Arsenic in Drinking Water and Urinary Tract Cancers: A Systematic Review Update
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Electronic Searches
2.3. Study Selection
2.4. Data Extraction
2.5. Data Analysis
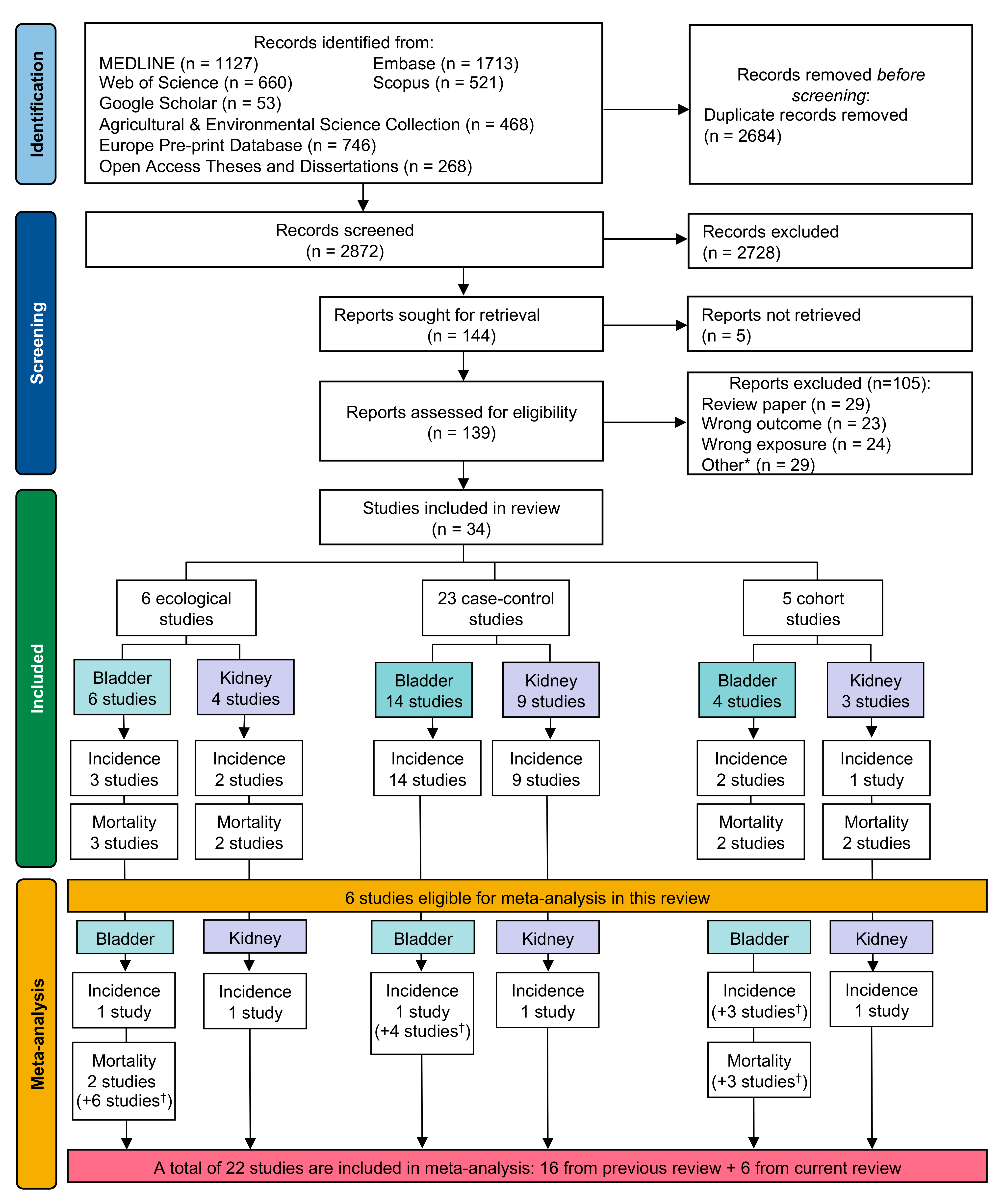
3. Results
3.1. Study Characteristics
3.2. Risk of Bias Assessment
| Study [Reference] (Table from Original Publication) | Study Locale | Outcome | Exposure 1 (Comments) | ICD 2 | Outcome Measure | Cases | Risk Estimate (95% CI) |
|---|---|---|---|---|---|---|---|
| Mendez et al., 2017 [45] (Table Three) | 1178 counties in 49 states, USA | Incidence 2006–2010 | Mean county–level groundwater As concentration (μg/L) (per one unit log of mean county As increase) (Approximately, 31,000 As measurements from ground water sources in 1178 counties in 49 states were collected between 1971–2000. The average proportion of population served by public groundwater in the counties was 76%.) | ICD–9: 188 | RRmale RRfemale (Adjusted for proportion groundwater dependence, education, household income, ethnicity, living in the same house, smoking status, rural–urban indicator, obesity, and age) | Counties 625 336 | 1.00 (0.99–1.02) ‡ 1.03 (1.00–1.06) |
| * Roh et al., 2018 [46] (Table One) | Region II and the rest of Chile | Mortality 2001–2010 | Annual average As concentration in drinking water for Antofagasta and Mejillones (Region II) of Chile ranging between <10 to 860 μg/L; compared with the rest of Chile; exposure generally < 10 μg/L. Data from historical records from 1950–2010. (Exposure data were based on where subjects died, without detailed residential history.) | ICD–9: 188 | SMRmale_birth SMRmale_1–10 SMRmale_11–20 SMRmale_21–30 SMRmale_31–40 SMRmale_≥40 SMRfemale_birth SMRfemale_1–10 SMRfemale_11–20 SMRfemale_21–30 SMRfemale_31–40 SMRfemale_≥40 (Age at potential first exposure to high levels of As in drinking water; SMRmale_birth = mortality for males first exposed at birth) | 17 28 32 65 39 13 7 7 27 47 41 9 | 16.8 (9.8–27.0) 7.9 (5.3–11.5) 4.8 (3.3–6.8) 5.9 (4.5–7.5) 4.4 (3.1–6.0) 4.9 (2.6–8.4) 13.6 (5.5–27.9) 5.3 (2.2–11.0) 9.9 (6.5–14.5) 8.8 (6.5–11.7) 6.4 (4.6–8.7) 3.6 (1.6–6.8) |
| * Saint–Jacques et al., 2018 [42] (Table Two) | Nova Scotia, Canada | Incidence 1998–2010 | As concentration in drinking water (μg/L) <2 (referent) 2–5 ≥5 <2 (referent) 2–5 ≥5 <2 (referent) 2–5 ≥5 (Residential addresses linked to As measurements from 10,498 private wells (1991–1999), at 901 unique locations and aggregated over a set of continuous 25 km2 cells. The maximum As level was 3900 µg/L and 17% of the wells had levels exceeding 10 µg/L.) | ICD-O: 188.0–188.9 ICD-O 2/3: C67.0–C67.9 | Posterior Mean RR RR-male RR-female RR-combined (Adjusted for material and social deprivation; SES used as a proxy for smoking) | 267 144 249 88 44 70 355 188 319 | 1.00 1.18 (0.91–1.51) 1.21 (0.96–1.49) 1.00 1.13 (0.73–1.69) 1.09 (0.74–1.55) 1.00 1.16 (0.91–1.45) 1.18 (0.95–1.44) |
| Smith et al., 2018 [47] (Table Two) | Region II in Chile, compared with the rest of Chile and unexposed Region V | Mortality 2001–2010 | Northern Chile (Region II) with population weighted average As concentration in drinking water before 1958 was 116.8 µg/L between 1958–1970 up to 600 µg/L, and after installation of the As removal plant fell to 108.9 µg/L in 1978, to 10 µg/L between 2005–2010. | ICD–9: 188 | RRsex_age at mortality RRmale_30–39 RRmale_40–49 RRmale_50–59 RRmale_60–69 RRmale_70–79 RRmale_80+ RRmale_all RRfemale_30–39 RRfemale_40–49 RRfemale_50–59 RRfemale_60–69 RRfemale_70–79 RRfemale_80+ RRfemale_all | 1 23 36 48 86 58 252 0 6 20 35 65 51 177 | 2.19 (0.28–16.8) 13.0 (7.94–21.4) 5.68 (3.98–8.11) 4.18 (3.10–5.63) 4.74 (3.79–5.93) 4.07 (3.11–5.32) 4.79 (4.20–5.46) 0 (Reference) 7.03 (2.90–17.0) 9.58 (5.83–15.7) 7.25 (5.05–10.4) 7.47 (5.74–9.74) 4.78 (3.58–6.38) 6.43 (5.49–7.54) |
| * Lopez et al., 2020 [48] (Text–Results) | Antofagasta (Region II) and the rest of Chile | Mortality 1990–2016 | From 1958 to 1971, As concentrations rose from 90 to up to 870 μg/L in Antofagasta compared to rest of Chile (mean concentration 570 vs. 50–178 μg/L) | ICD–9: 188, 189.1 and 189.2 ICD–10: C65, C66 and C67 | MRRBC † MRRUTUC § | N/A 257 | 5.5 (5.2–5.9) 17.6 (13.5−22.9) |
| Krajewski et al., 2021 [49] (Table Two) | 943 counties in 19 states, USA | Incidence 2011–2015 | Aggregated cumulative county–level As concentrations (μg/year) <3.83 (referent) 3.83–7.18 7.18–12.89 >12.89 (Annual As concentrations in public water supplies were collected from 73,035 samples from 18,320 community water systems between 2000–2010. The annual median As concentration was close to 1 μg/L (three outliers with over 100 μg/L).) | N/A | RR (Adjusted for county population of black residents, percent of county population of males, percent of county population that lived in the same county for at least the last 5 years, percent of county population that ever smoked, environmental quality index of water, air, land, build, and sociodemographic and overall environmental quality) | Counties 236 235 237 235 | 1.00 1.28 (1.08–1.53) 1.79 (1.47–2.18) 1.89 (1.53–2.35) |
| Study [Reference] (Table from Original Publication) | Study Locale | Outcome | Arsenic Exposure Assessment | Exposure (Comments) | Cases: Controls | All Participants | Never Smokers | Ever Smokers | Covariates Assessed | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | OR (95% CI) | n | OR (95% CI) | n | OR (95% CI) | |||||||
| Chung et al., 2013 [50] (Table Two) | Taiwan | Incidence 2007–2011 | Individual level ‘measured’ | As urine concentration (μg/g creatinine) <12.81 (referent) 12.81–23.30 ≥23.30 (Average As concentration of tap water was 0.7 μg/L (range: 0–4.0 μg/L). All cases and controls lived far way (200–300 km) from As–contaminated areas.) | 191:364 | 29 44 118 | 1.00 1.64 (0.95–2.82) 4.63 (2.80–7.65) | Age, sex | ||||
| Ferreccio et al., 2013 (a) [51] (Table Two, Supplementary Table Four) | Regions I and II, Northern Chile | Incidence 2007–2010 | Individual level ‘estimated’ | Water As concentration (μg/L) 0–59 (referent) 60–199 200–799 ≥800 Model 2 0–34 35–260 >260 (Average lifetime exposure up to 1971, when high exposure period in Antofagasta ended. As measurements were from the government, research and other sources (>97% of all drinking water sources in the study area.) | 232:640 | 23 27 60 122 | 1.00 0.84 (0.46–1.52) 2.50 (1.48–4.22) 4.44 (2.75–7.15) | 14 20 31 | 1.00 1.92 (0.90–4.11) 5.27 (2.51–11.07) | 19 18 41 | 2.83 (1.22–6.58) 2.28 (0.98–5.31) 15.30 (6.75–34.67) | Age, sex, socioeconomic status, and second–hand tobacco smoke exposure. |
| * Wang et al., 2013 [43] (Table Two) | Taipei, Taiwan | Incidence 1998–2009 | Group level | As water concentration (μg /L) Bladder Cancer <350 (referent) 350–1140 UUTUC § <350 (referent) 350–1140 (Period of As water samples collection not reported. Participants used artesian well water for > 10 years when recruited. The interview included history of well–water consumption, residential history, lifestyle factors. As water concentration in Peimen, Hsuechia, Putai and Ichu townships ranged between 350–1140 μg /L (BFD–endemic area), in surrounding areas was <350 μg /L. Core zone (350–1140 + BFD) Zone 1 (>350) Zone 2 (<350)) | 470:850 260:850 | 391 79 215 45 | 1.0 2.4 (1.6–3.4) 1.0 2.5 (1.6–3.8) | 174 36 146 27 | 1.0 2.5 (1.6–4.1) 1.0 2.3 (1.3–3.9) | 217 43 69 18 | 2.7 (1.9–3.6) 5.7 (3.1–10.3) 2.2 (1.4–3.4) 6.4 (3.1–13.3) | Age, sex, cigarette smoking, alcohol consumption, and hazardous chemical |
| Wu et al., 2013 (a) [52] (Table Two, Three) | Taipei, Taiwan | Incidence 2002–2009 | Individual level ‘measured’ | Total urinary As (μg /g creatinine) <11.50 (referent) 11.50–20.40 ≥20.40 No second–hand smoke exposure <15.40 (referent) ≥15.40 Second–hand smoke exposure <15.40 ≥15.40 (Average As concentration of tap water was 0.7 μg/L (range: 0–4.0 μg/L). All cases and controls lived far way (200–300 km) from As–contaminated areas.) | 261:672 | 36 55 170 | 1.00 1.50 (0.95–2.39) 4.68 (3.06–7.14) | 1.00 3.06 (1.55–6.01) 1.72 (0.75–3.94) 5.55 (2.72–11.3) | 2.93 (1.28–6.71) 5.55 (2.67–11.5) 3.00 (1.28–7.04) 10.8 (5.16–22.7) | Age, sex, educational level, and alcohol consumption | ||
| Wu et al., 2013 (b) [53] (Table Two) | Taipei, Taiwan | Incidence 2002–2009 | Individual level ‘measured’ | Total urinary As (μg /g creatinine) ≤11.74 (referent) 11.74–20.94 >20.94 (Average As concentration of tap water was 0.7 μg/L (range: 0–4.0 μg/L). All cases and controls lived far way (200–300 km) from As–contaminated areas.) | 299:594 | 44 63 192 | 1.00 1.42 (0.90–2.25) 4.13 (2.69–6.35) | Age, sex, education level, alcohol drinking, tea or coffee consumption, cumulative cigarette smoking, pesticide exposure, analgesic use and disease history | ||||
| Melak et al., 2014 [54] (Table Four) | Regions I and II, Northern Chile | Incidence 2007–2010 | Individual level ‘measured’ | Proportion of monomethylarsonic acid in urine (%) All: <12.5 (referent) ≥12.5 Water As <200 μg/L: <12.5 (referent) ≥12.5 Water As ≥200 μg/L: <12.5 ≥12.5 (Residential history was collected; each city/town was linked to a water As measurement for that city/town so that an As concentration could be assigned to each year of each subject’s life.) | 117:347 | 75 42 14 13 61 29 | 1.00 1.41 (0.89–2.23) 1.00 2.37 (1.01–5.57) 6.42 (3.29–12.53 6.96 (3.27–14.8) | Age, sex, smoking | ||||
| Steinmaus et al., 2014 [55] (Table Four) | Regions I and II, Northern Chile | Incidence 2007–2010 | Individual level ‘estimated’ | As water concentration (μg/L) Exposed only in utero or as children ≤110 (referent) 111–800 >800 Exposed only as adults ≤110 (referent) 111–800 >800 (Residential history was collected; each city/town was linked to a water As measurement for that city/town so that an As concentration could be assigned to each year of each subject’s life.) | 90:286 84:332 | 29 13 48 30 12 42 | 1.00 2.94 (1.29–6.70) 8.11 (4.31–15.3) 1.00 2.21 (1.03–4.74) 4.71 (2.61–8.48) | Age, sex and smoking | ||||
| Mostafa et al., 2015 [56] (Table Three) | Bangladesh | Incidence 2008–2011 | Individual level ‘estimated’ | As water concentration (μg/L) TCC £ ≤10 (referent) 10–50 50–100 100–200 200–300 ≥300 (3535 wells were sampled (1998–1999) in 61/64 districts in Bangladesh, 27% of hand–pumped tube wells contained >50 μg/L of As. The mean As concentration data was linked to where participants lived during a biopsy.) | 1446: 1078 | 238 319 204 278 251 156 | 1.00 1.52 (1.08–2.14) 1.07 (0.73–1.57) 0.99 (0.69–1.41) 1.63 (1.08–2.46) 0.89 (0.55–1.43) | Age, sex and smoking status | ||||
| Baris et al., 2016 [57] (Table Three) | Maine, New Hampshire, and Vermont states, USA | Incidence 2001–2004 | Individual level ‘estimated’ | Average drinking water As concentration (μg/L) Unlagged ≤ 0.5 (referent) >0.5–1.0 >1.0–2.1 >2.1–7.0 >7.0–10.4 >10.4 Lagged 40 years ≤ 0.4 >0.4–0.7 >0.7–1.6 >1.6–5.7 >5.7–8.7 >8.7 (Direct measurements of As in water samples collected between 2001–2004 (range 0–20.7 [95th percentile]). When direct estimates were unavailable historical records from 1971–2005 were collected (range 0–30.5 [95th percentile]). Residential history from interview combined with water sample measurements or prediction estimates.) | 1079: 1287 | 303 226 281 225 18 26 280 260 233 220 26 37 | 1.00 0.77 (0.60–0.98) 0.97 (0.76–1.24) 0.98 (0.74–1.28) 0.64 (0.33–1.23) 1.10 (0.61–2.00) 1.00 0.91 (0.71–1.17) 0.93 (0.72–1.20) 1.06 (0.81–1.40) 0.92 (0.51–1.66) 1.49 (0.85–2.61) | – – – – – – – – – – – | – – – – – – – – – – – | – – – – – – – – – – – | – – – – – – – – – – – | Age, sex, Hispanic ethnicity, state of residence, smoking, education, employment in a high–risk occupation, and exposure to disinfection by–products (total trihalomethanes) |
| Chang et al., 2016 [58] (Table Three) | Taichung, Taiwan | Incidence 2011–2013 | Individual level ‘measured’ | Urinary As concentration (μg/L) <46 (referent) 46–86.8 ≥86.8 (Average urinary As level in controls was 95.6 μg/L (range = 3.8–1312.4 μg/L); in cases was 68.1 μg/L (range = 3.8–2819.6 μg/L).) | 205:406 | 59 58 88 | 1.00 0.94 (0.59–1.50) 1.52 (0.98–2.37) | Age, sex, education level, cumulative cigarette smoking, herbal medicine use, exposure to dye and pesticide use | ||||
| Huang et al., 2016 (a) [59] (Table One) | Taipei, Taiwan | Incidence 2007–2009 | Individual level ‘measured’ | Urinary total As concentration (μg/g creatinine) ≤12.24 (referent) 12.24–21.80 >21.80 (Average As concentration of tap water was 0.7 μg/L (range: 0–4.0 μg/L). All cases and controls lived far way (200–300 km) from As–contaminated areas.) | 167:334 | 13 35 119 | 1.00 2.44 (1.19–5.02) 8.44 (4.25–16.8) | Age, sex, education level, and cigarette smoking status | ||||
| Huang et al., 2018 [60] (Table Two) | Taipei, Taiwan | Incidence 2007–2011 | Individual level ‘measured’ | Total urinary As concentration (μg/L) UTUC+ and bladder cancer: ≤9.78 (referent) 9.78–17.91 17.91–30.28 >30.28 Bladder cancer: ≤9.78 (referent) 9.78–17.91 17.91–30.28 >30.28 UTUC:+ ≤9.78 (referent) 9.78–17.91 17.91–30.28 >30.28 (Average As concentration of tap water was 0.7 μg/L (range: 0–4.0 μg/L). All cases and controls lived far way (200–300 km) from As–contaminated areas.) | 428:813 | 177 112 76 63 72 64 46 34 105 48 30 29 | 1.00 1.52 (1.01–2.26) 1.67 (1.05–2.67) 3.49 (2.01–6.06) 1.00 1.94 (1.18–3.20) 2.09 (1.18–3.69) 3.52 (1.77–6.96) 1.00 1.40 (0.83–2.38) 1.91 (0.99–3.69) 4.80 (2.22–10.4) | Age, sex, schools, father’s educational level, cigarette smoking, alcohol, tea and coffee drinking, pesticide contact, urinary tract calculus, hypertension and diabetes history, and urinary creatinine | ||||
| Koutros et al., 2018 [61] (Table One, Two, Three) | Maine, New Hampshire, and Vermont states, USA | Incidence 2001–2004 | Individual level ‘estimated’ | Cumulative As exposure (mg) Unlagged 0–15.7 (referent) >15.7–34.5 >34.5–77.0 >77.0–291.0 >291.0–483.6 >483.6 Lagged 40 years 0–3.52 (referent) >3.52–8.77 >8.77–22.42 >22.4–83.5 >83.5–124.8 >124.8 (Period of As water sample collection not reported. Residential history from interview combined with water sample measurements or prediction estimates.) | 1079: 1287 | 228 288 263 235 33 32 233 269 260 213 34 47 | 1.0 1.2 (0.92–1.5) 1.1 (0.87–1.5) 1.3 (1.00–1.7) 1.3 (0.7–2.3) 1.6 (0.90–2.9) 1.0 1.1 (0.87–1.5) 1.2 (0.92–1.6) 1.3 (0.95–1.7) 1.7 (0.96–3.1) 2.2 (1.3–3.9) | 50 41 36 37 48 33 40 37 | 1.0 1.2 (0.7–2.1) 0.9 (0.5–1.5) 1.1 (0.6–1.9) 1.0 1.0 (0.5–1.7) 1.3 (0.7–2.3) 1.1 (0.6–2.0) | 108 64 151 89 139 86 152 104 94 83 149 83 140 75 162 91 | 1.0† 1.0‡ 1.1 (0.8–1.6) † 1.5 (0.8–2.6) ‡ 1.3 (0.9–1.8) † 1.3 (0.7–2.3) ‡ 1.4 (0.9–2.0) † 1.6 (0.9–3.0) ‡ 1.0† 1.0c 1.1 (0.8–1.6) † 1.7 (0.9–3.0) ‡ 1.2 (0.8–1.7) † 1.3 (0.7–2.3) ‡ 1.6 (1.1–2.4) † 1.6 (0.9–3.0) ‡ | Age, sex, ethnicity, state of residence, smoking, disinfection by products and high–risk occupation |
| Lin et al., 2018 [62] (Table Four) | Taipei, Taiwan | Incidence 2007–2011 | Individual level ‘measured’ | Urinary total As concentration (μg/L) ≤9.71 (referent) 9.71–17.98 17.98–30.51 >30.51 (Average As concentration of tap water was 0.7 μg/L (range: 0–4.0 μg/L). All cases and controls lived far way (200–300 km) from As– contaminated areas.) | 216:648 | 72 64 46 34 | 1.00 2.02 (1.25–3.27) 2.36 (1.36–4.09) 3.23 (1.68–6.20) | Age, sex, education level, cigarette smoking, and urine creatinine level | ||||
| Study [Reference] (Table from Original Publication) | Study Locale | Outcome | ICD 1 | As Exposure Assessment | Exposure (Comments) | Outcome Measure | Cohort Size | Cases | Risk Estimate (95% CI) | Covariates Assessed |
|---|---|---|---|---|---|---|---|---|---|---|
| Yang et al., 2013 [63] (Table One, Two) | 18 villages in four townships in the Lanyang Basin, North–eastern Taiwan | Incidence 1991–1994 | ICD–9: 188, 189.0, 189.1–189.9 | Individual level ‘measured’ | As concentration (μg/L) <10 (referent) 10–99 ≥100 Non–Smokers <10 (referent) 10–99 ≥100 Smokers <10 (referent) 10–99 ≥100 Cumulative As exposure (μg/L*y) <500 (referent) 500–4999 ≥5000 Non–Smokers <500 (referent) 500–4999 ≥5000 Smokers <500 (referent) 500–4999 ≥5000 (As levels in shallow well ranging from < 0.15 to 3590 μg/L and collected from 3901 well water samples between 1991–1994.) | RR | 6876 | 3 9 17 2 3 6 1 6 11 4 11 16 3 3 5 1 8 11 | 1.00 2.18 (0.59–8.01) 8.71 (2.49–30.5) 1.00 1.14 (0.20–6.66) 4.64 (0.89–24.2) 1.00 4.19 (0.51–34.5) 16.50 (2.12–128.6) 1.00 2.46 (0.78–7.72) 9.36 (3.03–28.9) 1.00 0.91 (0.19–4.43) 4.95 (1.21–20.2) 1.00 7.14 (0.88–58.1) 23.45 (3.02–182.3) | Cigarette smoking |
| D’Ippoliti et al., 2015 [64] (Table Three) | 17 municipalities, Viterbo province, Lazio Region, Italy | Mortality 1990–2010 | ICD–9: 188 | Individual level ‘estimated’ | Cumulative As intake (μg) Males ≤ 204.9 (referent) 204.9–804.0 >804.0 Females ≤ 204.9 (referent) 204.9–804.0 >804.0 (Residential history combined with local water records used to assess exposure. As concentration data were only available for 2005–2010, it was assumed that the As concentrations were stable in the study period. As levels ranged 0.5 μg/L to 80.4 μg/L (mean=19.3 μg/L).) | HR | 68,758 70,042 | 13 56 88 5 18 15 | 1.00 0.82 (0.43–1.58) 1.32 (0.67–2.60) 1.00 0.91 (0.31–2.67) 0.71 (0.23–2.24) | Age, calendar period, socioeconomic level, occupation in the ceramic industry, smoking sales and radon exposure |
| Tsai et al., 2021 [65] (Table Four) | 18 villages in four townships in the Lanyang Basin, North–eastern Taiwan | Incidence 1991–1994 | ICD–9: 188 | Individual level ‘measured’ | Cumulative exposure to As in well water (μg/L) <874.2 (referent) ≥874.2 Total urinary As concentration (μg/g creatinine) <97.14 (referent) ≥97.14 (As levels in shallow well ranging from < 0.15 to 3590 μg/L and collected from 3901 well water samples between 1991–1994.) | HR | 771 | 1 11 | 1.00 11.38 (1.48–87.7) 1.00 2.78 (0.75–10.4) | Age, sex, education level and cigarette smoking. |
| Nuvolone et al., 2023 [66] (Table Five) | Five municipalities in Mt. Amiata area; Tuscany, Italy | Mortality 1998–2016 | N/A | Individual level ‘estimated’ | Time–weighted average As concentration in drinking water (μg/L) ≤ 5 (referent) 5–10 >10 (As concentration in tap water were available from 2005 to 2010. It was assumed that As concentrations were stable before 2005, given no mitigation action prior to 2010 and widely known levels in groundwater. Long–term exposure to As for each subject was analyzed by overlapping home coordinates with the water supply units and sampling points.) | HR | 30,910 | 11 27 34 | 1.00 0.56 (0.26–1.24) 0.63 (0.31–1.29) | Sex, socioeconomic status, calendar period |
| Study [Reference] (Table from Original Publication) | Study Locale | Outcome | Exposure 1 (Comments) | ICD 2 | Outcome Measure | Cases | Risk Estimate (95% CI) |
|---|---|---|---|---|---|---|---|
| Roh et al., 2018 [46] (Table One) | Region II and the rest of Chile | Mortality 2001–2010 | Annual average As concentration in drinking water for Antofagasta and Mejillones (Region II) of Chile ranging between <10 to 860 μg/L; compared with the rest of Chile; exposure generally < 10 μg/L. Data from historical records from 1950–2010. (Exposure data were based on where subjects died, without detailed residential history.) | ICD–9: 189 | SMR sex_age at exposure SMRmale_birth SMRmale_1–10 SMRmale_11–20 SMRmale_21–30 SMRmale_31–40 SMRmale_≥40 SMRfemale_birth SMRfemale_1–10 SMRfemale_11–20 SMRfemale_21–30 SMRfemale_31–40 SMRfemale_≥40 | 4 18 32 29 16 3 4 7 17 17 22 4 | 0.9 (0.2–2.3) 1.7 (1.0–2.6) 2.1 (1.4–3.0) 1.7 (1.2–2.5) 2.2 (1.2–3.5) 2.0 (0.4–5.9) 2.2 (0.6–5.6) 1.8 (0.7–3.7) 2.6 (1.5–4.2) 1.9 (1.1–3.0) 3.4 (2.1–5.1) 2.1 (0.6–5.4) |
| * Saint–Jacques et al., 2018 [42] (Table Three) | Nova Scotia, Canada | Incidence 1998–2010 | As concentration in drinking water (μg/L) <2 (referent) 2–5 ≥5 <2 (referent) 2–5 ≥5 <2 (referent) 2–5 ≥5 (Participant residential addresses were linked to As measurements collected from 10,498 private wells between 1991–1999, pooled at 901 unique locations and aggregated over a set of continuous 25 km2 cells. The maximum As level was 3900 µg/L and 17% of the wells had levels exceeding 10µg/L.) | ICD–O: 189.0 ICD–O 2/3: C64.9 | Posterior Mean RR RR-male RR-female RR-combined (Adjusted for material and social deprivation; SES used as a proxy for smoking) | 132 66 123 89 40 74 221 106 197 | 1.00 1.10 (0.78–1.51) 1.15 (0.86–1.51) 1.00 0.99 (0.66–1.43) 1.10 (0.79–1.51) 1.00 1.05 (0.79–1.37) 1.14 (0.89–1.44) |
| Smith et al., 2018 [47] (Table Two) | Region II in Chile, compared with the rest of Chile and unexposed Region V | Mortality 2001–2010 | Northern Chile (Region II) with population weighted average As concentration in drinking water before 1958 was 116.8 µg/L between 1958–1970 up to 600 µg/L, and after installation of the As removal plant fell to 108.9 µg/L in 1978, to 10 µg/L between 2005–2010. | ICD–9: 189 | RR sex_age at mortality RRmale_30–39 RRmale_40–49 RRmale_50–59 RRmale_60–69 RRmale_70–79 RRmale_80+ RRmale_all RRfemale_30–39 RRfemale_40–49 RRfemale_50–59 RRfemale_60–69 RRfemale_70–79 RRfemale_80+ RRfemale_all | 0 8 29 45 47 25 154 0 5 12 18 31 26 92 | 0 (Reference) 0.99 (0.49–2.00) 1.52 (1.05–2.21) 1.74 (1.29–2.35) 1.95 (1.46–2.61) 2.47 (1.65–3.70) 1.75 (1.49–2.05) 0 (Reference) 1.55 (0.63–3.81) 1.80 (1.00–3.21) 1.81 (1.13–2.90) 2.32 (1.61–3.33) 2.60 (1.75–3.86) 2.09 (1.69–2.57) |
| Krajewski et al., 2021 [49] (Table Two) | 943 counties in 19 states, USA | Incidence 2011–2015 | Aggregated cumulative county–level As concentrations (µg–year) <3.83 (referent) 3.83–7.18 7.18–12.89 >12.89 (Annual As concentrations in public water supplies were collected from 73,035 samples from 18,320 community water systems between 2000–2010. The annual median As concentration was close to 1 µg/L (three outliers with over 100 µg/L).) | N/A | RR (Adjusted for county population of black residents, percent of county population of males, percent of county population that lived in the same county for at least the last 5 years, percent of county population that ever smoked, environmental quality index of water, air, land, build, and sociodemographic and overall environmental quality.) | Counties 236 235 237 235 | 1.00 1.15 (0.97–1.37) 1.60 (1.32–1.94) 1.69 (1.37–2.09) |
| Study [Reference] (Table from Original Publication) | STUDY LOCALE | Outcome | ICD 1 | As Exposure Assessment | Exposure (Comments) | Cases: CONTROLS | n | OR, (95% CI) | Covariates Assessed |
|---|---|---|---|---|---|---|---|---|---|
| Ferreccio et al., 2013 (b) [67] (Table Four) | Regions I and II, Northern Chile | Incidence 2007–2010 | ICD–10: C64–66 | Individual level ‘estimated’ | Cumulative As exposure (mg) <10 (referent) 10–25 >25 (Exposure for cumulative As intake based on typical water consumptions and As water concentrations in the 3 main exposure areas of Arica/Iquique, Calama, and Antofagasta where historical average levels ranged from <10ug/L to 860 ug/L over the period of 1930 to 1995.) | 122:640 | 80 28 14 | 1.00 0.96 (0.59–1.55) 1.69 (0.87–3.26) | Sex, age, smoking, mining work, present body mass index, socioeconomic status. |
| * Mostafa et al., 2013 [68] (Table Three) | Bangladesh | Incidence 2008–2011 | N/A | Individual level ‘estimated’ | As water concentration (μg/L) RCC+TCC: † <10 (referent) 10–50 50–100 100–200 200–300 ≥300 RCC: <10 (referent) 10–50 50–100 100–200 200–300 ≥300 TCC: <10 (referent) 10–50 50–100 100–200 200–300 ≥300 (3535 wells were sampled between 1998–1999 in 61 out of 64 districts in Bangladesh, 27% of hand–pumped tube wells contained >50 μg/L of As. The mean As concentration data was linked to where participants lived during a biopsy.) | 986:503 | 216 149 123 150 197 151 197 144 108 130 180 137 19 5 15 20 17 14 | 1.00 1.29 (0.86–1.91) 2.12 (1.33–3.39) 2.41 (1.53–3.81) 3.84 (2.38–6.19) 6.00 (3.29–11.0) 1.00 1.37 (0.92–2.06) 2.05 (1.27–3.32) 2.28 (1.42–3.64) 3.95 (2.42–6.44) 6.00 (3.24–11.1) 1.00 0.51 (0.16–1.63) 4.59 (1.70–12.4) 4.94 (1.88–13.0) 4.83 (1.77–13.1) 7.70 (2.37–25.0) | Age, sex, ever smoked and for clustering within thana (smallest administrative area) |
| Yang et al., 2015 [69] (Table Two) | Taipei, Taiwan | Incidence 2006–2009 | N/A | Individual level ‘measured’ | Urinary total As (μg/L) ≤10.52 (referent) 10.52–24.23 >24.23 (Results shown for renal cell carcinoma) (Average As concentration of tap water was 0.7 μg/L (range: 0–4.0 μg/L). All cases and controls lived far way (200–300 km) from As–contaminated areas.) | 191:376 | 66 66 59 | 1.00 1.72 (0.96–3.08) 4.07 (2.02–8.19) | Age, sex, parental ethnicity, alcohol consumption, tea drinking, coffee drinking, and histories of hypertension, diabetes, urolithiasis, and creatinine |
| Huang et al., 2016 (b) [70] (Table Two) | Taipei, Taiwan | Incidence 2006–2012 | N/A | Individual level ‘measured’ | Total urinary As (μg/L) ≤12.35 (referent) 12.35–25.50 >25.50 (Average As concentration of tap water was 0.7 μg/L (range: 0–4.0 μg/L). All cases and controls lived far way (200–300 km) from As–contaminated areas.) | 398:756 | 204 110 84 | 1.00 1.32 (0.92–1.91) 1.90 (1.22–2.96) | Individual urine creatinine level, age, sex, education level, cigarette smoking, alcohol consumption, history of hypertension and diabetes. |
| Yang et al., 2016 [71] (Table Three) | Taipei, Taiwan | Incidence 2006–2014 | N/A | Individual level ‘measured’ | Urinary total As (μg/L) ≤10.80 (referent) 10.80–22.44 >22.44 (Results shown for clear cell renal carcinoma.) (Average As concentration of tap water was 0.7 μg/L (range: 0–4.0 μg/L). All cases and controls lived far way (200–300 km) from As–contaminated areas.) | 293:293 | 132 84 77 | 1.00 1.61 (0.94–2.77) 2.86 (1.49–5.50) | Age, sex, education level, parental ethnicity, BMI, cumulative cigarette smoking, alcohol consumption, tea drinking, coffee drinking, and histories of hypertension and diabetes |
| Hsueh et al., 2017 [72] (Table Two) | Taipei, Taiwan | Incidence 2006–2012 | N/A | Individual level ‘measured’ | Urinary total As (μg/g creatinine) ≤11.70 (referent) 11.70–19.59 >19.59 (Average As concentration of tap water was 0.7 μg/L (range: 0–4.0 μg/L). All cases and controls lived far way (200–300 km) from As–contaminated areas.) | 180:360 | 33 58 89 | 1.00 1.82 (1.08–3.07) 2.87 (1.73–4.76) | Age, sex, eGFR, diabetes and hypertension. |
| Hsueh et al., 2018 [73] (Table One, Six) | Taipei, Taiwan | Incidence 2006–2012 | N/A | Individual level ‘measured’ | Urinary total As (μg/L) Model 1: ≤9.29 (referent) 9.29–16.78 16.78–29.24 >29.24 Model 2: ≤16.78 (referent) >16.78 (Results shown for renal cell carcinoma) (Average As concentration of tap water was 0.7 μg/L (range: 0–4.0 μg/L). All cases and controls lived far way (200–300 km) from As–contaminated areas.) | 389:389 | 158 86 83 62 | 1.00 1.20 (0.77–1.85) 1.94 (1.19–3.15) 2.70 (1.52–4.79) 1.00 2.27 (1.49–3.44) | Model 1: age and sex Model 2: age, sex, education level, smoking, alcohol drinking, diabetes, hypertension, BMI, ADIPOQ rs182052, and urinary creatinine levels |
| Chen et al., 2021 [44] (Text– Results) | Taipei and Tainan, Taiwan | Incidence 2004–2011 | ICD–9: 189.1‚ 189.2 | Group Level | Arseniasis grades Grade 0 Grade 1 Grade 2 Grade 3 (As exposure was graded based on (1) As concentration >350 μg/L in well water; (2) Blackfoot disease cases; (3) signs of chronic arseniasis (skin lesions) in children. Grade 3—all three factors, Grade 2—factors 1 and 3; Grade 1—factor 1; Grade 0—As concentration <350 μg/L in well water.) (Results shown for upper tract urothelial carcinoma.) | 2921:11684 | 3.92 ‡ 4.71 5.31 8.35 | Age | |
| Hsueh et al., 2021 [74] (Table Two) | Taipei, Taiwan | Incidence 2006–2012 | N/A | Individual level ‘measured’ | Total urinary As (μg/L) per SD increment (Average As concentration of tap water was 0.7 μg/L (range: 0–4.0 μg/L). All cases and controls lived far way (200–300 km) from As–contaminated areas.) | 401:774 | 1.43 (1.19–1.72) | Age, cigarette use, second–hand smoking, alcohol drinking, coffee drinking, hypertension, diabetes mellitus, chronic kidney disease |
| Study [Reference] (Table from Original Publication) | Study Locale | Outcome | ICD 1 | As Exposure Assessment | Exposure (Comments) | Outcome Measure | Cohort Size | Cases | Risk Estimate (95% CI) | Covariates Assessed |
|---|---|---|---|---|---|---|---|---|---|---|
| García–Esquinas et al., 2013 [75] (Table Three) | Arizona, Oklahoma and North/ South Dakota, USA | Mortality 1989–2008 | ICD–9: 189 | Individual level ‘measured’ | Urinary As concentration (μg/g) <6.91 (referent) 6.91–13.32 >13.32 | HR | 3909 | 1.00 0.69 (0.25–1.90) 0.44 (0.14–1.40) | Sex, age, education, smoking, alcohol, BMI, glomerular filtration rate, hypertension | |
| D’Ippoliti et al., 2015 [64] (Table Three) | 17 municipalities, Viterbo province, Lazio Region, Italy | Mortality 1990–2010 | ICD–9: 189 | Individual level ‘estimated’ | Cumulative As intake (μg) Males ≤ 204.9 (referent) 204.9–804.0 >804.0 Females ≤204.9 (referent) 204.9–804.0 >804.0 (Residential history combined with local water records to assess exposure. As concentration data only available for 2005–2010 and assumed stable. As levels ranged 0.5 μg/L to 80.4 μg/L (mean = 19.3 μg/L).) | HR | 68758 70,042 | 4 34 30 1 18 | 1.00 1.95 (0.67–5.72) 1.93 (0.63–5.96) 1.00 4.51 (0.58–35.3) 3.03 (0.37–25.2) | Age, calendar period, socioeconomic level, occupation in the ceramic industry, smoking sales and radon exposure |
| *Tsai et al., 2021 [65] (Table Four) | 18 villages in four townships in the Lanyang Basin, North– eastern Taiwan | Incidence 1991–1994 | ICD–9: 189 | Individual level ‘measured’ | Well As exposure (μg/L) <24.30 (referent) ≥24.30 Cumulative exposure to As in well water (μg/L) <874.2 (referent) ≥874.2 Total urinary As concentration (μg/g creatinine) <97.14 (referent) ≥97.14 (As levels in shallow well ranging from < 0.15 to 3590 μg/L (95th percentile = 525.0 μg/L) and collected from 3901 well water samples between 1991–1994.) | HR | 775 | 7 9 6 10 | 1.00 1.66 (0.53–5.15) 1.00 2.37 (0.72–7.82) 1.00 0.93 (0.32–2.69) | Age, sex, education level and cigarette smoking. |
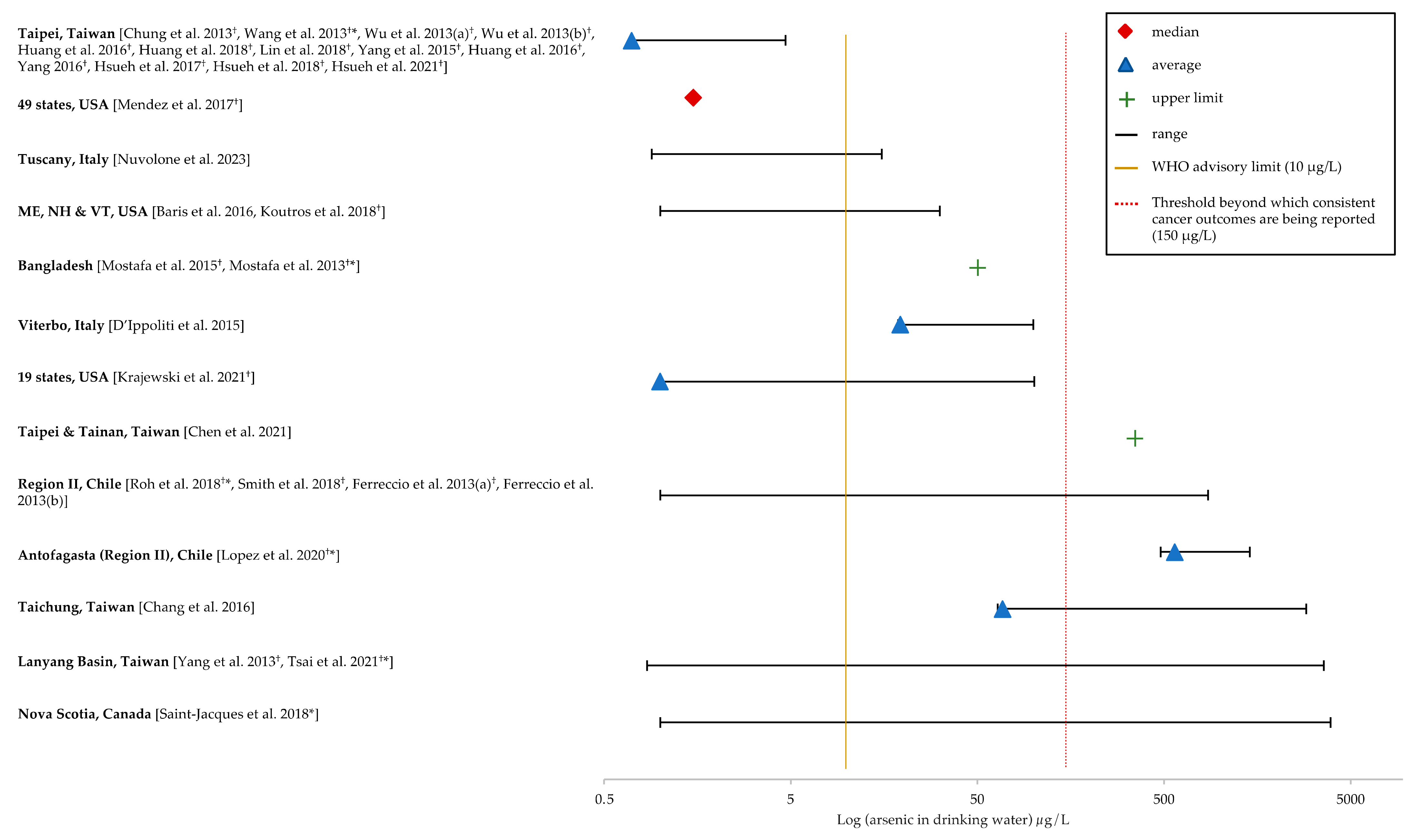
3.3. Arsenic Exposure and Bladder Cancer
3.3.1. Ecological Studies
3.3.2. Case–Control Studies
3.3.3. Cohort Studies
3.4. Arsenic Exposure and Kidney Cancer
3.4.1. Ecological Studies
3.4.2. Case–Control Studies
3.4.3. Cohort Studies
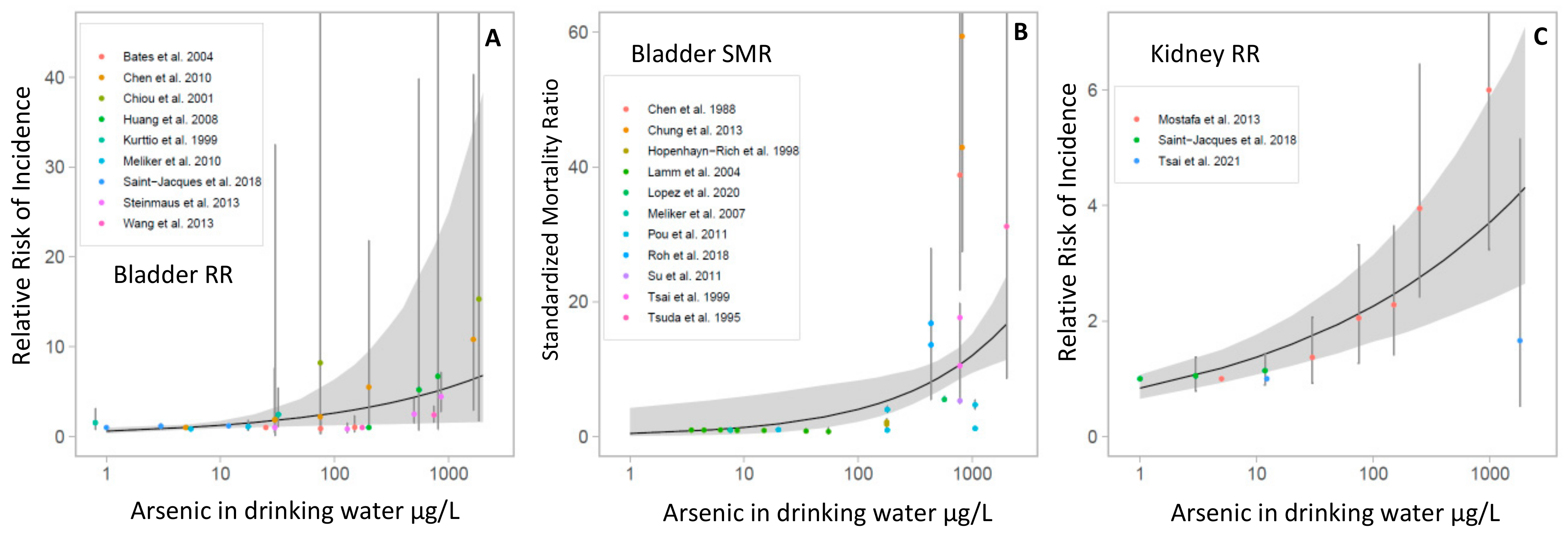
3.5. Meta-Analyses
4. Discussion
4.1. Summary of Findings
4.2. Quantitative Synthesis of the Association between As Exposure and Bladder and Kidney Cancers
4.3. Strengths and Weakness of the Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some Drinking-Water Disinfectants and Contaminants, Including Arsenic; IARC: Lyon, France, 2004; Volume 844. [Google Scholar]
- Singh, N.; Kumar, D.; Sahu, A.P. Arsenic in the environment: Effects on human health and possible prevention. J. Environ. Biol. 2007, 28. [Google Scholar]
- Cantor, K.P.; Lubin, J.H. Arsenic, internal cancers, and issues in inference from studies of low-level exposures in human populations. Toxicol. Appl. Pharmacol. 2007, 222, 252–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondal, D.; Banerjee, M.; Kundu, M.; Banerjee, N.; Bhattacharya, U.; Giri, A.; Ganguli, B.; Roy, S.S.; Polya, D.A. Comparison of drinking water, raw rice and cooking of rice as arsenic exposure routes in three contrasting areas of West Bengal, India. Environ. Geochem. Heal. 2010, 32, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Enterline, P.E.; Day, R.; Marsh, G.M. Cancers related to exposure to arsenic at a copper smelter. Occup. Environ. Med. 1995, 52, 28–32. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zheng, B.; Aposhian, H.; Zhou, Y.; Chen, M.; Zhang, A.; Waalkes, M.P. Chronic Arsenic Poisoning From Burning High-Arsenic-Containing Coal In Guizhou, China. J. Peripher. Nerv. Syst. 2002, 7, 208. [Google Scholar] [CrossRef]
- Silverman, D.T.; Devesa, S.S.; Moore, L.E.; Rothman, N. Bladder Cancer. In Cancer Epidemiology and Prevention; Schottenfeld, D., Fraumeni, J.F., Eds.; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- WHO. Arsenic—World Health Organizaiton. Fact Sheets. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/arsenic (accessed on 17 April 2023).
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic, metals, fibres, and dusts. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100, 11. [Google Scholar]
- Palma-Lara, I.; Martínez-Castillo, M.; Quintana-Pérez, J.; Arellano-Mendoza, M.; Tamay-Cach, F.; Valenzuela-Limón, O.; García-Montalvo, E.; Hernández-Zavala, A. Arsenic exposure: A public health problem leading to several cancers. Regul. Toxicol. Pharmacol. 2020, 110, 104539. [Google Scholar] [CrossRef]
- Lamm, S.H.; Boroje, I.J.; Ferdosi, H.; Ahn, J. A review of low-dose arsenic risks and human cancers. Toxicology 2021, 456, 152768. [Google Scholar] [CrossRef] [PubMed]
- Buchet, J.P.; Lauwerys, R.; Roels, H. Urinary excretion of inorganic arsenic and its metabolites after repeated ingestion of sodium metaarsenite by volunteers. Int. Arch. Occup. Environ. Heal. 1981, 48, 111–118. [Google Scholar] [CrossRef]
- Chu, H.-A.; Crawford-Brown, D.J. Inorganic Arsenic in Drinking Water and Bladder Cancer: A Meta-Analysis for Dose-Response Assessment. Int. J. Environ. Res. Public Heal. 2006, 3, 316–322. [Google Scholar] [CrossRef]
- Chen, C.-L.; Chiou, H.-Y.; Hsu, L.-I.; Hsueh, Y.-M.; Wu, M.-M.; Wang, Y.-H.; Chen, C.-J. Arsenic in Drinking Water and Risk of Urinary Tract Cancer: A Follow-up Study from Northeastern Taiwan. Cancer Epidemiology Biomarkers Prev. 2010, 19, 101–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, H.N.; Zu, K.; Kennedy, E.M.; Lam, T.; Liu, X.; Pizzurro, D.M.; Loftus, C.T.; Rhomberg, L.R. Quantitative assessment of lung and bladder cancer risk and oral exposure to inorganic arsenic: Meta-regression analyses of epidemiological data. Environ. Int. 2017, 106, 178–206. [Google Scholar] [CrossRef]
- Christoforidou, E.P.; Riza, E.; Kales, S.N.; Hadjistavrou, K.; Stoltidi, M.; Kastania, A.N.; Linos, A. Bladder cancer and arsenic through drinking water: A systematic review of epidemiologic evidence. J. Environ. Sci. Heal. Part A 2013, 48, 1764–1775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soria, F.; Marra, G.; Čapoun, O.; Soukup, V.; Gontero, P. Prevention of bladder cancer incidence and recurrence: Tobacco use. Curr. Opin. Urol. 2018, 28, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Mostafaei, H.; Abufaraj, M.; Yang, L.; Egawa, S.; Shariat, S.F. Smoking and bladder cancer: Review of the recent literature. Curr. Opin. Urol. 2020, 30, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xi, S. A review on arsenic carcinogenesis: Epidemiology, metabolism, genotoxicity and epigenetic changes. Regul. Toxicol. Pharmacol. 2018, 99, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Saintilnord, W.N.; Fondufe-Mittendorf, Y. Arsenic-induced epigenetic changes in cancer development. Semin. Cancer Biol. 2021, 76, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Zhao, L.; Wang, Y.; Lu-Yao, G.; Liu, L.-Z. Epigenetic Dysregulations in Arsenic-Induced Carcinogenesis. Cancers 2022, 14, 4502. [Google Scholar] [CrossRef]
- Shukla, V.; Chandrasekaran, B.; Tyagi, A.; Navin, A.K.; Saran, U.; Adam, R.M.; Damodaran, C. A Comprehensive Transcriptomic Analysis of Arsenic-Induced Bladder Carcinogenesis. Cells 2022, 11, 2435. [Google Scholar] [CrossRef]
- Chen, C.J.; Chuang, Y.C.; Lin, T.M.; Wu, H.Y. Malignant neoplasms among residents of a blackfoot disease-endemic area in Taiwan: High-arsenic artesian well water and cancers. Cancer Res. 1985, 45, 5895–5899. [Google Scholar]
- Chen, C.-J.; Kuo, T.-L.; Wu, M.-M. Arsenic and cancers. Lancet 1988, 331, 414–415. [Google Scholar] [CrossRef]
- Wu, M.-M.; Kuo, T.-L.; Hwang, Y.-H.; Chen, C.-J. Dose-response relation between arsenic concentration in well water and mortality from cancers and vascular diseases. Am. J. Epidemiol. 1989, 130, 1123–1132. [Google Scholar] [CrossRef]
- Tsai, S.-M.; Wang, T.-N.; Ko, Y.-C. Mortality for Certain Diseases in Areas with High Levels of Arsenic in Drinking Water. Arch. Environ. Heal. Int. J. 1999, 54, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.S.; Guo, H.R.; Hong, C.L.; Lin, S.M.; Lee, E.F. The Incidence of Bladder Cancer in the Black Foot Disease Endemic Area in Taiwan. BJU Int. 1993, 71, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Wu, M.M.; Lee, S.S.; Wang, J.D.; Cheng, S.H.; Wu, H.Y. Atherogenicity and carcinogenicity of high-arsenic artesian well water. Multiple risk factors and related malignant neoplasms of blackfoot disease. Arter. Off. J. Am. Hear. Assoc. Inc. 1988, 8, 452–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiou, H.Y.; Hsueh, Y.M.; Liaw, K.F.; Horng, S.F.; Chiang, M.H.; Pu, Y.-S.; Lin, J.S.; Huang, C.H.; Chen, C.J. Incidence of internal cancers and ingested inorganic arsenic: A seven-year follow-up study in Taiwan. Cancer Res 1995, 55, 1296–3000. [Google Scholar]
- Chiou, H.Y.; Chiou, S.-T.; Hsu, Y.-H.; Chou, Y.-L.; Tseng, C.-H.; Wei, M.-L.; Chen, C.-J. Incidence of Transitional Cell Carcinoma and Arsenic in Drinking Water: A Follow-up Study of 8,102 Residents in an Arseniasis-endemic Area in Northeastern Taiwan. Am. J. Epidemiol. 2001, 153, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Mink, P.J.; Alexander, D.D.; Barraj, L.M.; Kelsh, M.A.; Tsuji, J.S. Low-level arsenic exposure in drinking water and bladder cancer: A review and meta-analysis. Regul. Toxicol. Pharmacol. 2008, 52, 299–310. [Google Scholar] [CrossRef]
- Tsuji, J.S.; Alexander, D.D.; Perez, V.; Mink, P.J. Arsenic exposure and bladder cancer: Quantitative assessment of studies in human populations to detect risks at low doses. Toxicology 2014, 317, 17–30. [Google Scholar] [CrossRef]
- Saint-Jacques, N.; Parker, L.; Brown, P.; Dummer, T.J. Arsenic in drinking water and urinary tract cancers: A systematic review of 30 years of epidemiological evidence. Environ. Heal. 2014, 13, 44. [Google Scholar] [CrossRef] [Green Version]
- Issanov, A.; Adewusi, B.; Dummer, T.J.; Saint-Jacques, N. Arsenic in Drinking Water and Urinary Tract Cancers: A Systematic Review Update. PROSPERO 2022 CRD42022381522. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022381522 (accessed on 17 April 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- The Cochrane Public Health Group. Data Extraction and Assessment Template. 2011. Available online: https://ph.cochrane.org/sites/ph.cochrane.org/files/public/uploads/CPHG%20Data%20extraction%20template_0.docx (accessed on 17 April 2023).
- Sutton, A.J.; Abrams, K.R. Bayesian methods in meta-analysis and evidence synthesis. Stat. Methods Med. Res. 2001, 10, 277–303. [Google Scholar] [CrossRef] [PubMed]
- Gelman, A.; O’Reilly for Higher, E. Bayesian Data Analysis, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Bürkner, P.-C. brms: An R package for Bayesian multilevel models using Stan. J. Stat. Softw. 2017, 80, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Gelman, A.; Lee, D.; Guo, J. Stan: A probabilistic programming language for Bayesian inference and optimization. J. Educ. Behav. Stat. 2015, 40, 530–543. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 17 April 2023).
- Saint-Jacques, N.; Brown, P.; Nauta, L.; Boxall, J.; Parker, L.; Dummer, T.J. Estimating the risk of bladder and kidney cancer from exposure to low-levels of arsenic in drinking water, Nova Scotia, Canada. Environ. Int. 2018, 110, 95–104. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Yeh, S.-D.; Wu, M.-M.; Liu, C.-T.; Shen, C.-H.; Shen, K.-H.; Pu, Y.-S.; Hsu, L.-I.; Chiou, H.-Y.; Chen, C.-J. Comparing the joint effect of arsenic exposure, cigarette smoking and risk genotypes of vascular endothelial growth factor on upper urinary tract urothelial carcinoma and bladder cancer. J. Hazard. Mater. 2013, 262, 1139–1146. [Google Scholar] [CrossRef]
- Chen, C.-H.; Grollman, A.P.; Huang, C.-Y.; Shun, C.-T.; Sidorenko, V.S.; Hashimoto, K.; Moriya, M.; Turesky, R.J.; Yun, B.H.; Tsai, K.; et al. Additive Effects of Arsenic and Aristolochic Acid in Chemical Carcinogenesis of Upper Urinary Tract Urothelium. Cancer Epidemiology Biomarkers Prev. 2021, 30, 317–325. [Google Scholar] [CrossRef]
- Mendez, W.M.; Eftim, S.; Cohen, J.; Warren, I.; Cowden, J.; Lee, J.S.; Sams, R. Relationships between arsenic concentrations in drinking water and lung and bladder cancer incidence in U.S. counties. J. Expo. Sci. Environ. Epidemiol. 2016, 27, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Roh, T.; Steinmaus, C.; Marshall, G.; Ferreccio, C.; Liaw, J.; Smith, A.H. Age at Exposure to Arsenic in Water and Mortality 30–40 Years After Exposure Cessation. Am. J. Epidemiol. 2018, 187, 2297–2305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.H.; Marshall, G.; Roh, T.; Ferreccio, C.; Liaw, J.; Steinmaus, C. Lung, Bladder, and Kidney Cancer Mortality 40 Years After Arsenic Exposure Reduction. Gynecol. Oncol. 2017, 110, 241–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López, J.F.; Fernández, M.I.; Coz, L.F. Arsenic exposure is associated with significant upper tract urothelial carcinoma health care needs and elevated mortality rates. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 638.e7–638.e13. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, A.K.; Jimenez, M.P.; Rappazzo, K.M.; Lobdell, D.T.; Jagai, J.S. Aggregated cumulative county arsenic in drinking water and associations with bladder, colorectal, and kidney cancers, accounting for population served. J. Expo. Sci. Environ. Epidemiology 2021, 31, 979–989. [Google Scholar] [CrossRef]
- Chung, C.-J.; Huang, C.-Y.; Pu, Y.-S.; Shiue, H.-S.; Su, C.-T.; Hsueh, Y.-M. The effect of cigarette smoke and arsenic exposure on urothelial carcinoma risk is modified by glutathione S-transferase M1 gene null genotype. Toxicol. Appl. Pharmacol. 2013, 266, 254–259. [Google Scholar] [CrossRef]
- Ferreccio, C.; Yuan, Y.; Calle, J.; Benítez, H.; Parra, R.L.; Acevedo, J.; Smith, A.H.; Liaw, J.; Steinmaus, C. Arsenic, Tobacco Smoke, and Occupation. Epidemiology 2013, 24, 898–905. [Google Scholar] [CrossRef]
- Wu, C.-C.; Chen, M.-C.; Huang, Y.-K.; Huang, C.-Y.; Lai, L.-A.; Chung, C.-J.; Shiue, H.-S.; Pu, Y.-S.; Lin, Y.-C.; Han, B.-C.; et al. Environmental tobacco smoke and arsenic methylation capacity are associated with urothelial carcinoma. J. Formos. Med Assoc. 2013, 112, 554–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.-C.; Huang, Y.-K.; Chung, C.-J.; Huang, C.-Y.; Pu, Y.-S.; Shiue, H.-S.; Lai, L.-A.; Lin, Y.-C.; Su, C.-T.; Hsueh, Y.-M. Polymorphism of inflammatory genes and arsenic methylation capacity are associated with urothelial carcinoma. Toxicol. Appl. Pharmacol. 2013, 272, 30–36. [Google Scholar] [CrossRef]
- Melak, D.; Ferreccio, C.; Kalman, D.; Parra, R.; Acevedo, J.; Pérez, L.; Cortés, S.; Smith, A.H.; Yuan, Y.; Liaw, J.; et al. Arsenic methylation and lung and bladder cancer in a case-control study in northern Chile. Toxicol. Appl. Pharmacol. 2013, 274, 225–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinmaus, C.; Ferreccio, C.; Acevedo, J.; Yuan, Y.; Liaw, J.; Durán, V.; Cuevas, S.; García, J.; Meza, R.; Valdés, R.; et al. Increased Lung and Bladder Cancer Incidence in Adults after In Utero and Early-Life Arsenic Exposure. Cancer Epidemiology Biomarkers Prev. 2014, 23, 1529–1538. [Google Scholar] [CrossRef] [Green Version]
- Mostafa, M.G.; Cherry, N. Arsenic in Drinking Water, Transition Cell Cancer and Chronic Cystitis in Rural Bangladesh. Int. J. Environ. Res. Public Heal. 2015, 12, 13739–13749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baris, D.; Waddell, R.; Freeman, L.E.B.; Schwenn, M.; Colt, J.S.; Ayotte, J.D.; Ward, M.H.; Nuckols, J.; Schned, A.; Jackson, B.; et al. Elevated Bladder Cancer in Northern New England: The Role of Drinking Water and Arsenic. J. Natl. Cancer Inst. 2016, 108, djw099. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-H.; Liu, C.-S.; Liu, H.-J.; Huang, C.-P.; Huang, C.-Y.; Hsu, H.-T.; Liou, S.-H.; Chung, C.-J. Association between levels of urinary heavy metals and increased risk of urothelial carcinoma. Int. J. Urol. 2016, 23, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-Y.; Pu, Y.-S.; Shiue, H.-S.; Chen, W.-J.; Lin, Y.-C.; Hsueh, Y.-M. Polymorphisms of human 8-oxoguanine DNA glycosylase 1 and 8-hydroxydeoxyguanosine increase susceptibility to arsenic methylation capacity-related urothelial carcinoma. Arch. Toxicol. 2015, 90, 1917–1927. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Lin, Y.-C.; Shiue, H.-S.; Chen, W.-J.; Su, C.-T.; Pu, Y.-S.; Ao, P.-L.; Hsueh, Y.-M. Comparison of arsenic methylation capacity and polymorphisms of arsenic methylation genes between bladder cancer and upper tract urothelial carcinoma. Toxicol. Lett. 2018, 295, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Koutros, S.; Baris, D.; Waddell, R.; Freeman, L.E.B.; Colt, J.S.; Schwenn, M.; Johnson, A.; Ward, M.H.; Hosain, G.M.M.; Moore, L.E.; et al. Potential effect modifiers of the arsenic-bladder cancer risk relationship. Int. J. Cancer 2018, 143, 2640–2646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.-C.; Chen, W.-J.; Huang, C.-Y.; Shiue, H.-S.; Su, C.-T.; Ao, P.-L.; Pu, Y.-S.; Hsueh, Y.-M. Polymorphisms of Arsenic (+3 Oxidation State) Methyltransferase and Arsenic Methylation Capacity Affect the Risk of Bladder Cancer. Toxicol. Sci. 2018, 164, 328–338. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.-Y.; Hsu, L.-I.; Chen, H.-C.; Chiou, H.-Y.; Hsueh, Y.-M.; Wu, M.-M.; Chen, C.-L.; Wang, Y.-H.; Liao, Y.-T.; Chen, C.-J. Lifetime risk of urothelial carcinoma and lung cancer in the arseniasis-endemic area of Northeastern Taiwan. J. Asian Earth Sci. 2013, 77, 332–337. [Google Scholar] [CrossRef]
- D’ippoliti, D.; Santelli, E.; De Sario, M.; Scortichini, M.; Davoli, M.; Michelozzi, P. Arsenic in Drinking Water and Mortality for Cancer and Chronic Diseases in Central Italy, 1990-2010. PLOS ONE 2015, 10, e0138182. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-L.; Kuo, C.-C.; Hsu, L.-I.; Tsai, S.-F.; Chiou, H.-Y.; Chen, C.-J.; Hsu, K.-H.; Wang, S.-L. Association between arsenic exposure, DNA damage, and urological cancers incidence: A long-term follow-up study of residents in an arseniasis endemic area of northeastern Taiwan. Chemosphere 2020, 266, 129094. [Google Scholar] [CrossRef]
- Nuvolone, D.; Stoppa, G.; Petri, D.; Voller, F. Long-term exposure to low-level arsenic in drinking water is associated with cause-specific mortality and hospitalization in the Mt. Amiata area (Tuscany, Italy). BMC Public Heal. 2023, 23, 71. [Google Scholar] [CrossRef]
- Ferreccio, C.; Smith, A.H.; Durán, V.; Barlaro, T.; Benítez, H.; Valdés, R.; Aguirre, J.J.; Moore, L.E.; Acevedo, J.; Vásquez, M.I.; et al. Case-Control Study of Arsenic in Drinking Water and Kidney Cancer in Uniquely Exposed Northern Chile. Am. J. Epidemiology 2013, 178, 813–818. [Google Scholar] [CrossRef]
- Mostafa, M.; Cherry, N. Arsenic in drinking water and renal cancers in rural Bangladesh. Occup. Environ. Med. 2013, 70, 768–773. [Google Scholar] [CrossRef]
- Yang, S.-M.; Huang, C.-Y.; Shiue, H.-S.; Huang, S.-P.; Pu, Y.-S.; Chen, W.-J.; Lin, Y.-C.; Hsueh, Y.-M. Joint Effect of Urinary Total Arsenic Level and VEGF-A Genetic Polymorphisms on the Recurrence of Renal Cell Carcinoma. PLOS ONE 2015, 10, e0145410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.-Y.; Huang, Y.-L.; Pu, Y.-S.; Shiue, H.-S.; Chen, W.-J.; Chen, S.-S.; Lin, Y.-C.; Su, C.-T.; Hsueh, Y.-M. The joint effects of arsenic and risk diplotypes of insulin-like growth factor binding protein-3 in renal cell carcinoma. Chemosphere 2016, 154, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-M.; Huang, C.-Y.; Shiue, H.-S.; Pu, Y.-S.; Hsieh, Y.-H.; Chen, W.-J.; Lin, Y.-C.; Hsueh, Y.-M. Combined effects of DNA methyltransferase 1 and 3A polymorphisms and urinary total arsenic levels on the risk for clear cell renal cell carcinoma. Toxicol. Appl. Pharmacol. 2016, 305, 103–110. [Google Scholar] [CrossRef]
- Hsueh, Y.-M.; Lin, Y.-C.; Chen, W.-J.; Huang, C.-Y.; Shiue, H.-S.; Pu, Y.-S.; Chen, C.-H.; Su, C.-T. The polymorphism XRCC1 Arg194Trp and 8-hydroxydeoxyguanosine increased susceptibility to arsenic-related renal cell carcinoma. Toxicol. Appl. Pharmacol. 2017, 332, 1–7. [Google Scholar] [CrossRef]
- Hsueh, Y.-M.; Chen, W.-J.; Lin, Y.-C.; Huang, C.-Y.; Shiue, H.-S.; Yang, S.-M.; Ao, P.-L.; Pu, Y.-S.; Su, C.-T. Adiponectin gene polymorphisms and obesity increase the susceptibility to arsenic-related renal cell carcinoma. Toxicol. Appl. Pharmacol. 2018, 350, 11–20. [Google Scholar] [CrossRef]
- Hsueh, Y.-M.; Lin, Y.-C.; Huang, Y.-L.; Shiue, H.-S.; Pu, Y.-S.; Huang, C.-Y.; Chung, C.-J. Effect of plasma selenium, red blood cell cadmium, total urinary arsenic levels, and eGFR on renal cell carcinoma. Sci. Total. Environ. 2020, 750, 141547. [Google Scholar] [CrossRef]
- García-Esquinas, E.; Pollán, M.; Umans, J.G.; Francesconi, K.A.; Goessler, W.; Guallar, E.; Howard, B.; Farley, J.; Best, L.G.; Navas-Acien, A. Arsenic Exposure and Cancer Mortality in a US-Based Prospective Cohort: The Strong Heart Study. Cancer Epidemiol. Biomarkers Prev. 2013, 22, 1944–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, S.; Thomson, A.; Best, N.; Elliott, P. Interpreting posterior relative risk estimates in disease-mapping studies. Environ. Health Perspect. 2004, 112, 1016–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, P.; Mu, L.; Madden, M.; Vena, J.E. Hierarchical Bayesian modeling of spatio-temporal patterns of lung cancer incidence risk in Georgia, USA: 2000–2007. J. Geogr. Syst. 2014, 16, 387–407. [Google Scholar] [CrossRef] [Green Version]
- Bates, M.N.; Rey, O.A.; Biggs, M.L.; Hopenhayn, C.; Moore, L.E.; Kalman, D.; Steinmaus, C.; Smith, A.H. Case-Control Study of Bladder Cancer and Exposure to Arsenic in Argentina. Am. J. Epidemiology 2004, 159, 381–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.-K.; Huang, Y.-L.; Hsueh, Y.-M.; Yang, M.-H.; Wu, M.-M.; Chen, S.-Y.; Hsu, L.-I.; Chen, C.-J. Arsenic exposure, urinary arsenic speciation, and the incidence of urothelial carcinoma: A twelve-year follow-up study. Cancer Causes Control 2008, 19, 829–839. [Google Scholar] [CrossRef]
- Kurttio, P.; Pukkala, E.; Kahelin, H.; Auvinen, A.; Pekkanen, J. Arsenic concentrations in well water and risk of bladder and kidney cancer in Finland. Environ. Health Perspect. 1999, 107, 705–710. [Google Scholar] [CrossRef]
- Meliker, J.R.; Slotnick, M.J.; AvRuskin, G.A.; Schottenfeld, D.; Jacquez, G.M.; Wilson, M.L.; Goovaerts, P.; Franzblau, A.; Nriagu, J.O. Lifetime exposure to arsenic in drinking water and bladder cancer: A population-based case–control study in Michigan, USA. Cancer Causes Control. 2010, 21, 745–757. [Google Scholar] [CrossRef]
- Steinmaus, C.M.; Ferreccio, C.; Romo, J.A.; Yuan, Y.; Cortes, S.; Marshall, G.; Moore, L.E.; Balmes, J.R.; Liaw, J.; Golden, T. Drinking Water Arsenic in Northern Chile: High Cancer Risks 40 Years after Exposure CessationHigh Cancer Risks 40 Years after Arsenic Exposure. Cancer Epidemiol. Biomark. Prev. 2013, 22, 623–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, C.-J.; Huang, Y.-L.; Huang, Y.-K.; Wu, M.-M.; Chen, S.-Y.; Hsueh, Y.-M.; Chen, C.-J. Urinary arsenic profiles and the risks of cancer mortality: A population-based 20-year follow-up study in arseniasis-endemic areas in Taiwan. Environ. Res. 2013, 122, 25–30. [Google Scholar] [CrossRef]
- Hopenhayn-Rich, C.; Biggs, M.L.; Smith, A.H. Lung and kidney cancer mortality associated with arsenic in drinking water in Cordoba, Argentina. Leuk. Res. 1998, 27, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Lamm, S.H.; Engel, A.; Kruse, M.B.; Feinleib, M.; Byrd, D.M.; Lai, S.; Wilson, R. Arsenic in Drinking Water and Bladder Cancer Mortality in the United States: An Analysis Based on 133 U.S. Counties and 30 Years of Observation. J. Occup. Environ. Med. 2004, 46, 298–306. [Google Scholar] [CrossRef]
- Meliker, J.R.; Slotnick, M.J.; AvRuskin, G.A.; Kaufmann, A.; Fedewa, S.A.; Goovaerts, P.; Jacquez, G.J.; Nriagu, J.O. Individual lifetime exposure to inorganic arsenic using a space–time information system. Int. Arch. Occup. Environ. Heal. 2006, 80, 184–197. [Google Scholar] [CrossRef]
- Pou, S.A.; Osella, A.R.; Diaz, M.d.P. Bladder cancer mortality trends and patterns in Córdoba, Argentina (1986–2006). Cancer Causes Control. 2011, 22, 407–415. [Google Scholar] [CrossRef]
- Su, C.-C.; Lu, J.-L.; Tsai, K.-Y.; Lian, I.-B. Reduction in arsenic intake from water has different impacts on lung cancer and bladder cancer in an arseniasis endemic area in Taiwan. Cancer Causes Control. 2010, 22, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Babazono, A.; Yamamoto, E.; Kurumatani, N.; Mino, Y.; Ogawa, T.; Kishi, Y.; Aoyama, H. Ingested Arsenic and Internal Cancer: A Historical Cohort Study Followed for 33 Years. Am. J. Epidemiol. 1995, 141, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Freedman, N.D.; Silverman, D.T.; Hollenbeck, A.R.; Schatzkin, A.; Abnet, C.C. Association Between Smoking and Risk of Bladder Cancer Among Men and Women. JAMA 2011, 306, 737–745. [Google Scholar] [CrossRef]
- Boffetta, P.; Borron, C. Low-Level Exposure to Arsenic in Drinking Water and Risk of Lung and Bladder Cancer: A Systematic Review and Dose–Response Meta-Analysis. Dose-Response 2019, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaafarzadeh, N.; Poormohammadi, A.; Almasi, H.; Ghaedrahmat, Z.; Rahim, F.; Zahedi, A. Arsenic in drinking water and kidney cancer: A systematic review. Rev. Environ. Heal. 2022, 38, 255–263. [Google Scholar] [CrossRef]
- Podgorski, J.; Berg, M. Global threat of arsenic in groundwater. Science 2020, 368, 845–850. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic and arsenic compounds. In Arsenic, Metals, Fibres and Dusts; International Agency for Research on Cancer: Lyon, France, 2012. [Google Scholar]
- Yadav, M.K.; Saidulu, D.; Gupta, A.K.; Ghosal, P.S.; Mukherjee, A. Status and management of arsenic pollution in groundwater: A comprehensive appraisal of recent global scenario, human health impacts, sustainable field-scale treatment technologies. J. Environ. Chem. Eng. 2021, 9, 105203. [Google Scholar] [CrossRef]
- Punshon, T.; Jackson, B.P.; Meharg, A.A.; Warczack, T.; Scheckel, K.; Guerinot, M.L. Understanding arsenic dynamics in agronomic systems to predict and prevent uptake by crop plants. Sci. Total. Environ. 2016, 581–582, 209–220. [Google Scholar] [CrossRef]
- Flanagan, S.V.; Spayd, S.E.; Procopio, N.A.; Marvinney, R.G.; Smith, A.E.; Chillrud, S.N.; Braman, S.; Zheng, Y. Arsenic in private well water part 3 of 3: Socioeconomic vulnerability to exposure in Maine and New Jersey. Sci. Total. Environ. 2016, 562, 1019–1030. [Google Scholar] [CrossRef] [Green Version]
- Adeloju, S.B.; Khan, S.; Patti, A.F. Arsenic Contamination of Groundwater and Its Implications for Drinking Water Quality and Human Health in Under-Developed Countries and Remote Communities—A Review. Appl. Sci. 2021, 11, 1926. [Google Scholar] [CrossRef]
- Krupoff, M.; Mobarak, A.M.; van Geen, A. Evaluating Strategies to Reduce Arsenic Poisoning in South Asia: A View from the Social Sciences. Asian Dev. Rev. 2020, 37, 21–44. [Google Scholar] [CrossRef]
- Flanagan, S.V.; Spayd, S.E.; Procopio, N.A.; Chillrud, S.N.; Braman, S.; Zheng, Y. Arsenic in private well water part 1 of 3: Impact of the New Jersey Private Well Testing Act on household testing and mitigation behavior. Sci. Total. Environ. 2016, 562, 999–1009. [Google Scholar] [CrossRef] [Green Version]
- Flanagan, S.V.; Spayd, S.E.; Procopio, N.A.; Chillrud, S.N.; Ross, J.; Braman, S.; Zheng, Y. Arsenic in private well water part 2 of 3: Who benefits the most from traditional testing promotion? Sci. Total Environ. 2016, 562, 1010–1018. [Google Scholar] [CrossRef] [Green Version]
- Milton, A.H.; Hore, S.K.; Hossain, M.Z.; Rahman, M. Bangladesh arsenic mitigation programs: Lessons from the past. Emerg. Heal. Threat. J. 2012, 5, 7269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGowan, J.; Sampson, M.; Salzwedel, D.M.; Cogo, E.; Foerster, V.; Lefebvre, C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J. Clin. Epidemiology 2016, 75, 40–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B.; PRISMA-S Group. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- Vuorre, M. Bayesian Meta-Analysis with R, Stan, and brms. Meta-Analysis is a Special Case of Bayesian Multilevel Modeling. Available online: https://mvuorre.github.io/posts/2016-09-29-bayesian-meta-analysis/ (accessed on 18 April 2023).
- Hackenberger, B.K. Bayesian meta-analysis now–let’s do it. Croat. Med. J. 2020, 61, 564. [Google Scholar] [CrossRef]
- Bero, L.; Chartres, N.; Diong, J.; Fabbri, A.; Ghersi, D.; Lam, J.; Lau, A.; McDonald, S.; Mintzes, B.; Sutton, P.; et al. The risk of bias in observational studies of exposures (ROBINS-E) tool: Concerns arising from application to observational studies of exposures. Syst. Rev. 2018, 7, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
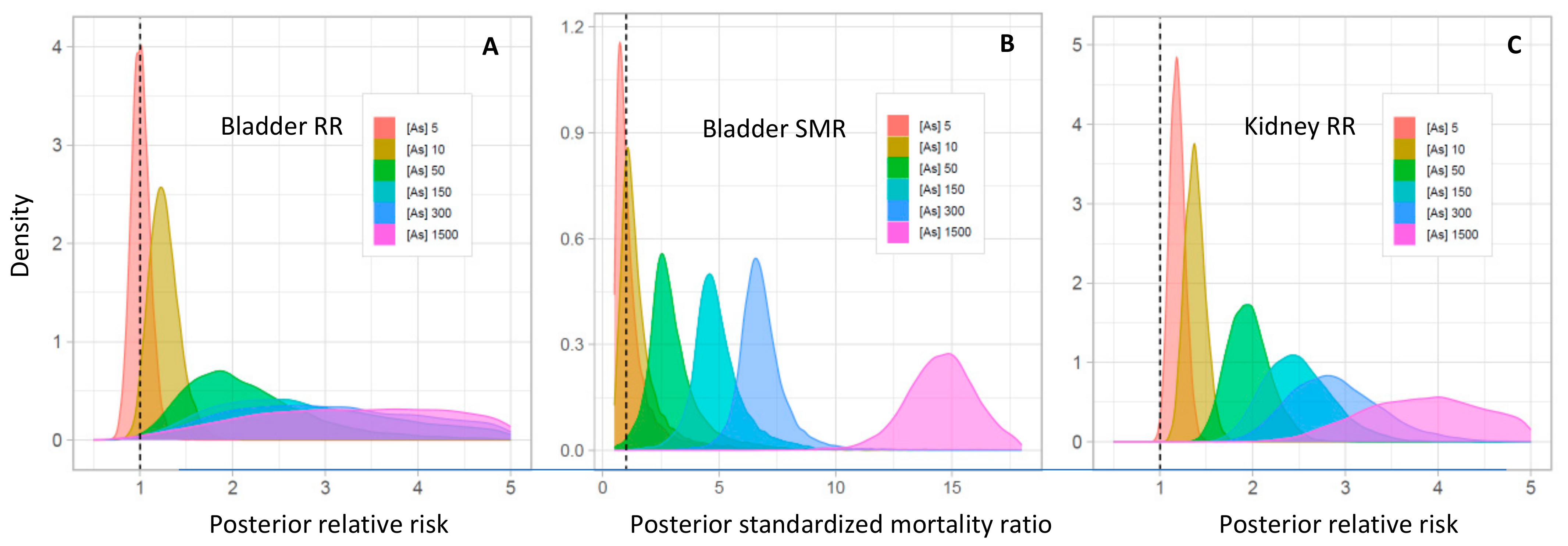
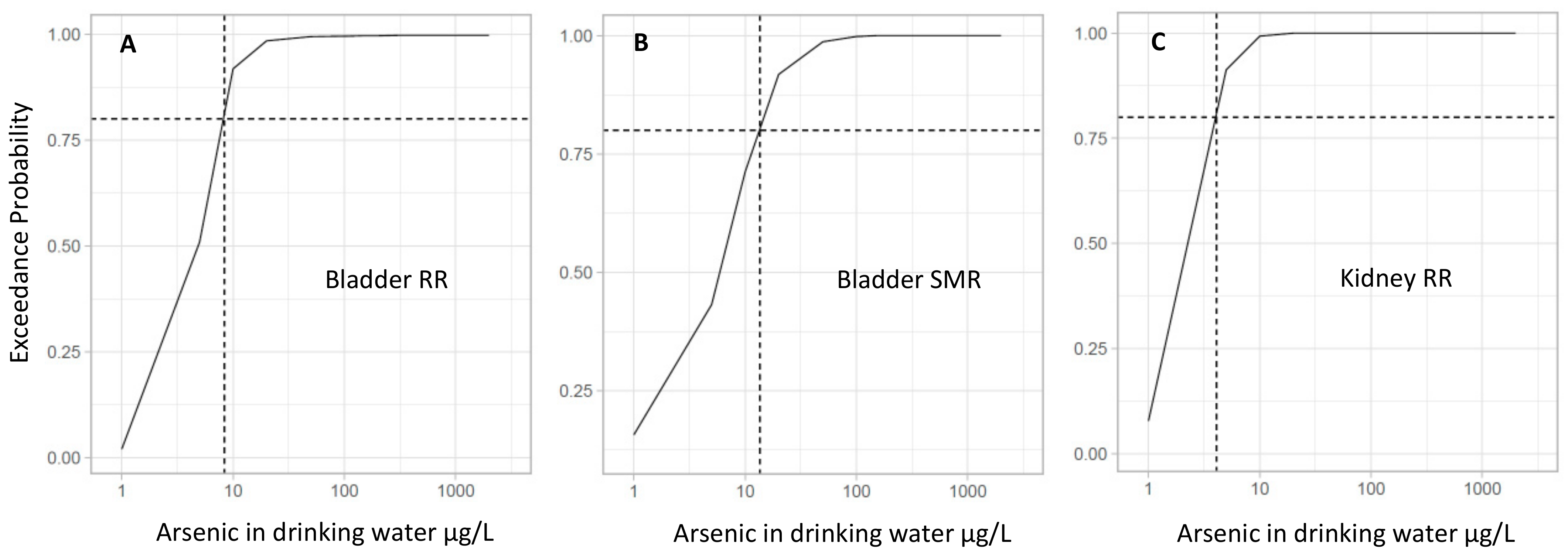
| Arsenic Concentration (μg/L) | Bladder Cancer Mean RR (95% CrI) | Exceedance Probability (RR > 1) * | Bladder Cancer Mean SMR (95% CrI) | Exceedance Probability (SMR > 1) * | Kidney Cancer Mean RR (95% CrI) | Exceedance Probability (RR > 1) * |
|---|---|---|---|---|---|---|
| 5 | 1.00 (0.76–1.32) | 0.50 | 0.98 (0.22–5.92) | 0.44 | 1.18 (0.94–1.51) | 0.91 |
| 10 | 1.25 (0.92–1.73) | 0.92 | 1.36 (0.35–6.39) | 0.72 | 1.37 (1.07–1.77) | 1.00 |
| 20 | 1.57 (1.04–2.46) | 0.99 | 1.89 (0.60–6.83) | 0.92 | 1.60 (1.23–2.09) | 1.00 |
| 50 | 2.11 (1.18–4.22) | 0.99 | 2.92 (1.24–7.82) | 0.99 | 1.95 (1.44–2.65) | 1.00 |
| 100 | 2.64 (1.26–6.37) | 1.00 | 4.04 (2.11–8.49) | 1.00 | 2.26 (1.61–3.15) | 1.00 |
| 150 | 3.01 (1.31–8.17) | 1.00 | 4.88 (2.83–9.03) | 1.00 | 2.47 (1.74–3.52) | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Issanov, A.; Adewusi, B.; Dummer, T.J.B.; Saint-Jacques, N. Arsenic in Drinking Water and Urinary Tract Cancers: A Systematic Review Update. Water 2023, 15, 2185. https://doi.org/10.3390/w15122185
Issanov A, Adewusi B, Dummer TJB, Saint-Jacques N. Arsenic in Drinking Water and Urinary Tract Cancers: A Systematic Review Update. Water. 2023; 15(12):2185. https://doi.org/10.3390/w15122185
Chicago/Turabian StyleIssanov, Alpamys, Betty Adewusi, Trevor J. B. Dummer, and Nathalie Saint-Jacques. 2023. "Arsenic in Drinking Water and Urinary Tract Cancers: A Systematic Review Update" Water 15, no. 12: 2185. https://doi.org/10.3390/w15122185
APA StyleIssanov, A., Adewusi, B., Dummer, T. J. B., & Saint-Jacques, N. (2023). Arsenic in Drinking Water and Urinary Tract Cancers: A Systematic Review Update. Water, 15(12), 2185. https://doi.org/10.3390/w15122185








