Colored Wastewater Treatment by Clathrate Hydrate Technique
Abstract
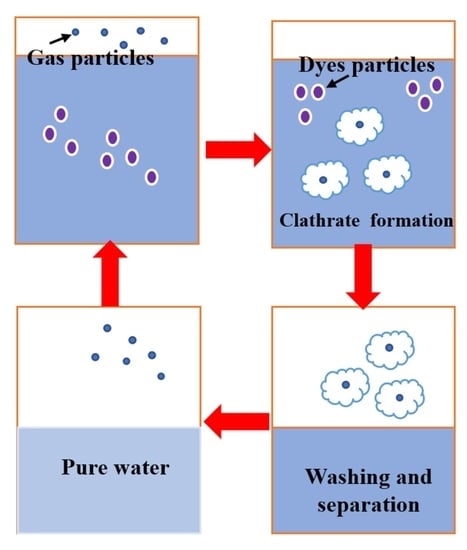
:1. Introduction
- gas hydrates formation;
- hydrates separation;
- improvement of the obtained water quality, by different operation like centrifugation, washing, etc.;
- hydrate dissociation, obtaining clean water and recovering the gas [35].
2. Materials and Methods
2.1. Chemicals
2.2. Apparatus
2.3. Preparation of Dye Solution
2.4. Experimental Procedure
2.5. Dye Removal Rate
3. Results and Discussion
3.1. Growth Rate of R134a Hydrate Formation
3.2. Thermodynamic Behaviour Study
3.3. Induction Time
3.4. Treatment Efficiency in Terms of Removal Rate
3.5. Gas Hydrate Formation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singha, K.; Pandit, P.; Maity, S.; Sharma, S.R. Harmful Environmental Effects for Textile Chemical Dyeing Practice. In Green Chemistry for Sustainable Textiles; Ibrahim, N., Hussain, C.M., Eds.; Woodhead Publishing: Cambridge, UK, 2021; pp. 153–164. [Google Scholar]
- Bayomie, O.S.; Kandeel, H.; Shoeib, T.; Yang, H.; Youssef, N.; El-Sayed, M.M.H. Novel approach for effective removal of methylene blue dye from water using fava bean peel waste. Sci. Rep. 2020, 10, 7824. [Google Scholar] [CrossRef]
- Saja, S.; Bouazizi, A.; Achiou, B.; Ouaddari, H.; Karim, A.; Ouammou, M.; Aaddane, A.; Bennazha, J.; Younssi, S.A. Fabrication of low-cost ceramic ultrafiltration membrane made from bentonite clay and its application for soluble dyes removal. J. Eur. Ceram. Soc. 2020, 40, 2453–2462. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef] [PubMed]
- Trott, A.T. Emergency Wound Care: An Overview. In Wounds and Lacerations, 4th ed.; Trott, A.T., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2012; pp. 1–3. [Google Scholar]
- Alardhi, S.M.; Abdalsalam, A.H.; Ati, A.A.; Abdulkareem, M.H.; Ramadhan, A.A.; Taki, M.M.; Abbas, Z.Y. Fabrication of polyaniline/zinc oxide nanocomposites: Synthesis, characterization and adsorption of methylene orange. Polym. Bull. 2023, 80, 1–27. [Google Scholar] [CrossRef]
- Alardhi, S.M.; Fiyadh, S.S.; Salman, A.D.; Adelikhah, M. Prediction of methyl orange dye (MO) adsorption using activated carbon with an artificial neural network optimization modeling. Heliyon 2023, 9, e12888. [Google Scholar] [CrossRef]
- Jamee, R.; Siddique, R. Biodegradation of synthetic dyes of textile effluent by microorganisms: An environmentally and economically sustainable approach. Eur. J. Microbiol. Immunol. 2019, 9, 114–118. [Google Scholar] [CrossRef]
- Gadekar, M.R.; Ahammed, M.M. Coagulation/flocculation process for dye removal using water treatment residuals: Modelling through artificial neural networks. Desalination Water Treat. 2016, 57, 26392–26400. [Google Scholar] [CrossRef]
- Sala, M.; Gutiérrez-Bouzán, M.C. Electrochemical Techniques in Textile Processes and Wastewater Treatment. Int. J. Photoenergy 2012, 2012, 629103. [Google Scholar] [CrossRef] [Green Version]
- Al-Jadir, T.; Alardhi, S.M.; Alheety, M.A.; Najim, A.A.; Salih, I.K.; Al-Furaiji, M.; Alsalhy, Q.F. Fabrication and Characterization of Polyphenylsulfone/Titanium Oxide Nanocomposite Membranes for Oily Wastewater Treatment. J. Ecol. Eng. 2022, 23, 1–13. [Google Scholar] [CrossRef]
- Alardhi, S.M.; Alrubaye, J.M.; Albayati, T.M. Hollow Fiber Ultrafiltration Membrane for Methyl Green Dye Removal. Eng. Technol. J. 2020, 38, 1077–1083. [Google Scholar] [CrossRef]
- Alardhi, S.M.; Alrubaye, J.M.; Albayati, T.M. Removal of Methyl Green Dye from simulated waste water using Hollow Fiber Ultrafiltration Membrane. IOP Conf. Ser. Mater. Sci. Eng. 2020, 928, 052020. [Google Scholar] [CrossRef]
- Elumalai, S.; Muthuraman, G. Removal and Recovery of Methyl Violet Dye from Industrial Wastewater by Liquid–Liquid Extraction; Springer: Singapore, 2018. [Google Scholar]
- Wijannarong, S.; Aroonsrimorakot, S.; Thavipoke, P.; Kumsopa, C.; Sangjan, S. Removal of Reactive Dyes from Textile Dyeing Industrial Effluent by Ozonation Process. APCBEE Procedia 2013, 5, 279–282. [Google Scholar] [CrossRef] [Green Version]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO2based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Fu, J.; Kyzas, G.Z. Wet air oxidation for the decolorization of dye wastewater: An overview of the last two decades. Chin. J. Catal. 2014, 35, 1–7. [Google Scholar] [CrossRef]
- Ganaie, R.J.; Rafiq, S.; Sharma, A. Recent Advances in Physico-chemical Methods for Removal of Dye from Wastewater. IOP Conf. Ser. Earth Environ. Sci. 2023, 1110, 012040. [Google Scholar] [CrossRef]
- Salman, S.; Sheikh, C.; Hasan, M.; Hasan, N.; Kubra, K.T.; Rehan, A.I.; Awual, E.; Rasee, A.I.; Waliullah, R.; Hossain, M.S.; et al. Chitosan-coated cotton fiber composite for efficient toxic dye encapsulation from aqueous media. Appl. Surf. Sci. 2023, 622, 157008. [Google Scholar] [CrossRef]
- Hassanpouryouzband, A.; Joonaki, E.; Farahani, M.V.; Takeya, S.; Ruppel, C.; Yang, J.; English, N.J.; Schicks, J.M.; Edlmann, K.; Mehrabian, H.; et al. Gas hydrates in sustainable chemistry. Chem. Soc. Rev. 2020, 49, 5225–5309. [Google Scholar] [CrossRef]
- Al-Hemeri, S.T.; Al-Mukhtar, R.S.; Mahmood, L.W. Thermodynamic and Kinetic Investigation of Desalination by Refrigerant Clathrate Hydrate Formation. Eng. Technol. J. 2019, 37, 29–44. [Google Scholar] [CrossRef]
- Marboeuf, U.; Schmitt, B.; Petit, J.-M.; Mousis, O.; Fray, N. A cometary nucleus model taking into account all phase changes of water ice: Amorphous, crystalline, and clathrate. Astron. Astrophys. 2012, 542, A82. [Google Scholar] [CrossRef]
- Lee, J.; Kenney, J.W. Clathrate Hydrates. In Solidification; IntechOpen: London, UK, 2018. [Google Scholar]
- Chen, G.-J.; Guo, T.-M. A new approach to gas hydrate modelling. Chem. Eng. J. 1998, 71, 145–151. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Bahadori, A. Gas Hydrates. In Fluid Phase Behavior for Conventional and Unconventional Oil and Gas Reservoirs; Gulf Professional Publishing: Boston, MA, USA, 2017; pp. 405–444. [Google Scholar]
- AL-Mukhtar, R.S.; Remedhan, S.T.; Hussin, M.N. Study of The Influence of Different Variables on Clathrate Practical Applications in Phenol Removal. Eng. Technol. J. 2020, 38, 1373–1383. [Google Scholar] [CrossRef]
- Thakre, N.; Jana, A.K. Physical and molecular insights to Clathrate hydrate thermodynamics. Renew. Sustain. Energy Rev. 2020, 135, 110150. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, L.; Wang, J. Formation, Exploration, and Development of Natural Gas Hydrates. Energies 2022, 15, 5951. [Google Scholar] [CrossRef]
- Yu, C.; Fan, S.; Lang, X.; Wang, Y.; Li, G.; Wang, S. Hydrogen and chemical energy storage in gas hydrate at mild conditions. Int. J. Hydrogen Energy 2020, 45, 14915–14921. [Google Scholar] [CrossRef]
- Marwa, N. Experimental Investigation to Study Clathrate Based for Removal Heavy Metals and Phenol from Wastewater. Master’s Thesis, Chemical Engineering Department—University of Technology, Baghdad, Iraq, 2019. [Google Scholar]
- Hegde, G.A.; Sum, A.K.; Danielson, T.J. Multiphase flow modeling for gas hydrates in flow assurance. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 4–7 May 2015. [Google Scholar]
- Max, M. Natural Gas Hydrate in Oceanic and Permafrost Environments; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Hai, S. Water Purifying by Gas Hydrate: Potential Applications to Desalination and Wastewater Treatments. Chem. Eng. Trans. 2020, 78, 67–72. [Google Scholar]
- Babu, P.; Nambiar, A.; He, T.; Karimi, I.A.; Lee, J.D.; Englezos, P.; Linga, P. A review of clathrate hydrate-based desalination to strengthen energy–water nexus. ACS Sustain. Chem. Eng. 2018, 6, 8093–8107. [Google Scholar] [CrossRef]
- Seo, S.D.; Hong, S.Y.; Sum, A.K.; Lee, K.H.; Lee, J.D.; Lee, B.R. Thermodynamic and kinetic analysis of gas hydratesfor desalination of saturated salinity water. Chem. Eng. J. 2019, 370, 980–987. [Google Scholar] [CrossRef]
- Gupta, A.; Baron, G.V.; Perreault, P.; Lenaerts, S.; Ciocarlan, R.-G.; Cool, P.; Mileo, P.G.; Rogge, S.; Van Speybroeck, V.; Watson, G.; et al. Hydrogen Clathrates: Next Generation Hydrogen Storage Materials. Energy Storage Mater. 2021, 41, 69–107. [Google Scholar] [CrossRef]
- Hussain, H.H.; Husin, H. Review on Application of Quaternary Ammonium Salts for Gas Hydrate Inhibition. Appl. Sci. 2020, 10, 1011. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.; Ko, G.; Seo, Y. Greenhouse Gas (CHF3) Separation by Gas Hydrate Formation. ACS Sustain. Chem. Eng. 2017, 5, 5485–5492. [Google Scholar] [CrossRef]
- Xu, H.; Khan, M.N.; Peters, C.J.; Sloan, E.D.; Koh, C.A. Hydrate-Based Desalination Using Cyclopentane Hydrates at Atmospheric Pressure. J. Chem. Eng. Data 2018, 63, 1081–1087. [Google Scholar] [CrossRef]
- Obara, S.; Mikawa, D. Electric power control of a power generator using dissociation expansion of a gas hydrate. Appl. Energy 2018, 222, 704–716. [Google Scholar] [CrossRef]
- Cha, J.-H.; Seol, Y. Increasing Gas Hydrate Formation Temperature for Desalination of High Salinity Produced Water with Secondary Guests. ACS Sustain. Chem. Eng. 2013, 1, 1218–1224. [Google Scholar] [CrossRef]
- Dicharry, C.; Duchateau, C.; Asbaï, H.; Broseta, D.; Torré, J.P. Carbon dioxide gas hydrate crystallisation in porous silica gel particles partially saturated with a surfactant solution. Chem. Eng. Sci. 2013, 98, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Al Hemeri, S.T.; Al Mukhtar, R.S.; Mohammed, M.S. Study the effect of organic promoters on thermodynamics behaviour for refrigerant gas clathrate hydrate. IOP Conf. Ser. Mater. Sci. Eng. 2019, 579, 012021. [Google Scholar] [CrossRef]
- Lee, S.; Seo, Y. Experimental Measurement and Thermodynamic Modeling of the Mixed CH4 + C3H8 Clathrate Hydrate Equilibria in Silica Gel Pores: Effects of Pore Size and Salinity. Langmuir 2010, 26, 9742–9748. [Google Scholar] [CrossRef]
- Kang, S.-P.; Lee, J.-W.; Ryu, H.-J. Phase behaviour of methane and carbon dioxide hydrates in meso-and macro-sized porous media. Fluid Phase Equilibria 2008, 274, 68–72. [Google Scholar] [CrossRef]
- Mondal, S. Methods of Dye Removal from Dye House Effluent—An Overview. Environ. Eng. Sci. 2008, 25, 383–396. [Google Scholar] [CrossRef]
- Salman, S.; Hasan, N.; Hasan, M.; Kubra, K.T.; Sheikh, C.; Rehan, A.I.; Waliullah, R.; Rasee, A.I.; Awual, E.; Hossain, M.S.; et al. Improving copper(II) ion detection and adsorption from wastewater by the ligand-functionalized composite adsorbent. J. Mol. Struct. 2023, 1282, 135259. [Google Scholar] [CrossRef]








| Dyes | Concentration (ppm) | Induction Time (min) | Dye Removal Efficacy (%) |
|---|---|---|---|
| Potassium permanganate | 10 | 35 | 86 |
| 50 | 32 | 88.5 | |
| 100 | 29 | 92 | |
| Povidone-iodine | 500 | 34 | 90 |
| 2500 | 31 | 92 | |
| 5000 | 30 | 95 |
| Promotors | Dye Removal Rate % | |||||
|---|---|---|---|---|---|---|
| Potassium Permanganate (ppm) | Povidone-Iodine (ppm) | |||||
| 10 | 50 | 100 | 500 | 2500 | 5000 | |
| Without | 86 | 90 | ||||
| Tween 80 | 87 | 88 | 89 | 90 | 91 | 91 |
| Cyclohexane | 88 | 89 | 90 | 91 | 93 | 94 |
| Silica gel | 89 | 91 | 92 | 92 | 94 | 95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, M.S.; Al-Humairi, S.T.; Al-Mukhtar, R.S.; Mohsen Alardhi, S.; Aliyu, A.; Dawood Salman, A.; Jakab, M.; Yasir AlJaberi, F.; Sluser, B.; Cretescu, I. Colored Wastewater Treatment by Clathrate Hydrate Technique. Water 2023, 15, 2227. https://doi.org/10.3390/w15122227
Mohammed MS, Al-Humairi ST, Al-Mukhtar RS, Mohsen Alardhi S, Aliyu A, Dawood Salman A, Jakab M, Yasir AlJaberi F, Sluser B, Cretescu I. Colored Wastewater Treatment by Clathrate Hydrate Technique. Water. 2023; 15(12):2227. https://doi.org/10.3390/w15122227
Chicago/Turabian StyleMohammed, Mustafa S., Shurooq T. Al-Humairi, Riyadh S. Al-Mukhtar, Saja Mohsen Alardhi, Auwal Aliyu, Ali Dawood Salman, Miklós Jakab, Forat Yasir AlJaberi, Brindusa Sluser, and Igor Cretescu. 2023. "Colored Wastewater Treatment by Clathrate Hydrate Technique" Water 15, no. 12: 2227. https://doi.org/10.3390/w15122227
APA StyleMohammed, M. S., Al-Humairi, S. T., Al-Mukhtar, R. S., Mohsen Alardhi, S., Aliyu, A., Dawood Salman, A., Jakab, M., Yasir AlJaberi, F., Sluser, B., & Cretescu, I. (2023). Colored Wastewater Treatment by Clathrate Hydrate Technique. Water, 15(12), 2227. https://doi.org/10.3390/w15122227