Two New Species, Mallomonas baicalensis sp. nov. and M. grachevii sp. nov. (Synurales Chrysophyceae), Found under the Ice of Lake Baikal
Abstract
:1. Introduction
2. Materials and Methods
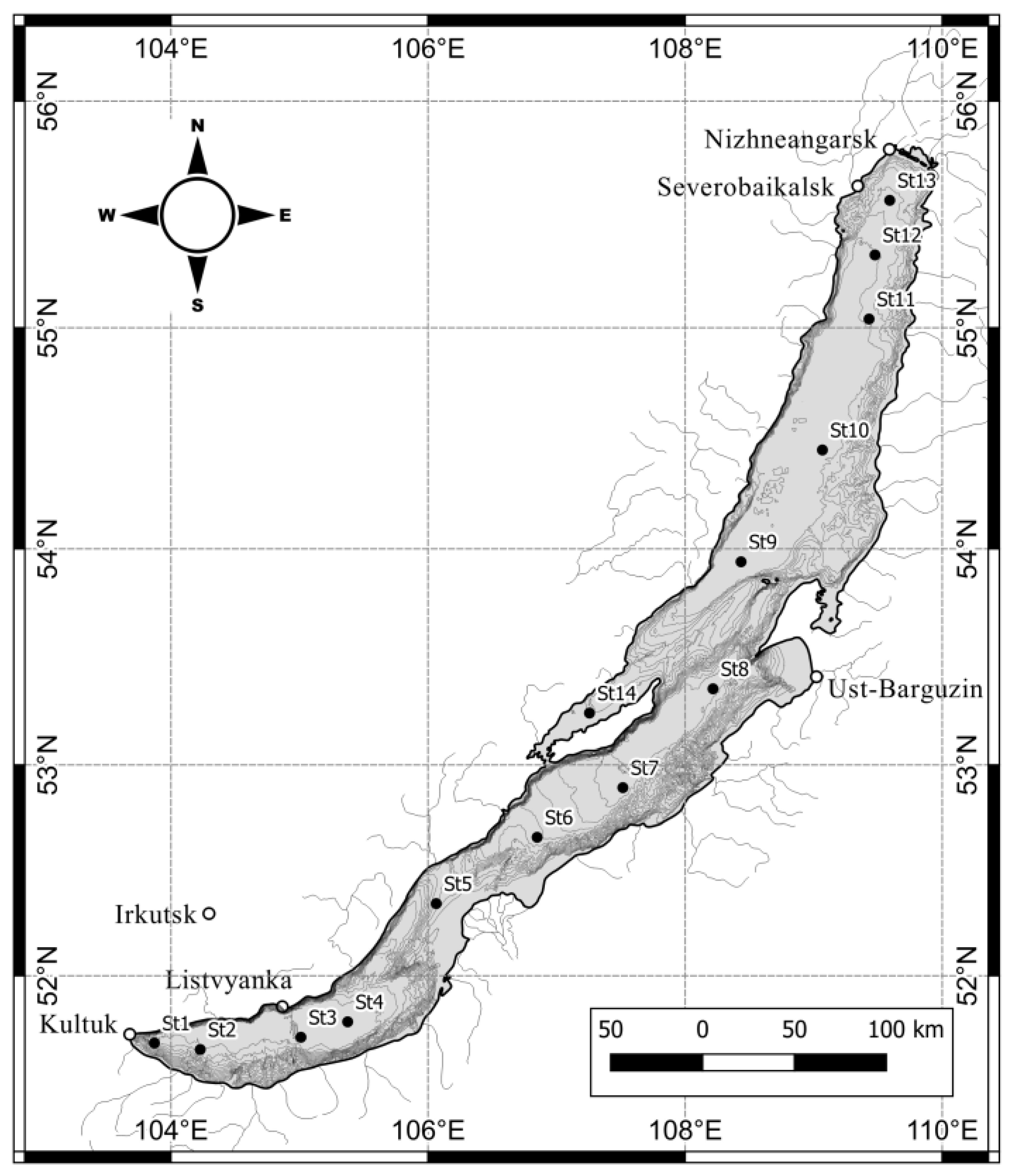
2.1. Study Site
2.2. Water Parameters
2.3. Scale Chrysophyte Sampling and Investigation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baikal., A.; Galazy, G.I. (Eds.) Federal Agency for Geodesy and Cartography: Moscow, Russia, 1993; 160p.
- Shimaraev, M.N. Hydrometeorological factors and oscillations of Baikal plankton abundance. In Limnology of Pre-Delta Areas of Lake Baikal; Nauka: Leningrad, Russia, 1971; pp. 259–267. [Google Scholar]
- Granin, N.G.; Jewson, D.H.; Gnatovsky, R.Y.; Levin, L.A.; Zhdanov, A.A.; Gorbunova, L.A.; Tsekhanovsky, V.V.; Doroschenko, L.M.; Mogilev, N.Y. Turbulent mixing under ice and the grow the of diatoms in Lake Baikal. Verh. Internat. Verein. Limnol. 2000, 27, 2812–2814. [Google Scholar]
- Jewson, D.H.; Granin, N.G.; Zhdanov, A.A.; Gnatovsky, R.Y. Effect of snow depth on under-ice irradiantce and growth of Aulacoseira baicalensis in Lake Baikal. Aquat. Ecol. 2009, 43, 673–679. [Google Scholar] [CrossRef]
- Grachev, M.A.; Domysheva, V.M.; Khodzher, T.V.; Korovyakova, N.V.; Golobokova, L.P.; Vereshagina, A.L.; Granin, N.G.; Gnatovsky, R.Y.; Kostornova, T.Y. Deep water of Lake Baikal as a standard of fresh water. Chem. Sust. Develop. 2004, 12, 417–429. [Google Scholar]
- Khodzher, T.V.; Domysheva, V.M.; Sorokovikova, L.M.; Sakirko, M.V.; Tomberg, I.V. Current chemical composition of LakeBaikal water. Inland Waters 2017, 7, 250–258. [Google Scholar] [CrossRef]
- Domysheva, V.M.; Sorokovikova, L.M.; Sinyukovich, V.N.; Onishchuk, N.A.; Sakirko, M.V.; Tomberg, I.V.; Zhuchenko, N.A.; Golobokova, L.P.; Khodzher, T.V. Ionic composition of water in Lake Baikal, its tributaries, and the Angara river source during the modern period. Russ. Meteorol. Hydrol. 2019, 44, 687–694. [Google Scholar] [CrossRef]
- Grachev, M.A. On Recent State of Ecological System of Lake Baikal; SB RAS: Novosibirsk, Russia, 2002; 153p. [Google Scholar]
- Granin, N.G.; Dzhuson, D.; Gnatovsky, R.Y.; Levin, L.A.; Zhdanov, A.A.; Averin, A.I.; Gorbunova, L.A.; Tsekhanovskiy, V.V.; Doroshchenko, L.F.; Min’ko, N.P.; et al. Turbulent mixing of Lake Baikal waters in the layer immediately adjacent to the ice and its role in the development of diatoms. Rep. Russ. Acad. Sci. 1999, 366, 835–839. [Google Scholar]
- Domysheva, V.M.; Usoltseva, M.V.; Sakirko, M.V.; Pestunov, D.A.; Shimaraev, M.N.; Popovskaya, G.I.; Panchenko, M.V. Spatial distribution of carbon dioxide fluxes, biogenic elements, and phytoplankton biomass in the pelagic zone of Lake Baikal in spring 2010–2012. Atmos. Ocean. Opt. 2014, 27, 539–545. [Google Scholar] [CrossRef]
- Votinsev, K.K. Hydrochemistry of Lake Baikal; Izdatelstvo Akademii Nauk SSSR: Moscow, Russia, 1961; 312p. [Google Scholar]
- Bashenkhaeva, M.V.; Zakharova, Y.R.; Petrova, D.P.; Khanaev, I.V.; Galachyants, Y.P.; Likhoshway, Y.V. Sub-ice microalgal and bacterial communities in fresh water Lake Baikal. Russia Microb. Ecol. 2015, 70, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Bashenkhaeva, M.V.; Galachyants, Y.P.; Khanaev, I.V.; Sakirko, M.V.; Petrova, D.P.; Likhoshway, Y.V.; Zakharova, Y.R. Comparative analysis of free-living and particle-associated bacterial communities of Lake Baikal during the ice-covered period. J. Great Lakes Res. 2020, 46, 508–518. [Google Scholar] [CrossRef]
- Bessudova, A.; Domysheva, V.M.; Firsova, A.D.; Likhoshway, Y.V. Silica-scaled chrysophytes of Lake Baikal. Acta Biol. Sib. 2017, 3, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Bessudova, A.; Firsova, A.D.; Tomberg, I.V.; Bayramova, E.; Hilkhanova, D.; Bedoshvili, E.L.; Bashenkhaeva, M.V.; Kopyrina, L.I.; Zakharova, Y.R.; Likhoshway, Y.V. Two new species of silica-scaled chrysophytes Mallomonas kicherica and M. sibirica inwater bodies of Eastern Siberia, Russia. Phytotaxa 2023, in press. [Google Scholar]
- Firsova, A.D.; Kuzmina, A.E.; Tomberg, I.V.; Potemkina, T.G.; Likhoshway, Y.V. Sezonnaya dinamica formirovaniya stomatocyst chrysophytovych vodorosley v planktone Yuzhnogo Baikala. Izv. RAN Seriya Biol. 2008, 5, 589–596. [Google Scholar]
- Firsova, A.D.; Bessudova, A.; Likhoshway, Y.V. Chrysophycean stomatosysts in tributaries of northern limit of Lake Baikal. Acta Biol. Sib. 2018, 4, 25–44. [Google Scholar] [CrossRef] [Green Version]
- Bondarenko, N.A.; Belykh, O.I.; Golobokova, L.P.; Artemyeva, O.V.; Logacheva, N.F.; Tikhonova, I.V.; Lipko, I.A.; Kostornova, T.Y.; Parfenova, V.V.; Khodzher, T.V.; et al. Stratified distribution of nutrients and extremophile biota with in fresh water icecovering the surface of Lake Baikal. J. Microbiol. 2012, 50, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, I.S.; Galachyants, Y.P.; Bukin, Y.S.; Petrova, D.P.; Bashenkhaeva, M.V.; Sakirko, M.V.; Blinov, V.V.; Titova, L.A.; Zakharova, Y.R.; Likhoshway, Y.V. Seasonal succession and coherence among bacteria and microeukaryotes in Lake Baikal. Microb. Ecol. 2022, 84, 404–422. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase; World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 2023; Available online: https://www.algaebase.org (accessed on 10 March 2023).
- Asmund, B.; Kristiansen, J. The Genus Mallomonas (Chrysophyceae). A taxonomic survey based on the ultrastructure of silica scales and bristles. Opera Bot. 1986, 85, 1–128. [Google Scholar]
- Kristiansen, J.; Preisig, H.R. Chrysophyta and Haptophyta algae, 2nd part: Synurophyceae. In Süsswasser Flora von Mitteleuropa (Freshwater Flora of Central Europe); Springer: Berlin, Germany, 2007; pp. 1–252. [Google Scholar]
- Voloshko, L.N. New taxa of the genus Mallomonas (Chrysophyta, Synurophyceae) from lakes of the Polar Ural. Russ. Bot. J. 2009, 94, 1068–1076. [Google Scholar]
- Nemcová, Y.; Rott, E. Diversity of silica-scaled chrysophytes inhigh-altitude alpinesites (North Tyrol, Austria) including a description of Mallomonas pechlaneri sp. nov. Cryptogam. Algol. 2018, 39, 63–83. [Google Scholar] [CrossRef]
- Gusev, E.; Němcová, Y.; Kulikovskiy, M. Mallomonas voloshkoae sp. nov. (Synurales, Chrysophyceae) and distribution of M. pechlaneri in mountain lakes of Siberia. Phytotaxa 2022, 530, 221–229. [Google Scholar] [CrossRef]
- Gusev, E.; Martynenko, N. Silica-scaled chrysophytes of Teletskoye lake and Adjacent area with a description of a new species from the genus Mallomonas. Diversity 2022, 14, 1040. [Google Scholar] [CrossRef]
- ISO 7890-3: 1988; Water Duality—Determination of Nitrate-Part 3: Spectrometric Method Using Sulfosalicylic Acid. International standard ISO: Geneva, Switzerland, 2004.
- Wetzel, R.; Likens, G. Limnological Analysis, 3rd ed.; Springer: New York, NY, USA, 2000; pp. 57–112. [Google Scholar]
- ISO 6878: 2004; Water Quality—Determination of Phosphorus—Ammonium Molybdate Spectrometric Method. International standard ISO: Geneva, Switzerland, 2004.
- ISO 15705: 2002; Water Quality—Determination of the Chemical Oxygen Demand Index (ST-COD)—Small-Scale Sealed-Tube Method. International standard ISO: Geneva, Switzerland, 2002.
- Lavau, S.; Saunders, G.W.; Wetherbee, R. A phylogenetic analysis of the Synurophyceae using molecular data and scale case morphology. J. Phycol. 1997, 33, 135–151. [Google Scholar] [CrossRef]
- Siver, P.A.; Wolfe, A.P.; Rohlf, F.J.; Shin, W.; Jo, B.Y. Combining geometric morphometrics, molecular phylogeny, and micropaleontology to assess evolutionary patterns in Mallomonas (Synurophyceae: Heterokontophyta). Geobiology 2013, 11, 127–138. [Google Scholar] [CrossRef]
- Siver, P.A. Synurophyte algae. In Freshwater Algae of North America: Ecology and Classification., 2nd ed.; Academic Press: Boston, MA, USA, 2015; pp. 607–651. [Google Scholar]
- Němcová, Y.; Bulant, P.; Kristiansen, J. Mallomonas solea-ferrea and Mallomonas siveri (Chrysophyceae/Synu-ophyceae): Twonew taxa from the Western Cape (South Africa). Nova Hedwig. 2011, 93, 375–384. [Google Scholar] [CrossRef]
- Nicholls, K.N. Form variation in Mallomonas asmundiue and a description of Mallomonas sphagniphila sp. nov. (series Comconticae, Mallomonadaceae). Can. J. Bot. 1987, 65, 627–634. [Google Scholar] [CrossRef]
- Harris, K.; Bradley, D.E. A taxonomic study of Mallomonas. J. Gen. Microbiol. 1960, 22, 750–777. [Google Scholar] [CrossRef] [Green Version]
- Jeong, M.; Kim, J.I.; Jo, B.Y.; Kim, H.S.; Siver, P.A.; Shin, W. Surviving the marine environment: Two new species of Mallomonas (Synurophyceae). Phycologia 2019, 58, 276–286. [Google Scholar] [CrossRef]
- Gusev, E.; Kulikovskiy, M. Two new species of genus Mallomonas from swamp localities in Vietnam. Phytotaxa 2020, 468, 121–129. [Google Scholar] [CrossRef]
- Obolkina, L.A.; Bondarenko, N.A.; Doroshenko, L.F.; Molozhavaya, O.A. Finding of a cryophilic community in Lake Baikal. DAN 2000, 371, 815–817. [Google Scholar]
- Popovskaya, G.I. Ecological monitoring of phytoplankton in Lake Baikal. Aquat. Ecosyst. Health. Manag. 2000, 3, 215–225. [Google Scholar] [CrossRef]
- Bordonsky, G.S.; Bondarenko, N.A.; Obolkina, L.A.; Timoshkin, O.A. Ice communities of Baikal. Priroda 2003, 7, 22–24. [Google Scholar]
- Bashenkhaeva, M.V.; Zakharova, Y.R.; Galachyants, Y.P.; Khanaev, I.V.; Likhoshway, Y.V. Bacterial communities during theperiod of massive under-ice dinoflagellate development in Lake Baikal. Microbiology 2017, 86, 524–532. [Google Scholar] [CrossRef]
- Bondarenko, N.A.; Timoshkin, O.A.; Röpstorf, P.; Melnik, N.G. Theunder-ice and bottom periods in the life of Aulacoseira baicalensis (K. Meyer) Simonsen, a principal Lake Baikal alga. Hydrobiol. J. 2006, 568, 107–109. [Google Scholar] [CrossRef]
- Skabitchevsky, A.P. On the biology of Melosira baicalensis (K. Meyer) Wisl. Russ. Hydrobiol. J. 1929, 8, 93–114. [Google Scholar]
- Yasnitsky, V.N. Results on observations of Baikal plankton in the region of biological station during 1926–1928. Proc. Biol. Geogr. 1930, 4, 191–234. [Google Scholar]
- Izmest’eva, L.R.; Moore, M.V.; Hampton, S.E. Seasonal dynamics of common phytoplankton in Lake Baikal. Proc. Russ. Acad. Sci. Sci. Cent. 2006, 8, 191–196. [Google Scholar]
- Bedoshvili, E.L.; Bondarenko, N.A.; Sakirko, M.V.; Khanaev, I.V.; Likhoshway, Y.V. Changes of colony length in the planktonic diatom Aulacoseira baicalensis at different stage sofits annual cyclein Lake Baikal. Hydrobiol. J. 2007, 43, 81–89. [Google Scholar] [CrossRef]
- Kozhova, O.M.; Izmest’eva, L.R. Lake Baikal: Evolution and Biodiversity; Backhuys Publish: Leiden, The Netherlands, 1998; 447p. [Google Scholar]
- Pomazkina, G.V.; Belykh, O.I.; Domysheva, V.M.; Sakirko, M.V.; Gnatovsky, R.Y. Structure and dynamics of phytoplankton of Southern Baikal (Russia). Intern. J. Algae 2010, 12, 64–79. [Google Scholar] [CrossRef]
- Annenkova, N.V. Phylogenetic relations of the dinoflagellate Gymnodinium baicalense from Lake Baikal. Cent. Eur. J. Biol. 2013, 8, 366–373. [Google Scholar] [CrossRef]





| St. No. | Coordinates N/E | Date of Sampling dd.mm.yy | Time of Sampling hh:mm | Ice Thickness, cm | Snow Cover Thickness, cm | T, °C | pH | EC25, μS·cm−1 | PAR, μmol·m−2·s−1 | Si, mg·L−1 | PO43−, mg·L−1 | NO3−, mg·L−1 | COD, mgO·L−1 | M. baicalensis | M. grachevii |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 51° 40.578′/ 103° 52.309′ | 17.03.22 | 14:25 | 63 | 12 | 0.044 | 7.80 | 141.1 | 59.808 | 0.47 | 0.028 | 0.41 | 6.6 | N.f. | + |
| 2. | 51° 38.710′/ 104° 13.715′ | 17.03.22 | 18:37 | 45 | 21 | 0.049 | 7.82 | 141.3 | 0.0 | 0.51 | 0.023 | 0.44 | 6.5 | N.f. | N.f. |
| 3. | 51° 42.262′/ 105° 00.720′ | 18.03.22 | 11:40 | 63 | 9 | 0.073 | 7.94 | 142.5 | 154.626 | 0.53 | 0.025 | 0.42 | 7.0 | N.f. | N.f. |
| 4. | 51° 46.731′/ 105° 22.528′ | 19.03.22 | 09:52 | 64 | 4 | 0.274 | 7.99 | 141.2 | 152.8 | 0.53 | 0.023 | 0.36 | 6.5 | + | N.f. |
| 5. | 52° 20.722′/ 106° 03.870′ | 19.03.22 | 14:33 | 65 | 7 | 0.651 | 8.08 | 141.8 | 96.177 | 0.60 | 0.021 | 0.36 | 7.4 | +++ | +++ |
| 6. | 52° 39.590′/ 106° 50.978′ | 19.03.22 | 18:03 | 85 | 4 | 0.243 | 7.93 | 141.5 | 0.0 | 0.60 | 0.028 | 0.40 | 6.7 | + | N.f. |
| 7. | 52° 53.630′/ 107° 31.001′ | 20.03.22 | 11:34 | 67 | 3.5 | 0.187 | 7.71 | 140.9 | 98.174 | 0.55 | 0.023 | 0.36 | 5.3 | ++ | ++ |
| 8. | 53° 21.278′/ 108° 13.078′ | 20.03.22 | 16:54 | 85 | 7 | 0.109 | 7.65 | 143.3 | 7.476 | 0.54 | 0.024 | 0.38 | 5.7 | +++ | +++ |
| 9. | 53° 56.472′/ 108° 26.178′ | 23.03.22 | 9:20 | 88 | 5 | 0.147 | 7.95 | 140.8 | 364.988 | 0.45 | 0.020 | 0.31 | 4.2 | +++ | +++ |
| 10. | 54° 27.052′/ 109° 04.164′ | 21.03.22 | 11:05 | 84 | 11 | 0.107 | 7.80 | 141.0 | 117.214 | 0.53 | 0.024 | 0.42 | 5.4 | N.f. | + |
| 11. | 55° 02.388′/ 109° 25.939′ | 22.03.22 | 12:56 | 84 | 8 | 0.102 | 8.04 | 146.1 | 76.415 | 0.60 | 0.025 | 0.40 | 6.1 | N.f. | + |
| 12. | 55° 19.487′/ 109° 28.707′ | 21.03.22 | 15:30 | 86 | 8 | 0.068 | 7.92 | 143.0 | 33.629 | 0.55 | 0.026 | 0.39 | 6.2 | +++ | ++ |
| 13. | 55° 33.968′/ 109° 35.597′ | 22.03.22 | 09:34 | 83 | 10 | 0.110 | 7.94 | 153.2 | 47.839 | 0.62 | 0.022 | 0.38 | 4.1 | + | +++ |
| 14. | 53° 14.500′/ 107° 15.416′ | 23.03.22 | 15:30 | 78 | 5 | 0.325 | 8.04 | 141.8 | 36.031 | 0.50 | 0.031 | 0.29 | 9.4 | + | N.f. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bessudova, A.; Firsova, A.; Hilkhanova, D.; Makarov, M.; Sakirko, M.; Bashenkhaeva, M.; Khanaev, I.; Zakharova, Y.; Likhoshway, Y. Two New Species, Mallomonas baicalensis sp. nov. and M. grachevii sp. nov. (Synurales Chrysophyceae), Found under the Ice of Lake Baikal. Water 2023, 15, 2250. https://doi.org/10.3390/w15122250
Bessudova A, Firsova A, Hilkhanova D, Makarov M, Sakirko M, Bashenkhaeva M, Khanaev I, Zakharova Y, Likhoshway Y. Two New Species, Mallomonas baicalensis sp. nov. and M. grachevii sp. nov. (Synurales Chrysophyceae), Found under the Ice of Lake Baikal. Water. 2023; 15(12):2250. https://doi.org/10.3390/w15122250
Chicago/Turabian StyleBessudova, Anna, Alena Firsova, Diana Hilkhanova, Mikhail Makarov, Maria Sakirko, Maria Bashenkhaeva, Igor Khanaev, Yulia Zakharova, and Yelena Likhoshway. 2023. "Two New Species, Mallomonas baicalensis sp. nov. and M. grachevii sp. nov. (Synurales Chrysophyceae), Found under the Ice of Lake Baikal" Water 15, no. 12: 2250. https://doi.org/10.3390/w15122250





