Fish Assemblages as Ecological Indicators in the Büyük Menderes (Great Meander) River, Turkey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Ichthyofaunal Knowledge
2.2. Sampling and Fish Inventory
2.3. Statistical Analyses and Fish Assemblage Delineations for River Typology
2.4. Reference Conditions
2.5. Assessment of Anthropogenic Pressures (On-Site Preclassification)
2.6. Fish Index Modification and Application
3. Results
3.1. Sampled Sites and Icthyological Results
| Taxon | Species Code | Local Taxonomic Reference | n | F.O. (%) | Mean Abundance (ind.) | CPUE (ind./m2) |
|---|---|---|---|---|---|---|
| Native species | ||||||
| Alburnoides smyrnae (Leuciscidae) | Alsm | [60] | 21 | 3.64 | 0.38 | 0.0022 |
| Alburnus demiri (Leuciscidae) | Alde | [60] | 80 | 18.18 | 1.45 | 0.0035 |
| Anatolichthys maeandricus (Aphaniidae) | Apme | [61] | 1 | 1.82 | 0.02 | 0.0000 |
| Barbus xanthos (Cyprinidae) | Bape | [34] | 1193 | 43.64 | 21.69 | 0.0737 |
| Capoeta aydinensis (Cyprinidae) | Cabe | [62] | 148 | 16.36 | 2.69 | 0.0069 |
| Chondrostoma turnai/meandrense (Leuciscidae) | Chme | [20] [63] | 1046 | 30.91 | 19.02 | 0.0865 |
| Cobitis afifeae (Cobitidae) | Cofa | [34] [64] | 191 | 20.00 | 3.47 | 0.0099 |
| Unidentified Cyprinidae | Cyprsp | 7 | 1.82 | 0.10 | 0.0003 | |
| Dicentrarchus labrax (Moronidae) | Dila | [34] | 6 | 3,62 | 0.1 | 0.0004 |
| Garra menderesensis (Cyprinidae) | Gaki | [65] [35] | 1 | 1.00 | 0.12 | 0.0001 |
| Gobio maeandricus (Gobionidae) | Gome | [34] | 19 | 7.27 | 0.35 | 0.0006 |
| Knipowitschia caucasica (Gobiidae) | Knca | [34] | 5 | 1.82 | 0.09 | 0.0004 |
| Chelon labrosus (Mugilidae) | Chela | 6 | 3.64 | 0.11 | 0.0009 | |
| Liza spp. (Mugilidae) | Liza1 | 88 | 5.45 | 1.60 | 0.0043 | |
| Liza2 | 29 | 5.45 | 0.53 | 0.0015 | ||
| Mugil cephalus (Mugilidae) | Muce | [34] | 639 | 5.45 | 11.62 | 0.0450 |
| Luciobarbus kottelati (Cyprinidae) | Luko | [66] | 300 | 23.64 | 5.45 | 0.0122 |
| Oxynoemacheilus germencicus (Nemacheilidae) | Oxynsp | [34] [35] | 3589 | 50.91 | 65.25 | 0.2747 |
| Petroleuciscus ninae (Cyprinidae) | Pesm | [66] | 90 | 14.55 | 1.64 | 0.0094 |
| Squalius fellowesi/carinus (Leuciscidae) | Squasp | [67] | 3608 | 54.55 | 65.60 | 0.2249 |
| Vimba mirabilis (Leuciscidae) | Vimi | [34] | 353 | 20.00 | 6.42 | 0.0127 |
| Non-native species | ||||||
| Carassius gibellio (Cyprinidae) | Cagi | [34] | 779 | 27.27 | 14.16 | 0.0705 |
| Cyprinus carpio (Cyprinidae) | Cyca | [34] | 2 | 3.64 | 0.04 | 0.0001 |
| Gambusia holbrooki (Poeciliidae) | Gaho | [34] | 837 | 20.00 | 15.22 | 0.1253 |
| Lepomis gibbosus (Centrarchidae) | Legi | [34] | 266 | 20.00 | 4.84 | 0.0215 |
| Pseudorasbora parva (Gobionidae) | Pspa | [34] | 7 | 7.27 | 0.13 | 0.0009 |
| Rhodeus amarus (Acheilognathidae) | Rham | [34] | 105 | 7.27 | 1.91 | 0.0044 |
| Tinca tinca (Tincidae) | Titi | [34] | 119 | 5.45 | 2.16 | 0.0037 |
| N | Water Body Name | No. Sites | No. Samples | No. Species Recorded | Species Recorded |
|---|---|---|---|---|---|
| 1 | Aşağı Sarıçay | 1 | 1 | 4 | Cagi, Cofa, Gaho, Pesm |
| 2 | Aşağı Çine1 | 2 | 2 | 2 | Pesm, Squasp |
| 3 | Dokuzsele-2 | 1 | 1 | 0 | FISHLESS |
| 4 | Hamam2 | 1 | 1 | 2 | Oxynsp, Squasp |
| 5 | Aşağı Çürüksu2 | 1 | 1 | 0 | FISHLESS |
| 6 | Gökpınar Deresi | 1 | 1 | 1 | Oxynsp |
| 7 | Yukarı Akçay1 | 1 | 2 | 6 | Bape, Cagi, Legi, Oxynsp, Squasp, Alde |
| 8 | Yukarı Dandalaz | 1 | 2 | 4 | Bape, Cabe, Oxynsp, Squasp |
| 9 | Aşağı Akçay | 1 | 1 | 12 | Alde, Bape, Cabe, Chme, Cofa, Cyprsp, Gaho, Luko, Oxynsp, Pesm, Squasp, Vimi |
| 10 | Aşağı Çine2 | 1 | 1 | 10 | Alde, Cagi, Chme, Cofa, Legi, Luko, Oxynsp, Pesm, Squasp, Vimi |
| 11 | Girme Deresi | 1 | 1 | 2 | Bape, Squasp |
| 12 | Yukarı Çine1 | 1 | 1 | 6 | Alsm, Bape, Cabe, Luko, Oxynsp, Squasp |
| 13 | Aşağı Dandalaz | 1 | 1 | 3 | Cofa, Oxynsp, Squasp |
| 14 | Aşağı Çine3 | 1 | 1 | 3 | Cabe, Legi, Squasp |
| 15 | Yukarı Akçay2 | 1 | 1 | 0 | FISHLESS |
| 16 | Çaykavuştu2 | 1 | 1 | 0 | FISHLESS |
| 17 | Çaykavuştu1 | 1 | 1 | 0 | FISHLESS |
| 18 | Yukarı Çürüksu | 1 | 1 | 0 | FISHLESS |
| 19 | Kufi4 | 1 | 1 | 9 | Vimi, Bape, Cagi, Chme, Gome, Squasp, Titi, Gaki, Apme |
| 20 | Yukari Akcay4 | 2 | 2 | 8 | Alde, Bape, Cabe, Chme, Luko, Oxynsp, Squasp, Vimi |
| 21 | Yukari Cine3 | 1 | 2 | 1 | Bape |
| 22 | Yukari Ikizdere2 | 1 | 2 | 4 | Bape, Cagi, Oxynsp, Squasp |
| 23 | Hamam1 | 1 | 1 | 0 | FISHLESS |
| 24 | Asagi Büyük Menderes1 | 1 | 2 | 13 | Alde, Cagi, Chme, Cofa, Cyca, Gaho, Legi, Luko, Oxynsp, Pesm, Pspa, Rham, Vimi |
| 25 | Yukarı Banaz | 2 | 2 | 3 | Bape, Oxynsp, Squasp |
| 26 | Aşağı Banaz1 | 2 | 2 | 0 | FISHLESS |
| 27 | Yukari Büyük Menderes 1 | 1 | 2 | 8 | Cagi, Chme, Cofa, Gome, Luko, Oxynsp, Squasp, Titi |
| 28 | Asagi Banaz2 | 1 | 1 | 6 | Alsm, Bape, Cagi, Oxynsp, Squasp, Cyprsp |
| 29 | Yukari Akcay3 | 2 | 2 | 5 | Bape, Cabe, Chme, Oxynsp, Squasp |
| 30 | Asagi Büyük Menderes2 | 4 | 5 | 15 | Alde, Cagi, Chme, Gaho, Legi, Chela, Muce, Vimi, Pspa, Rham, Dila, Liza1, Liza2, Cyca, Knca |
| 31 | Yalkı | 1 | 1 | 0 | FISHLESS |
| 32 | Asagi Ikizdere2 | 1 | 1 | 8 | Alde, Cagi, Chme, Gaho, Luko, Pesm, Rham, Vimi |
| 33 | Naipli Cayi | 1 | 1 | 4 | Bape, Cabe, Oxynsp, Squasp |
| 34 | Yukari Ikizdere1 | 1 | 2 | 3 | Bape, Oxynsp, Squasp |
| 35 | Yukari Büyük Menderes 2 | 1 | 2 | 9 | Bape, Cagi, Chme, Cofa, Gaho, Gome, Luko, Oxynsp, Squasp |
| 36 | Orta Büyük Menderes | 1 | 2 | 8 | Alde, Bape, Chme, Cofa, Luko, Oxynsp, Squasp, Vimi |
| 37 | Dokuzsele-1 | 1 | 1 | 0 | FISHLESS |
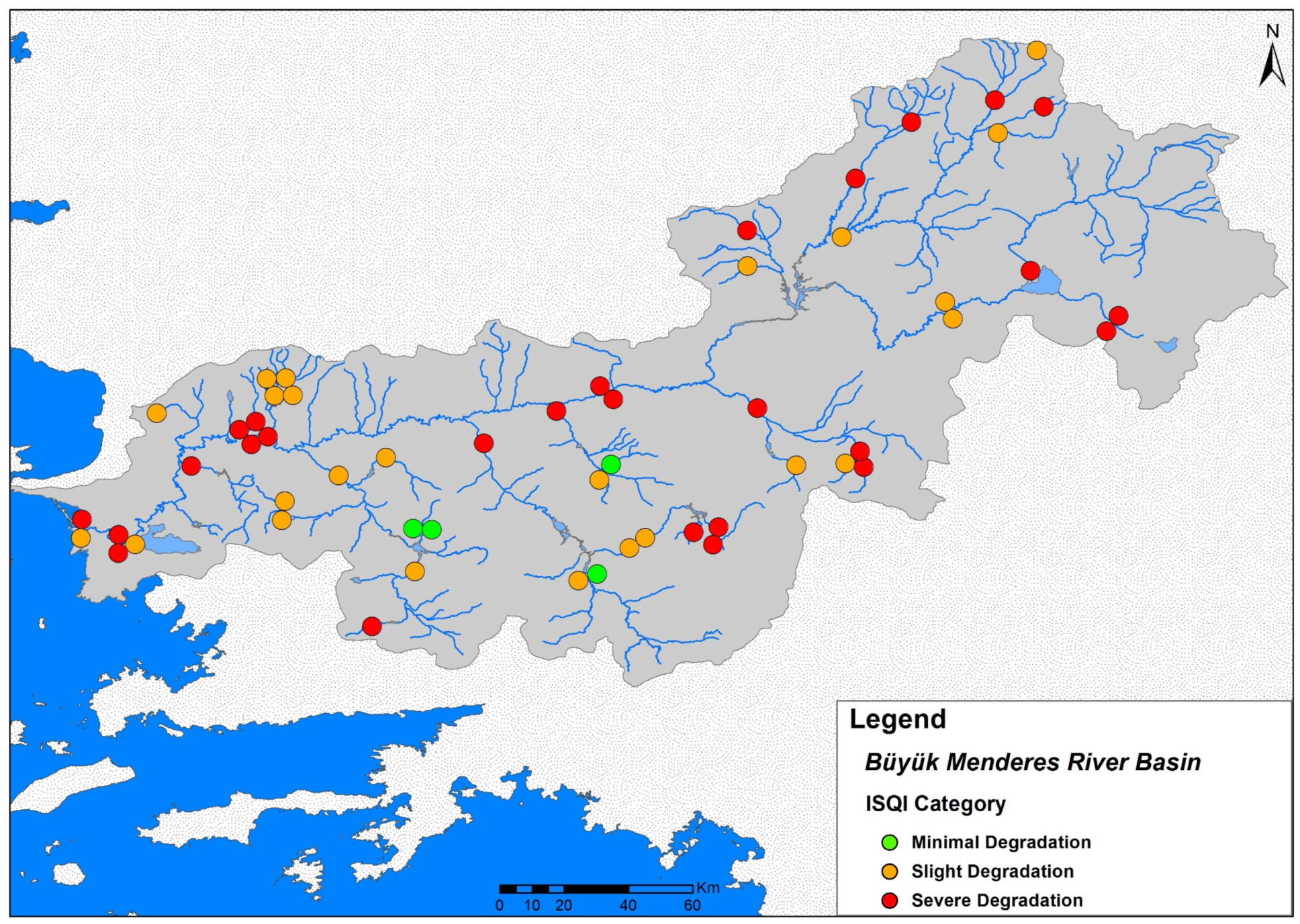
3.2. Environmental Assessment Results
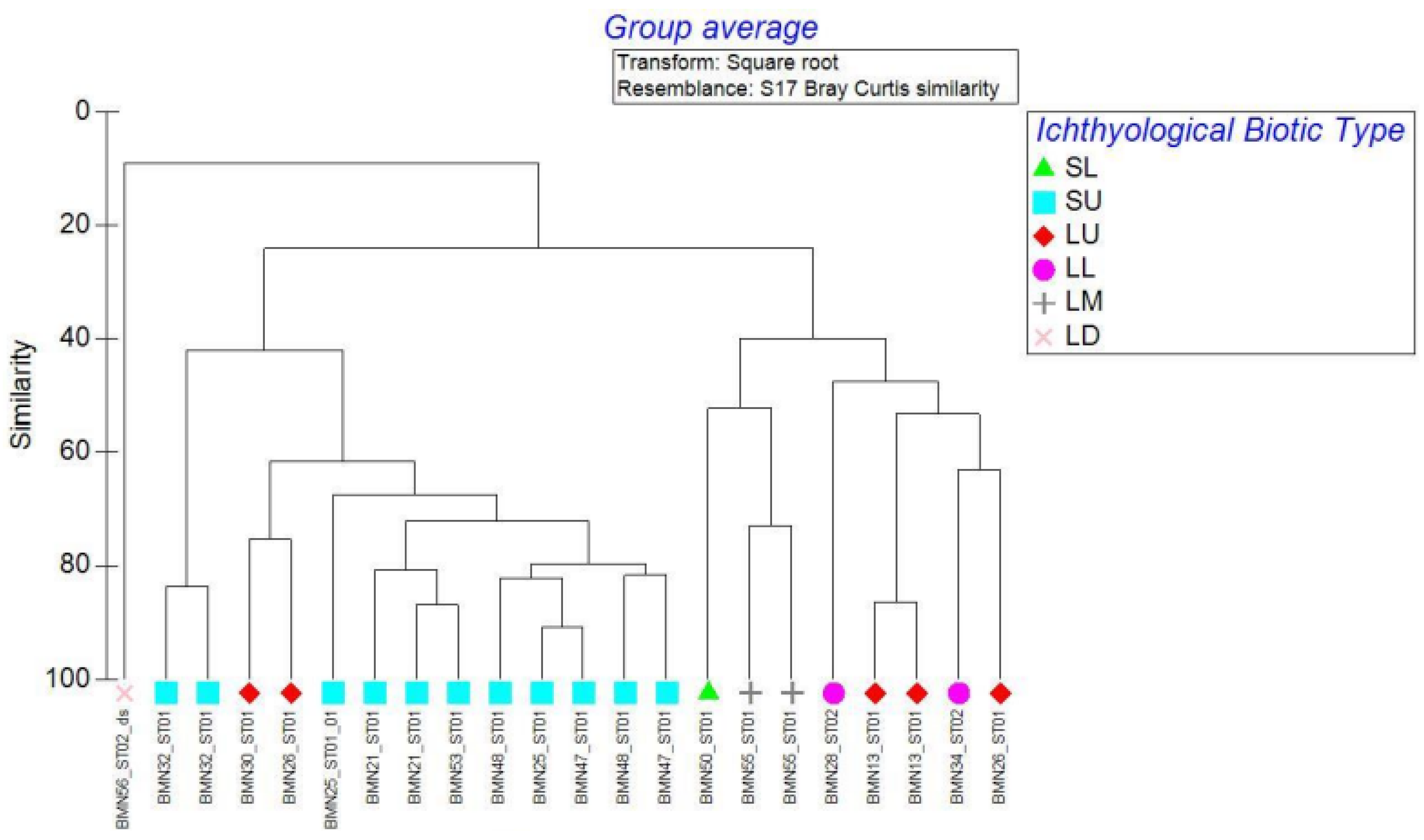
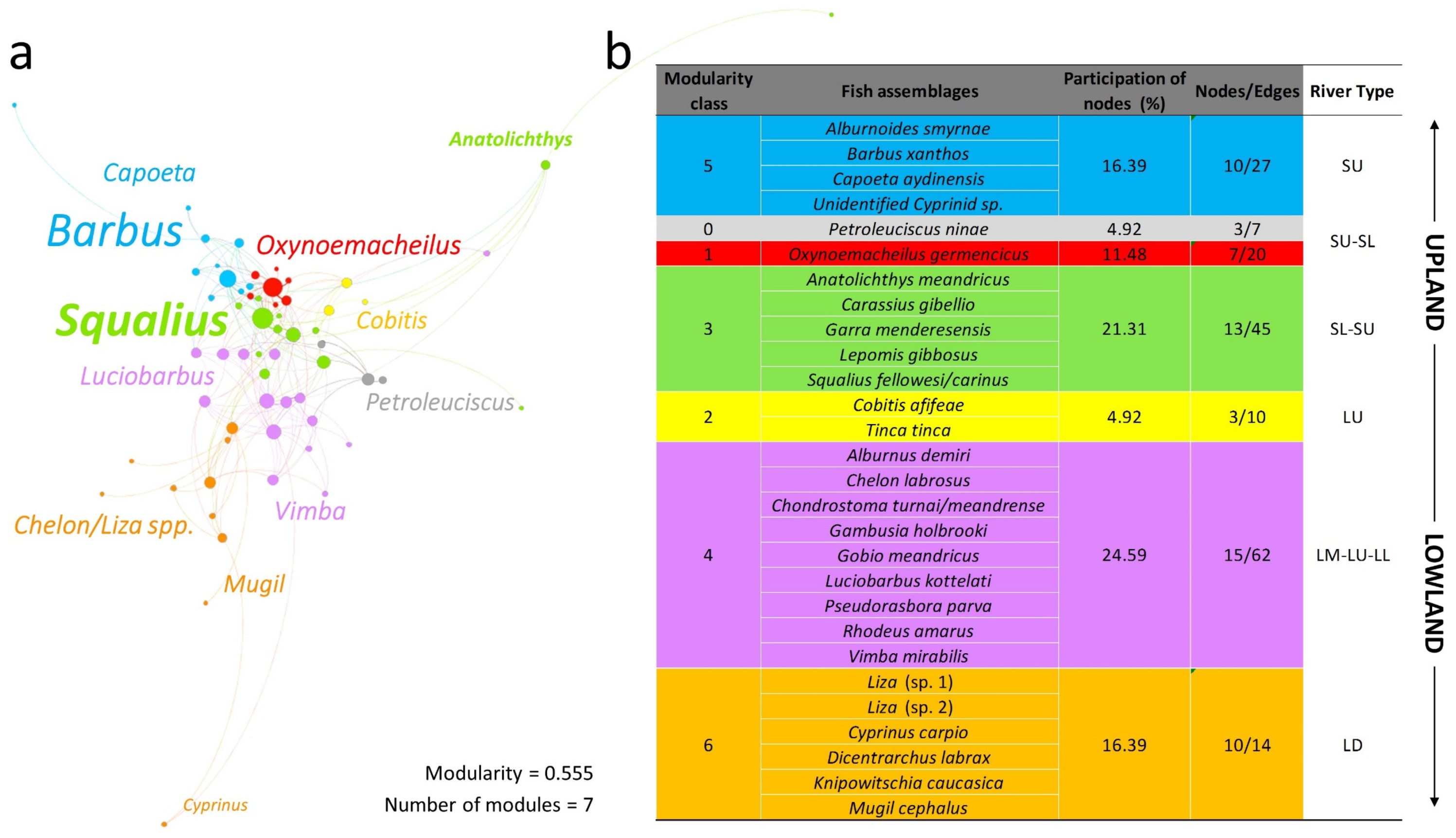
3.3. Biotic Typology Based on Fish Assemblage Types
- o
- SU = small upland (all small and very small tributaries);
- o
- SL = small lowland (small low-elevation main tributaries);
- o
- LU = large upland (mid-section main stem and two major low-land tributaries);
- o
- LL = large lowland;
- o
- LM = large main stem (the major meandering lower section of the river’s main stem);
- o
- LD = large delta (large channel reaches with free communication with the sea and surrounding lagoonal wetlands).
3.4. Application of the European Fish Index (EFI+)
4. Discussions
4.1. Achievements
4.2. Identifying Problems and Shortcomings
4.3. Insights and Recommendations
- A complete taxonomic inventory must finally complete the ichthyofaunal natural history knowledge of the river basin. The mapping of all species distributions and habitats is critical for understanding fish communities;
- A historical study of species distributions and human-induced changes must be investigated. This includes careful analysis of the history of habitat changes including a socioecological research approaches (e.g., engaging fishers and local communities).
- Fish-based monitoring techniques and a long-term monitoring initiative must be set in place in order to explore trends and patterns of change. This new research program must be integrated within a basin-wide biodiversity conservation strategy.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hershkovitz, Y.; Gasith, A. Resistance, resilience, and community dynamics in Mediterranean-climate streams. Hydrobiologia 2013, 719, 59–75. [Google Scholar] [CrossRef]
- Álvarez Cobelas, M.; Rojo, C.; Angeler, D.G. Mediterranean limnology: Current status, gaps and the future. J. Limnol. 2005, 64, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, T.; Oliveira, J.; Caiola, N.; De Sostoa, A.; Casals, F.; Cortes, R.; Economou, A.; Zogaris, S.; Garcia-Jalon, D.; Ilheu, M.; et al. Ecological traits of fish assemblages from Mediterranean Europe and their responses to human disturbance. Fish. Manag. Ecol. 2007, 14, 473–481. [Google Scholar] [CrossRef]
- Grapci-Kotori, L.; Vavalidis, T.; Zogaris, D.; Šanda, R.; Vukić, J.; Geci, D.; Ibrahimi, H.; Bilalli, A.; Zogaris, S. Fish distribution patterns in the White Drin (Drini i Bardhë) river, Kosovo. Knowl. Manag. Aquat. Ecosyst. 2020, 421, 29. [Google Scholar] [CrossRef]
- Tachos, V.; Dimitrakopoulos, P.G.; Zogaris, S. Multiple anthropogenic pressures in Eastern Mediterranean rivers: Insights from fish-based bioassessment in Greece. Ecohydrol. Hydrobiol. 2022, 22, 40–54. [Google Scholar] [CrossRef]
- Schmutz, S.; Cowx, I.; Haidvogl, G.; Pont, D. Fish-based methods for assessing European running waters: A synthesis. Fish. Manag. Ecol. 2007, 14, 369–380. [Google Scholar] [CrossRef]
- Schmutz, S.; Melcher, A.; Frangez, C.; Haidvogl, G.; Beier, U.; Böhmer, J.; Breine, J.; Caiola, N.; Sostoa, A.; Ferreira, M.; et al. Spatially based methods to assess the ecological status of riverine fish assemblages in European ecoregions. Fish. Manag. Ecol. 2007, 14, 441–452. [Google Scholar] [CrossRef]
- Gordon, N.D.; McMahon, T.A.; Finlayson, B.L.; Gippel, C.J.; Nathan, R.J. Stream Hydrology: An Introduction for Ecologists; John Wiley and Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Miranda, L.E.; Raborn, S.W. From zonation to connectivity: Fluvial ecology paradigms of the 20th century. Pol. Arch. Hydrobiol. 2000, 47, 5–19. [Google Scholar]
- Aarts, B.; Nienhuis, P. Fish zonations and guilds as the basis for assessment of ecological integrity of large rivers. Hydrobiologia 2003, 500, 157–178. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Methods for Evaluating Wetland Condition: Developing Metrics and Indexes of Biological Integrity; Office of Water, U.S. Environmental Protection Agency: Washington, DC, USA, 2002; Volume EPA-822-R-02-016.
- Karr, J.R.; Chu, E. Seven foundations of biological monitoring and assessment. Biol. Ambient. 2006, 20, 7–18. [Google Scholar]
- Karr, J.R.; Chu, E.W. Restoring Life in Running Waters; Island Press: Washington, DC, USA, 1999. [Google Scholar]
- Economou, A.; Zogaris, S.; Vardakas, L.; Koutsikos, N.; Chatzinikolaou, Y.; Kommatas, D.; Kapakos, Y.; Giakoumi, S.; Oikonomou, E.; Tachos, V. Developing policy-relevant river fish monitoring in Greece: Insights from a nation-wide survey. Mediterr. Mar. Sci. 2016, 17, 302–322. [Google Scholar] [CrossRef]
- Hering, D.; Johnson, R.K.; Kramm, S.; Schmutz, S.; Szoszkiewicz, K.; Verdonschot, P.F. Assessment of European streams with diatoms, macrophytes, macroinvertebrates and fish: A comparative metric-based analysis of organism response to stress. Freshw. Biol. 2006, 51, 1757–1785. [Google Scholar] [CrossRef]
- Poikane, S.; Ritterbusch, D.; Argillier, C.; Białokoz, W.; Blabolil, P.; Breine, J.; Jaarsma, N.; Krause, T.; Kubecka, J.; Lauridsen, T.; et al. Response of fish communities to multiple pressures: Development of a total anthropogenic pressure intensity index. Sci. Total Environ. 2017, 586, 502–511. [Google Scholar] [CrossRef]
- EFI Consortium. Manual for the Application of the New European Fish Index–EFI+. A Fish-Based Method to Assess the Ecological Status of European Running Waters in Support of the Water Framework Directive; EFI Consortium: Joensuu, Finland, 2009. [Google Scholar]
- Mostafavi, H.; Schinegger, R.; Melcher, A.; Moder, K.; Mielach, C.; Schmutz, S. A new fish-based multi-metric assessment index for cyprinid streams in the Iranian Caspian Sea Basin. Limnologica 2015, 51, 37–52. [Google Scholar] [CrossRef] [Green Version]
- Ergönül, M.B.; Breine, J.; Van den Bergh, E. A technical guide to develop a statistically valid fish-based index in compliance with the water framework directive: An evaluation for Turkish freshwaters. Int. Aquat. Res. 2018, 10, 303–319. [Google Scholar] [CrossRef] [Green Version]
- Küçük, F.; Çiftci, Y.; Güçlü, S.S.; Turan, D. Chondrostoma smyrnae, a new nase from the Tahtalı reservoir drainage in the Aegean Sea basin (Teleostei, Leuciscidae). Zoosystematics Evol. 2021, 97, 235–248. [Google Scholar] [CrossRef]
- Yilmaz, E.; Koç, C. A study about effects of river water quality on fish living in Büyük Menderes Basin, Turkey. J. Water Resour. Prot. 2016, 8, 1175. [Google Scholar] [CrossRef] [Green Version]
- Darwall, W.R.T.; Barrios, V.; Carrizosa, S.; Freyhof, J.; Numa, C.; Smith, K. Freshwater Key Biodiversity Areas in the Mediterranean Basin Hotspot: Informing Species Conservation and Development Planning in Freshwater Ecosystems; IUCN: Gland, Switzerland, 2014; Volume 52. [Google Scholar]
- Canoglu, H.; Aksu, I.; Turan, D.; Bektas, Y. DNA barcoding of the genus Alburnoides Jeitteles, 1861 (Actinopterygii, Cyprinidae) from Anatolia, Turkey. Zoosystematics Evol. 2023, 99, 185–194. [Google Scholar] [CrossRef]
- Duran, M.A.; Akyildiz, K.G. Büyük Menderes Havza Atlasi. In Yaşayan Nehirler Yaşayan Ege Projesi; WWF-Türkiye: Istanbul, Turkey, 2014. [Google Scholar]
- Akbulut, N.E.; Bayarı, S.; Akbulut, A.; Özyurt, N.N.; Sahin, Y. Rivers of Turkey. In Rivers of Europe; Elsevier: Amsterdam, The Netherlands, 2022; pp. 853–882. [Google Scholar]
- Seal, J. Meander: East to West Along a Turkish River; Random House: Manhattan, CA, USA, 2012. [Google Scholar]
- Gürbüz, A.; Kazancı, N. The Büyük Menderes River: Origin of meandering phenomenon. In Landscapes and Landforms of Turkey; Springer: Cham, Switzerland, 2019; pp. 509–519. [Google Scholar]
- Durmaz, E.; Kocagoz, R.; Bilacan, E.; Orhan, H. Metal pollution in biotic and abiotic samples of the Büyük Menderes River, Turkey. Environ. Sci. Pollut. Res. 2017, 24, 4274–4283. [Google Scholar] [CrossRef] [PubMed]
- Koca, S.; Koca, Y.B.; Yildiz, Ş.; Gürcü, B. Genotoxic and histopathological effects of water pollution on two fish species, Barbus capito pectoralis and Chondrostoma nasus in the Büyük Menderes River, Turkey. Biol. Trace Elem. Res. 2008, 122, 276–291. [Google Scholar] [CrossRef]
- Sarı, H.; Balik, S.; Türe, G.; Bilecenoglu, M. Recent changes in the fish fauna of Lake Bafa, Aegean Region of Turkey. Zool. Middle East 1999, 18, 67–76. [Google Scholar] [CrossRef]
- Abell, R.; Thieme, M.L.; Revenga, C.; Bryer, M.; Kottelat, M.; Bogutskaya, N.; Coad, B.; Mandrak, N.; Balderas, S.C.; Bussing, W. Freshwater ecoregions of the world: A new map of biogeographic units for freshwater biodiversity conservation. BioScience 2008, 58, 403–414. [Google Scholar] [CrossRef] [Green Version]
- Barlas, M.; Dirican, S. The fish fauna of the Dipsiz-Çine (Muğla-Aydin) stream. Gazi Univ. J. Sci. 2004, 17, 35–48. [Google Scholar]
- Pülhan, B. İkizdere (İncirliova-Aydın) Balık Faunası Üzerine Bir Araştırma [An Investigation on Fish Fauna of İkizdere Stream (Aydın-İncirliova); Muğla Üniversitesi: Mugla, Turkey, 2008. [Google Scholar]
- Güçlü, S.S.; Küçük, F.; Ertan, Ö.O.; Güçlü, Z. The fish fauna of the Büyük Menderes River (Turkey): Taxonomic and zoogeographic features. Turk. J. Fish. Aquat. Sci. 2013, 13, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Yoğurtçuoğlu, B.; Ekmekçi, F.; Bektas, Y.; Aksu, I.; Turan, D. The first record of Garra kemali (Teleostei: Cyprinidae) from the Black Sea basin with a re-description of the species. Ichthyol. Explor. Freshw. 2018, 28. [Google Scholar] [CrossRef]
- Geldiay, R.; Balık, S. Freshwater Fishes of Turkey; Ege Üniversitesi Su Ürünleri Fakültesi Yayınları: Erzene, Turkey, 2007. [Google Scholar]
- Innis, S.A.; Naiman, R.J.; Elliott, S.R. Indicators and assessment methods for measuring the ecological integrity of semi-aquatic terrestrial environments. Hydrobiologia 2000, 422, 111–131. [Google Scholar] [CrossRef]
- IMBRIW. Inland Waters Fish Monitoring Operations Manual: Electrofishing Health and Safety/HCMR Rapid Fish Sampling Protocol, 1st ed.; Zogaris, S.; Oikonomou, E.; Tachos, V.; Beaumont, B., Translators; Hellenic Centre for Marine Research Special Publication: Athens, Greece, 2013. [Google Scholar]
- EN 14011:2003; Water Quality—Sampling of Fish with Electricity. CEN: Brussels, Belgium, 2003; Volume CEN/TC 230.
- Stout, C.C.; Tan, M.; Lemmon, A.R.; Lemmon, E.M.; Armbruster, J.W. Resolving Cypriniformes relationships using an anchored enrichment approach. BMC Evol. Biol. 2016, 16, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Laan, R. Freshwater fish list. Almer. Neth. 2019, 20, 2019. [Google Scholar]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An open source software for exploring and manipulating networks. In Proceedings of the International AAAI Conference on Web and Social Media, San Jose, CA, USA, 17–20 May 2009; pp. 361–362. [Google Scholar]
- Vilhena, D.; Antonelli, A. Beyond similarity: A network approach for identifying and delimiting biogeographical regions. Nat. Commun. 2015, 6, 6848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koutsikos, N.; Vardakas, L.; Vavalidis, T.; Kalogianni, E.; Dimitriou, E.; Kalantzi, O.-I.; Zogaris, S. Defining non-indigenous fish assemblage types in Mediterranean rivers: Network analysis and management implications. J. Environ. Manag. 2021, 278, 111551. [Google Scholar] [CrossRef]
- Jacomy, M.; Venturini, T.; Heymann, S.; Bastian, M. ForceAtlas2, a continuous graph layout algorithm for handy network visualization designed for the Gephi software. PLoS ONE 2014, 9, e98679. [Google Scholar] [CrossRef]
- Stoddard, J.L.; Larsen, D.P.; Hawkins, C.P.; Johnson, R.K.; Norris, R.H. Setting Expectations for The Ecological Condition of Streams: The Concept of Reference Condition. Ecol. Appl. 2006, 16, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Whittier, T.R.; Stoddard, J.; Hughes, R.; Lomnicky, G. Associations among catchment- and site-scale disturbance indicators and biological assemblages at least- and most-disturbed stream and river sites in the western United States. Trans. Am. Fish. Soc. 2006, 48, 641–664. [Google Scholar]
- EU Twinning Project. Cooperation between the Republic of Turkey, The Netherlands, France and Spain European Union Twinning Project Capacity Building on Water Quality Monitoring. Twinning Project. 2011. Available online: http://www.monitoring.ormansu.gov.tr/ (accessed on 4 February 2021).
- Google Earth Pro., Büyük Menderes Basin. History Layers (2013–2014). Borders and Labels; Places Layers. Data SIO, NOAA, U.S. Navy, NGA, GEBCO, Image Landsat/Copernicus. 37°47′35.30″ N., 27°54′18.25″ E. 2013–2014. Available online: http://www.google.com/earth/index.html (accessed on 1 February 2023).
- Bjorkland, R.; Pringle, C.M.; Newton, B. A stream visual assessment protocol (SVAP) for riparian landowners. Environ. Monit. Assess. 2001, 68, 99–125. [Google Scholar] [CrossRef]
- Zogaris, S.; Chatzinikolaou, Y.; Dimopoulos, P. Riparian woodland flora in upland rivers of Western Greece. Mediterr. Mar. Sci. 2008, 9, 87–102. [Google Scholar] [CrossRef] [Green Version]
- Schinegger, R.; Trautwein, C.; Melcher, A.; Schmutz, S. Multiple human pressures and their spatial patterns in European running waters. Water Environ. J. 2012, 26, 261–273. [Google Scholar] [CrossRef] [Green Version]
- Angermeier, P.L.; Davideanu, G. Using fish communities to assess streams in Romania: Initial development of an index of biotic integrity. Hydrobiologia 2004, 511, 65–78. [Google Scholar] [CrossRef]
- Zogaris, S.; Tachos, V.; Economou, A.N.; Chatzinikolaou, Y.; Koutsikos, N.; Schmutz, S. A model-based fish bioassessment index for Eastern Mediterranean rivers: Application in a biogeographically diverse area. Sci. Total Environ. 2018, 622–623, 676–689. [Google Scholar] [CrossRef]
- QGIS.org. QGIS Geographic Information System. 2021. Available online: http://www.qgis.org (accessed on 12 October 2022).
- Vlami, V.; Morera Beita, C.; Zogaris, S. Landscape Conservation Assessment in the Latin American Tropics: Application and Insights from Costa Rica. Land 2022, 11, 514. [Google Scholar] [CrossRef]
- Çicek, E.; Sungur, S.; Fricke, R. Freshwater lampreys and fishes of Turkey; a revised and updated annotated checklist 2020. Zootaxa 2020, 4809, 241–270. [Google Scholar] [CrossRef] [PubMed]
- Geiger, M.F.; Herder, F.; Monaghan, M.T.; Almada, V.; Barbieri, R.; Bariche, M.; Berrebi, P.; Bohlen, J.; Casal-Lopez, M.; Delmastro, G.B. Spatial heterogeneity in the Mediterranean Biodiversity Hotspot affects barcoding accuracy of its freshwater fishes. Mol. Ecol. Resour. 2014, 14, 1210–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Froese, R.; Pauly, D. (Eds.) 2023. FishBase. World Wide Web Electronic Publication. Version (02/2023). 2022. Available online: www.fishbase.org (accessed on 4 June 2021).
- Bektas, Y.; Aksu, I.; Kaya, C.; Baycelebi, E.; Atasaral, S.; Ekmekci, F.G.; Turan, D. Phylogeny and phylogeography of the genus Alburnoides (Teleostei, Cyprinidae) in Turkey based on mitochondrial DNA sequences. Mitochondrial DNA Part A 2019, 30, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Freyhof, J.; Yoğurtçuoğlu, B. A proposal for a new generic structure of the killifish family Aphaniidae, with the description of Aphaniops teimorii (Teleostei: Cyprinodontiformes). Zootaxa 2020, 4810, zootaxa.4810.3.2. [Google Scholar] [CrossRef]
- Turan, D.; Küçük, F.; Kaya, C.; Güçlü, S.; Bektas, Y. Capoeta aydinensis, a new species of scraper from southwestern Anatolia, Turkey (Teleostei: Cyprinidae). Turk. J. Zool. 2017, 41, 436–442. [Google Scholar] [CrossRef]
- Küçük, F.n.; Çiftçi, Y.; Güçlü, S.m.S.; Mutlu, A.G.; Turan, D. Taxonomic review of the Chondrostoma (Teleostei, Leuciscidae) species from inland waters of Turkey: An integrative approach. Zoosystematics Evol. 2023, 99, 1–13. [Google Scholar] [CrossRef]
- Freyhof, J.; Baycelebi, E.; Geiger, M. Review of the genus Cobitis in the Middle East, with the description of eight new species (Teleostei: Cobitidae). Zootaxa 2018, 4535, 1–75. [Google Scholar] [CrossRef]
- Küçük, F.; Bayçelebi, E.; Güçlü, S.S.; Gülle, I. Description of a new species of Hemigrammocapoeta (Teleostei: Cyprinidae) from Lake Işıklı, Turkey. Zootaxa 2015, 4052, 359–365. [Google Scholar] [CrossRef] [Green Version]
- Turan, D.; Kalayci, G.; Kaya, C.; Bektas, Y.; Küçük, F. A new species of Petroleuciscus (Teleostei: Cyprinidae) from the Büyük Menderes River, southwestern Anatolia, Turkey. J. Fish Biol. 2018, 92, 875–887. [Google Scholar] [CrossRef]
- Özulug, M.; Freyhof, J. Revision of the genus Squalius in Western and Central Anatolia, with description of four new species (Teleostei: Cyprinidae). Ichthyol. Explor. Freshw. 2011, 22, 107. [Google Scholar]
- Yılmaz, E.; Koç, C. Organic pollution of the Büyük Menderes River, Turkey and effects on aquaculture. Environ. Sci. Pollut. Res. 2016, 23, 11493–11506. [Google Scholar] [CrossRef]
- Innal, D.; Erk’akan, F. Effects of exotic and translocated fish species in the inland waters of Turkey. Rev. Fish Biol. Fish. 2006, 16, 39–50. [Google Scholar] [CrossRef]
- Özcan, G. Büyük Menderes Nehir Havzası’ndaki egzotik balık türleri ve etkileri. Türk Bilimsel Derlemeler Derg. 2008, 2, 23–25. [Google Scholar]
- Sarı, H.; Bilecenoglu, M. Threatened Fishes of the World: Acanthobrama mirabilis Ladiges, 1960 (Cyprinidae). Environ. Biol. Fishes 2002, 65, 318. [Google Scholar] [CrossRef]
- Durdu, O.F. Effects of climate change on water resources of the Büyük Menderes river basin, western Turkey. Turk. J. Agric. For. 2010, 34, 319–332. [Google Scholar] [CrossRef]
- Safari, M.J.S.; Tayfur, G.; Vaheddoost, B.; Mersin, D. Drought Assessment in Büyük Menderes Basin of Turkey. In Proceedings of the 5th International Congress of Developing Agriculture, Natural Resources, Environment & Tourism of Iran, Tabriz, Iran, 10–12 August 2021. [Google Scholar]
- Skoulikidis, N.; Vardakas, L.; Karaouzas, I.; Economou, A.; Dimitriou, E.; Zogaris, S. Assessing water stress in Mediterranean lotic systems: Insights from an artificially intermittent river in Greece. Aquat. Sci. 2011, 73, 581–597. [Google Scholar] [CrossRef]
- Helfman, G.S. Fish Conservation: A Guide to Understanding and Restoring Global Aquatic Biodiversity and Fishery Resources; Island Press: Washington, DC, USA, 2007. [Google Scholar]
- Yegen, V.; Balik, S.; Bilcen, E.; Sari, H.; Uysal, R.; Yagci, A. Fish species in Denizli City rivers and their distribution in the region. J. Fish. Sci. 2008, 2, 301–311. [Google Scholar]
- Ozdemir, A.; Duran, M.; Akyildiz, K.G.; Sen, A. EROD and metallothionein in Limnodrilus profundicola (Oligochaeta: Tubifi cidae) as an indicator of pollution exposure in the Curuksu stream of Menderes river, Denizli–Turkey. Desalination Water Treat. 2011, 26, 98–103. [Google Scholar] [CrossRef] [Green Version]
- Küçük, S. Büyük Menderes nehri su kalite ölçümlerinin su ürünleri açisindan incelenmesi. Adnan Menderes Üniversitesi Ziraat Fakültesi Derg. 2007, 4, 7–13. [Google Scholar]
- Champ, W.S.T.; Kelly, F.L.; King, J.J. The Water Framework Directive: Using Fish as a Management Tool. Biol. Environ.-Proc. R. Ir. Acad. 2009, 109b, 191–206. [Google Scholar] [CrossRef]
- Ergönül, M.B.; Breine, J.; Van den Bergh, E.; Bahçeci, H. Biological assessment of some wadable rivers in Turkey using fish data: A statistical approach. Environ. Dev. Sustain. 2020, 22, 7385–7425. [Google Scholar] [CrossRef]
- Akyildiz, G.K.; Bakir, R.; Polat, S.; Duran, M. Mentum Deformities of Chironomid Larvae as an Indicator of Environmental Stress in Büyük Menderes River, Turkey. Inland Water Biol. 2018, 11, 515–522. [Google Scholar] [CrossRef]
- Akyildiz, G.K.; Duran, M. Evaluation of the impact of heterogeneous environmental pollutants on benthic macroinvertebrates and water quality by long-term monitoring of the Büyük Menderes river basin. Environ. Monit. Assess. 2021, 193, 280. [Google Scholar] [CrossRef]
- Innal, D.; Tocan, B.; Gülle, İ.; Çağlan, D.C.; Düğel, M.; Avşar, D. Diversity and distribution of the ichthyofauna in the Göksu River estuary, Turkey. Acta Zool. Bulg. 2020, 72, 667–676. [Google Scholar]
- Higgins, J.V. Maintaining the ebbs and flows of the landscape: Conservation planning for freshwater ecosystems. In Drafting a Conservation Blueprint: A Practitioner’s Guide to Planning for Biodiversity; Island Press: Washington, DC, USA, 2003; pp. 291–318. [Google Scholar]
- Haidvogl, G. Historic Milestones of Human River Uses and Ecological Impacts. Riverine Ecosyst. Manag. Sci. Gov. Towards A Sustain. Future 2018, 8, 19–39. [Google Scholar] [CrossRef] [Green Version]
- Labay, B.; Cohen, A.E.; Sissel, B.; Hendrickson, D.A.; Martin, F.D.; Sarkar, S. Assessing historical fish community composition using surveys, historical collection data, and species distribution models. PLoS ONE 2011, 6, e25145. [Google Scholar] [CrossRef]
- Hermoso, V.; Clavero, M. Revisiting ecological integrity 30 years later: Non-native species and the misdiagnosis of freshwater ecosystem health. Fish Fish. 2013, 14, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Karr, J.R.; Larson, E.R.; Chu, E.W. Ecological integrity is both real and valuable. Conserv. Sci. Pract. 2022, 4, e583. [Google Scholar] [CrossRef]
- Pauly, D. Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol. Evol. 1995, 10, 430. [Google Scholar] [CrossRef]
- Kennard, M.; Arthington, A.; Pusey, B.; Harch, B. Are alien fish a reliable indicator of river health? Freshw. Biol. 2005, 50, 174–193. [Google Scholar] [CrossRef] [Green Version]
- Aparicio, E.; Carmona-Catot, G.; Moyle, P.B.; Garcia-Berthou, E. Development and evaluation of a fish-based index to assess biological integrity of Mediterranean streams. Aquat. Conserv.-Mar. Freshw. Ecosyst. 2011, 21, 324–337. [Google Scholar] [CrossRef]
- Meador, M.R.; Carlisle, D.M. Quantifying tolerance indicator values for common stream fish species of the United States. Ecol. Indic. 2007, 7, 329–338. [Google Scholar] [CrossRef]
- Pont, D.; Valentini, A.; Rocle, M.; Maire, A.; Delaigue, O.; Jean, P.; Dejean, T. The future of fish-based ecological assessment of European rivers: From traditional EU Water Framework Directive compliant methods to eDNA metabarcoding-based approaches. J. Fish Biol. 2021, 98, 354–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, A. An index of naturalness. J. Nat. Conserv. 2004, 12, 95–110. [Google Scholar] [CrossRef]
- Mihov, S. Development of fish based index for assessing ecological status of Bulgarian rivers (BRI). Biotechnol. Biotechnol. Equip. 2010, 24, 247–256. [Google Scholar] [CrossRef] [Green Version]
- Reyjol, Y.; Argillier, C.; Bonne, W.; Borja, A.; Buijse, A.D.; Cardoso, A.C.; Daufresne, M.; Kernan, M.; Ferreira, M.T.; Poikane, S. Assessing the ecological status in the context of the European Water Framework Directive: Where do we go now? Sci. Total Environ. 2014, 497, 332–344. [Google Scholar] [CrossRef]
- Vlachopoulou, M.; Coughlin, D.; Forrow, D.; Kirk, S.; Logan, P.; Voulvoulis, N. The potential of using the Ecosystem Approach in the implementation of the EU Water Framework Directive. Sci. Total Environ. 2014, 470–471, 684–694. [Google Scholar] [CrossRef]
- Şekercioğlu, Ç.H.; Anderson, S.; Akçay, E.; Bilgin, R.; Can, Ö.E.; Semiz, G.; Tavşanoğlu, Ç.; Yokeş, M.B.; Soyumert, A.; İpekdal, K.; et al. Turkey’s globally important biodiversity in crisis. Biol. Conserv. 2011, 144, 2752–2769. [Google Scholar] [CrossRef]
- Maltby, E. The Wetlands Paradigm Shift in Response to Changing Societal Priorities: A Reflective Review. Land 2022, 11, 1526. [Google Scholar] [CrossRef]
- Moyle, P.B.; Randall, P.J. Evaluating the biotic integrity of watersheds in the Sierra Nevada, California. Conserv. Biol. 1998, 12, 1318–1326. [Google Scholar] [CrossRef]
- Logez, M.; Pont, D. Global warming and potential shift in reference conditions: The case of functional fish-based metrics. Hydrobiologia 2013, 704, 417–436. [Google Scholar] [CrossRef]
- Woodward, G.; Perkins, D.; Brown, L. Climate change and freshwater ecosystems: Impacts across multiple levels of organization. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2093–2106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beechie, T.J.; Sear, D.A.; Olden, J.D.; Pess, G.R.; Buffington, J.M.; Moir, H.; Roni, P.; Pollock, M.M. Process-based principles for restoring river ecosystems. BioScience 2010, 60, 209–222. [Google Scholar] [CrossRef] [Green Version]







| Anthropogenic Pressure Category | Pressure Element | Score Modalities | Assessment Method |
|---|---|---|---|
| Morphological alteration | 1.Channel alteration | (1, 2, 3, 4, 5) | Visual assessment on-site |
| 2. Instream/aquatic habitat alteration | (1, 2, 3) | Visual assessment on-site | |
| 3. Embankment restraining riverbed and riparian | (1, 2, 3, 4, 5) | Remote sensing | |
| Riparian conditions | 4. Riparian vegetation alteration | (1, 2, 3, 4, 5) | Visual assessment on-site |
| Barriers to fish movement | 5. Barrier upstream—within water body segment | (1, 2, 3) | Remote sensing |
| 6. Barrier downstream—within water body segment | (1, 2, 5) | Remote sensing | |
| 7. Barrier in the catchment downstream | (1, 3, 5) | Remote sensing | |
| Hydrological | 8. Water abstraction affecting site | (1, 3, 5) | Bibliographic references |
| 9. Hydrological modification of flow regime | (1, 3, 5) | Bibliographic references | |
| Hydropeaking | 10. Hydropeaking due to water development, irrigation regulation, and hydroelectric works | (1, 2, 5) | Visual assessment on-site |
| Impounding | 11. Impounding at site and/or segment | (1, 2, 5) | Visual assessment on-site |
| Pollution | 12. Pollution observed during fish and macroinvertebrate sampling or in recent chemical sampling (where available) | (1, 2, 5) | Assessment visually on-site; bibliographic references; physicochemical parameters recorded on-site |
| Metrics | Min. | 25% Quantile | Median | Mean | 95% Quantile | Max. |
|---|---|---|---|---|---|---|
| Ric.RH.Par | 0.000 | 0.70 | 0.80 | 0.77 | 0.86 | 1.000 |
| Ni.LITHO | 0.000 | 0.71 | 0.80 | 0.73 | 0.83 | 1.000 |
| N | Site Name | Water Body Name | Longitude | Latitude | Elevation | Samples |
|---|---|---|---|---|---|---|
| 1 | BMN01_N3 | Yukari Banaz | 38.747665 | 29.765062 | 916 | 1 |
| 2 | BMN02_N4 | Asagi Banaz1 | 38.654668 | 29.773069 | 945 | 1 |
| 3 | BMN01_ST02 | Yukari Banaz | 38.887430 | 29.882031 | 1264 | 1 |
| 4 | BMN02_ST02 | Asagi Banaz1 | 38.729145 | 29.901113 | 1187 | 1 |
| 5 | BMN03_ST02 | Asagi Banaz2 | 38.363521 | 29.336081 | 537 | 1 |
| 6 | BMN04_ST01 | Dokuzsele1 | 38.686412 | 29.530637 | 899 | 1 |
| 7 | BMN05_ST02 | Dokuzsele 2 | 38.527895 | 29.374914 | 809 | 1 |
| 8 | BMN06_ST01 | Hamam1 | 38.381997 | 29.071666 | 651 | 1 |
| 9 | BMN07_ST01 | Hamam 2 | 38.283191 | 29.072374 | 610 | 1 |
| 10 | BMN11_Bu | Kufi4 | 38.269653 | 29.864130 | 821 | 1 |
| 11 | BMN12_ST02 | Yukari Büyük Menderes 1 | 38.122981 | 30.095312 | 843 | 2 |
| 12 | BMN13_ST01 | Yukari Büyük Menderes 2 | 38.156494 | 29.640245 | 811 | 2 |
| 13 | BMN14_ST01 | Çaykavuştu1 | 37.729445 | 29.370022 | 1019 | 1 |
| 14 | BMN15_ST01 | Çaykavuştu2 | 37.719342 | 29.397376 | 988 | 1 |
| 15 | BMN16_ST01 | Yukarı Çürüksu | 37.763766 | 29.387657 | 860 | 1 |
| 16 | BMN17_ST01 | Gokpinar Deresi | 37.724315 | 29.208474 | 668 | 1 |
| 17 | BMN19_ST01 | Asagi Curuksu2 | 37.884179 | 29.100378 | 179 | 1 |
| 18 | BMN20_ST02 | Orta Büyük Menderes | 37.933321 | 28.687675 | 117 | 2 |
| 19 | BMN21_ST01 | Yukari Dandalaz | 37.700548 | 28.684416 | 451 | 2 |
| 20 | BMN22_ST01 | Asagi Dandalaz | 37.876850 | 28.537744 | 84 | 1 |
| 21 | BMN23_ST02 | Yukari Akcay1 | 37.521739 | 28.985097 | 894 | 2 |
| 22 | BMN24_ST01 | Yukari Akcay2 | 37.537270 | 28.921229 | 894 | 1 |
| 23 | BMN25_ST01 | Yukari Akcay3 | 37.521221 | 28.785840 | 591 | 1 |
| 24 | BMN26_ST01 | Yukari Akcay4 | 37.408976 | 28.625789 | 319 | 2 |
| 25 | BMN28_ST02 | Asagi Akcay | 37.786307 | 28.334788 | 60 | 1 |
| 26 | BMN29_ST01 | Girme Deresi | 37.273232 | 28.021417 | 499 | 1 |
| 27 | BMN30_ST01 | Yukari Cine1 | 37.426505 | 28.141515 | 266 | 1 |
| 28 | BMN32_ST01 | Yukari Cine3 | 37.547058 | 28.161884 | 556 | 2 |
| 29 | BMN33_ST01 | Asagi Cine1 | 37.595376 | 27.771665 | 296 | 1 |
| 30 | BMN33-2 | Asagi Cine1 | 37.601343 | 27.775746 | 310 | 1 |
| 31 | BMN34_ST02 | Asagi Cine2 | 37.695438 | 27.928781 | 40 | 1 |
| 32 | BMN35_ST01 | Asagi Cine3 | 37.745620 | 28.060388 | 140 | 1 |
| 33 | BMN37_ST01 | Asagi Saricay | 37.722100 | 27.515985 | 11 | 1 |
| 34 | BMN47_ST01 | Yukari Ikizdere1 | 37.928269 | 27.777246 | 195 | 2 |
| 35 | BMN48_ST01 | Yukari Ikizdere2 | 37.954009 | 27.758918 | 216 | 2 |
| 36 | BMN50_ST01 | Asagi Ikizdere2 | 37.809497 | 27.734028 | 23 | 1 |
| 37 | BMN51_ST01 | Yalki | 37.836625 | 27.695994 | 24 | 1 |
| 38 | BMN53_ST01 | Naipli Cayi | 37.870638 | 27.419755 | 164 | 1 |
| 39 | BMN55_ST01 | Asagi Büyük Menderes1 | 37.803416 | 27.677998 | 21 | 2 |
| 40 | BMN56_ST02 | Asagi Büyük Menderes2 | 37.505351 | 27.337874 | 3 | 2 |
| 41 | BMN56_ST02_ds | Asagi Büyük Menderes2 | 37.505606 | 27.342842 | 4 | 1 |
| 42 | BMN25_ST01_01 | Yukari Akcay3 | 37.492186 | 28.742547 | 541 | 1 |
| 43 | BMN56_ST02_m | Asagi Büyük Menderes2 | 37.541314 | 27.211556 | 0 | 1 |
| 44 | BMN56_ST02_nc | Asagi Büyük Menderes2 | 37.555730 | 27.215484 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zogaris, S.; Koutsikos, N.; Chatzinikolaou, Y.; Őzeren, S.C.; Yence, K.; Vlami, V.; Kohlmeier, P.G.; Akyildiz, G.K. Fish Assemblages as Ecological Indicators in the Büyük Menderes (Great Meander) River, Turkey. Water 2023, 15, 2292. https://doi.org/10.3390/w15122292
Zogaris S, Koutsikos N, Chatzinikolaou Y, Őzeren SC, Yence K, Vlami V, Kohlmeier PG, Akyildiz GK. Fish Assemblages as Ecological Indicators in the Büyük Menderes (Great Meander) River, Turkey. Water. 2023; 15(12):2292. https://doi.org/10.3390/w15122292
Chicago/Turabian StyleZogaris, Stamatis, Nicholas Koutsikos, Yorgos Chatzinikolaou, Saniye Cevher Őzeren, Kaan Yence, Vassiliki Vlami, Pinar Güler Kohlmeier, and Gürçay Kıvanç Akyildiz. 2023. "Fish Assemblages as Ecological Indicators in the Büyük Menderes (Great Meander) River, Turkey" Water 15, no. 12: 2292. https://doi.org/10.3390/w15122292
APA StyleZogaris, S., Koutsikos, N., Chatzinikolaou, Y., Őzeren, S. C., Yence, K., Vlami, V., Kohlmeier, P. G., & Akyildiz, G. K. (2023). Fish Assemblages as Ecological Indicators in the Büyük Menderes (Great Meander) River, Turkey. Water, 15(12), 2292. https://doi.org/10.3390/w15122292









