Enhanced PMS Activation by Highly Dispersed Mn-Ce Bimetallic Oxide on Carbon Nanotubes for Degradation of Phenol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of MCC
2.3. Characterization
2.4. Phenol Removal Tests
2.5. Analysis
3. Results and Discussion
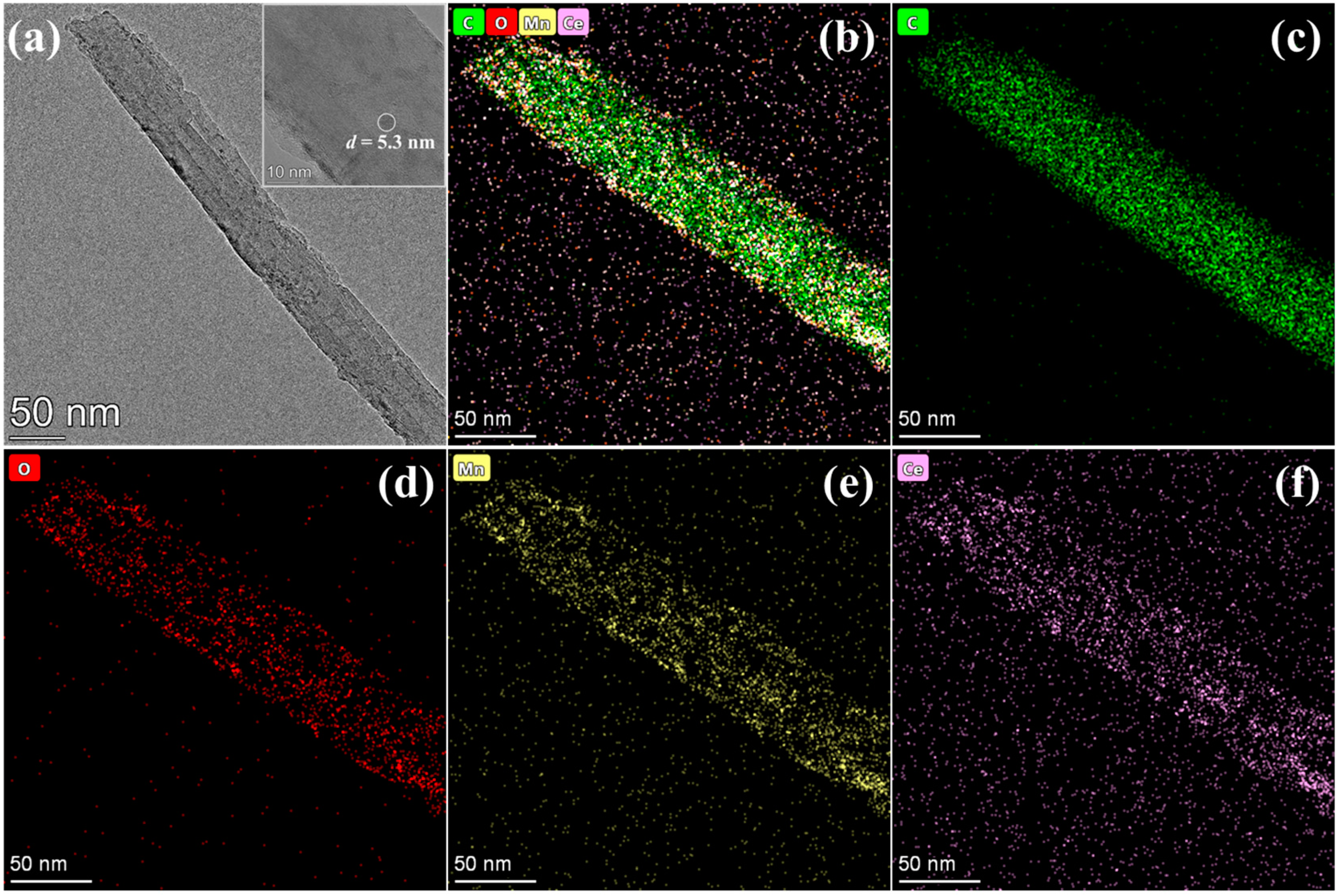
3.1. Physicochemical Characteristics of Catalysts
3.2. Catalytic Performance of Catalysts
3.3. Active Species Generated during the Catalytic System
3.4. The Synergistic Effect between Bimetallic Oxide and CNT
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, G.; Chen, S.; Quan, X.; Yu, H.; Zhang, Y. Enhanced activation of peroxymonosulfate by nitrogen doped porous carbon for effective removal of organic pollutants. Carbon 2017, 115, 730–739. [Google Scholar] [CrossRef]
- Li, J.; Zhu, W.; Gao, Y.; Lin, P.; Huang, T. The catalyst derived from the sulfurized Co-doped metal-organic framework (MOF) for peroxymonosulfate (PMS) activation and its application to pollutant removal. Sep. Purif. Technol. 2021, 285, 120362. [Google Scholar] [CrossRef]
- Oh, W.D.; Dong, Z.L.; Lim, T.T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Cai, H.; Zou, J.; Lin, J.; Li, J.; Huang, Y.; Zhang, S.; Yuan, B.; Ma, J. Sodium hydroxide-enhanced acetaminophen elimination in heat/peroxymonosulfate system: Production of singlet oxygen and hydroxyl radical. Chem. Eng. J. 2022, 429, 132438. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Z.; Kang, J.; Zhao, S.; Chu, W. UV/peroxymonosulfate process for degradation of chloral hydrate: Pathway and the role of radicals. J. Hazard. Mater. 2021, 401, 123837. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Wang, S.Z. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Kohantorabi, G. A review of the innovations in metal- and carbon-based catalysts explored for heterogeneous peroxymonosulfate (PMS) activation, with focus on radical vs. non-radical degradation pathways of organic contaminants. Chem. Eng. J. 2021, 411, 127957. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Zhao, C.; Shao, B.; Yan, M.; Liu, Z.; Tang, L. Activation of peroxymonosulfate by biochar-based catalysts and applications in the degradation of organic contaminants: A review. Chem. Eng. J. 2021, 416, 128829. [Google Scholar] [CrossRef]
- Wang, W.X.; Liu, Y.; Yue, Y.F.; Wang, H.H.; Cheng, G.; Gao, C.Y.; Chen, C.L.; Ai, Y.J.; Chen, Z.; Wang, X.K. The confined interlayer growth of ultrathin two-dimensional Fe3O4 nanosheets with enriched oxygen vacancies for peroxymonosulfate activation. ACS Catal. 2021, 11, 11256–11265. [Google Scholar] [CrossRef]
- He, D.; Li, Y.; Lyu, C.; Song, L.; Feng, W.; Zhang, S. New insights into MnOOH/peroxymonosulfate system for catalytic oxidation of 2,4-dichlorophenol: Morphology dependence and mechanisms. Chemosphere 2020, 255, 126961. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Huang, Y.H. Behavioral evidence of the dominant radicals and intermediates involved in bisphenol a degradation using an efficient Co2+/PMS oxidation process. J. Hazard. Mater. 2009, 167, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, J.; Qiao, X.; Wang, D.; Cai, X. Performance of nano-Co3O4/peroxymonosulfate system: Kinetics and mechanism study using acid orange 7 as a model compound. Appl. Catal. B Environ. 2008, 80, 116–121. [Google Scholar] [CrossRef]
- Yang, X.; Wei, G.L.; Wu, P.Q.; Liu, P.; Liang, X.L.; Chu, W. Controlling oxygen vacancies of CoMn2O4 by loading on planar and tubular clay minerals and its application for boosted PMS activation. J. Hazard. Mater. 2022, 436, 129060. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhang, G.; Liu, M.; Wang, Q.; Wang, P. Optimization of the catalytic activity of a ZnCo2O4 catalyst in peroxymonosulfate activation for bisphenol A removal using response surface methodology. Chemosphere 2018, 212, 152–161. [Google Scholar] [CrossRef]
- Huang, J.Z.; Zhang, H.C. Mn-based catalysts for sulfate radical-based advanced oxidation processes: A review. Environ. Int. 2019, 133, 105141. [Google Scholar] [CrossRef]
- Saputra, E.; Muhammad, S.; Sun, H.Q.; Ang, H.M.; Tadé, M.O.; Wang, S.B. Manganese oxides at different oxidation states for heterogeneous activation of peroxymonosulfate for phenol degradation in aqueous solutions. Appl. Catal. B Environ. 2013, 142–143, 729–735. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Wang, H.B.; Liu, Y.; Jawad, A.; Ifthikar, J.; Liao, Z.W.; Wang, T.; Chen, Z.Q. Highly efficient α-Mn2O3@α-MnO2-500 nanocomposite for peroxymonosulfate activation: Comprehensive investigation of manganese oxides. J. Mater. Chem. A. 2018, 6, 1590–1600. [Google Scholar] [CrossRef]
- Zhou, X.Q.; Luo, C.G.; Luo, M.G.; Wang, Q.L.; Wang, J.; Liao, Z.W.; Chen, Z.L.; Chen, Z.Q. Understanding the synergetic effect from foreign metals in bimetallic oxides for PMS activation: A common strategy to increase the stoichiometric efficiency of oxidants. Chem. Eng. J. 2020, 381, 122587. [Google Scholar] [CrossRef]
- Deng, J.; Xu, M.Y.; Qiu, C.G.; Chen, Y.; Ma, X.Y.; Gao, N.Y.; Li, X.Y. Magnetic MnFe2O4 activated peroxymonosulfate processes for degradation of bisphenol A: Performance, mechanism and application feasibility. Appl. Surf. Sci. 2018, 459, 138–147. [Google Scholar] [CrossRef]
- Ke, M.K.; Huang, G.X.; Mei, S.C.; Wang, Z.H.; Zhang, Y.J.; Hua, T.W.; Zheng, L.R.; Yu, H.Q. Interface-promoted direct oxidation of p-Arsanilic acid and removal of total arsenic by the coupling of peroxymonosulfate and Mn-Fe-Mixed oxide. Environ. Sci. Technol. 2021, 55, 7063–7071. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Tian, X.K.; Nie, Y.L.; Yang, C.; Zhou, Z.X.; Li, Y. Enhanced 2, 4-dichlorophenol degradation at pH 3-11 by peroxymonosulfate via controlling the reactive oxygen species over Ce substituted 3D Mn2O3. Chem. Eng. J. 2019, 355, 448–456. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Gao, Q.; Han, B.; Xia, K.; Zhou, C. Hierarchical flower-like Co2TiO4 nanosheets with unique structural and compositional advantages to boost peroxymonosulfate activation for degradation of organic pollutants. J. Mater. Chem. A. 2020, 8, 20953–20962. [Google Scholar] [CrossRef]
- Zhang, H.; An, Q.; Su, Y.; Quan, X.; Chen, S. Co3O4 with upshifted d-band center and enlarged specific surface area by single-atom Zr doping for enhanced PMS activation. J. Hazard. Mater. 2023, 448, 130987. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, W.; Croué, J.P. Catalytic ozonation of oxalate with a cerium supported palladium oxide: An efficient degradation not relying on hydroxyl radical oxidation. Environ. Sci. Technol. 2011, 45, 9339–9346. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.X.; Wang, C.Y.; Yang, C.W.; Guo, P.C.; Yu, H.Q. Degradation of bisphenol a by peroxymonosulfate catalytically activated with Mn1.8Fe1.2O4 nanospheres: Synergism between Mn and Fe. Environ. Sci. Technol. 2017, 51, 12611–12618. [Google Scholar] [CrossRef]
- Wu, M.H.; Shi, J.; Deng, H.P. Metal doped manganese oxide octahedral molecular sieve catalysts for degradation of diclofenac in the presence of peroxymonosulfate. Arab. J. Chem. 2018, 11, 924–934. [Google Scholar] [CrossRef]
- Wang, J.; Ma, L.; Pan, Z.W.; Li, T.Y. Catalytic ozonation of phenol by magnetic Mn0.7Ce0.3Ox/CNT@Fe3C. Mater. Res. Express. 2022, 9, 126101. [Google Scholar] [CrossRef]
- Rastogi, A.S.; Al-Abed, R.; Dionysiou, D.D. Sulfate radical-based ferrous-peroxymonosulfate oxidative system for PCBs degradation in aqueous and sediment systems. Appl. Catal. B Environ. 2009, 85, 171–179. [Google Scholar] [CrossRef]
- Liu, B.M.; Song, W.B.; Wu, H.X.; Liu, Z.Y.; Teng, Y.; Sun, Y.J.; Xu, Y.H.; Zheng, H.L. Degradation of norfloxacin with peroxymonosulfate activated by nanoconfinement Co3O4@CNT nanocomposite. Chem. Eng. J. 2020, 398, 125498. [Google Scholar] [CrossRef]
- Gong, C.; Chen, F.; Yang, Q.; Luo, K.; Yao, F.B.; Wang, S.N.; Wang, X.L.; Wu, J.W.; Li, X.M.; Wang, D.B.; et al. Heterogeneous activation of peroxymonosulfate by Fe-Co layered doubled hydroxide for efficient catalytic degradation of Rhoadmine B. Chem. Eng. J. 2017, 321, 222–232. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, G.Z.; Xu, Y.H.; Yu, P.; Sun, Y.J. Nitrogen doped cobalt anchored on the used resin-based catalyst to activate peroxymonosulfate for the removal of ibuprofen. Water 2022, 14, 3754. [Google Scholar] [CrossRef]
- Guo, Z.Y.; Li, C.X.; Gao, M.; Han, X.; Zhang, Y.J.; Zhang, W.J.; Li, W.W. Mn-O covalency governs the intrinsic activity of Co-Mn spinel oxides for boosted peroxymonosulfate activation. Angewandte 2021, 60, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Alhamd, M.; Tabatabaie, T.; Parseh, I.; Amiri, F.; Mengelizadeh, N. Magnetic CuNiFe2O4 nanoparticles loaded on multi-walled carbon nanotubes as a novel catalyst for peroxymonosulfate activation and degradation of reactive black 5. Environ. Sci. Pollut. Res. 2021, 28, 57099–57114. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.P.; Soares, O.S.G.P.; Bakker, J.J.W.; Pereira, M.F.R.; Órfão, J.J.M.; Gascon, J.; Kapteijn, F.; Figueiredo, J.L. Structural and chemical disorder of cryptomelane promoted by alkali doping: Influence on catalytic properties. J. Catal. 2012, 293, 165–174. [Google Scholar] [CrossRef]











| Materials | SSAs (m2 g−1) | Average Pore Diameter (nm) | Pore Volume (cm3 g−1) |
|---|---|---|---|
| pristine CNT | 127.7 | 11.2 | 0.36 |
| MCC | 177.1 | 7.5 | 0.33 |
| MnCeO | 158.0 | 6.6 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wang, Q.; Gao, P.; Sun, D.; Jin, L.; Ma, L.; Yang, L.; Zhao, J. Enhanced PMS Activation by Highly Dispersed Mn-Ce Bimetallic Oxide on Carbon Nanotubes for Degradation of Phenol. Water 2023, 15, 2243. https://doi.org/10.3390/w15122243
Wang J, Wang Q, Gao P, Sun D, Jin L, Ma L, Yang L, Zhao J. Enhanced PMS Activation by Highly Dispersed Mn-Ce Bimetallic Oxide on Carbon Nanotubes for Degradation of Phenol. Water. 2023; 15(12):2243. https://doi.org/10.3390/w15122243
Chicago/Turabian StyleWang, Jing, Quanfeng Wang, Pei Gao, Da Sun, Libo Jin, Li Ma, Lan Yang, and Jujiao Zhao. 2023. "Enhanced PMS Activation by Highly Dispersed Mn-Ce Bimetallic Oxide on Carbon Nanotubes for Degradation of Phenol" Water 15, no. 12: 2243. https://doi.org/10.3390/w15122243







