.; Friedrichsen, H. 3He evidence for a wide zone of active mantle melting beneath the central andes. Earth Planet. Sci. Lett. 1994, 128, 341–355. [Google Scholar] [CrossRef]
- Klemperer, S.L.; Kennedy, B.M.; Sastry, S.R.; Makovsky, Y.; Harinarayana, T.; Leech, M.L. Mantle fluids in the Karakoram fault: Helium isotope evidence. Earth Planet. Sci. Lett. 2013, 366, 59–70. [Google Scholar] [CrossRef]
- Torgersen, T.; Drenkard, S.; Stute, M.; Schlosser, P.; Shapiro, A. Mantle helium in ground waters of eastern North-America: Time and space constraints on sources. Geology 1995, 23, 675–678. [Google Scholar] [CrossRef]
- Ballentine, C.J.; Burnard, P.G. Production, Release and transport of noble gases in the continental crust. Rev. Mineral. Geo-Chem. 2002, 47, 481–538. [Google Scholar] [CrossRef]
- Sano, Y.; Wakita, H. Geographical distribution of 3He/4He ratios in Japan: Implications for arc tectonics and incipient magmatism. J. Geophys. Res. -Solid Earth Planets 1985, 90, 8729–8741. [Google Scholar] [CrossRef]
- Ma, L.; Castro, M.C.; Hall, C.M. Crustal noble gases in deep brines as natural tracers of vertical transport processes in the Michigan Basin. Geochem. Geophys. Geosystems 2009, 10, Q06001. [Google Scholar] [CrossRef]
- Tolstikhin, I.; Waber, H.N.; Kamensky, I.; Loosli, H.H.; Skiba, V.; Gannibal, M. Production, redistribution and loss of helium and argon isotopes in a thick sedimentary aquitard-aquifer system (Molasse Basin, Switzerland). Chem. Geol. 2011, 286, 48–58. [Google Scholar] [CrossRef]
- Kharaka, Y.K.; Lico, M.S.; Law, L.M. Chemical geothermometers applied to formation waters, gulf-of-mexico and california basins. Aapg Bull.-Am. Assoc. Pet. Geol. 1982, 66, 588. [Google Scholar]
- Ahmad, M.; Akram, W.; Ahmad, N.; Tasneem, M.A.; Rafiq, M.; Latif, Z. Assessment of reservoir temperatures of thermal springs of the northern areas of Pakistan by chemical and isotope geothermometry. Geothermics 2002, 31, 613–631. [Google Scholar] [CrossRef]
- Arnorsson, S. The use of mixing models and chemical geothermometers for estimating underground temperatures in geothermal systems. J. Volcanol. Geotherm. Res. 1985, 23, 299–335. [Google Scholar] [CrossRef]
- Pirlo, M.C. Hydrogeochemistry and geothermometry of thermal groundwaters from the Birdsville Track Ridge, Great Artesian Basin, South Australia. Geothermics 2004, 33, 743–774. [Google Scholar] [CrossRef]
- Chenaker, H.; Houha, B.; Vincent, V. Hydrogeochemistry and geothermometry of thermal water from northeastern Algeria. Geothermics 2018, 75, 137–145. [Google Scholar] [CrossRef]
- Reed, M.; Spycher, N. Calculation of ph and mineral equilibria in hydrothermal waters with application to geothermometry and studies of boiling and dilution. Geochim. Cosmochim. Acta 1984, 48, 1479–1492. [Google Scholar] [CrossRef]
- Pang, Z.H.; Reed, M. Theoretical chemical thermometry on geothermal waters: Problems and methods. Geochim. Cosmochim. Acta 1998, 62, 1083–1091. [Google Scholar] [CrossRef]
- Spycher, N.; Peiffer, L.; Sonnenthal, E.L.; Saldi, G.; Reed, M.H.; Kennedy, B.M. Integrated multicomponent solute geothermometry. Geothermics 2014, 51, 113–123. [Google Scholar] [CrossRef]






| Sample ID | Longitude | Latitude | SO42− (mg/L) | Cl− (mg/L) | NO3− (mg/L) | F− (mg/L) | HCO3− (mg/L) | CO32− (mg/L) | Ca2+ (mg/L) | Mg2+ (mg/L) | Na+ (mg/L) | K+ (mg/L) | TDS (mg/L) | PH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 101.3020 | 35.9683 | 567 | 313 | 0.005 | 4.19 | 15.40 | 24.20 | 55.80 | 0.17 | 429 | 17.50 | 1488 | 8.97 |
| S2 | 101.3010 | 35.9677 | 541 | 307 | 0.005 | 4.47 | 24.60 | 18.10 | 53.80 | 0.23 | 448 | 17.90 | 1474 | 8.87 |
| S3 | 101.3010 | 35.9683 | 546 | 329 | 0.005 | 4.49 | 55.30 | 6.04 | 58.00 | 0.42 | 429 | 18.70 | 1470 | 8.72 |
| S4 | 101.3020 | 35.9681 | 539 | 326 | 0.005 | 4.48 | 49.10 | 9.06 | 59.90 | 0.54 | 438 | 18.70 | 1454 | 8.64 |
| S5 | 101.3890 | 35.6253 | 115 | 19.30 | 0.60 | 4.54 | 280 | 6.04 | 37.90 | 6.05 | 130 | 5.96 | 478 | 8.46 |
| S6 | 101.3900 | 35.6253 | 234 | 17.20 | 1.26 | 8.77 | 169 | 9.06 | 11.80 | 0.53 | 169 | 7.35 | 524 | 8.57 |
| H1 | 101.3890 | 35.6253 | 41.70 | 3.55 | 1.59 | 0.55 | 280 | 3.02 | 64.90 | 11.80 | 26.20 | 2.39 | 302 | 8.58 |
| H2 | 101.3840 | 36.0403 | 70.90 | 9.13 | 1.84 | 0.33 | 249 | 3.02 | 61.30 | 23.30 | 28.10 | 2.58 | 330 | 8.45 |
| H3 | 101.2780 | 36.0992 | 59.40 | 12.20 | 2.20 | 0.17 | 221 | 3.02 | 49.20 | 16.90 | 30.00 | 3.18 | 288 | 8.52 |
| H4 | 101.2690 | 36.0692 | 51.20 | 12.20 | 2.16 | 0.16 | 218 | 3.02 | 47.00 | 17.60 | 30.00 | 3.38 | 290 | 8.48 |
| H5 | 101.2790 | 36.0622 | 53.60 | 12.20 | 0.78 | 0.21 | 237 | 6.04 | 44.50 | 23.90 | 24.30 | 2.19 | 300 | 8.48 |
| H6 | 101.3910 | 36.0394 | 46.00 | 10.10 | 0.80 | 0.21 | 227 | 6.04 | 42.80 | 23.30 | 20.00 | 2.19 | 274 | 8.46 |
| Sample ID | Longitude | Latitude | Sampling Elevation (m) | δ18O (‰) | δD (‰) | δ13C (‰) | 3H (TU) | 87Sr/86Sr | Reference |
|---|---|---|---|---|---|---|---|---|---|
| S1 | 101.301660 | 35.968333 | 2511 | −11.1 | −84.3 | −13.80 | <3 | 0.714475 | |
| S2 | 101.301380 | 35.967777 | 2511 | −11.2 | −85.7 | −15.20 | <3 | 0.714370 | |
| S3 | 101.301110 | 35.968333 | 2509 | −11.1 | −84.4 | −6.00 | <3 | 0.714410 | |
| S4 | 101.301940 | 35.968055 | 2508 | −11.1 | −84.6 | −10.40 | <3 | 0.714328 | |
| S5 | 101.389440 | 35.625277 | 3240 | −10.4 | −71.2 | −12.10 | 9.6 | 0.712943 | |
| S6 | 101.389720 | 35.625277 | 3242 | −12.4 | −89.9 | −9.10 | <3 | 0.714421 | |
| S7 | 101.036430 | 36.140809 | 2557 | −10.4 | −83.3 | −5.40 | — | 0.712577 | |
| S8 | 101.038070 | 36.141305 | 2577 | −10.1 | −81.5 | −5.60 | — | 0.712627 | |
| S9 | 100.690410 | 36.207874 | 2644 | −9.6 | −66.7 | −10.50 | — | 0.711735 | |
| S10 | 100.683490 | 36.203893 | 2655 | −9.4 | −65.4 | — | — | 0.711801 | |
| G18 | — | — | 2700 | −10.6 | −74.6 | — | — | — | [29] |
| G20 | — | — | 2886 | −8.7 | −77.8 | — | — | — | [29] |
| GH18 | — | — | 2796 | −8.9 | −78.0 | — | — | — | [30] |
| GH48 | — | — | 2660 | −8.8 | −64.0 | — | — | — | [30] |
| G29 | — | — | — | −10.4 | −86.4 | — | — | — | [31] |
| GD-06 | — | — | — | −9.6 | −68.0 | — | — | — | [32] |
| GD-07 | — | — | — | −10.0 | −69.0 | — | — | — | [32] |
| GD-01 | — | — | — | −9.0 | −64.0 | — | — | — | [32] |
| GD-04 | — | — | — | −8.7 | −65.0 | — | — | — | [32] |
| GD-05 | — | — | — | −7.9 | −59.0 | — | — | — | [32] |
| Sample ID | Longitude | Latitude | R/Ra | 3He/4He | 4He/20Ne |
|---|---|---|---|---|---|
| G1 | 101.301666 | 35.968333 | 0.05 | 7.26 × 10−8 | 24.0 |
| G2 | 101.301388 | 35.967777 | 0.49 | 6.87 × 10−7 | 1.3 |
| G3 | 101.301111 | 35.968333 | 0.04 | 5.87 × 10−8 | 48.0 |
| G4 | 101.301944 | 35.968055 | 0.07 | 1.01 × 10−7 | 6.2 |
| G5 | 101.389444 | 35.625277 | 0.84 | 1.18 × 10−6 | 1.0 |
| G6 | 101.389722 | 35.625277 | 0.03 | 4.53 × 10−8 | 58.0 |
| Sample ID | Sites | Elevation (m) | δ18O | δD | Recharge Elevation (m) | Recharge Elevation (m) | Average (m) |
|---|---|---|---|---|---|---|---|
| S1 | Zhacang | 2511 | −11.08 | −84.34 | — | 3782 | 3782 |
| S2 | 2511 | −11.26 | −85.67 | — | 3833 | 3833 | |
| S3 | 2509 | −11.12 | −84.36 | — | 3781 | 3781 | |
| S4 | 2508 | −11.12 | −84.61 | — | 3789 | 3789 | |
| S5 | Xinjie | 3240 | −10.39 | −71.21 | 3883 | 4006 | 3944 |
| S6 | 3242 | −12.45 | −89.89 | 4400 | 4726 | 4563 | |
| S7 | Qunaihai | 2557 | −10.40 | −83.31 | — | 3788 | 3788 |
| S8 | 2577 | −10.06 | −81.50 | — | 3739 | 3739 | |
| S9 | Qiabuqia | 2644. | −9.60 | −66.70 | 3097 | 3236 | 3166 |
| S10 | 2655 | −9.50 | −65.50 | 3063 | 3200 | 3131 | |
| G18 | 2700 | −10.60 | −74.60 | 3395 | 3596 | 3496 | |
| G20 | 2886 | −8.70 | −77.80 | — | 3905 | 3905 | |
| GH18 | 2796 | −8.90 | −78.00 | — | 3823 | 3823 | |
| GH48 | 2660 | −8.80 | −64.00 | 2905 | 3148 | 3027 |
| Sample ID | δ18O (‰) | δD (‰) | d |
|---|---|---|---|
| S1 | −11.08 | −84.34 | 4.30 |
| S2 | −11.26 | −85.67 | 4.41 |
| S3 | −11.12 | −84.36 | 4.60 |
| S4 | −11.12 | −84.61 | 4.35 |
| S5 | −10.39 | −71.21 | 11.91 |
| S6 | −12.45 | −89.89 | 9.71 |
| S7 | −10.40 | −83.31 | −0.11 |
| S8 | −10.06 | −81.50 | −1.02 |
| S9 | −9.63 | −66.69 | 10.35 |
| S10 | −9.45 | −65.46 | 10.14 |
| Sample ID | Sites | γNa+/γCl− | 100 × γSO42−/γCl− | γCl−/(γHCO3− + CO32−) |
|---|---|---|---|---|
| S1 | Zhacang | 1.37 | 181.15 | 7.90 |
| S2 | 1.46 | 176.22 | 7.19 | |
| S3 | 1.30 | 165.96 | 5.36 | |
| S4 | 1.34 | 165.34 | 5.61 | |
| S5 | Xinjie | 6.74 | 595.85 | 0.07 |
| S6 | 9.83 | 1360.47 | 0.10 |
| Areas | 3H | Age |
|---|---|---|
| continental areas | <0.8 | Submodern groundwater, replenished before 1952 |
| 0.8–5 | Mixed water of submodern and recent replenishment | |
| 5–15 | Modern water (5–10 yr) | |
| 15–30 | Groundwater containing some nuclear tritium | |
| 30–50 | Contains significant amounts of water replenished in the 1960s or 1970s | |
| >50 | Mainly from the 1960s |
| Sample ID | 14C (pMC) | Uncorrected Age | Corrected Age |
|---|---|---|---|
| S1 | 43.24 | 6931 | 3433 |
| S2 | 35.06 | 8665 | 6097 |
| S3 | 101.40 | Modern | Modern |
| S4 | 45.36 | 6535 | 217 |
| S5 | 85.39 | 1306 | Modern |
| S6 | 38.39 | 7915 | 199 |
| S7 | 2.30 | 31,185 | 17,332 |
| S8 | 2.73 | 29,768 | 16,393 |
| S9 | 32.68 | 9246 | 3026 |
| Type | Geothermometer | Code | Expression |
|---|---|---|---|
| silica geothermometer | SiO2 | Geothermometer 1 | T = 1315/(5.205 − lg (SiO2)) − 273.15 |
| SiO2 | Geothermometer 2 | T = 1309/(5.19 − lg (SiO2)) − 273.15 | |
| SiO2 | Geothermometer 3 | T = −1107/(0.0254 + lg (SiO2)) − 273.15 | |
| Chalcedonite | Geothermometer 4 | T = 1032/(4.69 − lg (SiO2)) − 273.15 | |
| Cation geothermometer | Na-K | Geothermometer 5 | T = 856/(0.857 + lg (Na/K)) − 273.15 |
| Na-K | Geothermometer 6 | T = 1217/(1.483 + lg (Na/K)) − 273.15 | |
| Na-K | Geothermometer 7 | T = 933/(0.933 + lg (Na/K)) − 273.15 | |
| Na-K | Geothermometer 8 | T = 1390/(1.75 + lg (Na/K)) − 273.15 | |
| K-Mg | Geothermometer 9 | T = 4410/(13.95 − lg (K2/Mg)) − 273.15 |
| Sample ID | Geothermometer 1 | Geothermometer 2 | Geothermometer 3 | Geothermometer 4 | Geothermometer 5 | Geothermometer 6 | Geothermometer 7 | Geothermometer 8 | Geothermometer 9 |
|---|---|---|---|---|---|---|---|---|---|
| S1 | 163 | 163 | 227 | 139 | 108 | 151 | 129 | 170 | 139 |
| S2 | 163 | 163 | 227 | 139 | 106 | 149 | 127 | 168 | 135 |
| S3 | 169 | 169 | 218 | 146 | 113 | 155 | 134 | 174 | 127 |
| S4 | 171 | 171 | 215 | 149 | 111 | 153 | 132 | 172 | 123 |
| S5 | 121 | 121 | 311 | 93 | 117 | 158 | 138 | 177 | 61 |
| S6 | 144 | 144 | 260 | 118 | 113 | 155 | 133 | 174 | 96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Tang, X.; Han, X.; Zhang, D.; Wang, G. Hydrochemistry of the Geothermal in Gonghe Basin, Northeastern Tibetan Plateau: Implications for Hydro-Circulation and the Geothermal System. Water 2023, 15, 1971. https://doi.org/10.3390/w15111971
Liu S, Tang X, Han X, Zhang D, Wang G. Hydrochemistry of the Geothermal in Gonghe Basin, Northeastern Tibetan Plateau: Implications for Hydro-Circulation and the Geothermal System. Water. 2023; 15(11):1971. https://doi.org/10.3390/w15111971
Chicago/Turabian StyleLiu, Shasha, Xianchun Tang, Xiaomeng Han, Dailei Zhang, and Guiling Wang. 2023. "Hydrochemistry of the Geothermal in Gonghe Basin, Northeastern Tibetan Plateau: Implications for Hydro-Circulation and the Geothermal System" Water 15, no. 11: 1971. https://doi.org/10.3390/w15111971







