Simultaneous Removal of Nitrate and Phosphate in a Pyrrhotite and Sulfur-Circulating Packed Bed Reactor
Abstract
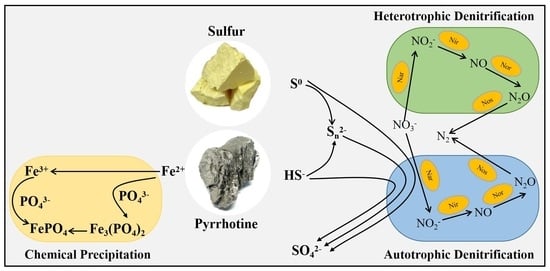
:1. Introduction
+ 1.16H+ + 0.5N2
2. Materials and Methods
2.1. Materials
2.1.1. Pyrrhotite and Sulfur
2.1.2. Inoculation Sludge and Culture Medium
2.1.3. Synthetic Wastewater
2.1.4. Reactor Setup
2.2. Methods
2.2.1. Reactor Start-Up and Operation Method
2.2.2. Conventional Index Determination Method
2.2.3. Precipitation Substance Analysis Method
2.2.4. Analyses of Biofilm
2.2.5. The Detection Methods of Community Structure and Functional Gene
2.2.6. Data Processing Method
3. Results
3.1. Performance of the PS-CPBR
3.1.1. The Removal Efficiency of NO3−-N and PO43−-P
3.1.2. Sulfate Generation and Alkalinity Consumption
3.2. Mechanism of NO3−-N Removal
3.2.1. Electron Transfer Mechanism
3.2.2. Evolution of Microbial Community Structures and Functional Bacteria
3.2.3. Denitrification Functional Genes and Denitrification Pathways
3.3. PO43−-P Removal Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kitsiou, D.; Karydis, M. Coastal marine eutrophication assessment: A review on data analysis. Environ. Int. 2011, 37, 778–801. [Google Scholar] [CrossRef] [PubMed]
- Poikane, S.; Kelly, M.G.; Salas Herrero, F.; Pitt, J.A.; Jarvie, H.P.; Claussen, U.; Leujak, W.; Lyche Solheim, A.; Teixeira, H.; Phillips, G. Nutrient criteria for surface waters under the European Water Framework Directive: Current state-of-the-art, challenges and future outlook. Sci. Total Environ. 2019, 695, 133888. [Google Scholar] [CrossRef] [PubMed]
- Di Capua, F.; Mascolo, M.C.; Pirozzi, F.; Esposito, G. Simultaneous denitrification, phosphorus recovery and low sulfate production in a recirculated pyrite-packed biofilter (RPPB). Chemosphere 2020, 255, 126977. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wei, D.; Wang, W.; Zhang, Y. Pyrrhotite-sulfur autotrophic denitrification for deep and efficient nitrate and phosphate removal: Synergistic effects, secondary minerals and microbial community shifts. Bioresour. Technol. 2020, 308, 123302. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, F.; Chen, N.; Peng, T.; Dong, S.; Feng, C. Denitrification performance and mechanism of biofilter constructed with sulfur autotrophic denitrification composite filler in engineering application. Bioresour. Technol. 2021, 340, 125699. [Google Scholar] [CrossRef] [PubMed]
- Di Capua, F.; Ahoranta, S.H.; Papirio, S.; Lens, P.N.L.; Esposito, G. Impacts of sulfur source and temperature on sulfur-driven denitrification by pure and mixed cultures of Thiobacillus. Process Biochem. 2016, 51, 1576–1584. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Wan, D.; Li, B.; Zhang, P.; Wang, H. Pilot-scale application of sulfur-limestone autotrophic denitrification biofilter for municipal tailwater treatment: Performance and microbial community structure. Bioresour. Technol. 2020, 300, 122682. [Google Scholar] [CrossRef]
- Qiu, Y.Y.; Gong, X.; Zhang, L.; Zhou, S.; Li, G.; Jiang, F. Achieving a Novel Polysulfide-Involved Sulfur-Based Autotrophic DenitrificationProcess for High-Rate Nitrogen Removal in Elemental Sulfur-Packed Bed Reactors. ACS EST Eng. 2022, 2, 1504–1513. [Google Scholar] [CrossRef]
- Sahinkaya, E.; Dursun, N. Use of elemental sulfur and thiosulfate as electron sources for water denitrification. Bioprocess Biosyst. Eng. 2015, 38, 531–541. [Google Scholar] [CrossRef]
- Li, R.; Morrison, L.; Collins, G.; Li, A.; Zhan, X. Simultaneous nitrate and phosphate removal from wastewater lacking organic matter through microbial oxidation of pyrrhotite coupled to nitrate reduction. Water Res. 2016, 96, 32–41. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, T.; Morrison, L.; Gerrity, S.; Collins, G.; Porca, E.; Li, R.; Zhan, X. Nanostructured pyrrhotite supports autotrophic denitrification for simultaneous nitrogen and phosphorus removal from secondary effluents. Chem. Eng. J. 2017, 328, 511–518. [Google Scholar] [CrossRef]
- Vaclavkova, S.; Jørgensen, C.J.; Jacobsen, O.S.; Aamand, J.; Elberling, B. The Importance of Microbial Iron Sulfide Oxidation for Nitrate Depletion in Anoxic Danish Sediments. Aquat. Geochem. 2014, 20, 419–435. [Google Scholar] [CrossRef]
- Lu, X.; Wan, Y.; Zhong, Z.; Liu, B.; Zan, F.; Zhang, F.; Wu, X. Integrating sulfur, iron(II), and fixed organic carbon for mixotrophic denitrification in a composite filter bed reactor for decentralized wastewater treatment: Performance and microbial community. Sci. Total Environ. 2021, 795, 148825. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.Y.; Zhang, L.; Mu, X.; Li, G.; Guan, X.; Hong, J.; Jiang, F. Overlooked pathways of denitrification in a sulfur-based denitrification system with organic supplementation. Water Res. 2020, 169, 115084. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Guo, J.; Song, Y.; Hou, Y.; Lu, C.; Han, Y.; Shen, X.; Liu, B. Effect of calcinated pyrite on simultaneous ammonia, nitrate and phosphorus removal in the BAF system and the Fe(2+) regulatory mechanisms: Electron transfer and biofilm properties. Environ. Res. 2021, 194, 110708. [Google Scholar] [CrossRef]
- Li, R.; Feng, C.; Hu, W.; Xi, B.; Chen, N.; Zhao, B.; Liu, Y.; Hao, C.; Pu, J. Woodchip-sulfur based heterotrophic and autotrophic denitrification (WSHAD) process for nitrate contaminated water remediation. Water Res. 2016, 89, 171–179. [Google Scholar] [CrossRef]
- APHA. Standard Methods for Water and Wastewater Examination; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Shi, Y.; Huang, J.; Zeng, G.; Gu, Y.; Chen, Y.; Hu, Y.; Tang, B.; Zhou, J.; Yang, Y.; Shi, L. Exploiting extracellular polymeric substances (EPS) controlling strategies for performance enhancement of biological wastewater treatments: An overview. Chemosphere 2017, 180, 396–411. [Google Scholar] [CrossRef]
- Hou, J.; You, G.X.; Xu, Y.; Wang, C.; Wang, P.F.; Miao, L.Z.; Li, Y.; Ao, Y.H.; Lv, B.W.; Yang, Y.Y. Long-term effects of CuO nanoparticles on the surface physicochemical properties of biofilms in a sequencing batch biofilm reactor. Appl. Microbiol. Biotechnol. 2016, 100, 9629–9639. [Google Scholar] [CrossRef]
- Feng, X.; Wu, Q.; Che, L.; Ren, N. Analyzing the inhibitory effect of metabolic uncoupler on bacterial initial attachment and biofilm development and the underlying mechanism. Environ. Res. 2020, 185, 109390. [Google Scholar] [CrossRef]
- Li, K.; Guo, J.; Li, H.; Han, Y.; Chen, Z.; Song, Y.; Xing, Y.; Zhang, C. A combined heterotrophic and sulfur-based autotrophic process to reduce high concentration perchlorate via anaerobic baffled reactors: Performance advantages of a step-feeding strategy. Bioresour. Technol. 2019, 279, 297–306. [Google Scholar] [CrossRef]
- Fang, Y.; Deng, C.; Chen, J.; Lu, J.; Chen, S.; Zhou, S. Accelerating the start-up of the cathodic biofilm by adding acyl-homoserine lactone signaling molecules. Bioresour. Technol. 2018, 266, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Li, L.; Feng, C.; Dong, S.; Chen, N. Comparative investigation on integrated vertical-flow biofilters applying sulfur-based and pyrite-based autotrophic denitrification for domestic wastewater treatment. Bioresour. Technol. 2016, 211, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Stocks, J.L.; Rodriguez-Gonzalez, L.C.; Feng, C.; Ergas, S.J. Effect of oyster shell medium and organic substrate on the performance of a particulate pyrite autotrophic denitrification (PPAD) process. Bioresour. Technol. 2017, 244, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Wei, D.; Ngo, H.H.; Guo, W.; Xu, W.; Du, B.; Wei, Q. Performance, microbial community and fluorescent characteristic of microbial products in a solid-phase denitrification biofilm reactor for WWTP effluent treatment. J. Environ. Manag. 2018, 227, 375–385. [Google Scholar] [CrossRef]
- Kluepfel, L.; Keiluweit, M.; Kleber, M.; Sander, M. Redox Properties of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2014, 48, 5601–5611. [Google Scholar] [CrossRef]
- Zeng, C.P.; Li, Y.; Lu, A.H.; Ding, H.R.; Wang, X.; Wang, C.Q. Electrochemical Interaction of a Heterotrophic Bacteria Alcaligenes faecalis with a Graphite Cathode. Geomicrobiol. J. 2012, 29, 244–249. [Google Scholar] [CrossRef]
- Li, L.; Dong, Y.; Qian, G.; Hu, X.; Ye, L. Performance and microbial community analysis of bio-electrocoagulation on simultaneous nitrification and denitrification in submerged membrane bioreactor at limited dissolved oxygen. Bioresour. Technol. 2018, 258, 168–176. [Google Scholar] [CrossRef]
- Zhang, R.C.; Xu, X.J.; Chen, C.; Xing, D.F.; Shao, B.; Liu, W.Z.; Wang, A.J.; Lee, D.J.; Ren, N.Q. Interactions of functional bacteria and their contributions to the performance in integrated autotrophic and heterotrophic denitrification. Water Res. 2018, 143, 355–366. [Google Scholar] [CrossRef]
- Yan, L.; Liu, S.; Liu, Q.; Zhang, M.; Liu, Y.; Wen, Y.; Chen, Z.; Zhang, Y.; Yang, Q. Improved performance of simultaneous nitrification and denitrification via nitrite in an oxygen-limited SBR by alternating the DO. Bioresour. Technol. 2019, 275, 153–162. [Google Scholar] [CrossRef]
- Li, J. Long-Term Operation of a Pilot-Scale Sulfur-Based Autotrophic Denitrification System for Deep Nitrogen Removal. Water 2023, 15, 428. [Google Scholar]
- Zhang, L.; Zhang, C.; Hu, C.; Liu, H.; Bai, Y.; Qu, J. Sulfur-based mixotrophic denitrification corresponding to different electron donors and microbial profiling in anoxic fluidized-bed membrane bioreactors. Water Res. 2015, 85, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, C.; Jiang, L.; Qi, J.; Wang, J.; He, X. Impact of dissolved oxygen on the microbial community structure of an intermittent biological aerated filter (IBAF) and the removal efficiency of gasification wastewater. Bioresour. Technol. 2018, 255, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Beller, H.R.; Chain, P.S.G.; Letain, T.E.; Chakicherla, A.; Larimer, F.W.; Richardson, P.M.; Coleman, M.A.; Wood, A.P.; Kelly, D.P. The genome sequence of the obligately chemolithoautotrophic, facultatively anaerobic bacterium Thiobacillus denitfificans. J. Bacteriol. 2006, 188, 1473–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, R.; Zhang, L.; Zhang, Z.; Chen, G.H.; Jiang, F. Realizing high-rate sulfur reduction under sulfate-rich conditions in a biological sulfide production system to treat metal-laden wastewater deficient in organic matter. Water Res. 2018, 131, 239–245. [Google Scholar] [CrossRef]
- Yang, J.; Feng, L.; Pi, S.; Cui, D.; Ma, F.; Zhao, H.P.; Li, A. A critical review of aerobic denitrification: Insights into the intracellular electron transfer. Sci. Total Environ. 2020, 731, 139080. [Google Scholar] [CrossRef]
- Chen, Y.N.; Zhou, W.; Li, Y.P.; Zhang, J.C.; Zeng, G.M.; Huang, A.Z.; Huang, J.X. Nitrite reductase genes as functional markers to investigate diversity of denitrifying bacteria during agricultural waste composting. Appl. Microbiol. Biotechnol. 2014, 98, 4233–4243. [Google Scholar] [CrossRef]
- Jia, L.; Sun, H.; Zhou, Q.; Dai, R.; Wu, W. Integrated evaluation for advanced removal of nitrate and phosphorus in novel PHBV/ZVI-based biofilters: Insight into functional genes and key enzymes. J. Clean. Prod. 2022, 349, 131199. [Google Scholar] [CrossRef]
- Hao, W.; Zhang, J.; Duan, R.; Liang, P.; Huang, X. Organic carbon coupling with sulfur reducer boosts sulfur based denitrification by Thiobacillus denitrificans. Sci. Total Environ. 2020, 748, 142445. [Google Scholar] [CrossRef]
- Morales-Gallardo, M.V.; Ayala, A.M.; Pal, M.; Cortes Jacome, M.A.; Toledo Antonio, J.A.; Mathews, N.R. Synthesis of pyrite FeS2 nanorods by simple hydrothermal method and its photocatalytic activity. Chem. Phys. Lett. 2016, 660, 93–98. [Google Scholar] [CrossRef]
- Amend, J.P.; Edwards, K.J.; Lyons, T.W. Sulfur Biogeochemistry–Past and Present. Geol. Soc. Am. Spec. Pap. 2004, 379, 195–205. [Google Scholar]
- Chen, X.; Shi, T.; Zhong, K.; Wu, G.; Lu, Y. Capacitive behavior of MoS2 decorated with FeS2@carbon nanospheres. Chem. Eng. J. 2020, 379, 122240. [Google Scholar] [CrossRef]
- Li, X.; Hu, Y.; Zhang, C.; Xiao, C.; Cheng, J.; Chen, Y. Electro-activating of peroxymonosulfate via boron and sulfur co-doped macroporous carbon nanofibers cathode for high-efficient degradation of levofloxacin. J. Hazard. Mater. 2023, 442, 130016. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, F.Y.; Hu, W.W. Performance and mechanism of synchronous nitrate and phosphorus removal in constructed pyrite-based mixotrophic denitrification system from secondary effluent. Environ. Sci. Pollut. Res. 2020, 27, 36816–36825. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S. Mixotrophic Denitrification of Glucose Polymer-Based Pyrite Tailings for Enhanced Nitrogen and Phosphorus Removal of Municipal Tailwater. Water 2022, 14, 1868. [Google Scholar]







| Phases | I | II | III | IV | V | VI |
|---|---|---|---|---|---|---|
| Days | 1–14 | 15–28 | 29–42 | 43–56 | 57–70 | 71–84 |
| HRT | 12 | 8 | 4 | 4 | 4 | 4 |
| NO3−-N (mg/L) | 30 | 30 | 30 | 30 | 30 | 30 |
| PO43−-P (mg/L) | 3 | 3 | 3 | 3 | 3 | 3 |
| Influent alkalinity (mg/L CaCO3) | 476 | 476 | 476 | 476 | 476 | 238 |
| COD (provided by sodium acetate) /NO3−-N | – | – | – | 1.0 | 2.0 | 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Hu, Y.; Liang, D.; Wang, G.; Zhu, X.; Xie, J. Simultaneous Removal of Nitrate and Phosphate in a Pyrrhotite and Sulfur-Circulating Packed Bed Reactor. Water 2023, 15, 2158. https://doi.org/10.3390/w15122158
Yu M, Hu Y, Liang D, Wang G, Zhu X, Xie J. Simultaneous Removal of Nitrate and Phosphate in a Pyrrhotite and Sulfur-Circulating Packed Bed Reactor. Water. 2023; 15(12):2158. https://doi.org/10.3390/w15122158
Chicago/Turabian StyleYu, Meiling, Yongyou Hu, Donghui Liang, Guobin Wang, Xiaoqiang Zhu, and Jieyun Xie. 2023. "Simultaneous Removal of Nitrate and Phosphate in a Pyrrhotite and Sulfur-Circulating Packed Bed Reactor" Water 15, no. 12: 2158. https://doi.org/10.3390/w15122158
APA StyleYu, M., Hu, Y., Liang, D., Wang, G., Zhu, X., & Xie, J. (2023). Simultaneous Removal of Nitrate and Phosphate in a Pyrrhotite and Sulfur-Circulating Packed Bed Reactor. Water, 15(12), 2158. https://doi.org/10.3390/w15122158