Photocatalytic Degradation of Methylene Blue Dye from Wastewater by Using Doped Zinc Oxide Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
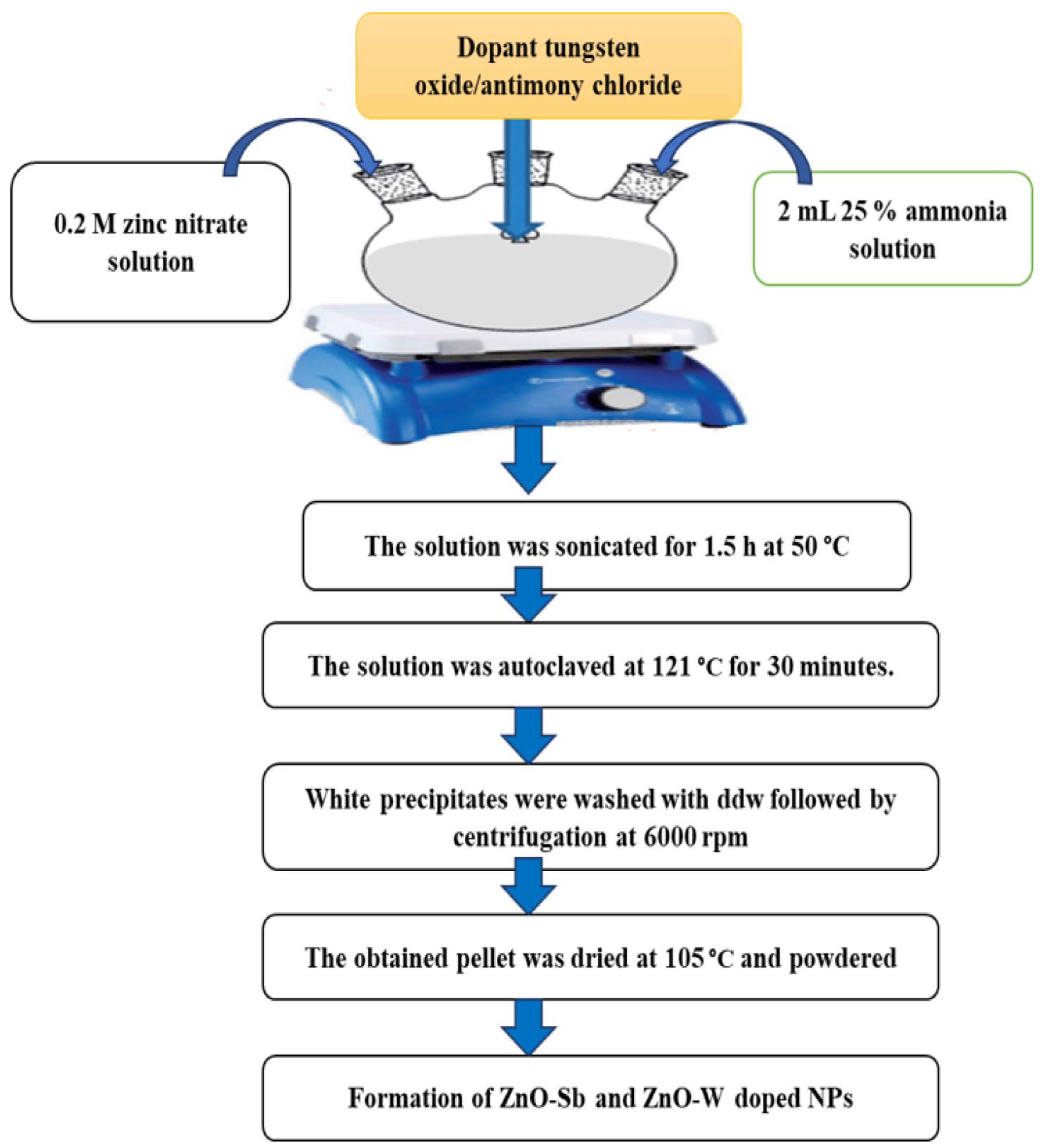
2.2. Synthesis of ZnO-NPs
2.3. Synthesis Procedure for Doped ZnO
2.4. Photocatalytic Degradation of MB Dye

3. Characterization of Pristine and Doped Zinc Oxide Nanoparticles
4. Results and Discussion
4.1. UV-Visible Spectroscopic Analysis
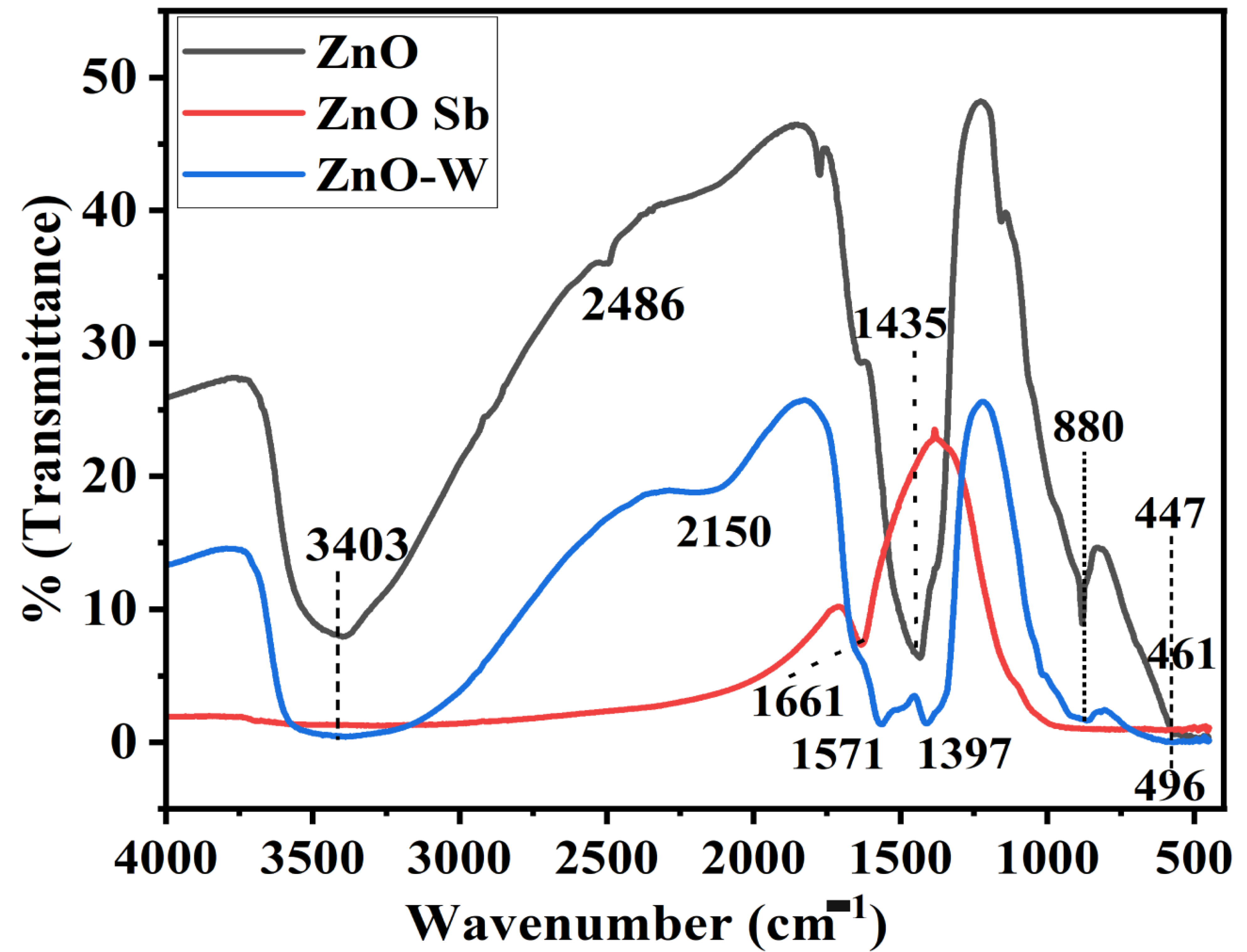
4.2. Identification of Functional Groups by FT-IR
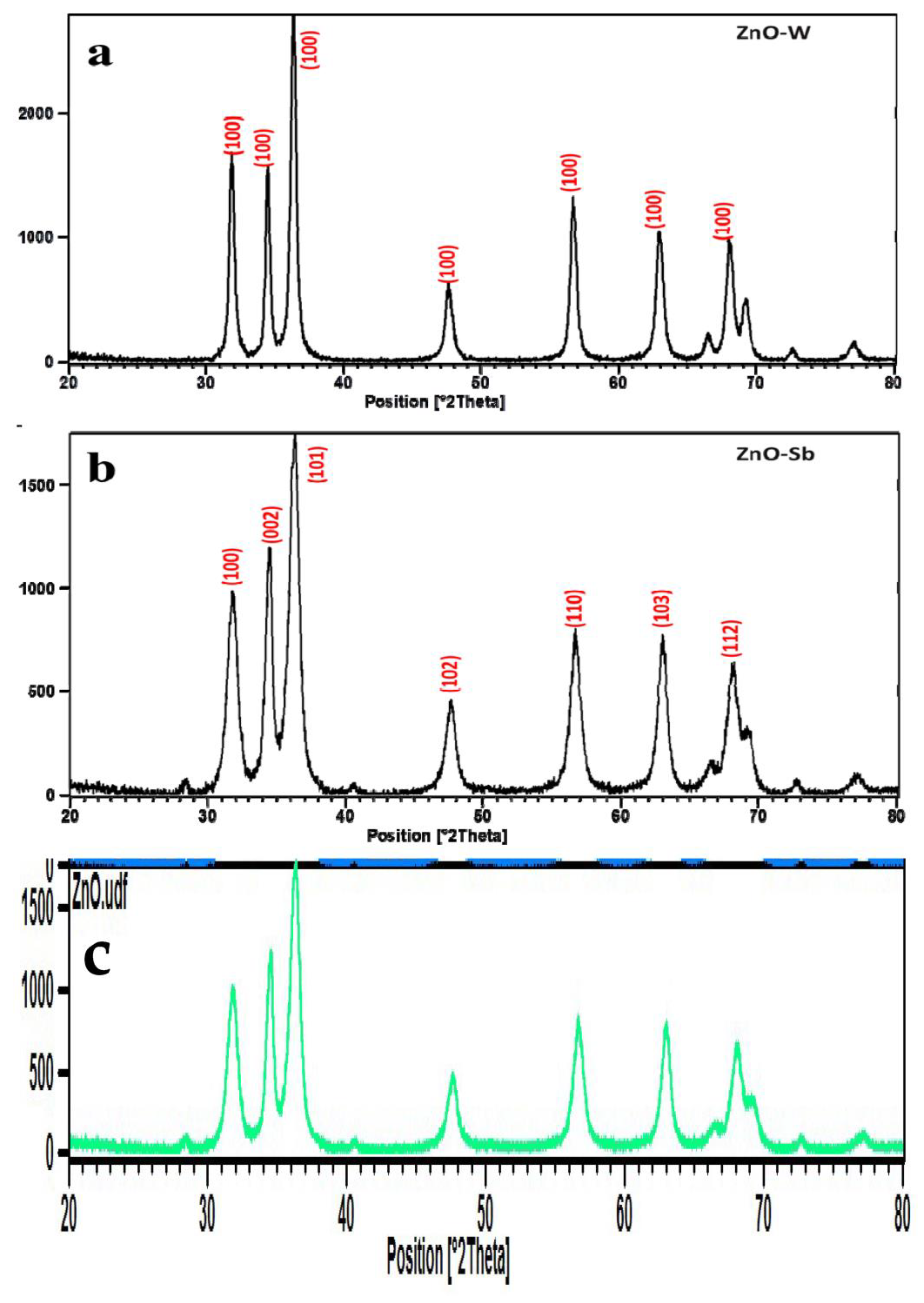
4.3. Phase Identification by X-ray Diffraction (XRD)
4.4. Morphological Analysis by Microscopic Techniques
4.4.1. Size Analysis of As-Synthesized and Doped ZnO-NPs by TEM
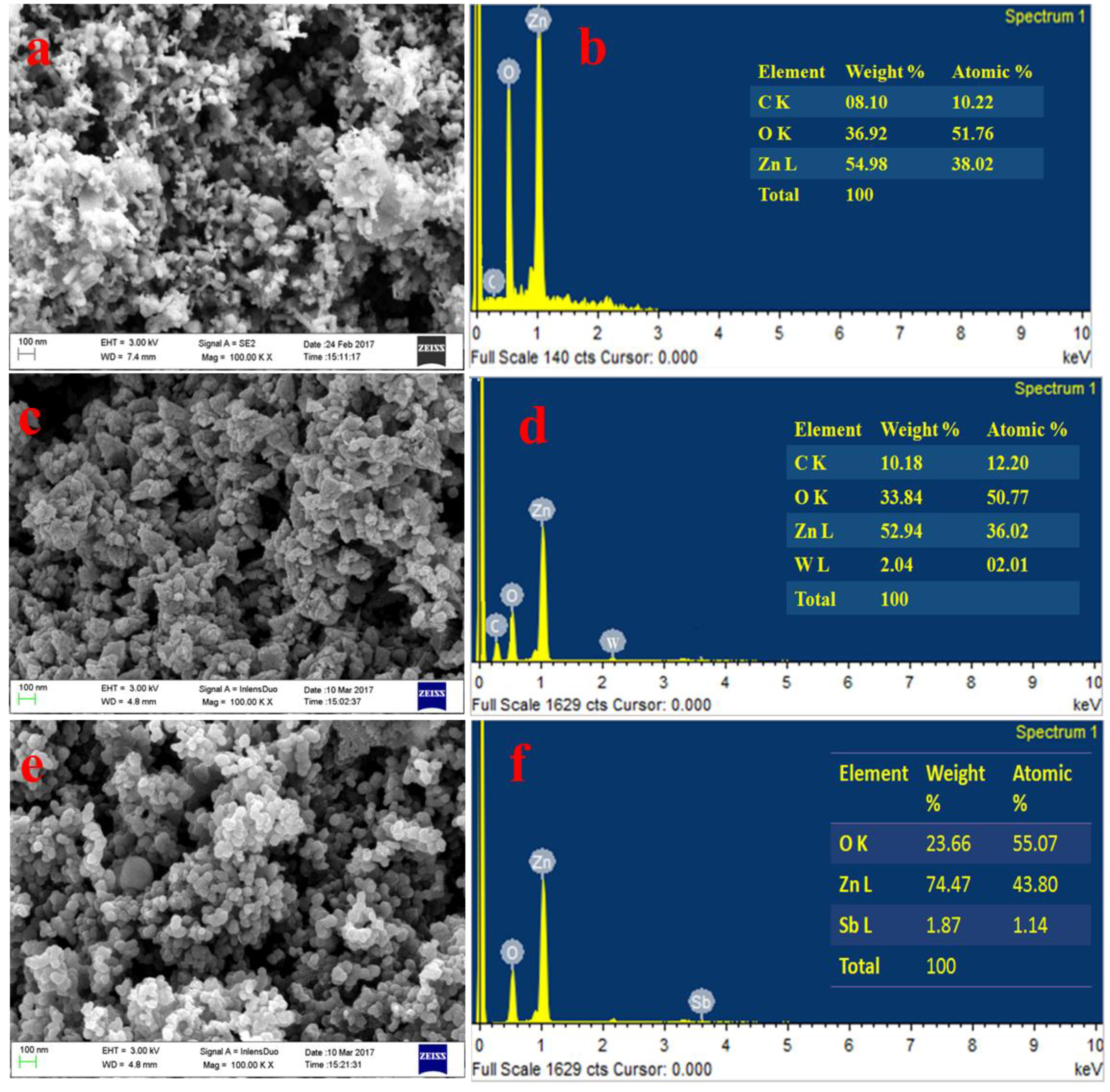
4.4.2. Morphological Analysis of As-Synthesized and Doped ZnO-NPs by FESEM
4.5. Band-Gap Study by UV-DRS
4.6. Surface Area Analysis by BET
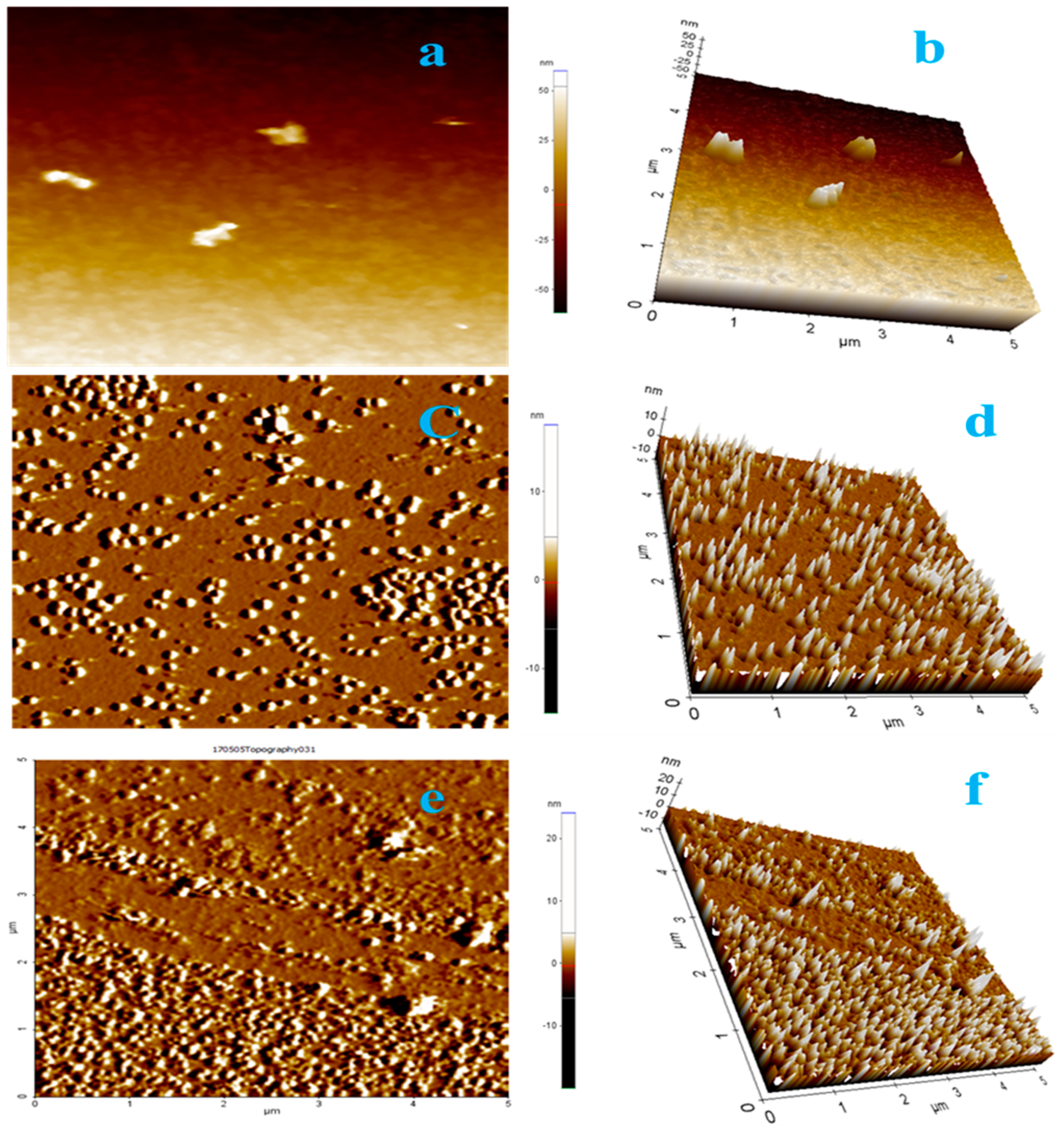
4.7. AFM for 3D Structure and Surface Topography
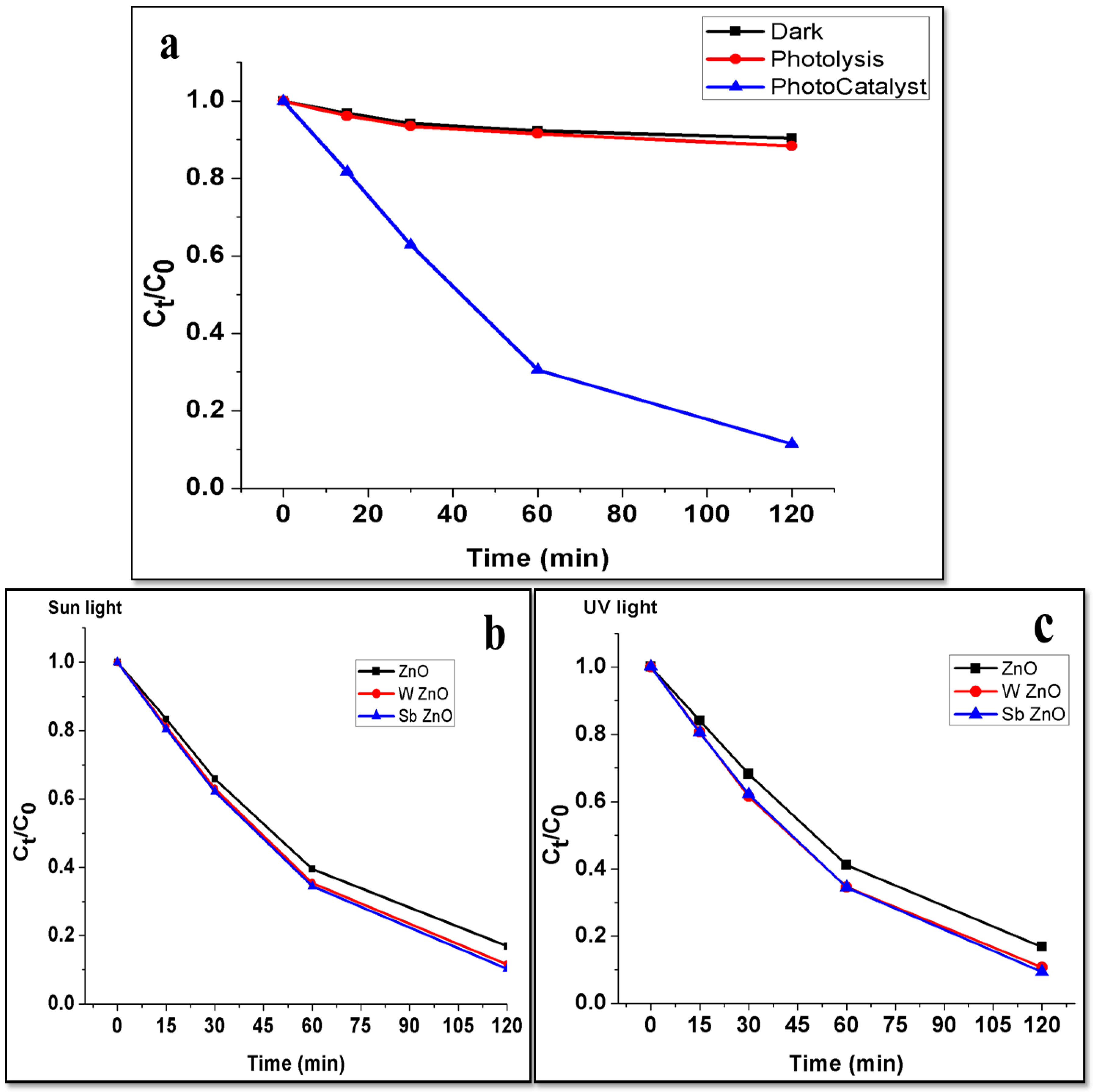
5. Degradation Study of MB Dye
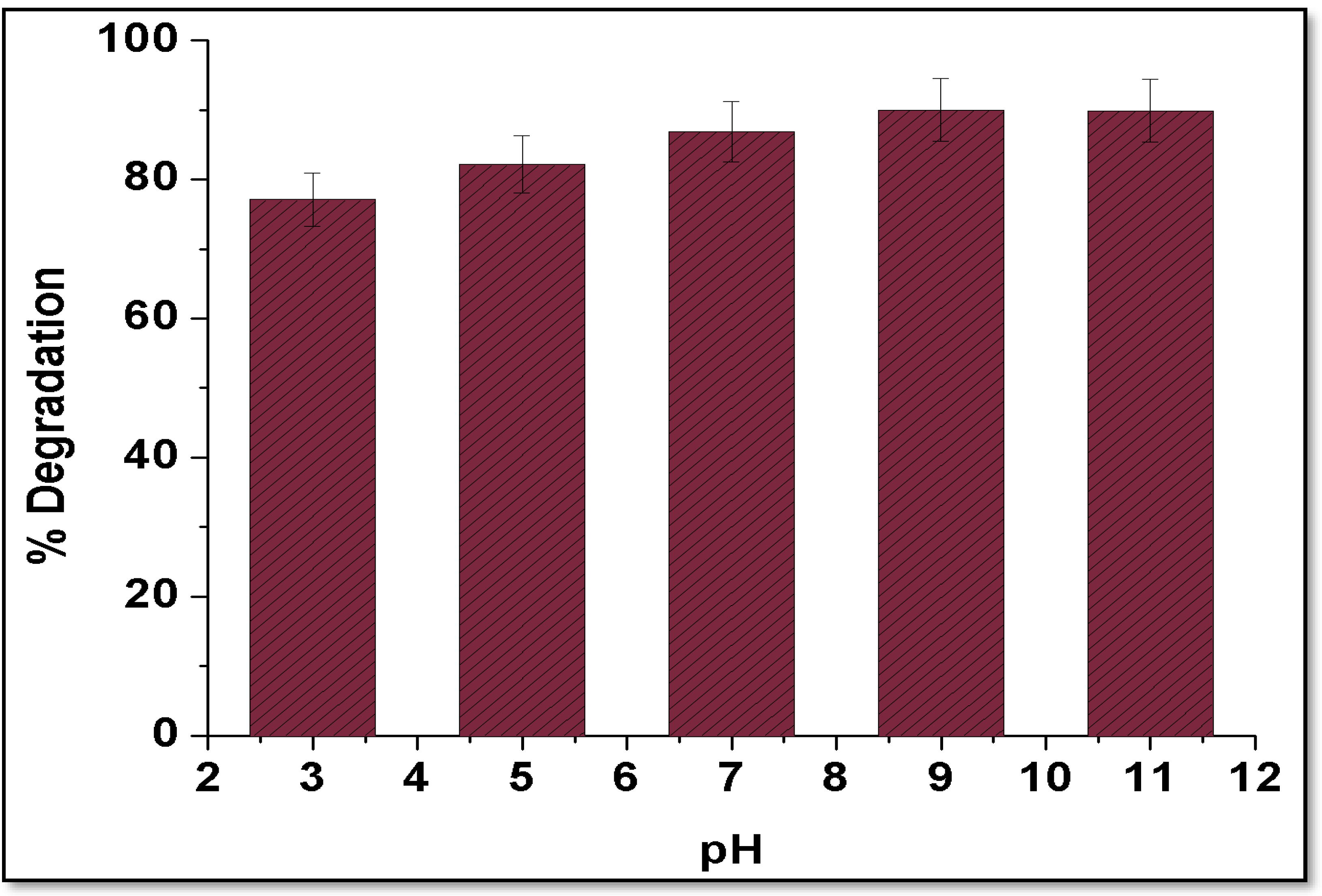
5.1. Effect of pH
5.2. Dosage Effect of Nano Photocatalyst
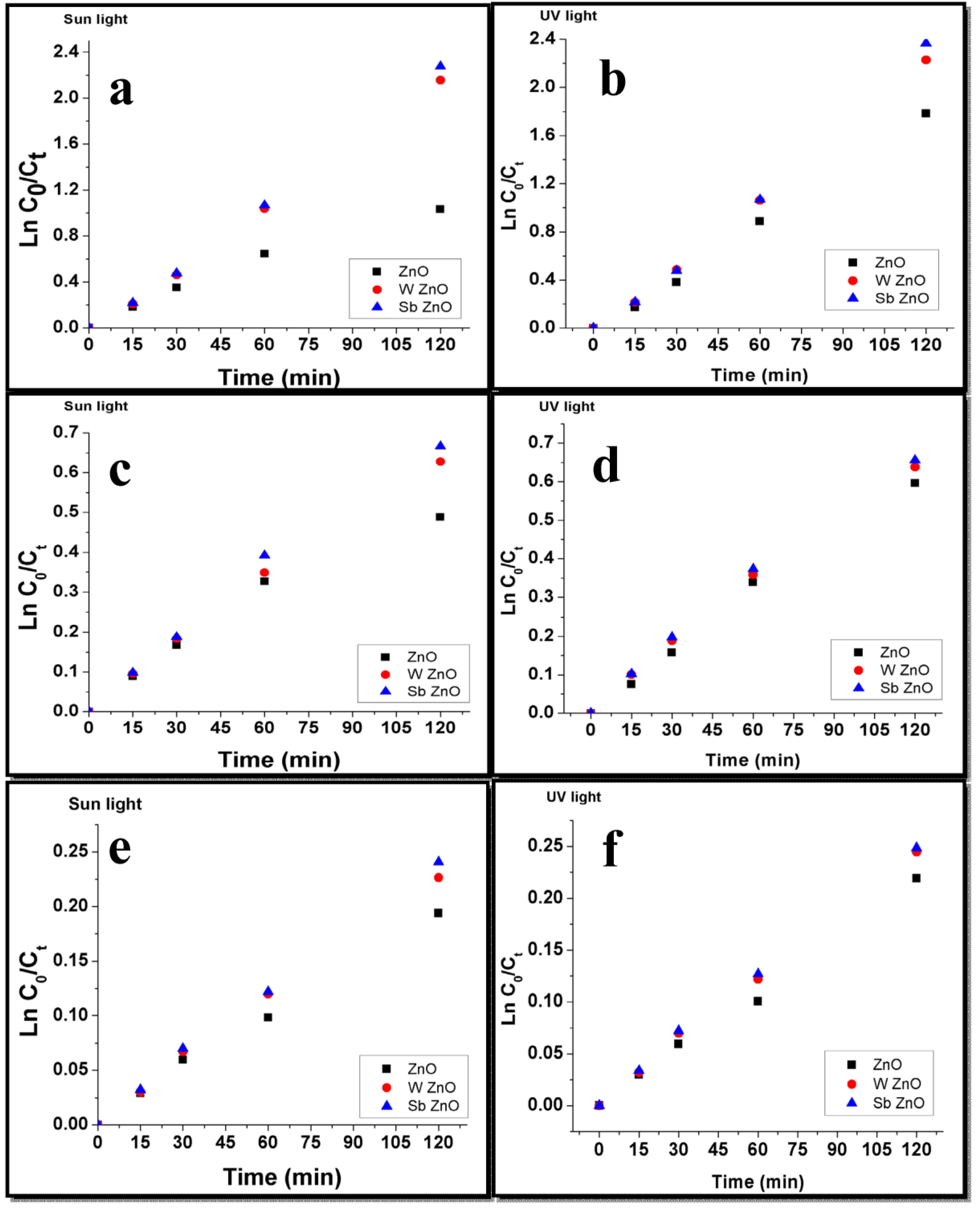
5.3. Dye Degradation Kinetics
5.4. Photocatalytic Degradation of Methylene Blue Dye
5.5. Degradation Mechanism of MB Dye
5.6. Reusability Study of NPC
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- González-González, R.B.; Parra-Saldívar, R.; Alsanie, W.F.; Iqbal, H.M.N. Nanohybrid catalysts with porous structures for environmental remediation through photocatalytic degradation of emerging pollutants. Environ. Res. 2022, 214, 113955. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, A.E.; Montero-Muñoz, M.; López, L.L.; Ramos-Ibarra, J.E.; Coaquira, J.A.H.; Heinrichs, B.; Paez, C.A. Significantly enhancement of sunlight photocatalytic performance of ZnO by doping with transition metal oxides. Sci. Rep. 2021, 11, 2804. [Google Scholar] [CrossRef] [PubMed]
- Fakhari, S.; Jamzad, M.; Kabiri Fard, H. Green synthesis of zinc oxide nanoparticles: A comparison. Green Chem. Lett. Rev. 2019, 12, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Nitrogen-doped titanium dioxide (N-doped TiO2) for visible light photocatalysis. New J. Chem. 2016, 40, 3000–3009. [Google Scholar] [CrossRef]
- Shenoy, R.U.K.; Rama, A.; Govindan, I.; Naha, A. The purview of doped nanoparticles: Insights into their biomedical applications. OpenNano 2022, 8, 100070. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, H.; Song, Q.; Ding, Z.; Mao, J.; Ren, Z.; Chen, G. Mobility enhancement in heavily doped semiconductors via electron cloaking. Nat. Commun. 2022, 13, 2482. [Google Scholar] [CrossRef] [PubMed]
- Rai, H.; Prashant Kondal, N. A review on defect related emissions in undoped ZnO nanostructures. Mater. Today Proc. 2022, 48, 1320–1324. [Google Scholar] [CrossRef]
- Gurylev, V.; Perng, T.P. Defect engineering of ZnO: Review on oxygen and zinc vacancies. J. Eur. Ceram. Soc. 2021, 41, 4977–4996. [Google Scholar] [CrossRef]
- Panžić, I.; Capan, I.; Brodar, T.; Bafti, A.; Mandić, V. Structural and electrical characterization of pure and al-doped zno nanorods. Materials 2021, 14, 7454. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, J.; Zhang, X.; Yuan, D.; Duan, G.; Li, Y. Robust and multifunctional natural polyphenolic composites for water remediation. Mater. Horiz. 2022, 9, 2496–2517. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, J.; Liu, H.; Huang, J. Design, modification and application of semiconductor photocatalysts. J. Taiwan Inst. Chem. Eng. 2018, 93, 590–602. [Google Scholar] [CrossRef]
- Yang, P.; Bai, W.; Zou, Y.; Zhang, X.; Yang, Y.; Duan, G.; Wu, J.; Xu, Y.; Li, Y. A melanin-inspired robust aerogel for multifunctional water remediation. Mater. Horiz. 2023, 10, 1020–1029. [Google Scholar] [CrossRef]
- Stefaniuk, I.; Cieniek, B.; Rogalska, I.; Virt, I.S.; Kosciak, A. Magnetic properties of ZnO:Co layers obtained by pulsed laser deposition method. Mater. Sci. Pol. 2018, 36, 439–444. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.B.; Khan, M.I.; Nisar, J. Microwave-Assisted Green Synthesis of Pure and Mn-Doped ZnO Nanocomposites: In Vitro Antibacterial Assay and Photodegradation of Methylene Blue. Front. Mater. 2022, 8, 710155. [Google Scholar] [CrossRef]
- Celik, O.; Baturay, Ş.; Ocak, Y.S. Sb doping influence on structural properties of ZnO thin films. Mater. Res. Express. 2020, 7, 3574–3577. [Google Scholar] [CrossRef]
- Sinornate, W.; Mimura, H.; Pecharapa, W. Structural, Morphological, Optical, and Electrical Properties of Sol–Gel Derived Sb-Doped ZnO Thin Films Annealed under Different Atmospheres. Phys. Status Solidi (A) 2021, 218, 2000233. [Google Scholar] [CrossRef]
- Khan, S.H.; R, S.; Pathak, B.; Fulekar, M.H. Development of zinc oxide nanoparticle by sonochemical method and study of their physical and optical properties. AIP Conf. Proc. 2016, 1724, 020108. [Google Scholar] [CrossRef]
- Jamshaid, M.; Nazir, M.A.; Najam, T.; Shah, S.S.A.; Khan, H.M.; Rehman, A.U. Facile synthesis of Yb3+-Zn2+ substituted M type hexaferrites: Structural, electric and photocatalytic properties under visible light for methylene blue removal. Chem. Phys. Lett. 2022, 805, 139939. [Google Scholar] [CrossRef]
- Shahzad, K.; Nazir, M.A.; Jamshaid, M.; Kumar, O.P.; Najam, T.; Shah, S.S.A.; Rehman, A.U. Synthesis of nanoadsorbent entailed mesoporous organosilica for decontamination of methylene blue and methyl orange from water. Int. J. Environ. Anal. Chem. 2021, 1–14. [Google Scholar] [CrossRef]
- Nazir, M.A.; Khan, N.A.; Cheng, C.; Shah, S.S.A.; Najam, T.; Arshad, M.; Sharif, A.; Akhtar, S.; Rehman, A.U. Surface induced growth of ZIF-67 at Co-layered double hydroxide: Removal of methylene blue and methyl orange from water. Appl. Clay Sci. 2020, 190, 105564. [Google Scholar] [CrossRef]
- Modi, S.; Yadav, V.K.; Gacem, A.; Ali, I.H.; Dave, D.; Khan, S.H.; Yadav, K.K.; Rather, S.-U.; Ahn, Y.; Son, C.T.; et al. Recent and Emerging Trends in Remediation of Methylene Blue Dye from Wastewater by Using Zinc Oxide Nanoparticles. Water 2022, 14, 1749. [Google Scholar] [CrossRef]
- Modi, S.; Yadav, V.K.; Amari, A.; Osman, H.; Igwegbe, C.A.; Fulekar, M.H. Nanobioremediation: A bacterial consortium-zinc oxide nanoparticle-based approach for the removal of methylene blue dye from wastewater. Environ. Sci. Pollut. Res. 2023, 30, 72641–72651. [Google Scholar] [CrossRef] [PubMed]
- Salih, A.M.; Al-Qurainy, F.; Khan, S.; Tarroum, M.; Nadeem, M.; Shaikhaldein, H.O.; Gaafar, A.-R.Z.; Alfarraj, N.S. Biosynthesis of zinc oxide nanoparticles using Phoenix dactylifera and their effect on biomass and phytochemical compounds in Juniperus procera. Sci. Rep. 2021, 11, 19136. [Google Scholar] [CrossRef] [PubMed]
- Naseer, M.; Aslam, U.; Khalid, B.; Chen, B. Green route to synthesize Zinc Oxide Nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential. Sci. Rep. 2020, 10, 9055. [Google Scholar] [CrossRef]
- Syahir, A.; Sulaiman, S.; Mel, M.; Othman, M.; Zubaidah Sulaiman, S. An Overview: Analysis of ultrasonic-assisted extraction’s parameters and its process. IOP Conf. Ser. Mater. Sci. Eng. 2020, 778. [Google Scholar] [CrossRef]
- Niazi, S.; Olsen, E.; Nygård, H.S. Hydrolysis of eutectic compositions in the ZnCl2, KCl:NaCl ternary system and effect of adding ZnO. J. Mol. Liq. 2020, 317, 114069. [Google Scholar] [CrossRef]
- Ozdemir, Z.; Hernandez-Nava, E.; Tyas, A.; Warren, J.A.; Fay, S.D.; Goodall, R.; Todd, I.; Askes, H. Energy absorption in lattice structures in dynamics: Experiments. Int. J. Impact Eng. 2016, 89, 49–61. [Google Scholar] [CrossRef] [Green Version]
- Bonetto, L.R.; Crespo, J.S.; Guégan, R.; Esteves, V.I.; Giovanela, M. Removal of methylene blue from aqueous solutions using a solid residue of the apple juice industry: Full factorial design, equilibrium, thermodynamics and kinetics aspects. J. Mol. Struct. 2021, 1224, 129296. [Google Scholar] [CrossRef]
- Yadav, V.K.; Choudhary, N.; Ali, D.; Kumar, G.; Gnanamoorthy, G.; Khan, A.U.; Kumar, P.; Kumar, S.H.; Tizazu, B.Z. Determination of Adsorption of Methylene Blue Dye by Incense Stick Ash Waste and Its Toxicity on RTG-2 Cells. Adsorpt. Sci. Technol. 2022, 2022, 8565151. [Google Scholar] [CrossRef]
- Gnanamoorthy, G.; Karthikeyan, V.; Ali, D.; Kumar, G.; Yadav, V.K.; Narayanan, V. Global popularization of CuNiO2 and their rGO nanocomposite loveabled to the photocatalytic properties of methylene blue. Environ. Res. 2022, 204, 112338. [Google Scholar] [CrossRef]
- Choudhary, N.; Yadav, V.K.; Yadav, K.K.; Almohana, A.I.; Almojil, S.F.; Gnanamoorthy, G.; Kim, D.-H.; Islam, S.; Kumar, P.; Jeon, B.-H. Application of green synthesized mmt/ag nanocomposite for removal of methylene blue from aqueous solution. Water 2021, 13, 3206. [Google Scholar] [CrossRef]
- Amari, A.; Yadav, V.K.; Pathan, S.K.; Singh, B.; Osman, H.; Choudhary, N.; Khedher, K.M.; Basnet, A. Remediation of Methyl Red Dye from Aqueous Solutions by Using Biosorbents Developed from Floral Waste. Adsorpt. Sci. Technol. 2023, 2023, 1532660. [Google Scholar] [CrossRef]
- Haque, M.J.; Bellah, M.M.; Hassan, M.R.; Rahman, S. Synthesis of ZnO nanoparticles by two different methods & comparison of their structural, antibacterial, photocatalytic and optical properties. Nano Express. 2020, 1, 010007. [Google Scholar] [CrossRef]
- Modi, S.; Fulekar, M.H. Synthesis and characterization of zinc oxide nanoparticles and zinc oxide/cellulose nanocrystals nanocomposite for photocatalytic degradation of Methylene blue dye under solar light irradiation. Nanotechnol. Environ. Eng. 2020, 5, 18. [Google Scholar] [CrossRef]
- Modi Shreya Fulekar, M.H. Green synthesis of zinc oxide nanoparticles using garlic skin extract and its characterization. J. Nanostructures 2020, 10, 20–27. [Google Scholar] [CrossRef]
- Modi, S.; Yadav, V.K.; Choudhary, N.; Alswieleh, A.M.; Sharma, A.K.; Bhardwaj, A.K.; Khan, S.H.; Yadav, K.K.; Cheon, J.K.; Jeon, B.H. Onion Peel Waste Mediated-Green Synthesis of Zinc Oxide Nanoparticles and Their Phytotoxicity on Mung Bean and Wheat Plant Growth. Materials 2022, 15, 2393. [Google Scholar] [CrossRef]
- Jayachandran, A.; T R, A.; Nair, A.S. Green synthesis and characterization of zinc oxide nanoparticles using Cayratia pedata leaf extract. Biochem. Biophys. Rep. 2021, 26, 100995. [Google Scholar] [CrossRef] [PubMed]
- Ghamsari, M.S.; Alamdari, S.; Han, W.; Park, H.H. Impact of nanostructured thin ZnO film in ultraviolet protection. Int. J. Nanomed. 2017, 12, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Sadak, M.S.; Bakry, B.A. Zinc-oxide and nano ZnO oxide effects on growth, some biochemical aspects, yield quantity, and quality of flax (Linum uitatissimum L.) in absence and presence of compost under sandy soil. Bull. Natl. Res. Cent. 2020, 44, 98. [Google Scholar] [CrossRef]
- Aldalbahi, A.; Alterary, S.; Almoghim, R.A.A.; Awad, M.A.; Aldosari, N.S.; Alghannam, S.F.; Alabdan, A.N.; Alharbi, S.; Alateeq, B.A.M.; Al Mohsen, A.A.; et al. Greener Synthesis of Zinc Oxide Nanoparticles: Characterization and Multifaceted Applications. Molecules 2020, 25, 4198. [Google Scholar] [CrossRef]
- Carofiglio, M.; Barui, S.; Cauda, V.; Laurenti, M. Doped zinc oxide nanoparticles: Synthesis, characterization and potential use in nanomedicine. Appl. Sci. 2020, 10, 5194. [Google Scholar] [CrossRef]
- Soltani, S.; Gacem, A.; Choudhary, N.; Yadav, V.K.; Alsaeedi, H.; Modi, S.; Patel, A.; Khan, S.H.; Cabral-Pinto, M.M.S.; Yadav, K.K.; et al. Scallion Peel Mediated Synthesis of Zinc Oxide Nanoparticles and Their Applications as Nano fertilizer and Photocatalyst for Removal of Organic Pollutants from Wastewater. Water 2023, 15, 1672. [Google Scholar] [CrossRef]
- Shamhari, N.M.; Wee, B.S.; Chin, S.F.; Kok, K.Y. Synthesis and characterization of zinc oxide nanoparticles with small particle size distribution. Acta Chim. Slov. 2018, 65, 578–585. [Google Scholar] [CrossRef]
- Alamdari, S.; Ghamsari, M.S.; Lee, C.; Han, W.; Park, H.-H.; Tafreshi, M.J.; Afarideh, H.; Ara, M.H.M. Preparation and characterization of zinc oxide nanoparticles using leaf extract of sambucus ebulus. Appl. Sci. 2020, 10, 3620. [Google Scholar] [CrossRef]
- Rajendran, N.K.; George, B.P.; Houreld, N.N.; Abrahamse, H. Synthesis of zinc oxide nanoparticles using rubus fairholmianus root extract and their activity against pathogenic bacteria. Molecules 2021, 26, 3029. [Google Scholar] [CrossRef]
- El-Belely, E.F.; Farag, M.M.S.; Said, H.A.; Amin, A.S.; Azab, E.; Gobouri, A.A.; Fouda, A. Green Synthesis of Zinc Oxide Nanoparticles (ZnO-NPs) Using Arthrospira platensis (Class: Cyanophyceae) and Evaluation of their Biomedical Activities. Nanomaterials 2021, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Thangapandiyan, S.; Monika, S. Green Synthesized Zinc Oxide Nanoparticles as Feed Additives to Improve Growth, Biochemical, and Hematological Parameters in Freshwater Fish Labeo rohita. Biol. Trace Elem. Res. 2020, 195, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, R.; Hossienzadeh, K.; Maleki, A.; Ghanbari, R.; Rezaee, R.; Safari, M.; Shahmoradi, B.; Daraei, H.; Jafari, A.; Yetilmezsoy, K. Effects of doping zinc oxide nanoparticles with transition metals (Ag, Cu, Mn) on photocatalytic degradation of Direct Blue 15 dye under UV and visible light irradiation. J. Environ. Health Sci. Eng. 2019, 17, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Adrees, M.; Khan, Z.S.; Hafeez, M.; Rizwan, M.; Hussain, K.; Asrar, M.; Alyemeni, M.N.; Wijaya, L.; Ali, S. Foliar exposure of zinc oxide nanoparticles improved the growth of wheat (Triticum aestivum L.) and decreased cadmium concentration in grains under simultaneous Cd and water deficient stress. Ecotoxicol. Environ. Saf. 2021, 208, 111627. [Google Scholar] [CrossRef]
- Degefa, A.; Bekele, B.; Jule, L.T.; Fikadu, B.; Ramaswamy, S.; Dwarampudi, L.P.; Nagaprasad, N.; Ramaswamy, K. Green Synthesis, Characterization of Zinc Oxide Nanoparticles, and Examination of Properties for Dye-Sensitive Solar Cells Using Various Vegetable Extracts. J. Nanomater. 2021, 2021, 3941923. [Google Scholar] [CrossRef]
- Mallikarjunaswamy, C.; Parameswara, P.; Pramila, S.; Nagaraju, G.; Deepakumari, H.N.; Lakshmi Ranganatha, V. Green and facile synthesis of zinc oxide nanoparticles for enhanced photocatalytic organic pollutant degradation. J. Mater. Sci. Mater. Electron. 2022, 33, 20361–20372. [Google Scholar] [CrossRef]
- Kabir, R.; Saifullah, M.A.K.; Ahmed, A.Z.; Masum, S.M.; Molla, M.A.I. Synthesis of n-doped zno nanocomposites for sunlight photocatalytic degradation of textile dye pollutants. J. Compos. Sci. 2020, 4, 49. [Google Scholar] [CrossRef]
- Khalafi, T.; Buazar, F.; Ghanemi, K. Phycosynthesis and Enhanced Photocatalytic Activity of Zinc Oxide Nanoparticles Toward Organosulfur Pollutants. Sci. Rep. 2019, 9, 43368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, M.; Heib, F. About the possibility of calibrating optical detectors by solar radiation. RSC Adv. 2014, 4, 17639–17647. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Nguyen, V.A. Synthesis, Characterization, and Photocatalytic Activity of ZnO Nanomaterials Prepared by a Green, Nonchemical Route. J. Nanomater. 2020, 2020, 1768371. [Google Scholar] [CrossRef]
- Chijioke-Okere, M.O.; Okorocha, N.J.; Anukam, B.N.; Oguzie, E.E. Photocatalytic Degradation of a Basic Dye Using Zinc Oxide Nanocatalyst. Int. Lett. Chem. Phys. Astron. 2019, 81, 18–26. [Google Scholar] [CrossRef]
- Balcha, A.; Yadav, O.P.; Dey, T. Photocatalytic degradation of methylene blue dye by zinc oxide nanoparticles obtained from precipitation and sol-gel methods. Environ. Sci. Pollut. Res. 2016, 23, 25485–25493. [Google Scholar] [CrossRef]
- Yang, D.; Ni, X.; Chen, W.; Weng, Z. The observation of photo-Kolbe reaction as a novel pathway to initiate photocatalytic polymerization over oxide semiconductor nanoparticles. J. Photochem. Photobiol. A Chem. 2008, 195, 323–329. [Google Scholar] [CrossRef]









| 2 Theta Degree | hkl | FWHM | D-Value | Size (nm) | Lattice Strain |
|---|---|---|---|---|---|
| ZnO NPs | |||||
| 31.5 | 100 | 0.501 | 2.83 | 17.22 | 0.0077 |
| 34.18 | 002 | 0.313 | 2.61 | 27.75 | 0.0044 |
| 36.03 | 101 | 0.496 | 2.48 | 17.6 | 0.0066 |
| 47.34 | 102 | 0.456 | 1.91 | 19.88 | 0.0045 |
| 56.41 | 110 | 0.498 | 1.62 | 18.91 | 0.004 |
| 62.68 | 103 | 0.5 | 1.48 | 18.84 | 0.0041 |
| 66.21 | 112 | 0.47 | 1.38 | 21.28 | 0.0031 |
| ZnO-W | |||||
| 31.8 | 100 | 0.467 | 2.8114 | 18.48 | 0.0072 |
| 34.44 | 002 | 0.272 | 2.6018 | 31.95 | 0.0038 |
| 36.28 | 101 | 0.486 | 2.4738 | 17.97 | 0.0065 |
| 47.33 | 102 | 0.614 | 1.1989 | 14.76 | 0.0061 |
| 56.6 | 110 | 0.567 | 1.6248 | 17.49 | 0.0042 |
| 62.85 | 103 | 0.589 | 1.6248 | 16.5 | 0.0042 |
| ZnO-Sb | |||||
| 31.74 | 100 | 0.817 | 2.8163 | 10.55 | 0.0125 |
| 34.43 | 102 | 0.556 | 2.6026 | 15.63 | 0.0078 |
| 36.23 | 101 | 0.772 | 2.4770 | 11.30 | 0.0103 |
| 47.55 | 102 | 0.811 | 1.9105 | 11.18 | 0.008 |
| 56.59 | 110 | 0.813 | 1.8248 | 11.59 | 0.0066 |
| 62.88 | 103 | 0.713 | 1.4786 | 13.64 | 0.0051 |
| 67.99 | 112 | 0.513 | 1.3787 | 12.11 | 0.0072 |
| Nanomaterial | SA (m2 g−1) | BJH | |
|---|---|---|---|
| PD (Å) | Pore Volume | ||
| ZnO-NPs | 13.014 | 15.20 | 0.023 |
| ZnO-W | 18.999 | 80.67 | 0.039 |
| ZnO-Sb | 22.83 | 81.63 | 0.049 |
| Nanomaterial | Concentration of Dye (ppm) | R2 | Final % Degradation | % COD Reduction | % TOC Reduction | ||
|---|---|---|---|---|---|---|---|
| Sun Light | UV Light | Sun Light | UV Light | Sunlight | |||
| ZnO | 10 | 0.980 | 0.988 | 64.39 | 82.01 | 52.2 ± 0.02 | 74.12 ± 0.023 |
| 25 | 0.968 | 0.946 | 38. 63 | 44.02 | 30.59 ± 0.48 | 50.87 ± 0.22 | |
| 50 | 0.992 | 0.941 | 18.2 | 18.92 | 14.99 ± 0.29 | 31.1 ± 0.04 | |
| ZnO-Sb | 10 | 0.987 | 0.962 | 91.22 | 90.06 | 88.5 ± 0.12 | 95. 34 ± 0.22 |
| 25 | 0.993 | 0.994 | 48.61 | 46.49 | 48.92 ± 0.21 | 69.22 ± 0.08 | |
| 50 | 0.992 | 0.995 | 22.12 | 20.7 | 27.03 ± 0.33 | 48.32 ± 0.4 | |
| ZnO-W | 10 | 0.993 | 0.988 | 86.04 | 88.21 | 85.12 ± 0.22 | 92.04 ± 0.09 |
| 25 | 0.994 | 0.993 | 45.6 | 48.1 | 44.99 ± 0.08 | 66.98 ± 0.06 | |
| 50 | 0.998 | 0.921 | 20.12 | 27.98 | 25.02 ± 0.30 | 46.5 ± 0.33 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modi, S.; Yadav, V.K.; Amari, A.; Alyami, A.Y.; Gacem, A.; Harharah, H.N.; Fulekar, M.H. Photocatalytic Degradation of Methylene Blue Dye from Wastewater by Using Doped Zinc Oxide Nanoparticles. Water 2023, 15, 2275. https://doi.org/10.3390/w15122275
Modi S, Yadav VK, Amari A, Alyami AY, Gacem A, Harharah HN, Fulekar MH. Photocatalytic Degradation of Methylene Blue Dye from Wastewater by Using Doped Zinc Oxide Nanoparticles. Water. 2023; 15(12):2275. https://doi.org/10.3390/w15122275
Chicago/Turabian StyleModi, Shreya, Virendra Kumar Yadav, Abdelfattah Amari, Abeer Yousef Alyami, Amel Gacem, Hamed N. Harharah, and Madhusudan Hiraman Fulekar. 2023. "Photocatalytic Degradation of Methylene Blue Dye from Wastewater by Using Doped Zinc Oxide Nanoparticles" Water 15, no. 12: 2275. https://doi.org/10.3390/w15122275






