Performance Assessment of Household Water Treatment and Safe Storage in Kathmandu Valley, Nepal
Abstract
:1. Introduction
2. Materials and Methods
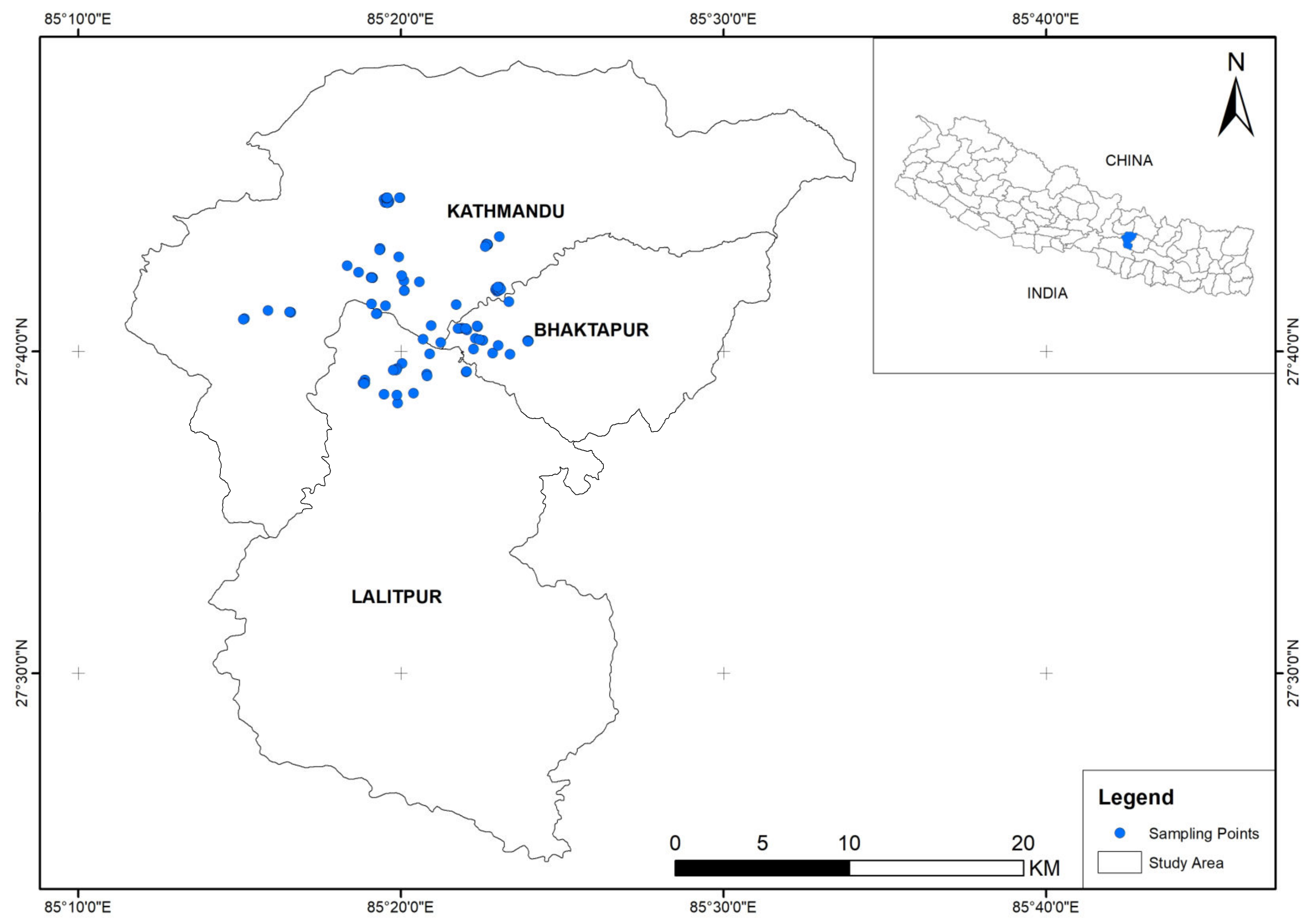
2.1. Study Area
2.2. Sampling Points
2.3. Water Quality Analysis
2.4. Questionnaire Survey
2.5. Statistical Analysis
3. Results
3.1. Raw Water Quality
3.1.1. Physicochemical Parameters
3.1.2. Microbial Parameters
3.2. Treatment Performance of HWTS
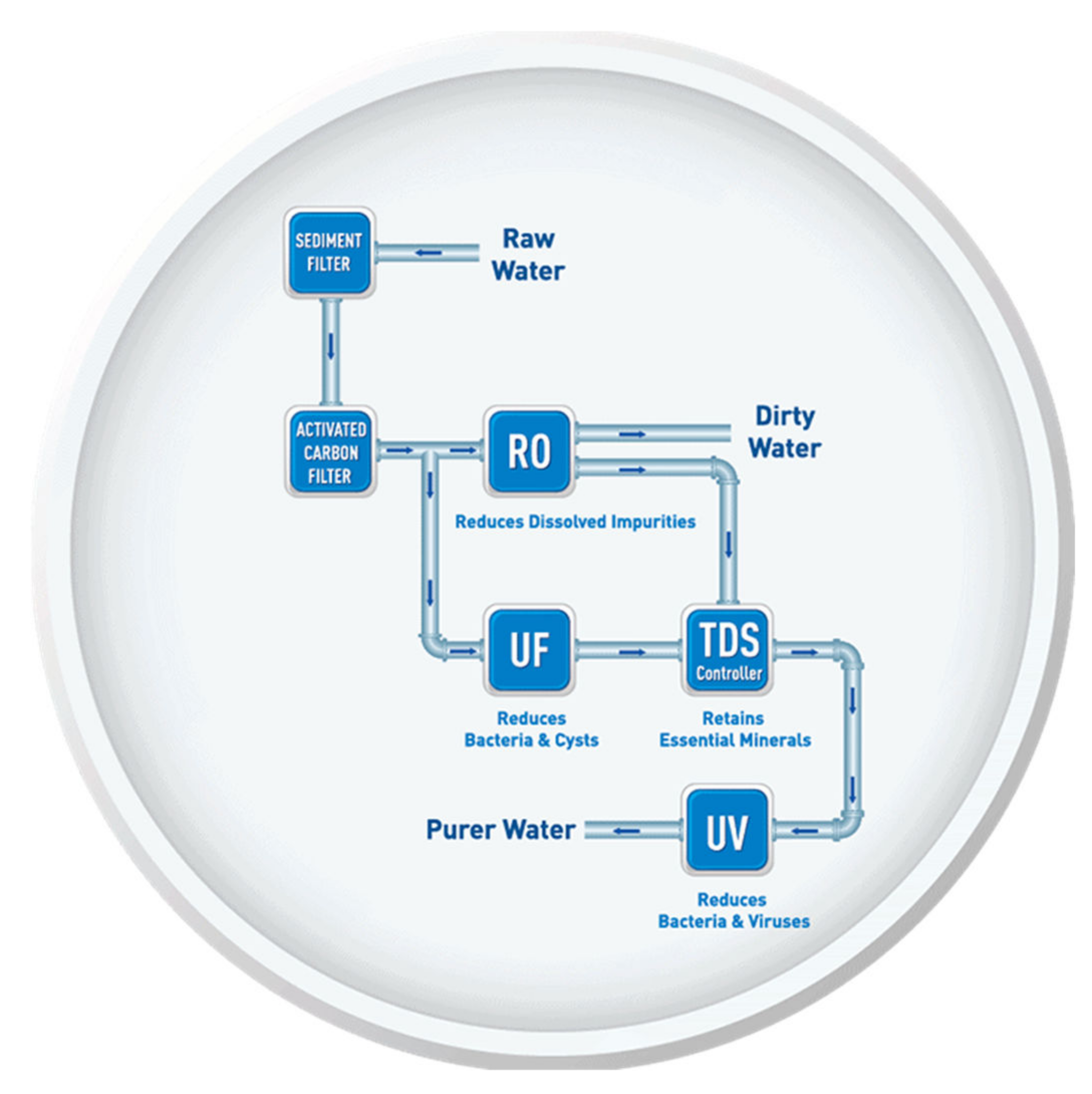
3.2.1. Selection of HWTS for Different Water Sources
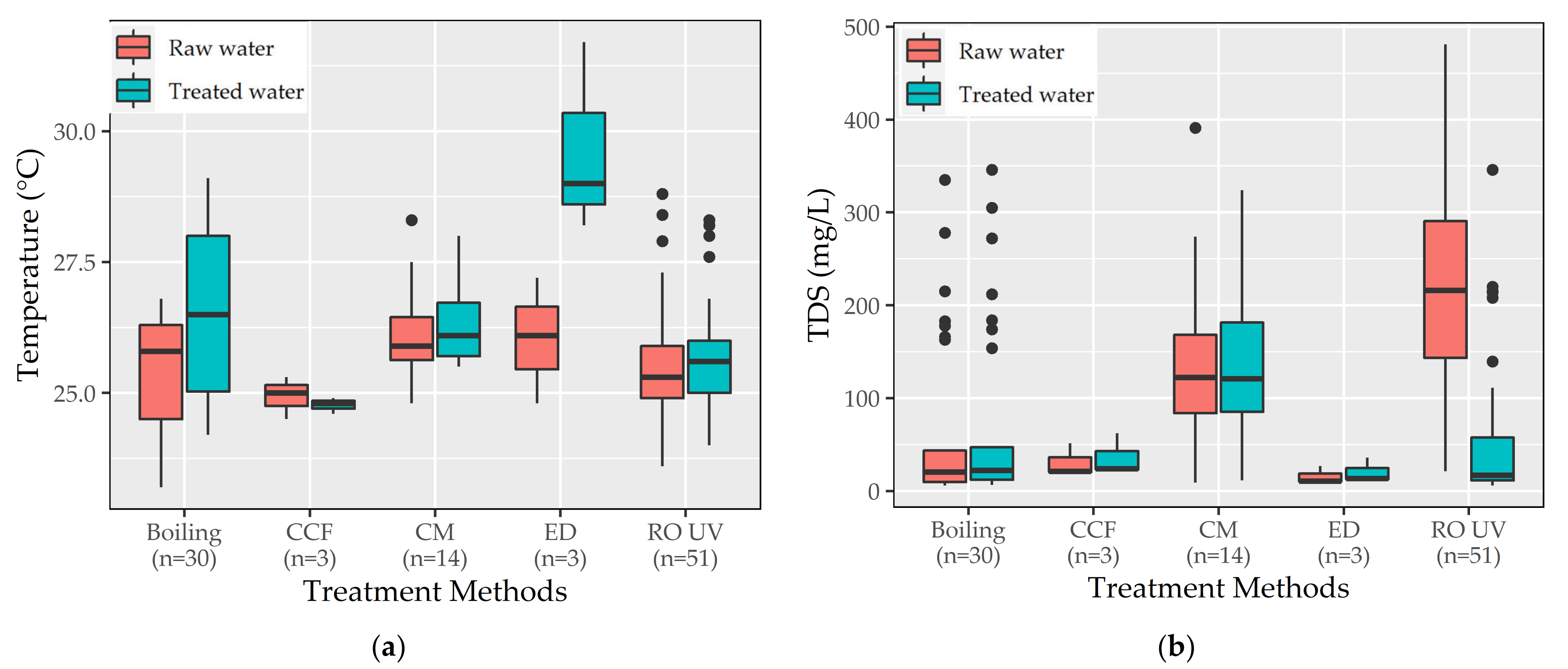
3.2.2. Physicochemical Parameters
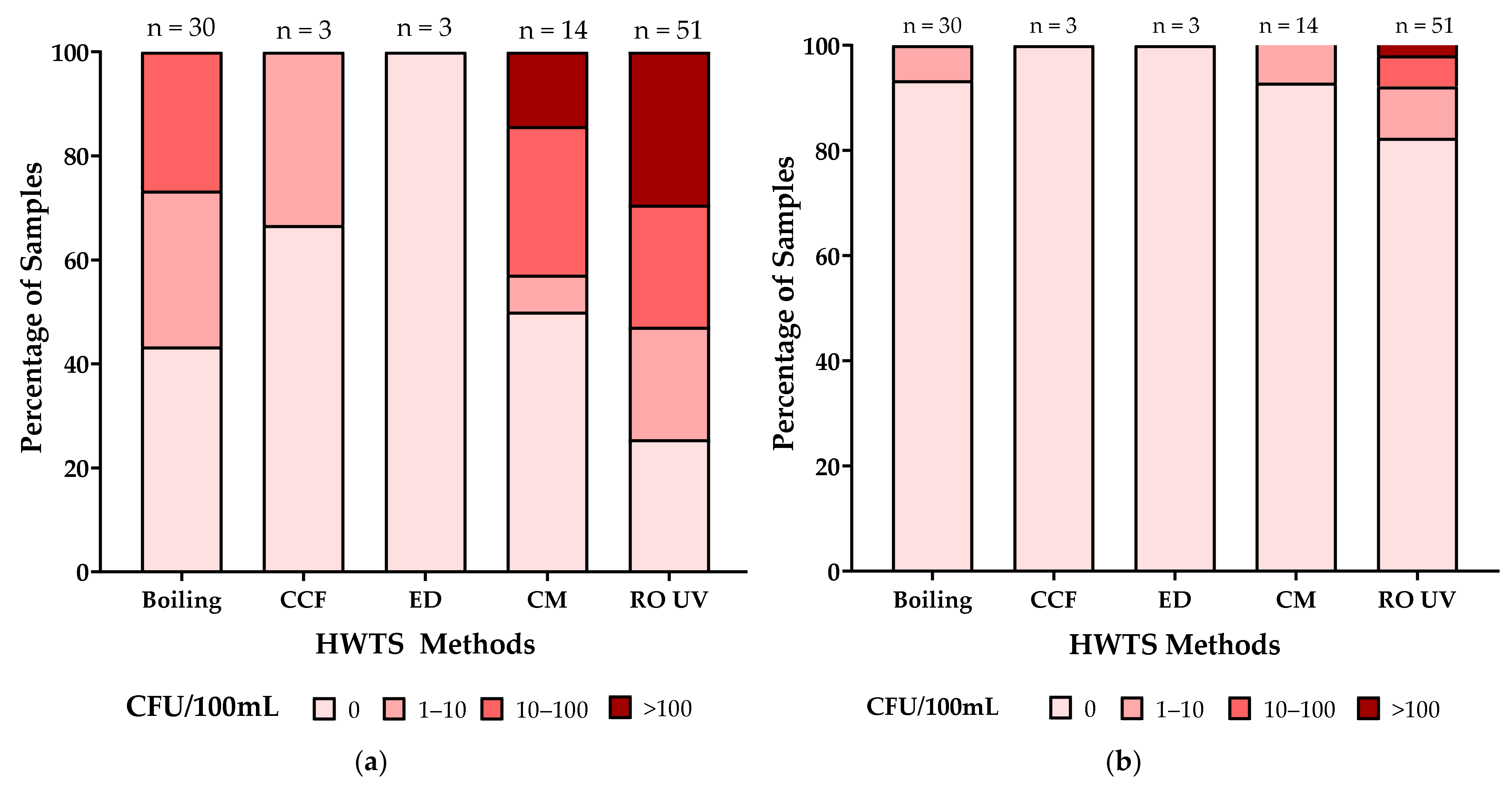
3.2.3. Microbial Parameters
3.3. Reduction in Proportions of Samples Noncompliant with NDWQS by the HWTS Treatments
3.4. Questionnaire Survey
4. Discussion
4.1. Selection of HWTS Based on the Removal of Physicochemical Contaminants
4.2. Selection of HWTS Based on Bacterial Reduction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HWTS | Household water treatment and safe storage |
| POU | Point of use |
| POE | Point of entry |
| TC | Total coliforms |
| NDWQS | National Drinking Water Quality Standards |
| CCF | Ceramic candle filter |
| ED | Electric dispenser |
| CM | Combined methods |
| RO-UV | Reverse osmosis with ultraviolet irradiation |
| TDS | Total dissolved solids |
| LDL | Lower detection limit |
| UDL | Upper detection limit |
| TMTC | Too many to count |
| CFU | Colony-forming units |
Appendix A
| No. | HWTS Methods | Market Price Range (NPR) | Average Monthly Cost (NPR) | |
|---|---|---|---|---|
| Minimum Price | Maximum Price | |||
| 1 | Boiling | 600 | 3000 | 340 |
| 2 | CCF | 1500 | 13,000 | 56.7 |
| 3 | RO-UV | 15,000 | 57,000 | 799 |
| 4 | ED | 2200 | 9000 | 340 |
| 5 | Chemicals | 25 | 55 | 22.5 |
| No. | Parameter | Unit | Device/Method | LDL * | UDL ** | Sensitivity |
|---|---|---|---|---|---|---|
| 1 | Temperature | °C | Horiba Compact EC meter: LAQUAtwin EC-33B | 0 | 50 | 0.1 |
| 2 | TDS | mg/L | 0 | 9900 | 0.1–1 | |
| 3 | pH | - | Horiba Compact pH meter: LAQUAtwin pH-22B | 0 | 14 | 0.1 |
| 4 | Ammonia-nitrogen | mg/L | Hach® DR 900/salicylate method | 0.4 | 50 | 0.3 |
| 5 | Manganese | mg/L | Hach® DR 900/USEPA periodate oxidation method | 0.1 | 20 | 0.1 |
| 6 | Arsenic | mg/L | Pack test and arsenic set Kyoritsu Laboratories SPK-As(D) | 0.009 | 0.2 | 0.001 |
| 7 | E. coli | CFU/100 mL | Portable incubator/USEPA Method 10029 | NA | NA | NA |
| 8 | Total coliforms | CFU/100 mL |
| HWTS Method | Net Samples Detected with TC | Samples with Increased TC | Mean TC Raw * | Mean TC Treated * | LRV ** |
|---|---|---|---|---|---|
| Boiling | 29 | 2 | 116 | 180 | −0.18 |
| CCF | 3 | 1 | 133 | 275 | −0.31 |
| ED | 3 | 0 | 0 | 0 | NA |
| CM | 14 | 3 | 35 | 111 | −0.49 |
| RO-UV | 50 | 7 | 48 | 180 | −0.57 |
References
- United Nations General Assembly. 64/292: The Human Right to Water and Sanitation; United Nations: New York City, NY, USA, 2010; Available online: https://digitallibrary.un.org/record/687002?ln=en (accessed on 28 February 2023).
- UNICEF-WHO. 1 in 3 People Globally Do Not Have Access to Safe Drinking Water. 2019. Available online: https://www.who.int/news/item/18-06-2019-1-in-3-people-globally-do-not-have-access-to-safe-drinking-water-unicef-who (accessed on 28 February 2023).
- UN-Habitat. Water, A Shared Responsibility: The United Nations World Water Development Report 2; UN-Habitat: Nairobi, Kenya, 2006; Available online: https://unhabitat.org/water-a-shared-responsibility-the-united-nations-world-water-development-report-2-2 (accessed on 28 February 2023).
- Mwabi, J.K.; Adeyemo, F.E.; Mahlangu, T.O.; Mamba, B.B.; Brouckaert, B.M.; Swartz, C.D.; Offringa, G.; Mpenyana-Monyatsi, L.; Momba, M.N.B. Household water treatment systems: A solution to the production of safe drinking water by the low-income communities of Southern Africa. Phys. Chem. Earth Parts A/B/C 2011, 36, 1120–1128. [Google Scholar] [CrossRef]
- Prüss-Ustün, A.; Wolf, J.; Bartram, J.; Clasen, T.; Cumming, O.; Freeman, M.C.; Gordon, B.; Hunter, P.R.; Medlicott, K.; Johnston, R. Burden of disease from inadequate water, sanitation and hygiene for selected adverse health outcomes: An updated analysis with a focus on low-and middle-income countries. Int. J. Hyg. Environ. Health 2019, 222, 765–777. [Google Scholar] [CrossRef] [PubMed]
- WHO. Diarrhoeal Disease. 2017. Available online: https://www.who.int/en/news-room/fact-sheets/detail/diarrhoeal-disease (accessed on 3 April 2023).
- Clasen, T.F. Household water treatment and the millennium development goals: Keeping the focus on health. Environ. Sci. Technol. 2010, 44, 7357–7360. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.; Gundry, S.; Conroy, R.M. Household drinking water in developing countries: A systematic review of microbiological contamination between source and point-of-use. Trop. Med. Int. Health 2004, 9, 106–117. [Google Scholar] [CrossRef] [Green Version]
- Masanyiwa, Z.S.; Zilihona, I.J.; Kilobe, B.M. Users’ perceptions on drinking water quality and household water treatment and storage in small towns in Northwestern Tanzania. Open J. Soc. Sci. 2019, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- WHO. Combating Waterborne Disease at the Household Level; World Health Organization: Geneva, Switzerland, 2007; Available online: https://apps.who.int/iris/handle/10665/43621 (accessed on 10 April 2023).
- Mwabi, J.K.; Mamba, B.B.; Momba, M.N. Removal of Escherichia coli and faecal coliforms from surface water and groundwater by household water treatment devices/systems: A sustainable solution for improving water quality in rural communities of the Southern African development community region. Int. J. Environ. Res. Public Health 2012, 9, 139–170. [Google Scholar] [CrossRef]
- Xiao, R.; Duan, Y.; Chu, W. The effectiveness of household water treatment and safe storage in improving drinking water quality: A disinfection by-product (DBP) perspective. J. Water Supply Res. Technol.-Aqua 2020, 69, 785–806. [Google Scholar] [CrossRef]
- Global Water Treatment Systems Market Size, Trends & Growth Opportunity, by Installation, by Technology, by End-Use by Region and Forecast to 2028. 2022. Available online: https://www.reportsanddata.com/report-detail/point-of-use-water-treatment-systems-market (accessed on 1 March 2023).
- Rosa, G.; Huaylinos, M.L.; Gil, A.; Lanata, C.; Clasen, T. Assessing the consistency and microbiological effectiveness of household water treatment practices by urban and rural populations claiming to treat their water at home: A case study in Peru. PLoS ONE 2014, 9, e114997. [Google Scholar] [CrossRef] [Green Version]
- Alsulaili, A.; Al-Harbi, M.; Elsayed, K. The influence of household filter types on the quality of drinking water. Process Saf. Environ. Prot. 2020, 143, 204–211. [Google Scholar] [CrossRef]
- Stubbé, S.M.; Pelgrim-Adams, A.; Szántó, G.L.; Van Halem, D. Household water treatment and safe storage–effectiveness and economics. Drink. Water Eng. Sci. 2016, 9, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Oswald, W.E.; Lescano, A.G.; Bern, C.; Calderon, M.M.; Cabrera, L.; Gilman, R.H. Fecal contamination of drinking water within peri-urban households, Lima, Peru. Am. J. Trop. Med. Hyg. 2007, 77, 699–704. [Google Scholar] [CrossRef] [Green Version]
- Massieux, B.; Boivin, M.E.; Van Den Ende, F.P.; Langenskiöld, J.; Marvan, P.; Barranguet, C.; Admiraal, W.; Laanbroek, H.J.; Zwart, G. Analysis of structural and physiological profiles to assess the effects of Cu on biofilm microbial communities. Appl. Environ. Microbiol. 2004, 70, 4512–4521. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, K.B.; Thapa, B.R.; Aihara, Y.; Shrestha, S.; Bhattarai, A.P.; Bista, N.; Kazama, F.; Shindo, J. Hidden Cost of Drinking Water Treatment and Its Relation with Socioeconomic Status in Nepalese Urban Context. Water 2018, 10, 607. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, S.; Haramoto, E.; Malla, R.; Nishida, K. Risk of diarrhoea from shallow groundwater contaminated with enteropathogens in the Kathmandu Valley, Nepal. J. Water Health 2015, 13, 259–269. [Google Scholar] [CrossRef]
- Japan International Cooperation Agency. Institutional Framework Assessment of KUKL: Final Report; JICA: Kathmandu, Nepal, 2016. Available online: https://www.jica.go.jp/nepal/english/office/others/c8h0vm0000bjww96-att/publications_04.pdf (accessed on 1 March 2023).
- Katuwal, H.; Bohara, A.K. Coping with poor water supplies: Empirical evidence from Kathmandu, Nepal. J. Water Health 2011, 9, 143–158. [Google Scholar] [CrossRef] [Green Version]
- Raina, A. Equity in Urban Water Service Delivery and the Role of Informal Water Vendors: The Case of Kathmandu Valley, Nepal; NUS Libraries: Singapore, 2016; Available online: https://scholarbank.nus.edu.sg/handle/10635/135477 (accessed on 2 March 2023).
- Molden, O.C.; Khanal, A.; Pradhan, N. The pain of water, a household perspective of water insecurity and inequity in the Kathmandu Valley. Water Policy 2018, 22, 130–145. [Google Scholar] [CrossRef]
- Pattanayak, S.K.; Yang, J.C.; Whittington, D.; Balkumar, K.C. Coping with unreliable public water supplies: Averting expenditures by households in Kathmandu, Nepal. Water Resour. Res. 2005, 41, W02012. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, S.; Aihara, Y.; Kondo, N.; Rajbhandari, S.; Bhattarai, A.P.; Bista, N.; Kazama, F.; Nishida, K.; Timilsina, H.P.; Shindo, J. Household Water Use in the Kathmandu Valley: A Dry Season Survey; Interdisciplinary Center for River Basin Environment (ICRE): Kofu, Japan, 2016. [Google Scholar] [CrossRef]
- Shrestha, S.; Kobayashi, Y.; Ito, Y.; Yokomichi, H.; Nishida, K. Relationship between diarrhea risk and the combinations of drinking water sources in the Kathmandu Valley, Nepal. Int. Health 2022, 14, 195–200. [Google Scholar] [CrossRef]
- Malla, B.; Ghaju Shrestha, R.; Tandukar, S.; Bhandari, D.; Inoue, D.; Sei, K.; Tanaka, Y.; Sherchand, J.B.; Haramoto, E. Identification of Human and Animal Fecal Contamination in Drinking Water Sources in the Kathmandu Valley, Nepal, Using Host-Associated Bacteroidales Quantitative PCR Assays. Water 2018, 10, 1796. [Google Scholar] [CrossRef] [Green Version]
- Maharjan, S.; Joshi, T.P.; Shrestha, S.M. Poor quality of treated water in Kathmandu: Comparison with Nepal Drinking Water Quality Standards. Tribhuvan Univ. J. Microbiol. 2018, 5, 83–88. [Google Scholar] [CrossRef]
- Subedi, M.; Aryal, M. Public perception about drinking jar water and its bacteriological analysis. Nepal Med. Coll. J. 2010, 12, 110–114. Available online: https://europepmc.org/article/med/21222409 (accessed on 2 March 2023). [PubMed]
- Guragai, B.; Takizawa, S.; Hashimoto, T.; Oguma, K. Effects of inequality of supply hours on consumers’ coping strategies and perceptions of intermittent water supply in Kathmandu Valley, Nepal. Sci. Total Environ. 2017, 599, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Government of Nepal. A Country Report Based on the Global Burden of Disease Study; Ministry of Health and Population: Kathmandu, Nepal, 2017. Available online: https://nhrc.gov.np/wp-content/uploads/2019/04/NBoD-2017_NHRC-MoHP.pdf (accessed on 1 March 2023).
- Lin, W.; Ye, C.; Guo, L.; Hu, D.; Yu, X. Analysis of microbial contamination of household water purifiers. Appl. Microbiol. Biotechnol. 2020, 104, 4533–4545. Available online: https://link.springer.com/article/10.1007%2Fs00253-020-10510-5 (accessed on 12 March 2023). [CrossRef] [PubMed]
- Rosa, G.; Kelly, P.; Clasen, T. Consistency of use and effectiveness of household water treatment practices among urban and rural populations claiming to treat their drinking water at home: A case study in Zambia. Am. J. Trop. Med. Hyg. 2016, 94, 445. [Google Scholar] [CrossRef]
- Koju, N.K.; Prasai, T.; Shrestha, S.M.; Raut, P. Drinking water quality of Kathmandu Valley. Nepal J. Sci. Technol. 2014, 15, 115–120. [Google Scholar] [CrossRef]
- Pant, P.R.; Dongo, D. Kathmandu Valley Profile; Governance and Infrastructure Development Challenges in Kathmandu Valley: Kathmandu, Nepal, 2009; Available online: https://silo.tips/download/kathmandu-valley-profile-briefing-paper (accessed on 1 March 2023).
- Chinnasamy, P.; Shrestha, S.R. Melamchi Water Supply Project: Potential to replenish Kathmandu’s groundwater status for dry season access. Water Policy 2019, 21, 29–49. [Google Scholar] [CrossRef]
- Adhikari, B.R.; Shrestha, S.D.; Shakya, N.M. Future urban water crisis in mountain regions: Example of Kathmandu Valley, Nepal. In Urban Drought: Emerging Water Challenges in Asia; Springer: Singapore, 2019; pp. 169–182. [Google Scholar] [CrossRef]
- Raina, A.; Zhao, J.; Wu, X.; Kunwar, L.; Whittington, D. The structure of water vending markets in Kathmandu, Nepal. Water Policy 2019, 21, 50–75. [Google Scholar] [CrossRef]
- Government of Nepal. Annual Report (15th Anniversary); Kathmandu Upatyaka Khanepani Limited: Kathmandu, Nepal, 2023; Available online: https://kathmanduwater.org/wp-content/uploads/2023/02/KUKL_Annual_Report_2079.pdf (accessed on 1 April 2023).
- Government of Nepal. National Population and Housing Census; Central Bureau of Statistics: Kathmandu, Nepal, 2011; Available online: https://unstats.un.org/unsd/demographic/sources/census/wphc/nepal/nepal-census-2011-vol1.pdf (accessed on 30 April 2023).
- Thapa, K.; Shrestha, S.M.; Rawal, D.S.; Pant, B.R. Quality of drinking water in Kathmandu valley, Nepal. Sustain. Water Resour. Manag. 2019, 5, 1995–2000. [Google Scholar] [CrossRef]
- Malla, B.; Ghaju Shrestha, R.; Tandukar, S.; Bhandari, D.; Thakali, O.; Sherchand, J.B.; Haramoto, E. Detection of pathogenic viruses, pathogen indicators, and fecal-source markers within tanker water and their sources in the Kathmandu Valley, Nepal. Pathogens 2019, 8, 81. [Google Scholar] [CrossRef] [Green Version]
- Government of Nepal. National Drinking Water Quality Standard 2023: Implementation Directive; Department of Water Supply and Sewerage: Kathmandu, Nepal, 2023. Available online: https://dwssm.gov.np/storage/listies/March2023/khanepani-mapdanda-kitab-final.pdf (accessed on 1 April 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 22 April 2023).
- Gautam, B.; Gyanwali, G.; Ussery, D.W. Assessment of bacterial load in polyethylene terephthalate (PET) bottled water marketed in Kathmandu Valley, Nepal. Int. J. Polym. Sci. 2021, 2021, 6681249. [Google Scholar] [CrossRef]
- Emerman, S.H.; Prasai, T.; Anderson, R.B.; Palmer, M.A. Arsenic contamination of groundwater in the Kathmandu Valley, Nepal, as a consequence of rapid erosion. J. Nepal Geol. Soc. 2010, 40, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Shakya, S.; Sherstha, P.K.; Pradhanana, A.R.; Shakya, R.K.; Shrestha, M.; Pant, D.R.; Yadav, P.K.; Ghimire, N.G.; Shakya, P. R Assessment of drinking water quality of Kathmandu municipality area in pre-monsoon season. Res. J. Recent Sci. 2019, 8, 54–62. Available online: http://www.isca.in/rjrs/archive/v8/i4/7.ISCA-RJRS-2019-029.php (accessed on 3 March 2023).
- Burlakoti, N.; Upadhyaya, J.; Ghimire, N.; Bajgai, T.R.; Chhetri, A.B.; Rawal, D.S.; Koirala, N.; Pant, B.R. Physical, chemical and microbiological characterization of processed drinking water in central Nepal: Current state study. J. Water Sanit. Hyg. Dev. 2020, 10, 157–165. [Google Scholar] [CrossRef]
- Gwachha, S.; Acharya, B.N.; Dhakal, A.; Shrestha, S.M.; Joshi, T.P. Assessment of Arsenic Content in Deep Groundwater of Kathmandu Valley, Nepal. Nepal J. Sci. Technol. 2020, 19, 69–77. [Google Scholar] [CrossRef]
- Wang, B.B. Research on drinking water purification technologies for household use by reducing total dissolved solids (TDS). PLoS ONE 2021, 16, e0257865. [Google Scholar] [CrossRef]
- Murindababisha, D.; Havugimana, V.; Mutezinka, H.; Nimukuze, D. Performance of Locally Produced Ceramic Pot Filters for Drinking Water Treatment in Rwanda. Int. J. Appl. Chem. 2018, 5, 10–14. [Google Scholar] [CrossRef]
- Pourjamali, R.; Sadrabad, E.K.; Hashemi, S.A.; Shekofteh, H.; Mokhtari, M.; Heydari, A.; Mohajeri, F.A. Evaluation of Point-of-Use Drinking Water Treatment Systems Efficiency in Reducing or Removing Physicochemical Parameters and Heavy Metals. J. Environ. Health Sustain. Dev. 2019, 4, 717–726. Available online: http://dorl.net/dor/20.1001.1.24766267.2019.4.1.8.6 (accessed on 2 March 2023). [CrossRef]
- Bae, M.; Watanabe, C.; Inaoka, T.; Sekiyama, M.; Sudo, N.; Bokul, M.H.; Ohtsuka, R. Arsenic in cooked rice in Bangladesh. Lancet 2002, 360, 1839–1840. [Google Scholar] [CrossRef]
- Tamene, A. A qualitative analysis of factors influencing household water treatment practices among consumers of self-supplied water in rural Ethiopia. Risk Manag. Healthc. Policy 2021, 14, 1129–1139. [Google Scholar] [CrossRef]
- Figueroa, M.E.; Kincaid, D.L. Social, Cultural and Behavioral Correlates of Household Water Treatment and Storage; Center Publication HCI 2010-1; Johns Hopkins Bloomberg School of Public Health, Center for Communication Programs: Baltimore, MD, USA, 2010; Available online: https://ccp.jhu.edu/wp-content/uploads/2014/11/Household-Water-Treatment-and-Storage-2010.pdf (accessed on 6 April 2023).
- Koyuncu, I.; Topacik, D.; Turan, M.; Celik, M.S.; Sarikaya, H.Z. Application of the membrane technology to control ammonia in surface water. Water Sci. Technol. 2001, 1, 117–124. [Google Scholar] [CrossRef]
- How To Remove Manganese from Water: Ultimate Guide. 2022. Available online: https://waterfilterguru.com/how-to-remove-manganese-from-water/ (accessed on 1 April 2023).
- Barbeau, B.; Carrière, A.; Bouchard, M.F. Spatial and temporal variations of manganese concentrations in drinking water. J. Environ. Sci. Health Part A 2011, 46, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Boil Water Response—Information for the Public Health Professional. Department of Health: New York State. Available online: https://www.health.ny.gov/environmental/water/drinking/boilwater/response_information_public_health_professional.htm (accessed on 22 April 2023).
- Mahlangu, T.O.; Mamba, B.B.; Momba, M.N. A comparative assessment of chemical contaminant removal by three household water treatment filters. Water SA 2012, 38, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Pratson, E.; Vengosh, A.; Dwyer, G.; Pratson, L.; Klein, E. The effectiveness of arsenic remediation from groundwater in a private home. Groundw. Monit. Remediat. 2010, 30, 87–93. [Google Scholar] [CrossRef]
- George, C.M.; Smith, A.H.; Kalman, D.A.; Steinmaus, C.M. Reverse osmosis filter use and high arsenic levels in private well water. Arch. Environ. Occup. Health 2006, 61, 171–175. [Google Scholar] [CrossRef]
- Walker, M.; Seiler, R.L.; Meinert, M. Effectiveness of household reverse-osmosis systems in a Western US region with high arsenic in groundwater. Sci. Total Environ. 2008, 389, 245–252. [Google Scholar] [CrossRef]
- Saturday, A.; Makokha, G.L.; Macharia, A. Performance of household water treatment methods for microbial removal under household conditions in Kabale district, Uganda. J. Environ. Health Sci. 2016, 2, 1–9. [Google Scholar] [CrossRef]
- Daniel, D. Factors Influencing the Household Water Treatment Adoption in Rural Areas in Developing Countries. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2021; p. 6. [Google Scholar] [CrossRef]
- Psutka, R.; Peletz, R.; Michelo, S.; Kelly, P.; Clasen, T. Assessing the microbiological performance and potential cost of boiling drinking water in urban Zambia. Environ. Sci. Technol. 2011, 45, 6095–6101. [Google Scholar] [CrossRef] [Green Version]
- Al Moosa, M.E.; Khan, M.A.; Alalami, U.; Hussain, A. Microbiological quality of drinking water from water dispenser machines. Int. J. Environ. Sci. Dev. 2015, 6, 710. [Google Scholar] [CrossRef] [Green Version]
- Murphy, H.M.; McBean, E.A.; Farahbakhsh, K. A critical evaluation of two point-of-use water treatment technologies: Can they provide water that meets WHO drinking water guidelines? J. Water Health 2010, 8, 611–630. [Google Scholar] [CrossRef] [Green Version]
- Al-Haddad, A.; Al-Qallaf, H.; Rashid, T. Effectiveness of domestic water filters. Desalination Water Treat. 2015, 53, 649–657. [Google Scholar] [CrossRef]
- Fengyi, S.; Mingfang, L.; Zhang, F.; Peng, L.I.; Kai, L.O.U.; Xing, X. Performance of microbiological control by a point-of-use filter system for drinking water purification. J. Environ. Sci. 2009, 21, 1237–1246. [Google Scholar] [CrossRef]
- Malan, A.; Sharma, H.R. Assessment of drinking water quality and various household water treatment practices in rural areas of Northern India. Arab. J. Geosci. 2023, 16, 96. [Google Scholar] [CrossRef]
- Fahiminia, M.; Mosaferi, M.; Taadi, R.A.; Pourakbar, M. Evaluation of point-of-use drinking water treatment systems’ performance and problems. Desalin. Water Treat. 2014, 52, 1855–1864. [Google Scholar] [CrossRef]
- Labhasetwar, P.K.; Yadav, A. Membrane Based Point-of-Use Drinking Water Systems. IWA Publ. 2023, 35, 131–150. [Google Scholar] [CrossRef]
- What is TDS Controller/MTDS and Mineralizer? Which One to Choose? 2023. Available online: https://www.bestrowaterpurifier.in/blog/tds-controller/ (accessed on 7 April 2023).






| District | Water Sources | HWTS | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Piped Water | Jarred Water | Groundwater | Tanker Water | Boiling | CCF | ED | CM | RO-UV | ||
| Kathmandu | 27 | 8 | 20 | 3 | 14 | 0 | 2 | 9 | 33 | 58 |
| Lalitpur | 4 | 10 | 4 | 5 | 8 | 3 | 1 | 3 | 8 | 23 |
| Bhaktapur | 6 | 7 | 7 | 0 | 8 | 0 | 0 | 2 | 10 | 20 |
| Total | 37 | 25 | 31 | 8 | 30 | 3 | 3 | 14 | 51 | 101 |
| Parameter | Description | Piped Water (n = 37) | Groundwater (n = 31) | Jarred Water (n = 25) | Tanker Water (n = 8) | NDWQS |
|---|---|---|---|---|---|---|
| Temperature (°C) | Mean ± SD | 25.7 ± 1.1 | 25.6 ± 0.9 | 25.4 ± 1.0 | 25.0 ± 0.8 | NA |
| Range | 24.2 to 28.8 | 23.6 to 28.3 | 23.2 to 27.2 | 23.8 to 26.1 | ||
| pH * | Mean ± SD | 7.38 ± 0.33 | 7.14 ± 0.36 | 6.77 ± 0.75 | 7.89 ± 0.46 | 6.50–8.50 |
| Range | 6.82 to 7.95 | 6.5 to 8.11 | 5.88 to 8.54 | 7.00 to 8.29 | ||
| TDS (mg/L) | Mean ± SD | 130.1 ± 93.3 | 269.1 ± 89.1 | 16.0 ± 11.4 | 172.3 ± 8.8 | 1000 |
| Range | 19.5 to 322.0 | 115.0 to 481.0 | 6.0 to 51.2 | 158.0 to 183.0 | ||
| Ammonia Nitrogen * (mg/L) | Mean ± SD | 0.54 ± 0.88 | 1.43 ± 5.41 | 0.56 ± 0.81 | 1.00 ± 1.55 | 1.50 |
| Range | LDL ** to 3.00 | LDL to 26.00 | LDL to 3.00 | LDL to 3.00 | ||
| Arsenic (mg/L) | Mean ± SD | 0.022 ± 0.014 | 0.043 ± 0.044 | 0.019 ± 0.01 | 0.041 ± 0.048 | 0.050 |
| Range | LDL to 0.061 | LDL to UDL ** | LDL to 0.039 | LDL to 0.126 | ||
| Manganese (mg/L) | Mean ± SD | 0.26 ± 0.32 | 0.46 ± 0.47 | 0.24 ± 0.23 | 0.38 ± 0.43 | 0.20 |
| Range | LDL to 1.41 | LDL to 1.60 | LDL to 1.0 | LDL to 1.21 |
| Parameter | Piped Water (n = 37) | Groundwater (n = 31) | Jarred Water (n = 25) | Tanker Water (n = 8) | Total (n = 101) |
|---|---|---|---|---|---|
| TDS | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| pH | 0 (0.0) | 0 (0.0) | 9 (56.2) | 0 (0.0) | 9 (13.0) |
| Ammonia nitrogen | 4 (16.7) | 3 (13.0) | 1 (6.2) | 2 (33.3) | 10 (14.5) |
| Arsenic | 2 (5.4) | 10 (32.2) | 0 (0.0) | 2 (25.0) | 14 (13.8) |
| Manganese | 12 (32.4) | 17 (54.8) | 10 (40.0) | 4 (50.0) | 43 (42.5) |
| Raw Water | No. of Samples | E. coli (CFU/100 mL) | TC (CFU/100 mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean * | Min | Max | Positive ** | Mean * | Min | Max | Positive ** | ||
| Piped water | 37 | 7 | 0 | TMTC *** | 72.9% | 135 | 6 | TMTC | 100.0% |
| Groundwater | 31 | 11 | 0 | TMTC | 67.7% | 163 | 0 | TMTC | 96.7% |
| Jarred water | 25 | 1 | 0 | 53 | 32.0% | 150 | 0 | TMTC | 96.0% |
| Tanker water | 8 | 20 | 0 | 228 | 87.5% | 179 | 87 | TMTC | 100.0% |
| Total | 101 | 64.3% | 98.1% | ||||||
| Raw Water | Number of Households | HWTS Methods, n (%) | ||||
|---|---|---|---|---|---|---|
| Boiling | CCF | ED | CM | RO-UV | ||
| Piped water | 37 | 10 (27.1) | 0 (0.0) | 0 (0.0) | 8 (21.6) | 19 (51.3) |
| Groundwater | 31 | 1 (3.2) | 0 (0.0) | 0 (0.0) | 2 (6.4) | 28 (90.4) |
| Jarred water | 25 | 17 (68.0) | 3 (12.0) | 3 (12.0) | 2 (8.0) | 0 (0.0) |
| Tanker water | 8 | 2 (25.0) | 0 (0.0) | 0 (0.0) | 2 (25.0) | 4 (50.0) |
| Total | 101 | 30 | 3 | 3 | 14 | 51 |
| HWTS Methods | Net Samples Detected with TC | Samples with Reduced TC | Mean TC Raw * | Mean TC Treated * | LRV | Percentage of Samples > 2 LRV | Overall TC Contamination in Treated Water (%) |
|---|---|---|---|---|---|---|---|
| Boiling | 29 | 27 | 192 | 2 | 1.8 | 51.8 | 56.6 |
| CCF | 3 | 2 | 100 | 2 | 1.6 | 33.3 | 66.6 |
| ED | 3 | 3 | 248 | 5 | 1.6 | 33.3 | 66.6 |
| CM | 14 | 11 | 225 | 6 | 1.5 | 36.3 | 71.4 |
| RO UV | 50 | 43 | 201 | 11 | 1.2 | 25.5 | 76.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanal, S.; Kazama, S.; Benyapa, S.; Takizawa, S. Performance Assessment of Household Water Treatment and Safe Storage in Kathmandu Valley, Nepal. Water 2023, 15, 2305. https://doi.org/10.3390/w15122305
Khanal S, Kazama S, Benyapa S, Takizawa S. Performance Assessment of Household Water Treatment and Safe Storage in Kathmandu Valley, Nepal. Water. 2023; 15(12):2305. https://doi.org/10.3390/w15122305
Chicago/Turabian StyleKhanal, Shekhar, Shinobu Kazama, Sawangjang Benyapa, and Satoshi Takizawa. 2023. "Performance Assessment of Household Water Treatment and Safe Storage in Kathmandu Valley, Nepal" Water 15, no. 12: 2305. https://doi.org/10.3390/w15122305







