Can the Invasive Seaweed Caulerpa cylidracea Represent a New Trophic Resource in the Mediterranean Sea?
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions, Future Directions, and Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pysek, P.; Winter, M.; Arianoutsou, M.; et al. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef] [PubMed]
- Simberloff, D.; Martin, J.-L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; Garcia-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef]
- Cassey, P.; García-Díaz, P.; Lockwood, J.L.; Blackburn, T.M. Invasion biology: Searching for predictions and prevention, and avoiding lost causes. Invasion. Biol. Hypotheses Evid. 2018, 9, 3–13. [Google Scholar] [CrossRef]
- Claudet, J.; Fraschetti, S. Human-driven impacts on marine habitats: A regional meta-analysis in the Mediterranean Sea. Biol. Conserv. 2010, 143, 2195–2206. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Lasram, F.B.R.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T. The biodiversity of the Mediterranean Sea: Estimates, patterns, and threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef] [PubMed]
- Lacoue-Labarthe, T.; Nunes, P.A.; Ziveri, P.; Cinar, M.; Gazeau, F.; Hall-Spencer, J.M.; Hilmi, N.; Moschella, P.; Safa, A.; Sauzade, D. Impacts of ocean acidification in a warming Mediterranean Sea: An overview. Reg. Stud. Mar. Sci. 2016, 5, 1–11. [Google Scholar] [CrossRef]
- Liquete, C.; Piroddi, C.; Macías, D.; Druon, J.-N.; Zulian, G. Ecosystem services sustainability in the Mediterranean Sea: Assessment of status and trends using multiple modelling approaches. Sci. Rep. 2016, 6, 34162. [Google Scholar] [CrossRef]
- Essl, F.; Lenzner, B.; Bacher, S.; Bailey, S.; Capinha, C.; Daehler, C.; Dullinger, S.; Genovesi, P.; Hui, C.; Hulme, P.E.; et al. Drivers of future alien species impacts: An expert-based assessment. Glob. Chang. Biol. 2020, 26, 4880–4893. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Zenetos, A.; Belchior, C.; Cardoso, A.C. Invading European Seas: Assessing pathways of introduction of marine aliens. Ocean Coast Manag. 2013, 76, 64–74. [Google Scholar] [CrossRef]
- Pusceddu, A.; Fraschetti, S.; Scopa, M.; Rizzo, L.; Danovaro, R. Meiofauna communities, nematode diversity and C degradation rates in seagrass (Posidonia oceanica L.) and unvegetated sediments invaded by the algae Caulerpa cylindracea (Sonder). Mar. Environ. Res. 2016, 119, 88–99. [Google Scholar] [CrossRef]
- Rizzo, L.; Pusceddu, A.; Bianchelli, S.; Fraschetti, S. Potentially combined effect of the invasive seaweed Caulerpa cylindracea (Sonder) and sediment deposition rates on organic matter and meiofaunal assemblages. Mar. Environ. Res. 2020, 159, 104966. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Pusceddu, A.; Stabili, L.; Alifano, P.; Fraschetti, S. Potential effects of an invasive seaweed (Caulerpa cylindracea, Sonder) on sedimentary organic matter and microbial metabolic activities. Sci. Rep. 2017, 7, 12113. [Google Scholar] [CrossRef] [PubMed]
- Krijgsman, W.; Hilgen, F.J.; Raffi, I.; Sierro, F.J.; Wilson, D.S. Chronology, causes and progression of the Messinian salinity crisis. Nature 1999, 400, 652–655. [Google Scholar] [CrossRef]
- Morales-Muñiz, A.; Roselló-Izquierdo, E. Twenty thousand years of fishing in the strait. In Human Impacts on Ancient Marine Ecosystems, a Global Perspective; Rick, T.C., Erlandson, J.M., Eds.; University of California Press: Berkeley, CA, USA, 2008; pp. 243–277. [Google Scholar]
- Theodoropoulou, T. Fishing together, fishing on its own: Fish exploitation patterns at the Neolithic Alepotrypa cave (Diros, Greece) and Aegean prehistoric fishing traditions. Int. J. Osteoarchaeol. 2019, 29, 395–406. [Google Scholar] [CrossRef]
- Boudouresque, C.-F. Marine biodiversity in the Mediterranean: Status of species, populations and communities. Trav. Sci. Parc. Natl. Port-Cros 2004, 20, 97–146. [Google Scholar]
- Kalogirou, S.; Azzurro, E.; Bariche, M. The ongoing shift of Mediterranean coastal fish assemblages and the spread of non-indigenous species. In Biodiversity Enrichment in a Diverse World; Lameed, G.A., Ed.; InTech: Rijeka, Croatia, 2012; p. 11. [Google Scholar]
- Givan, O.; Edelist, D.; Sonin, O.; Belmaker, J. Thermal affinity as the dominant factor changing Mediterranean fish abundances. Glob. Chang. Biol. 2018, 24, e80–e89. [Google Scholar] [CrossRef]
- Azzurro, E.; Sbragaglia, V.; Cerri, J.; Bariche, M.; Bolognini, L.; Souissi, J.B.; Busoni, G.; Coco, S.; Chryssanthi, A.; Garrabou, J. The shifting distribution of Mediterranean fishes: A spatio-temporal assessment based on Local Ecological Knowledge. Glob. Chang. Biol. 2019, 25, 2779–2792. [Google Scholar] [CrossRef]
- Last, P.R.; White, W.T.; Gledhill, D.C.; Hobday, A.J.; Brown, R.; Edgar, G.J.; Pecl, G. Long-term shifts in abundance and distribution of a temperate fish fauna: A response to climate change and fishing practices. Glob. Ecol. Biogeogr. 2011, 20, 58–72. [Google Scholar] [CrossRef]
- Cheung, W.W.; Watson, R.; Pauly, D. Signature of ocean warming in global fisheries catch. Nature 2013, 497, 365. [Google Scholar] [CrossRef]
- Galil, B.S.; Marchini, A.; Occhipinti-Ambrogi, A. Mare Nostrum, Mare Quod Invaditur—The History of Bioinvasions in the Mediterranean Sea. In Histories of Bioinvasions in the Mediterranean; Queiroz, A., Pooley, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 21–49. [Google Scholar]
- Galil, B.S.; Marchini, A.; Occhipinti-Ambrogi, A. East is east and West is west? Management of marine bioinvasions in the Mediterranean Sea. Estuar. Coast Shelf Sci. 2018, 201, 7–16. [Google Scholar] [CrossRef]
- Galil, B.S.; Danovaro, R.; Rothman, S.; Gevili, R.; Goren, M. Invasive biota in the deep-sea Mediterranean: An emerging issue in marine conservation and management. Biol. Invasions 2019, 21, 281–288. [Google Scholar] [CrossRef]
- Dobler, J.P. Analysis of shipping patterns in the Mediterranean and Black seas. In Proceedings of the Alien Marine Organisms Introduced by Ships, Istanbul, Turkey, 6–9 November 2002; CIESM Workshop Monographs. 2002; Volume 20, pp. 19–28. [Google Scholar]
- Kalogirou, S. Alien Fish Species in the Eastern Mediterranean Sea: Invasion Biology in Coastal Ecosystems; Department of marine ecology, University of Gothenburg: Gothenburg, Sweden, 2011; p. 140. [Google Scholar]
- Hulme, P.E. Invasion pathways at a crossroad: Policy and research challenges for managing alien species introductions. J. Appl. Ecol. 2015, 52, 1418–1424. [Google Scholar] [CrossRef]
- Gewing, M.-T.; Shenkar, N. Monitoring the magnitude of marine vessel infestation by non-indigenous ascidians in the Mediterranean. Mar. Pollut. Bull. 2017, 121, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Williamson, M.; Fitter, A. The varying success of invaders. Ecology 1996, 77, 1661–1666. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Verlaque, M. Biological pollution in the Mediterranean Sea: Invasive versus introduced macrophytes. Mar. Pollut. Bull. 2002, 44, 32–38. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M. Invasive species, environmental change and management, and health. Annu. Rev. Environ. Resour. 2010, 35, 25–55. [Google Scholar] [CrossRef]
- Gaertner, M.; Biggs, R.; Te Beest, M.; Hui, C.; Molofsky, J.; Richardson, D.M. Invasive plants as drivers of regime shifts: Identifying high-priority invaders that alter feedback relationships. Divers. Distrib. 2014, 20, 733–744. [Google Scholar] [CrossRef]
- Tsiamis, K.; Azzurro, E.; Bariche, M.; Çinar, M.E.; Crocetta, F.; De Clerck, O.; Galil, B.; Gomez, F.; Hoffman, R.; Jensen, K.R. Prioritizing marine invasive alien species in the European Union through horizon scanning. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 794–845. [Google Scholar] [CrossRef]
- Giakoumi, S.; Katsanevakis, S.; Albano, P.G.; Azzurro, E.; Cardoso, A.C.; Cebrian, E.; Deidun, A.; Edelist, D.; Francour, P.; Jimenez, C. Management priorities for marine invasive species. Sci. Total Environ. 2019, 688, 976–982. [Google Scholar] [CrossRef]
- Belton, G.S.; Phomme van Reine, W.F.; Huisman, J.M.; Draisma, S.G.A.; Gurgel, C.F.D. Resolving phenotypic plasticity and species designation in the morphology challenging Caulerpa racemosa-peltata complex (Caulerpaceae, Chlorophyta). J. Phycol. 2014, 50, 32–54. [Google Scholar] [CrossRef]
- Streftaris, N.; Zenetos, A. Alien marine species in the Mediterranean-the 100 ‘Worst Invasives’ and their impact. Mediterr. Mar. Sci. 2006, 7, 87–118. [Google Scholar] [CrossRef]
- Verlaque, M.; Afonso-Carrillo, J.; Gil-Rodriguez, M.C.; Durand, C.; Boudouresque, C.F.; Le Parco, Y. Blitzkrieg in a marine invasion: Caulerpa racemosa var. cylindracea (Bryopsidales, Chlorophyta) reaches the Canary Islands (north-east Atlantic). Biol. Invasions 2004, 6, 269–281. [Google Scholar] [CrossRef]
- Klein, J.; Verlaque, M. The Caulerpa racemosa invasion: A critical review. Mar. Pollut. Bull. 2008, 56, 205–225. [Google Scholar] [CrossRef]
- Piazzi, L.; Balata, D.; Bulleri, F.; Gennaro, P.; Ceccherelli, G. The invasion of Caulerpa cylindracea in the Mediterranean: The known, the unknown and the knowable. Mar. Biol. 2016, 163, 1–14. [Google Scholar] [CrossRef]
- Papini, A.; Mosti, S.; Santosuosso, U. Tracking the origin of the invading Caulerpa (Caulerpales, Chlorophyta) with Geographic Profiling, a criminological technique for a killer alga. Biol. Invasions 2013, 15, 1613–1621. [Google Scholar] [CrossRef]
- Bulleri, F.; Balata, D.; Bertocci, I.; Tamburello, L.; Benedetti-Cecchi, L. The seaweed Caulerpa racemosa on Mediterranean rocky reefs: From passenger to driver of ecological change. Ecology 2010, 91, 2205–2212. [Google Scholar] [CrossRef] [PubMed]
- Bulleri, F.; Badalamenti, F.; Iveša, L.; Mikac, B.; Musco, L.; Jaklin, A.; Rattray, A.; Vega Fernández, T.; Benedetti-Cecchi, L. The effects of an invasive seaweed on native communities vary along a gradient of land-based human impacts. PeerJ 2016, 4, e179. [Google Scholar] [CrossRef]
- van Kleunen, M.; Weber, E.; Fischer, M. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol. Lett. 2010, 13, 235–245. [Google Scholar] [CrossRef]
- Parker, J.D.; Burkepile, D.E.; Hay, M.E. Opposing effects of native and exotic herbivores on plant invasions. Science 2006, 311, 1459–1461. [Google Scholar] [CrossRef]
- Chaffin, B.C.; Garmestani, A.S.; Angeler, D.G.; Herrmann, D.L.; Stow, C.A.; Nystrom, M.; Sendzimir, J.; Hopton, M.E.; Kolasa, J.; Allen, C.R. Biological invasions, ecological resilience and adaptive governance. J. Environ. Manag. 2016, 183, 399–407. [Google Scholar] [CrossRef]
- Sfriso, A.; Buosi, A.; Wolf, M.A.; Sfriso, A.A. Invasion of alien macroalgae in the Venice Lagoon, a pest or a resource? Aquat. Invasions 2020, 15, 245–270. [Google Scholar] [CrossRef]
- Noe, S.; Badalamenti, F.; Bonaviri, C.; Musco, L.; Vega Fernandez, T.; Vizzini, S.; Gianguzza, P. Food selection of a generalist herbivore exposed to native and alien seaweeds. Mar. Pollut. Bull. 2018, 129, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Holmer, M.; Marbà, N.; Lamote, M.; Duarte, C.M. Deterioration of Sediment Quality in Seagrass Meadows (Posidonia oceanica) Invaded by Macroalgae (Caulerpa sp.). Estuar. Coast. 2009, 32, 456–466. [Google Scholar] [CrossRef]
- Cebrian, E.; Linares, C.; Marchal, C.; Garrabou, J. Exploring the effects of invasive algae on the persistence of gorgonian populations. Biol. Invasions 2012, 14, 2647–2656. [Google Scholar] [CrossRef]
- Felline, S.; Caricato, R.; Cutignano, A.; Gorbi, S.; Lionetto, M.G.; Mollo, E.; Regoli, F.; Terlizzi, A. Subtle effects of biological invasions: Cellular and physiological responses of fish eating the exotic pest Caulerpa racemosa. PLoS ONE 2012, 7, e38763. [Google Scholar] [CrossRef]
- Schwede, J.G.; Cardellina, J.H.; Grode, S.H.; James, T.R.; Blackman, A.J. Distribution of the pigment caulerpin in species of the green alga Caulerpa. Phytochemistry 1986, 26, 155–158. [Google Scholar] [CrossRef]
- Box, A.; Sureda, A.; Tauler, P.; Terrados, J.; Marbà, N.; Pons, A.; Deudero, S. Seasonality of caulerpenyne content in native Caulerpa prolifera and invasive C. taxifolia and C. racemosa var. cylindracea in the western Mediterranean Sea. Bot. Mar. 2010, 53, 367–375. [Google Scholar] [CrossRef]
- Paul, V.J.; Puglisi, M.P. Chemical mediation of interactions among marine organisms. Nat. Prod. Rep. 2004, 21, 189–209. [Google Scholar] [CrossRef]
- Deudero, S.; Box, A.; Alos, J.; Arroyo, N.L.; Marba, N. Functional changes due to invasive species: Food web shifts at shallow Posidonia oceanica seagrass beds colonized by the alien macroalga Caulerpa racemosa. Estuar. Coast. Shelf Sci. 2011, 93, 106–116. [Google Scholar] [CrossRef]
- Casu, D.; Ceccherelli, G.; Sechi, N.; Rumolo, P.; Sara, G. Caulerpa racemosa var. cylindracea as a potential source of organic matter for benthic consumers: Evidences from a stable isotope analysis. Aquat. Ecol. 2009, 43, 1023–1029. [Google Scholar] [CrossRef]
- Maric, M.; De Troch, M.; Occhipinti-Ambrogi, A.; Olenin, S. Trophic interactions between indigenous and non-indigenous species in Lampedusa Island, Mediterranean Sea. Mar. Environ. Res. 2016, 120, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Bulleri, F.; Tamburello, L.; Benedetti-Cecchi, L. Loss of consumers alters the effects of resident assemblages on the local spread of an introduced macroalga. Oikos 2009, 118, 269–279. [Google Scholar] [CrossRef]
- Pusceddu, A.; Mikhno, M.; Giglioli, A.; Secci, M.; Pasquini, V.; Moccia, D.; Addis, P. Foraging of the sea urchin Paracentrotus lividus (Lamarck, 1816) on invasive allochthonous and autochthonous algae. Mar. Environ. Res. 2021, 170, 105428. [Google Scholar] [CrossRef] [PubMed]
- Vega Fernandez, T.; Badalamenti, F.; Bonaviri, C.; Di Trapani, F.; Gianguzza, P.; Noe, S.; Musco, L. Synergistic reduction of a native key herbivore performance by two non-indigenous invasive algae. Mar. Pollut. Bull. 2019, 141, 649–654. [Google Scholar] [CrossRef]
- Tejada, S.; Deudero, S.; Box, A.; Sureda, A. Physiological response of the sea urchin Paracentrotus lividus fed with the seagrass Posidonia oceanica and the alien algae Caulerpa racemosa and Lophocladia lallemandii. Mar. Environ. Res. 2013, 83, 48–53. [Google Scholar] [CrossRef]
- Tomas, F.; Box, A.; Terrados, J. Effects of invasive seaweeds on feeding preference and performance of a keystone Mediterranean herbivore. Biol. Invasions 2011, 13, 1559–1570. [Google Scholar] [CrossRef]
- Cebrian, E.; Ballesteros, E.; Linares, C.; Tomas, F. Do native herbivores provide resistance to Mediterranean marine bioinvasions? A seaweed example. Biol. Invasions 2011, 13, 1397–1408. [Google Scholar] [CrossRef]
- Zuljevic, A.; Nikolic, V.; Despalatovic, M.; Antolic, B. Experimental in situ feeding of the sea urchin Paracentrotus lividus with invasive algae Caulerpa racemosa var. cylindracea and Caulerpa taxifolia in the Adriatic sea. Fresenius Environ. Bull. 2008, 17, 2098–2102. [Google Scholar]
- Ruitton, S.; Verlaque, M.; Aubin, G.; Boudouresque, C.F. Grazing on Caulerpa racemosa var. cylindracea (Caulerpales, Chlorophyta) in the Mediterranean Sea by herbivorous fishes and sea urchins. Vie Milieu-Life Environ. 2006, 56, 33–41. [Google Scholar]
- Santamaria, J.; Tomas, F.; Ballesteros, E.; Cebrian, E. Herbivory on the Invasive Alga Caulerpa cylindracea: The Role of Omnivorous Fishes. Front. Mar. Sci. 2021, 8, 702492. [Google Scholar] [CrossRef]
- Miccoli, A.; Mancini, E.; Boschi, M.; Provenza, F.; Lelli, V.; Tiralongo, F.; Renzi, M.; Terlizzi, A.; Bonamano, S.; Marcelli, M. Trophic, Chemo-Ecological and Sex-Specific Insights on the Relation Between Diplodus sargus (Linnaeus, 1758) and the Invasive Caulerpa cylindracea (Sonder, 1845). Front. Mar. Sci. 2021, 8, 680787. [Google Scholar] [CrossRef]
- Magliozzi, L.; Maselli, V.; Almada, F.; Di Cosmo, A.; Mollo, E.; Polese, G. Effect of the algal alkaloid caulerpin on neuropeptide Y (NPY) expression in the central nervous system (CNS) of Diplodus sargus. J. Comp. Physiol. 2019, 205, 203–210. [Google Scholar] [CrossRef]
- Vitale, R.M.; D’Aniello, E.; Gorbi, S.; Martella, A.; Silvestri, C.; Giuliani, M.E.; Fellous, T.; Gentile, A.; Carbone, M.; Cutignano, A.; et al. Fishing for Targets of Alien Metabolites: A Novel Peroxisome Proliferator-Activated Receptor (PPAR) Agonist from a Marine Pest. Mar. Drugs 2018, 16, 431. [Google Scholar] [CrossRef] [PubMed]
- Del Coco, L.; Felline, S.; Girelli, C.R.; Angile, F.; Magliozzi, L.; Almada, F.; D’Aniello, B.; Mollo, E.; Terlizzi, A.; Fanizzi, F.P. H-1 NMR Spectroscopy and MVA to Evaluate the Effects of Caulerpin-Based Diet on Diplodus sargus Lipid Profiles. Mar. Drugs 2018, 16, 390. [Google Scholar] [CrossRef] [PubMed]
- Magliozzi, L.; Almada, F.; Robalo, J.; Mollo, E.; Polese, G.; Goncalves, E.J.; Felline, S.; Terlizzi, A.; D’Aniello, B. Cryptic effects of biological invasions: Reduction of the aggressive behaviour of a native fish under the influence of an invasive biomolecule. PLoS ONE 2017, 12, e0185620. [Google Scholar] [CrossRef] [PubMed]
- De Pascali, S.A.; Del Coco, L.; Felline, S.; Mollo, E.; Terlizzi, A.; Fanizzi, F.P. H-1 NMR Spectroscopy and MVA Analysis of Diplodus sargus Eating the Exotic Pest Caulerpa cylindracea. Mar. Drugs 2015, 13, 3550–3566. [Google Scholar] [CrossRef] [PubMed]
- Felline, S.; Mollo, E.; Ferramosca, A.; Zara, V.; Regoli, F.; Gorbi, S.; Terlizzi, A. Can a marine pest reduce the nutritional value of Mediterranean fish flesh? Mar. Biol. 2014, 161, 1275–1283. [Google Scholar] [CrossRef]
- Gorbi, S.; Giuliani, M.E.; Pittura, L.; d’Errico, G.; Terlizzi, A.; Felline, S.; Grauso, L.; Mollo, E.; Cutignano, A.; Regoli, F. Could molecular effects of Caulerpa racemosa metabolites modulate the impact on fish populations of Diplodus sargus? Mar. Environ. Res. 2014, 96, 2–11. [Google Scholar] [CrossRef]
- Terlizzi, A.; Felline, S.; Lionetto, M.G.; Caricato, R.; Perfetti, V.; Cutignano, A.; Mollo, E. Detrimental physiological effects of the invasive alga Caulerpa racemosa on the Mediterranean white seabream Diplodus sargus. Aquat. Biol. 2011, 12, 109–117. [Google Scholar] [CrossRef]
- Felline, S.; Mollo, E.; Cutignano, A.; Grauso, L.; Andaloro, F.; Castriota, L.; Consoli, P.; Falautano, M.; Sinopoli, M.; Terlizzi, A. Preliminary observations of caulerpin accumulation from the invasive Caulerpa cylindracea in native Mediterranean fish species Aquat. Biol. 2017, 26, 27–31. [Google Scholar] [CrossRef]
- Turhan, S.; Cavas, L. The threat on your plate: Do we just eat Sarpa salpa or more? Reg. Stud. Mar. Sci. 2019, 29, 100697. [Google Scholar] [CrossRef]
- Marco-Mendez, C.; Ferrero-Vicente, L.M.; Prado, P.; Sanchez-Lizaso, J.L. Epiphytes and nutrient contents influence Sarpa salpa herbivory on Caulerpa spp vs. seagrass species in Mediterranean meadows. Estuar. Coast. Shelf Sci. 2017, 184, 54–66. [Google Scholar] [CrossRef]
- Tomas, F.; Cebrian, E.; Ballesteros, E. Differential herbivory of invasive algae by native fish in the Mediterranean Sea. Estuar. Coast. Shelf Sci. 2011, 92, 27–34. [Google Scholar] [CrossRef]
- Santamaría, J.; Golo, R.; Verdura, J.; Tomas, F.; Ballesteros, E.; Alcoverro, T.; Arthur, R.; Cebrian, E. Learning takes time: Biotic resistance by native herbivores increases through the invasion process. Ecol. Lett. 2022, 25, 2525–2539. [Google Scholar] [CrossRef] [PubMed]
- Bariche, M. Diet of the Lessepsian Fishes, Siganus rivulatus and S. luridus (Siganidae) in the Eastern Mediterranean: A Bibliographic Analysis. Cybium 2006, 30, 41–49. Available online: https://sfi-cybium.fr/sites/default/files/pdfs-cybium/10-Bariche%20275.pdf (accessed on 30 December 2022).
- Azzurro, E.; Fanelli, E. Preliminary data on feeding habits of dusky spinefoot Siganus luridus in the Sicily Channel (Central Mediterranean). Biol. Mar. Mediterr. 2004, 11, 145. [Google Scholar]
- Lundberg, B.; Payiatas, G.; Argyrou, M. Notes on the diet of the Lessepsian migrant herbivorous fishes, Siganus luridus and S. rivulatus, in Cyprus. Isr. J. Zool. 1999, 45, 127–134. [Google Scholar]
- Box, A.; Deudero, S.; Sureda, A.; Blanco, A.; Alos, J.; Terrados, J.; Grau, A.M.; Riera, F. Diet and physiological responses of Spondyliosoma cantharus (Linnaeus, 1758) to the Caulerpa racemosa var. cylindracea invasion. J. Exp. Mar. Biol. Ecol. 2009, 380, 11–19. [Google Scholar] [CrossRef]
- Vazquez-Luis, M.; Sanchez-Jerez, P.; Bayle-Sempere, J.T. Effects of Caulerpa racemosa var. cylindracea on prey availability: An experimental approach to predation of amphipods by Thalassoma pavo (Labridae). Hydrobiologia 2010, 654, 147–154. [Google Scholar] [CrossRef]
- Alomar, C.; Deudero, S.; Andaloro, F.; Castriota, L.; Consoli, P.; Falautano, M.; Sinopoli, M. Caulerpa cylindracea Sonder invasion modifies trophic niche in in-fralittoral rocky benthic community. Mar. Environ. Res. 2016, 120, 86–92. [Google Scholar] [CrossRef]
- Sinopoli, M.; Allegra, A.; Andaloro, F.; Consoli, P.; Esposito, V.; Falautano, M.; Mangano, M.C.; Nicastro, A.; Scotti, G.; Castriota, L. Assessing the effect of the alien seaweed Caulerpa cylindracea on infralittoral rocky benthic invertebrate community: Evidence from a Mediterranean Marine Protected Area. Reg. Stud. Mar. Sci. 2020, 38, 101372. [Google Scholar] [CrossRef]
- Kimbro, D.L.; Cheng, B.S.; Grosholz, E.D. Biotic resistance in marine environments. Ecol. Lett. 2013, 16, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Papacostas, K.J.; Freestone, A.L. Stronger predation in a subtropical community dampens an invasive species-induced trophic cascade. Biol. Invasions 2019, 21, 203–215. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Hughes, T.P.; Hoey, A.S. Sleeping functional group drives coral-reef recovery. Curr. Biol. 2006, 16, 2434–2439. [Google Scholar] [CrossRef]
- Mendes, T.C.; Cordeiro, C.A.M.M.; Ferreira, C.E.L. An experimental evaluation of macroalgal consumption and selectivity by nominally herbivorous fishes on subtropical rocky reefs. J. Exp. Mar. Bio. Ecol. 2015, 471, 146–152. [Google Scholar] [CrossRef]
- Mendes, T.C.; Quimbayo, J.P.; Bouth, H.F.; Silva, L.P.S.; Ferreira, C.E.L. The omnivorous triggerfish Melichthys niger is a functional herbivore on an isolated Atlantic oceanic island. J. Fish Biol. 2019, 95, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Tebbett, S.B.; Hoey, A.S.; Depczynski, M.; Wismer, S.; Bellwood, D.R. Macroalgae removal on coral reefs: Acemose ecosystem functions transcend biogeographic locations. Coral Reefs 2020, 39, 203–214. [Google Scholar] [CrossRef]
- Caronni, S.; Calabretti, C.; Delaria, M.A.; Bernardi, G.; Navone, A.; Occhipinti-Ambrogi, A.; Panzalis, P.; Ceccherelli, G. Consumer depletion alters seagrass resistance to an invasive macroalga. PLoS ONE 2015, 10, e0115858. [Google Scholar] [CrossRef]
- Bulleri, F.; Malquori, F. High tolerance to simulated herbivory in the clonal seaweed, Caulerpa cylindracea. Mar. Environ. Res. 2015, 107, 61–65. [Google Scholar] [CrossRef]
- Tamburello, L.; Bulleri, F.; Balata, D.; Benedetti-Cecchi, L. The role of overgrazing and anthropogenic disturbance in shaping spatial patterns of distribution of an invasive seaweed. J. Appl. Ecol. 2014, 51, 406–414. [Google Scholar] [CrossRef]
- Ceccherelli, G.; Pinna, S.; Cusseddu, V.; Bulleri, F. The role of disturbance in promoting the spread of the invasive seaweed Caulerpa racemosa in seagrass meadows. Biol. Invasions 2014, 16, 2737–2745. [Google Scholar] [CrossRef]
- Kiparissis, S.; Fakiris, E.; Papatheodorou, G.; Geraga, M.; Kornaros, M.; Kapareliotis, A.; Ferentinos, G. Illegal trawling and induced invasive algal spread as collaborative factors in a Posidonia oceanica meadow degradation. Biol. Inv. 2011, 13, 669–678. [Google Scholar] [CrossRef]
- Montefalcone, M.; Lasagna, R.; Bianchi, C.N.; Morri, C.; Albertelli, G. Anchoring damage on Posidonia oceanica meadow cover: A case study in Prelo Cove (Ligurian Sea, NW Mediterranean). Chem. Ecol. 2006, 22 (Suppl. S1), S207–S217. [Google Scholar] [CrossRef]
- Stabili, L.; Rizzo, L.; Pizzolante, G.; Alifano, P.; Fraschetti, S. Spatial distribution of the culturable bacterial community associated with the invasive alga Caulerpa cylindracea in the Mediterranean Sea. Mar. Environ. Res. 2017, 125, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Fraschetti, S.; Alifano, P.; Tredici, M.S.; Stabili, L. Association of Vibrio community with the Atlantic Mediterranean invasive alga Caulerpa cylindracea. J. Exp. Mar. Biol. Ecol. 2016, 475, 129–136. [Google Scholar] [CrossRef]
- Tsirintanis, K.; Azzurro, E.; Crocetta, F.; Dimiza, M.; Froglia, C.; Gerovasileiou, V.; Langeneck, J.; Mancinelli, G.; Rosso, A.; Stern, N. Bioinvasion impacts on biodiversity, ecosystem services, and human health in the Mediterranean Sea. Aquat. Invasions 2022, 3, 308–352. [Google Scholar] [CrossRef]
- Tiralongo, F.; Messina, G.; Lombardo, B.M. Invasive Species Control: Predation on the Alien Crab Percnon gibbesi (H. Milne Edwards, 1853) (Malacostraca: Percnidae) by the Rock Goby, Gobius paganellus Linnaeus, 1758 (Actinopterygii: Gobiidae). J. Mar. Sci. Eng. 2021, 9, 393. [Google Scholar] [CrossRef]
- Ceccherelli, G.; Piazzi, L.; Balata, D. Spread of introduced Caulerpa species in macroalgal habitats. J. Exp. Mar. Biol. Ecol. 2002, 280, 1–11. [Google Scholar] [CrossRef]
- Piazzi, L.; Ceccherelli, G.; Balata, D.; Cinelli, F. Early patterns of Caulerpa racemosa recovery in the Mediterranean Sea: The influence of algal turfs. J. Mar. Biol. Assoc. 2003, 83, 27–29. [Google Scholar] [CrossRef]
- Gennaro, P.; Piazzi, L. The indirect role of nutrients in enhancing the invasion of Caulerpa racemosa var. cylindracea. Biol. Invasion 2014, 16, 1709–1717. [Google Scholar] [CrossRef]
- Galil, B.S. A Sea, a Canal, a Disaster: The Suez Canal and the Transformation of the Mediterranean Biota. In The Suez Canal: Past Lessons and Future Challenges; Springer International Publishing: Berlin/Heidelberg, Germany, 2023; pp. 199–215. [Google Scholar] [CrossRef]
- Jardas, I. Adriatic Ichthyofauna; Školska knjiga: Zagreb, Croatia, 1996; 535p. (In Croatian) [Google Scholar]
- Buñuel, X.; Alcoverro, T.; Romero, J.; Ruiz, J.M.; Arthur, R. The dominant seagrass herbivore Sarpa salpa shifts its shoaling and feeding strategies as they grow. Sci. Rep. 2020, 10, 10622. [Google Scholar] [CrossRef] [PubMed]
- Pallaoro, A.; Dulčić, J.; Matić-Skoko, S.; Kraljević, M.; Jardas, I. Biology of the salema, Sarpa salpa (L. 1758) (Pisces, Sparidae) from the middle-eastern Adriatic. J. Appl. Ichthyol. 2008, 24, 276–281. [Google Scholar] [CrossRef]
- Casadevall, M.; Rodríguez-Prieto, C.; Pueyo, J.; Martí, C.; Merciai, R.; Verlaque, M.; Real, E.; Torres, J.; Richir, J. The strange case of tough white seabream (Diplodus sargus, Teleostei: Sparidae): A first approach to the extent of the phenomenon in the mediterranean. Front. Mar. Sci. 2020, 7, 387. [Google Scholar] [CrossRef]
- Demirel, N.; Ulman, A.; Yıldız, T.; Ertör-Akyazi, P. A moving target: Achieving good environmental status and social justice in the case of an alien species, Rapa whelk in the Black Sea. Mar. Pol. 2021, 132, 104687. [Google Scholar] [CrossRef]
- Jimenez-Munoz, L.; Quintanilla, M.; Filomena, A. Managing the lionfish: Influence of high intensity ultrasound and binders on textural and sensory properties of lionfish (Pterois volitans) surimi patties. J. Food Sci. Technol. 2019, 56, 2167–2174. [Google Scholar] [CrossRef]
- Kleitou, P.; Crocetta, F.; Giakoumi, S.; Giovos, I.; Hall-Spencer, J.M.; Kalogirou, S.; Kletou, D.; Moutopoulos, D.K.; Rees, S. Fishery reforms for the management of non-indigenous species. J. Environ. Manag. 2021, 280, 111690. [Google Scholar] [CrossRef]
- Angilè, F.; Del Coco, L.; Girelli, C.R.; Basso, L.; Rizzo, L.; Piraino, S.; Stabili, L.; Fanizzi, F.P. 1H NMR metabolic profile of scyphomedusa Rhizostoma pulmo (Scyphozoa, Cnidaria) in Female gonads and somatic tissues: Preliminary results. Molecules 2020, 25, 806. [Google Scholar] [CrossRef]
- Stabili, L.; Rizzo, L.; Fanizzi, F.P.; Angilè, F.; Del Coco, L.; Girelli, C.R.; Lomartire, S.; Piraino, S.; Basso, L. The jellyfish Rhizostoma pulmo (Cnidaria): Biochemical composition of ovaries and antibacterial lysozyme-like activity of the oocyte lysate. Mar. Drugs 2018, 17, 17. [Google Scholar] [CrossRef]
- Basso, L.; Papadia, P.; Rizzo, L.; Migoni, D.; Fanizzi, F.P.; Piraino, S. Trace metals do not accumulate over time in the edible Mediterranean jellyfish Rhizostoma pulmo (Cnidaria, Scyphozoa) from urban coastal waters. Water 2021, 13, 1410. [Google Scholar] [CrossRef]
- Bel Mabrouk, S.; Reis, M.; Sousa, M.L.; Ribeiro, T.; Almeida, J.R.; Pereira, S.; Antunes, J.; Rosa, F.; Vasconcelos, V.; Achour, L. The marine seagrass Halophila stipulacea as a source of bioactive metabolites against obesity and biofouling. Mar. Drugs 2020, 18, 88. [Google Scholar] [CrossRef]
- Gianguzza, P.; Musco, L.; Russo, D.; Bonaviri, C.; Vega Fernández, T.; Arizza, V. Stress response, induced by the invasive algae Caulerpa taxifolia var. distichophylla and C. cylindracea, in the sea urchin Paracentrotus lividus. In Proceedings of the XXIV Congresso Società Italiana di Ecologia, Ferrara, Italy, 15–17 September 2014; p. 101. [Google Scholar]
- Schiano, V.; Cutignano, A.; Maiello, D.; Carbone, M.; Ciavatta, M.L.; Polese, G.; Fioretto, F.; Attanasio, C.; Palladino, A.; Felline, S.; et al. An Alkaloid from a Highly Invasive Seaweed Increases the Voracity and Reproductive Output of a Model Fish Species. Mar. Drugs 2022, 20, 513. [Google Scholar] [CrossRef] [PubMed]
- Lejeusne, C.; Chevaldonné, P.; Pergent-Martini, C.; Boudouresque, C.F.; Pérez, T. Climate change effects on a miniature ocean: The highly diverse, highly impacted Mediterranean Sea. Trends Ecol. Evol. 2010, 25, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Roveri, M.; Flecker, R.; Krijgsman, W.; Lofi, J.; Lugli, S.; Manzi, V.; Sierro, F.J.; Bedrtini, A.; Camerlenghi, A.; De Lange, G.; et al. The Messinian Salinity Crisis: Past and future of a great challenge for marine sciences. Mar. Geol. 2014, 352, 25–58. [Google Scholar] [CrossRef]
- Schøyen, H.; Bråthen, S. The Northern Sea Route versus the Suez Canal: Cases from bulk shipping. J. Transp. Geogr. 2011, 19, 977–983. [Google Scholar] [CrossRef]
- Macaraig, C.E.; Fenton, A.J. Analyzing the causes and effects of the South China Sea dispute: Natural resources and freedom of navigation. J. Territ. Marit. Stud. 2021, 8, 42–58. [Google Scholar]
- Regoli, F.; Giuliani, M.E.; Benedetti, M.; Arukwe, A. Molecular and biochemical biomarkers in environmental monitoring: A comparison of biotransformation and antioxidant defense systems in multiple tissues. Aquat. Toxicol. 2011, 105 (Suppl. S3–S4), 56–66. [Google Scholar] [CrossRef]
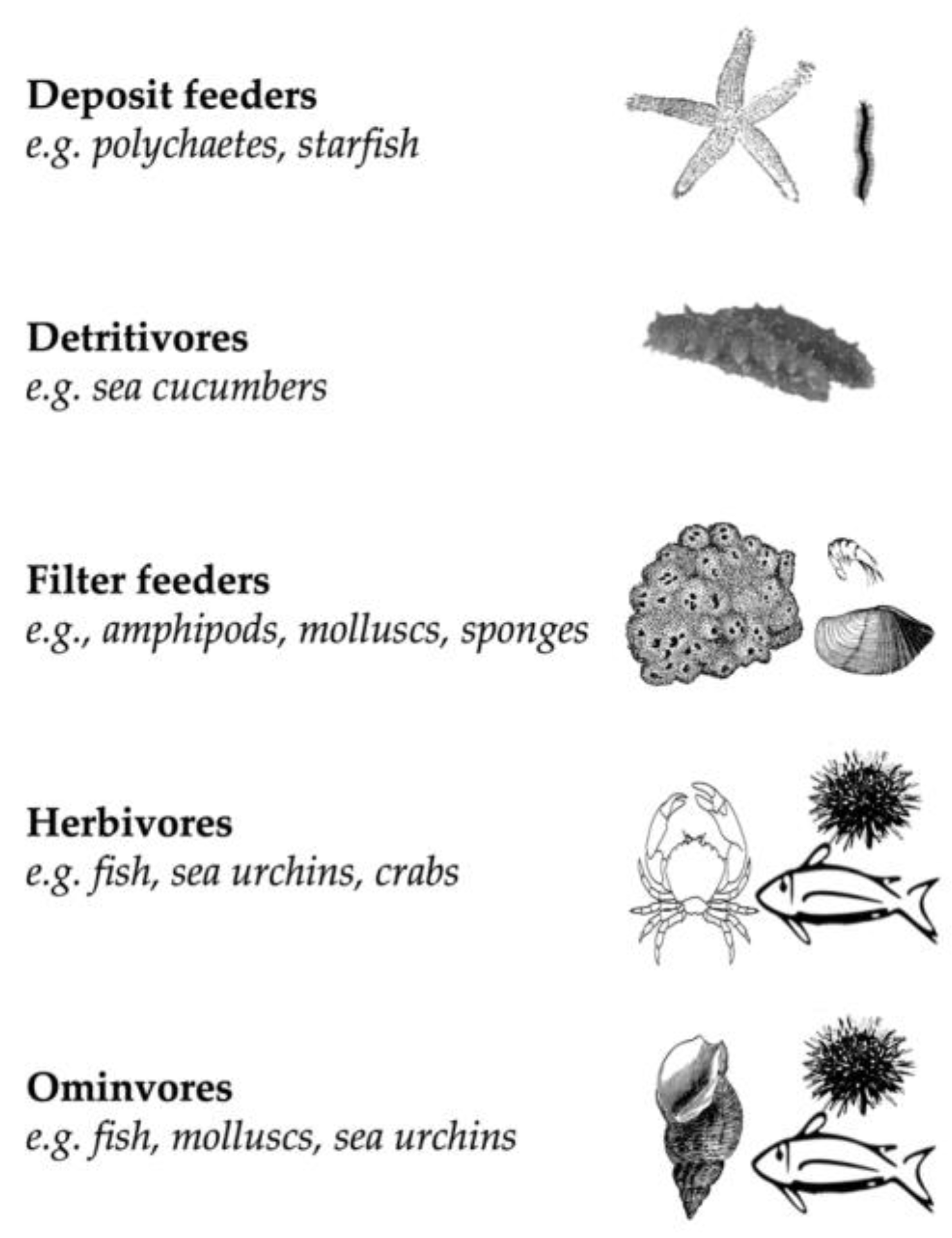

| Species | Authority | Class | Order | Family | Reference |
|---|---|---|---|---|---|
| Porifera | |||||
| Sarcotragus fasciculatus | (Pallas, 1766) | Demospongiae | Dictyoceratida | Irciniidae | [54] |
| Annelida | |||||
| Eunice vittata | (Delle Chiaje, 1828) | Polychaeta | Eunicida | Eunicidae | [54] |
| Pelogenia arenosa | (Delle Chiaje, 1830) | Polychaeta | Phyllodocida | Sigalionidae | [54] |
| Pontogenia chrysocoma | (Baird, 1865) | Polychaeta | Phyllodocida | Aphroditidae | [54] |
| Scoletoma fragilis | (O.F. Müller, 1776) | Polychaeta | Eunicida | Lumbrineridae | [54] |
| Syllis prolifera | Krohn, 1852 | Polychaeta | Phyllodocida | Syllidae | [55] |
| Species | Authority | Class | Order | Family | Reference |
|---|---|---|---|---|---|
| Mollusca | |||||
| Arca noae | Linnaeus, 1758 | Bivalvia | Arcida | Arcidae | [54] |
| Cerithium vulgatum | Bruguière, 1792 | Gastropoda | Caenogastropoda * | Cerithiidae | [54,55] |
| Glans trapezia | (Linnaeus, 1767) | Bivalvia | Carditida | Carditidae | [54] |
| Hexaplex trunculus | (Linnaeus, 1758) | Gastropoda | Neogastropoda | Muricidae | [54] |
| Pisinna glabrata | (Megerle von Mühlfeld, 1824) | Gastropoda | Littorinimorpha | Anabathridae | [55] |
| Arthropoda | |||||
| Monocorophium sextonae | (Crawford, 1937) | Malacostraca | Amphipoda | Corophiidae | [55] |
| Percnon gibbesi | (H. Milne Edwards, 1853) | Malacostraca | Decapoda | Percnidae | [56] |
| Species | Authority | Class | Order | Family | References |
|---|---|---|---|---|---|
| Echinodermata | |||||
| Arbacia lixula | (Linnaeus, 1758) | Echinoidea | Arbacioida | Arbaciidae | [57] |
| Echinaster (Echinaster) sepositus | (Retzius, 1783) | Asteroidea | Spinulosida | Echinasteridae | [54] |
| Holothuria (Panningothuria) forskali | Delle Chiaje, 1823 | Holothuroidea | Holothuriida | Holothuriidae | [54] |
| Holothuria (Roweothuria) poli | Delle Chiaje, 1824 | Holothuroidea | Holothuriida | Holothuriidae | [54] |
| Ophioderma longicaudum | (Bruzelius, 1805) | Ophiuroidea | Ophiacanthida | Ophiodermatidae | [54] |
| Paracentrotus lividus | (Lamarck, 1816) | Echinoidea | Camarodonta | Parechinidae | [47,54,57,58,59,60,61,62,63,64] |
| Sphaerechinus granularis | (Lamarck, 1816) | Echinoidea | Camarodonta | Toxopneustidae | [64] |
| Species | Authority | Class | Order | Family | References |
|---|---|---|---|---|---|
| Chordata | |||||
| Boops boops | (Linnaeus, 1758) | Actinopteri | Eupercaria * | Sparidae | [64] |
| Diplodus annularis | (Linnaeus, 1758) | Actinopteri | Eupercaria * | Sparidae | [65] |
| Diplodus sargus | (Linnaeus, 1758) | Actinopteri | Eupercaria * | Sparidae | [50,65,66,67,68,69,70,71,72,73,74] |
| Diplodus vulgaris | (Geoffroy Saint-Hilaire, 1817) | Actinopteri | Eupercaria * | Sparidae | [75] |
| Sarpa salpa | (Linnaeus, 1758) | Actinopteri | Eupercaria * | Sparidae | [64,75,76,77,78,79] |
| Siganus luridus | (Rüppell, 1829) | Actinopteri | Acanthuriformes | Siganidae | [75,80,81] |
| Siganus rivulatus | Forsskål & Niebuhr, 1775 | Actinopteri | Acanthuriformes | Siganidae | [82] |
| Spondyliosoma cantharus | (Linnaeus, 1758) | Actinopteri | Eupercaria * | Sparidae | [65,75,83] |
| Thalassoma pavo | (Linnaeus, 1758) | Actinopteri | Eupercaria * | Labridae | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, L.; Vega Fernández, T. Can the Invasive Seaweed Caulerpa cylidracea Represent a New Trophic Resource in the Mediterranean Sea? Water 2023, 15, 2115. https://doi.org/10.3390/w15112115
Rizzo L, Vega Fernández T. Can the Invasive Seaweed Caulerpa cylidracea Represent a New Trophic Resource in the Mediterranean Sea? Water. 2023; 15(11):2115. https://doi.org/10.3390/w15112115
Chicago/Turabian StyleRizzo, Lucia, and Tomás Vega Fernández. 2023. "Can the Invasive Seaweed Caulerpa cylidracea Represent a New Trophic Resource in the Mediterranean Sea?" Water 15, no. 11: 2115. https://doi.org/10.3390/w15112115
APA StyleRizzo, L., & Vega Fernández, T. (2023). Can the Invasive Seaweed Caulerpa cylidracea Represent a New Trophic Resource in the Mediterranean Sea? Water, 15(11), 2115. https://doi.org/10.3390/w15112115







