Removal of NOMs by Carbon Nanotubes/Polysulfone Nanocomposite Hollow Fiber Membranes for the Control of Disinfection Byproducts (DBPs)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Natural Water Samples
2.3. Fabrication of Hollow Fiber Nanocomposite Membranes
2.4. Filtration Test
2.5. THMs Analysis
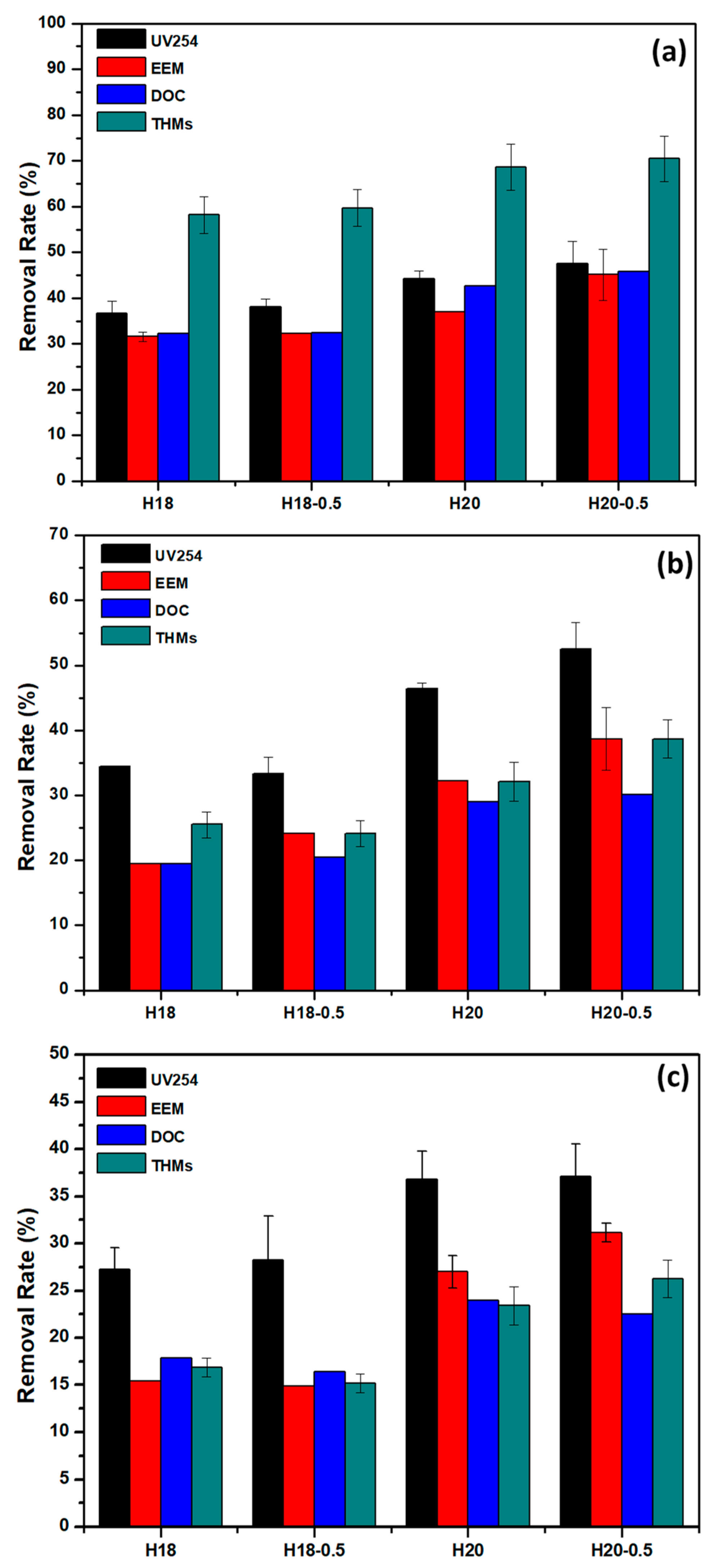
3. Results
3.1. Basic Parameters of Water Samples
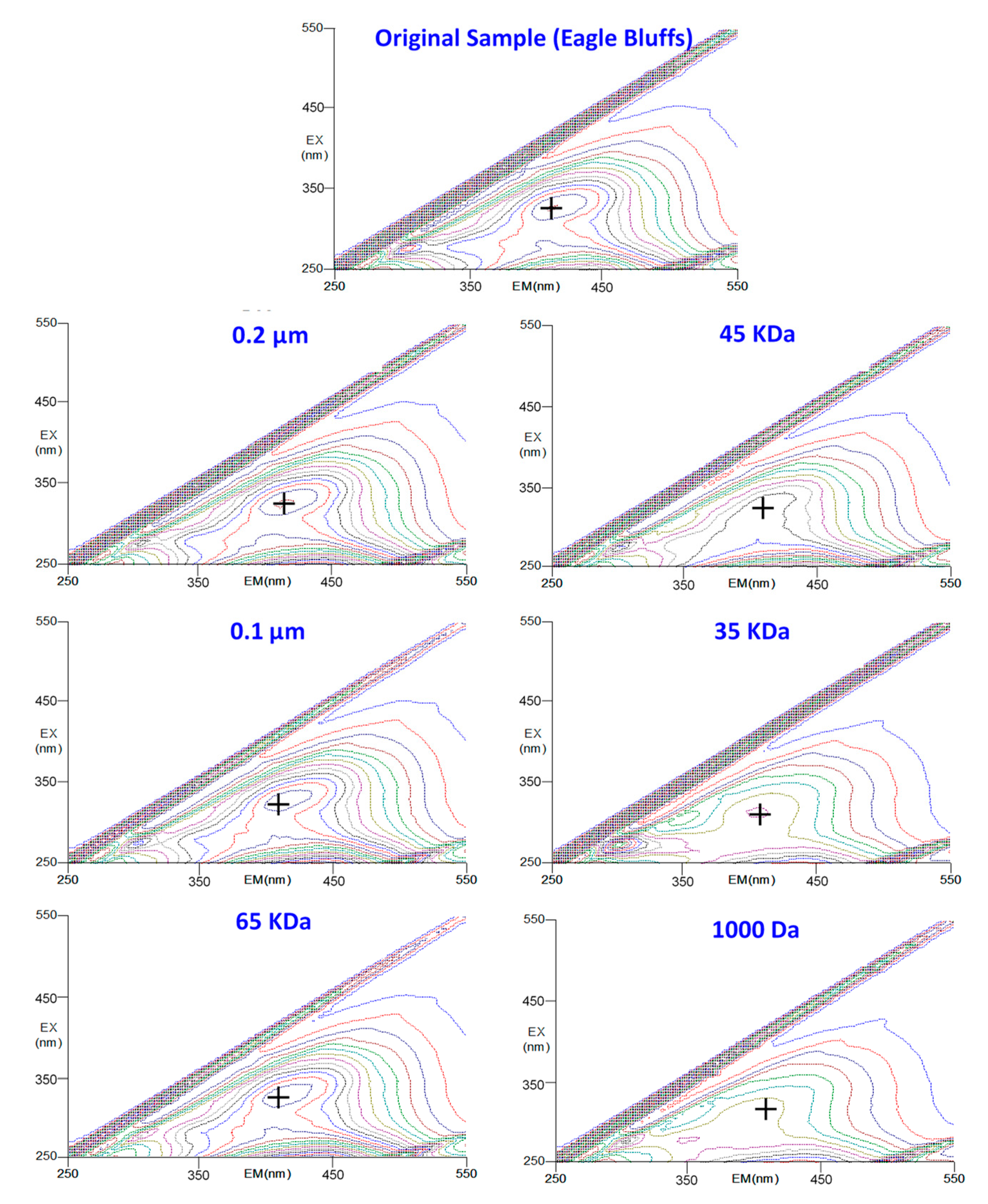
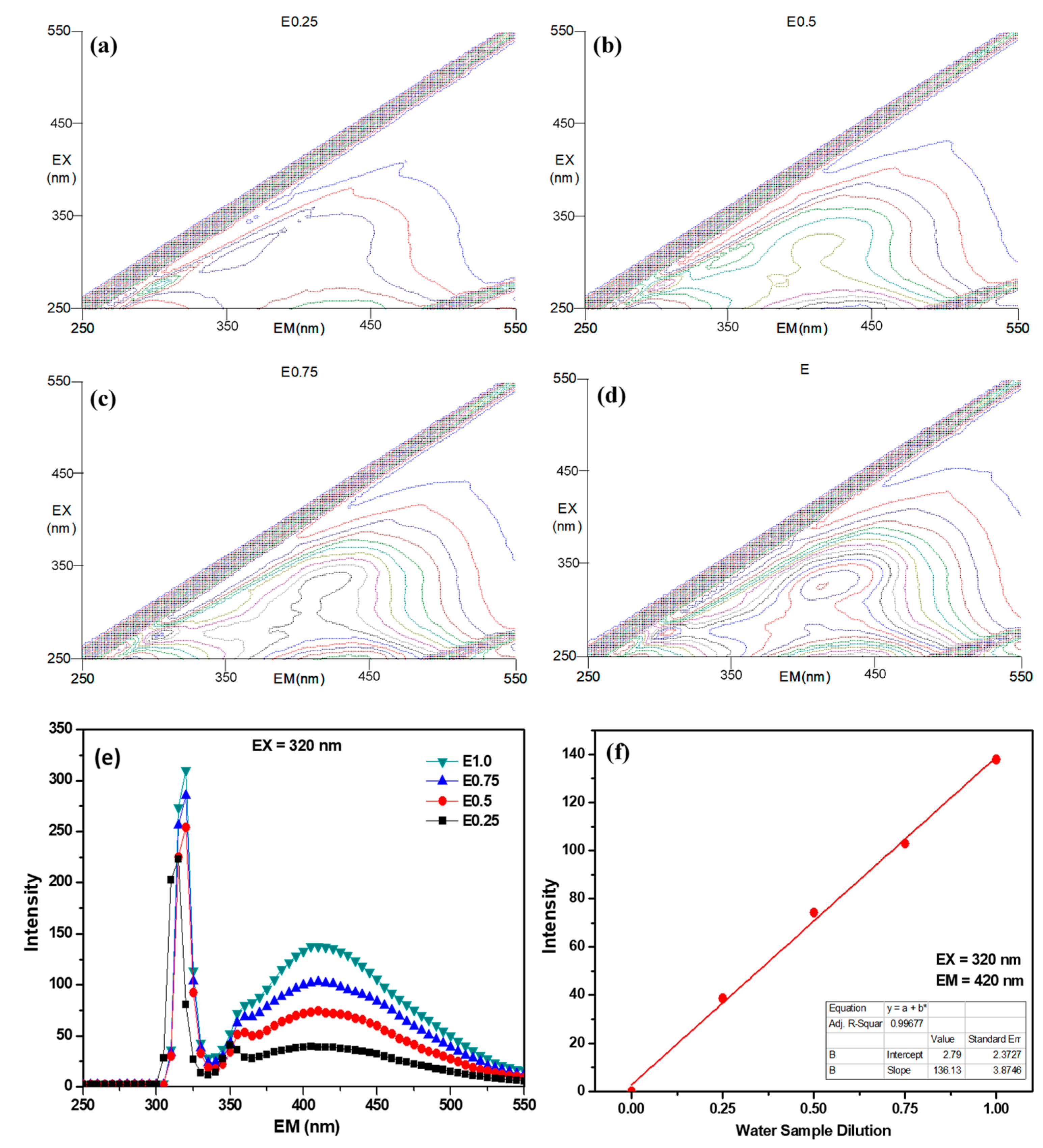
3.2. Effects of MWCO on the UV254 and EEM Removal Rates
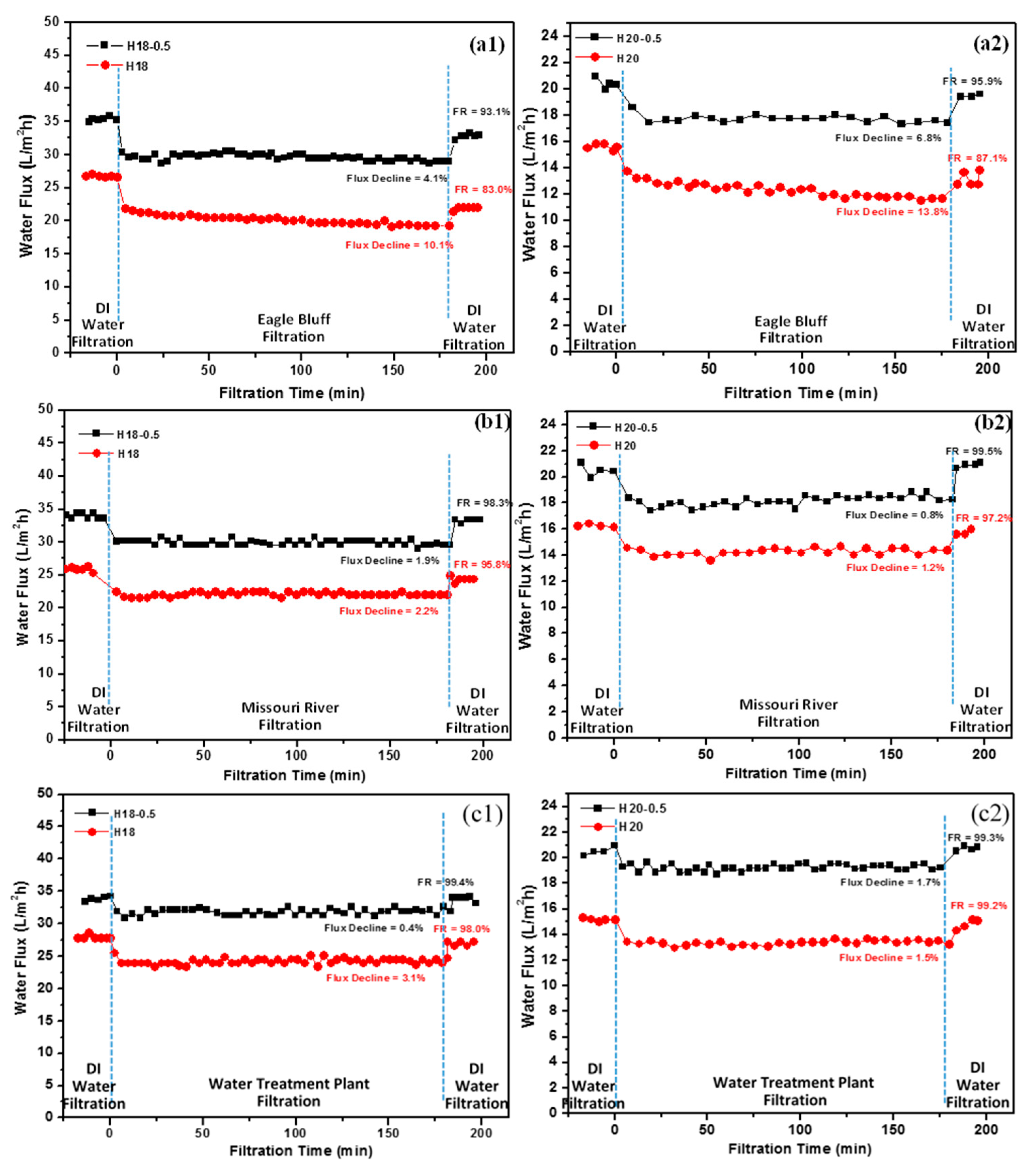
3.3. Performance of OMWNTs/PSU HFMs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singer, P.C. Control of Disinfection By-Products in Drinking Water. J. Environ. Eng. 1994, 120, 727–744. [Google Scholar] [CrossRef]
- Hua, B.; Veum, K.; Koirala, A.; Jones, J.; Clevenger, T.; Deng, B. Fluorescence fingerprints to monitor total trihalomethanes and N-nitrosodimethylamine formation potentials in water. Environ. Chem. Lett. 2006, 5, 73–77. [Google Scholar] [CrossRef]
- Kim, J.; Clevenger, T.E. Prediction of N-nitrosodimethylamine (NDMA) formation as a disinfection by-product. J. Hazard. Mater. 2007, 145, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Bei, E.; Liu, C.; Deng, Y.-L.; Miao, Y.; Qiu, Y.; Lu, W.-Q.; Chen, C.; Zeng, Q. Spatial, temporal variability and carcinogenic health risk assessment of nitrosamines in a drinking water system in China. Sci. Total Environ. 2020, 736, 139695. [Google Scholar] [CrossRef] [PubMed]
- Trussell, R.R.; Umphres, M. The Formation of Trihalomethanes. J. Am. Water Work. Assoc. 1978, 70, 604–612. [Google Scholar] [CrossRef]
- Xie, Y. Disinfection Byproducts in Drinking Water: Formation, Analysis, and Control; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Hua, B.; Veum, K.; Yang, J.; Jones, J.; Deng, B. Parallel factor analysis of fluorescence EEM spectra to identify THM precursors in lake waters. Environ. Monit. Assess. 2009, 161, 71–81. [Google Scholar] [CrossRef]
- MacCarthy, P.; Suffet, I.H. Introduction: Aquatic Humic Substances and Their Influence on the Fate and Treatment of Pollutants. In Aquatic Humic Substances; Suffet, I.H., MacCarthy, P., Eds.; American Chemical Society: Washington, DC, USA, 1989. [Google Scholar]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry; Wiley-Interscience: New York, NY, USA, 1996. [Google Scholar]
- Uyak, V.; Toroz, I. Disinfection by-product precursors reduction by various coagulation techniques in Istanbul water supplies. J. Hazard. Mater. 2007, 141, 320–328. [Google Scholar] [CrossRef]
- Bolto, B.; Dixon, D.; Eldridge, R. Ion exchange for the removal of natural organic matter. React. Funct. Polym. 2004, 60, 171–182. [Google Scholar] [CrossRef]
- Matilainen, A.; Vieno, N.; Tuhkanen, T. Efficiency of the activated carbon filtration in the natural organic matter removal. Environ. Int. 2006, 32, 324–331. [Google Scholar] [CrossRef]
- Siddiqui, M.; Amy, G.; Ryan, J.; Odem, W. Membranes for the control of natural organic matter from surface waters. Water Res. 2000, 34, 3355–3370. [Google Scholar] [CrossRef]
- Matilainen, A.; Sillanpää, M. Removal of natural organic matter from drinking water by advanced oxidation processes. Chemosphere 2010, 80, 351–365. [Google Scholar] [CrossRef]
- Judd, S. The MBR Book: Principles and Applications of Membrane Bioreactors for Water and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Pelekani, C.; Newcombe, G.; Snoeyink, V.L.; Hepplewhite, C.; Assemi, S.; Beckett, R. Characterization of Natural Organic Matter Using High Performance Size Exclusion Chromatography. Environ. Sci. Technol. 1999, 33, 2807–2813. [Google Scholar] [CrossRef]
- Tadanier, C.J.; Berry, D.F.; Knocke, W.R. Dissolved Organic Matter Apparent Molecular Weight Distribution and Number-Average Apparent Molecular Weight by Batch Ultrafiltration. Environ. Sci. Technol. 2000, 34, 2348–2353. [Google Scholar] [CrossRef]
- Taylor, J.S.; Mulford, L.; Duranceau, S.; Barrett, W. Cost and Performance of a Membrane Pilot Plant. J. AWWA 1989, 81, 52–60. [Google Scholar] [CrossRef]
- Chellam, S.; Jacangelo, J.G.; Bonacquisti, T.P.; Schauer, B.A. Effect of pretreatment on surface water nanofiltration. J. AWWA 1997, 89, 77–89. [Google Scholar] [CrossRef]
- Koyuncu, I.; Wiesner, M.R.; Bele, C.; Coriton, G.; Djafer, M.; Cavard, J. Bench-scale assessment of pretreatment to reduce fouling of salt-rejecting membranes. Desalination 2006, 197, 94–105. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Yu, S.; Dong, B.; Gao, N.; Wang, X. A review of advances in EDCs and PhACs removal by nanofiltration: Mechanisms, impact factors and the influence of organic matter. Chem. Eng. J. 2021, 406, 126722. [Google Scholar] [CrossRef]
- Yin, J.; Deng, B. Polymer-matrix nanocomposite membranes for water treatment. J. Membr. Sci. 2015, 479, 256–275. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, P.-F.; Li, X.; Gan, B.; Wang, L.; Song, X.; Park, H.-D.; Tang, C.Y. A Critical Review on Thin-Film Nanocomposite Membranes with Interlayered Structure: Mechanisms, Recent Developments, and Environmental Applications. Environ. Sci. Technol. 2020, 54, 15563–15583. [Google Scholar] [CrossRef]
- Yin, J.; Zhu, G.; Deng, B. Multi-walled carbon nanotubes (MWNTs)/polysulfone (PSU) mixed matrix hollow fiber membranes for enhanced water treatment. J. Membr. Sci. 2013, 437, 237–248. [Google Scholar] [CrossRef]
- APHA; AWWA; WPCF. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Epperson, J.E. Missouri Wetlands: A Vanishing Resource; (No. 39); Citeseer: State College, PA, USA, 1992. [Google Scholar]
- Duan, S.; He, Y.; Kaushal, S.S.; Bianchi, T.S.; Ward, N.D.; Guo, L. Impact of Wetland Decline on Decreasing Dissolved Organic Carbon Concentrations along the Mississippi River Continuum. Front. Mar. Sci. 2017, 3, 280. [Google Scholar] [CrossRef]
- Guo, W.; Xu, J.; Wang, J.; Wen, Y.; Zhuo, J.; Yan, Y. Characterization of dissolved organic matter in urban sewage using excitation emission matrix fluorescence spectroscopy and parallel factor analysis. J. Environ. Sci. 2010, 22, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Mayer, L.M.; Schick, L.L.; Loder, T.C., III. Dissolved protein fluorescence in two Maine estuaries. Mar. Chem. 1999, 64, 171–179. [Google Scholar] [CrossRef]
- Jönsson, C.; Jönsson, A.-S. Influence of the membrane material on the adsorptive fouling of ultrafiltration membranes. J. Membr. Sci. 1995, 108, 79–87. [Google Scholar] [CrossRef]
- Cornelissen, E.; Boomgaard, T.V.D.; Strathmann, H. Physicochemical aspects of polymer selection for ultrafiltration and microfiltration membranes. Colloids Surfaces A Physicochem. Eng. Asp. 1998, 138, 283–289. [Google Scholar] [CrossRef]
- Liu, F.; Xu, Y.-Y.; Zhu, B.-K.; Zhang, F.; Zhu, L.-P. Preparation of hydrophilic and fouling resistant poly(vinylidene fluoride) hollow fiber membranes. J. Membr. Sci. 2009, 345, 331–339. [Google Scholar] [CrossRef]
- Zhu, G.; Yin, J.; Zhang, P.; Wang, X.; Fan, G.; Hua, B.; Ren, B.; Zheng, H.; Deng, B. DOM removal by flocculation process: Fluorescence excitation–emission matrix spectroscopy (EEMs) characterization. Desalination 2014, 346, 38–45. [Google Scholar] [CrossRef]
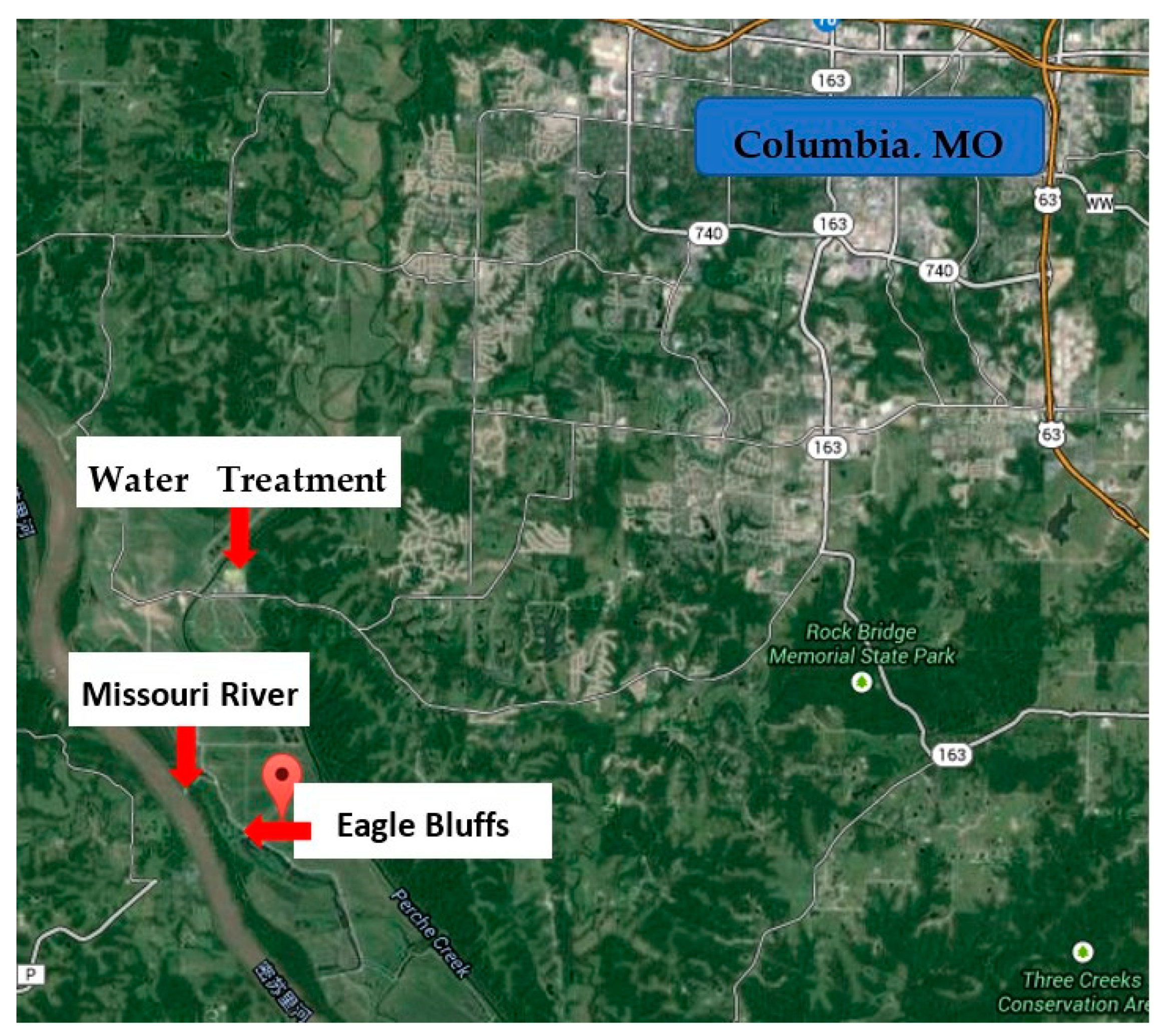




| Samples | pH | DOC | UV254 | |
|---|---|---|---|---|
| A | Missouri River | 7.56 | 7.44 mg/L | 0.104 |
| B | Eagle Bluffs | 7.65 | 9.78 mg/L | 0.174 |
| C | Water Treatment Plant | 7.68 | 5.72 mg/L | 0.054 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, J.; Fidalgo, M.; Deng, B. Removal of NOMs by Carbon Nanotubes/Polysulfone Nanocomposite Hollow Fiber Membranes for the Control of Disinfection Byproducts (DBPs). Water 2023, 15, 2054. https://doi.org/10.3390/w15112054
Yin J, Fidalgo M, Deng B. Removal of NOMs by Carbon Nanotubes/Polysulfone Nanocomposite Hollow Fiber Membranes for the Control of Disinfection Byproducts (DBPs). Water. 2023; 15(11):2054. https://doi.org/10.3390/w15112054
Chicago/Turabian StyleYin, Jun, Maria Fidalgo, and Baolin Deng. 2023. "Removal of NOMs by Carbon Nanotubes/Polysulfone Nanocomposite Hollow Fiber Membranes for the Control of Disinfection Byproducts (DBPs)" Water 15, no. 11: 2054. https://doi.org/10.3390/w15112054
APA StyleYin, J., Fidalgo, M., & Deng, B. (2023). Removal of NOMs by Carbon Nanotubes/Polysulfone Nanocomposite Hollow Fiber Membranes for the Control of Disinfection Byproducts (DBPs). Water, 15(11), 2054. https://doi.org/10.3390/w15112054








