Microalgae Cultivation Using Municipal Wastewater and Anaerobic Membrane Effluent: Lipid Production and Nutrient Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Municipal Wastewater and Anaerobic Digestate
2.2. Microalgae
2.3. Microalgae Cultivation Processes
2.4. Analytical Methods
2.4.1. General Parameters
2.4.2. Determination of Microalgae Growth
2.4.3. Lipid Extraction and Analysis Processes
2.5. Statistical Analysis
3. Results and Discussions
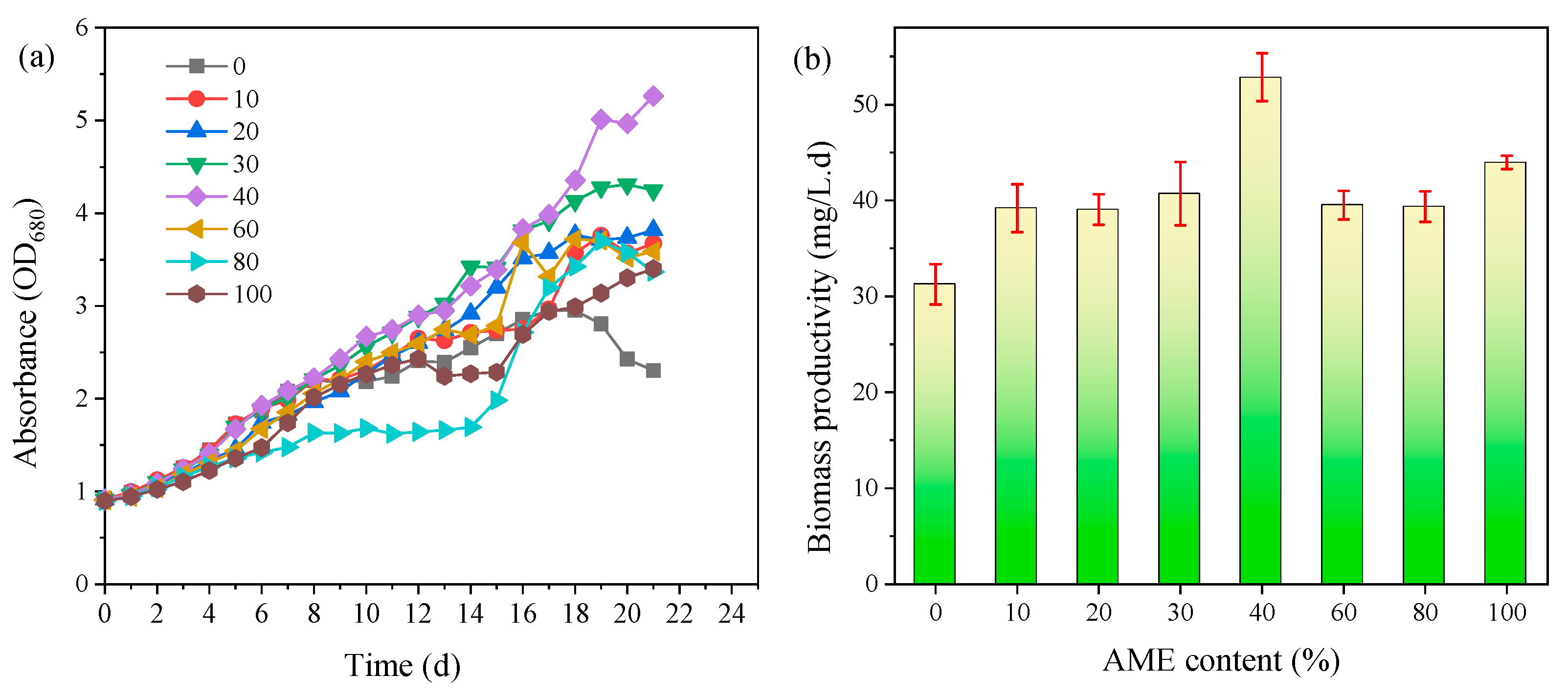
3.1. Microalgae Growth Properties
3.2. Lipid Production
3.3. Pollutant Removal through Microalgae Cultivation
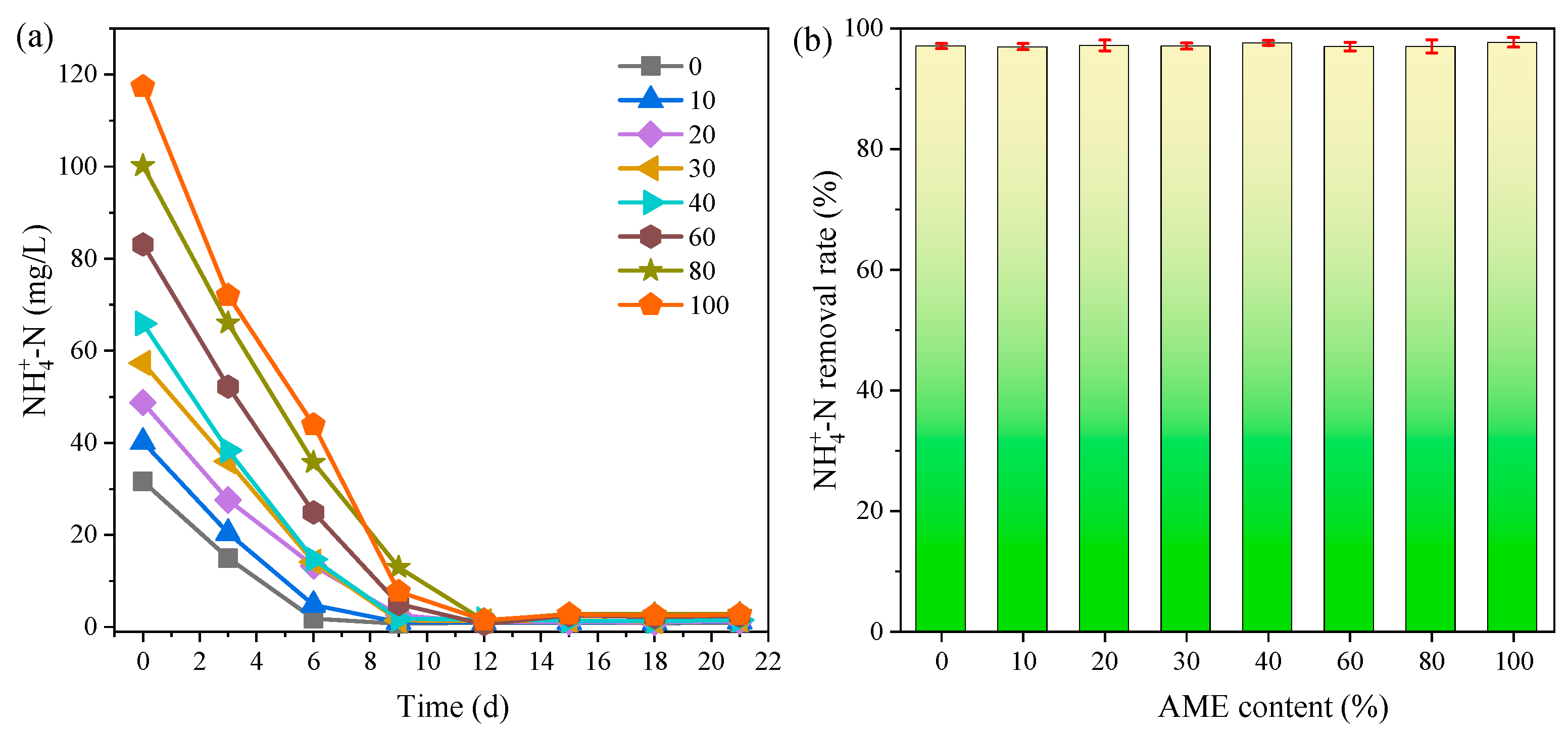
3.3.1. Ammonia Removal
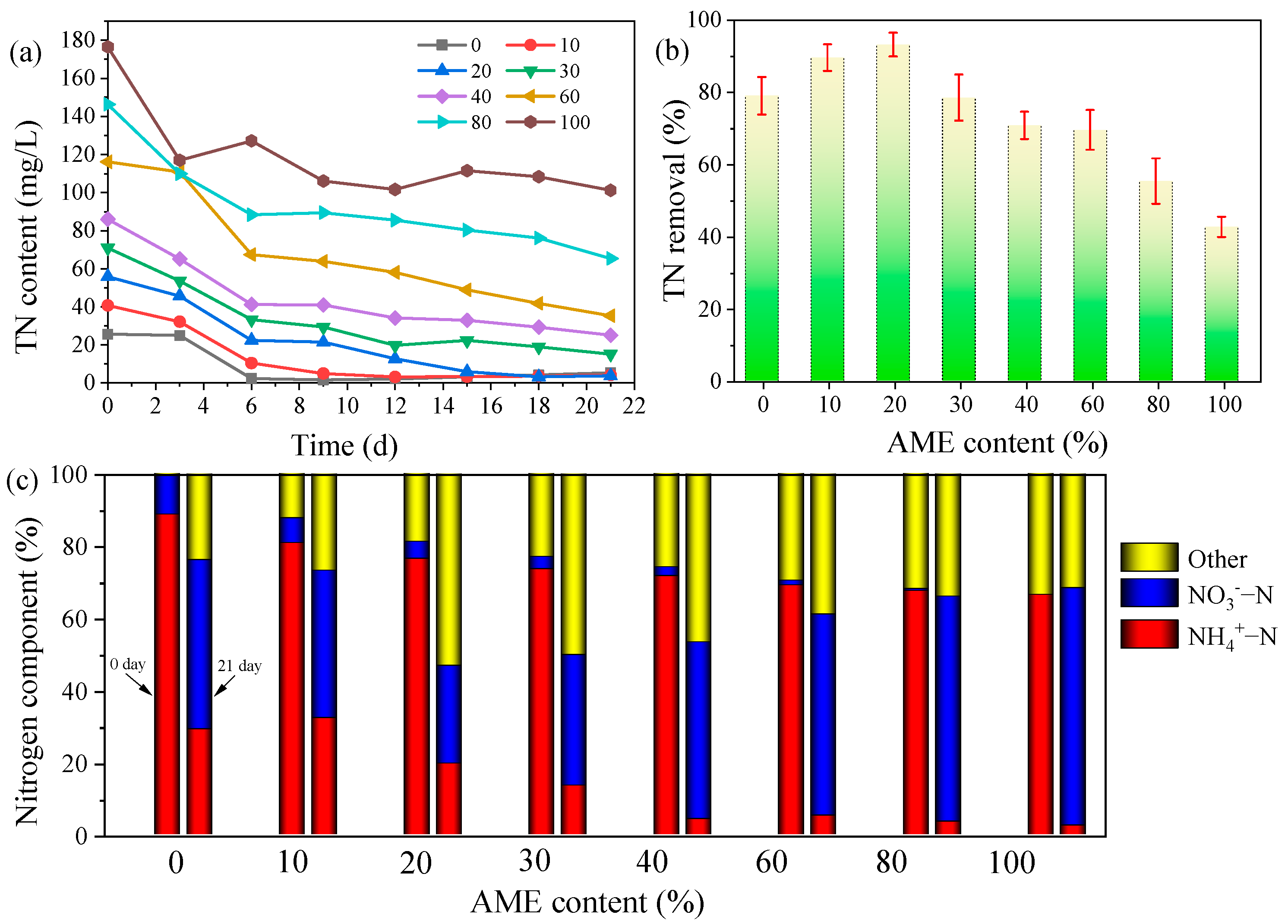
3.3.2. TN Removal
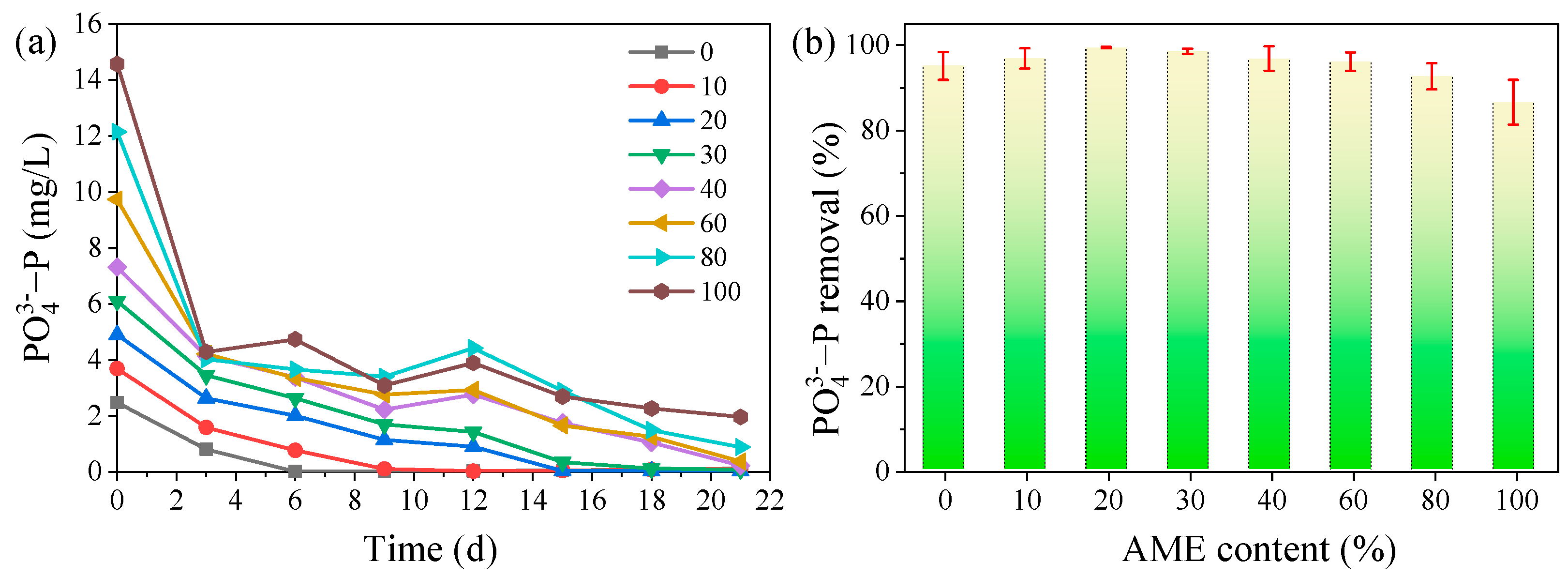
3.3.3. Phosphate Removal
3.3.4. COD Removal
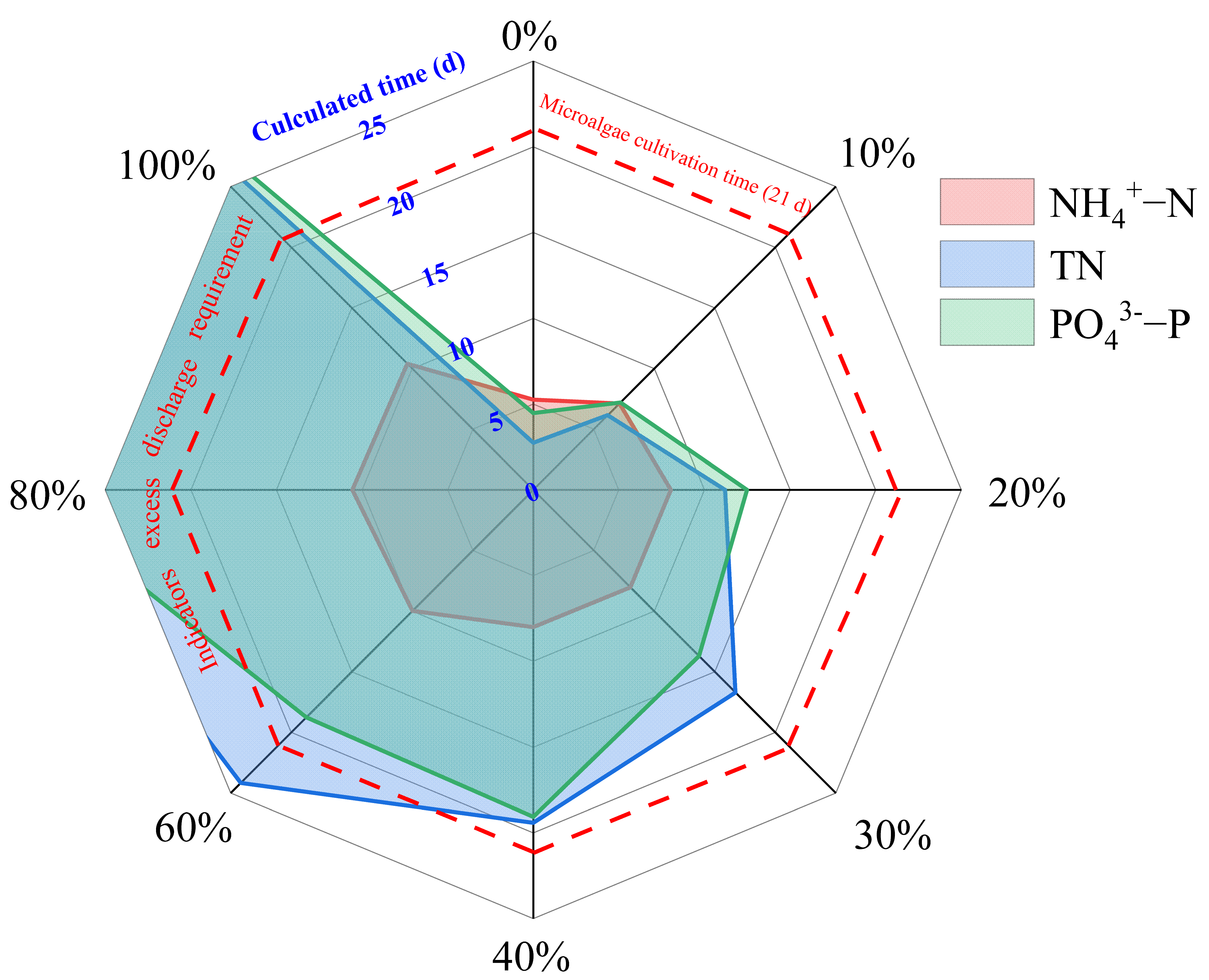
3.4. Evaluation of Wastewater Treatment through Microalgae Cultivation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lai, C.-W.; Bhuyar, P.; Shen, M.-Y.; Chu, C.-Y. A Two-stage strategy for polyhydroxybutyrate (PHB) production by continuous Biohydrogen fermenter and sequencing batch reactor from food industry wastewater. Sustain. Energy Technol. Assess. 2022, 53, 102445. [Google Scholar] [CrossRef]
- Juárez, J.M.; Vladic, J.; Rodríguez, S.B.; Vidovic, S. Sequential valorisation of microalgae biomass grown in pig manure treatment photobioreactors. Algal Res. 2020, 50, 101972. [Google Scholar] [CrossRef]
- Bhatt, A.; Khanchandani, M.; Rana, M.S.; Prajapati, S.K. Techno-economic analysis of microalgae cultivation for commercial sustainability: A state-of-the-art review. J. Clean. Prod. 2022, 370, 133456. [Google Scholar] [CrossRef]
- Liu, K.; Lv, L.; Li, W.; Ren, Z.; Wang, P.; Liu, X.; Gao, W.; Sun, L.; Zhang, G. A comprehensive review on food waste anaerobic co-digestion: Research progress and tendencies. Sci. Total Environ. 2023, 878, 163155. [Google Scholar] [CrossRef] [PubMed]
- Dalke, R.; Demro, D.; Khalid, Y.; Wu, H.; Urgun-Demirtas, M. Current status of anaerobic digestion of food waste in the United States. Renew. Sustain. Energy Rev. 2021, 151, 111554. [Google Scholar] [CrossRef]
- Jadhav, P.; Muhammad, N.; Bhuyar, P.; Krishnan, S.; Razak, A.S.A.; Zularisam, A.W.; Nasrullah, M. A review on the impact of conductive nanoparticles (CNPs) in anaerobic digestion: Applications and limitations. Environ. Technol. Innov. 2021, 23, 101526. [Google Scholar] [CrossRef]
- Aslam, A.; Khan, S.J.; Shahzad, H.M.A. Anaerobic membrane bioreactors (AnMBRs) for municipal wastewater treatment- potential benefits, constraints, and future perspectives: An updated review. Sci. Total Environ. 2022, 802, 149612. [Google Scholar] [CrossRef]
- Hu, Y.; Cai, X.; Xue, Y.; Du, R.; Ji, J.; Chen, R.; Sano, D.; Li, Y.-Y. Recent developments of anaerobic membrane bioreactors for municipal wastewater treatment and bioenergy recovery: Focusing on novel configurations and energy balance analysis. J. Clean. Prod. 2022, 356, 131856. [Google Scholar] [CrossRef]
- Yang, Y.; Zang, Y.; Hu, Y.; Wang, X.C.; Ngo, H.H. Upflow anaerobic dynamic membrane bioreactor (AnDMBR) for wastewater treatment at room temperature and short HRTs: Process characteristics and practical applicability. Chem. Eng. J. 2020, 383, 123186. [Google Scholar] [CrossRef]
- Tang, J.; Pu, Y.; Zeng, T.; Hu, Y.; Huang, J.; Pan, S.; Wang, X.C.; Li, Y.; Abomohra, A.E.-F. Enhanced methane production coupled with livestock wastewater treatment using anaerobic membrane bioreactor: Performance and membrane filtration properties. Bioresour. Technol. 2022, 345, 126470. [Google Scholar] [CrossRef]
- O’Connor, J.; Mickan, B.S.; Rinklebe, J.; Song, H.; Siddique, K.H.M.; Wang, H.; Kirkham, M.B.; Bolan, N.S. Environmental implications, potential value, and future of food-waste anaerobic digestate management: A review. J. Environ. Manag. 2022, 318, 115519. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Tang, J.; Zeng, T.; Hu, Y.; Yang, J.; Wang, X.; Huang, J.; Abomohra, A. Pollutant Removal and Energy Recovery from Swine Wastewater Using Anaerobic Membrane Bioreactor: A Comparative Study with up-Flow Anaerobic Sludge Blanket. Water 2022, 14, 2438. [Google Scholar] [CrossRef]
- Tan, X.-B.; Yang, L.-B.; Zhang, W.-W.; Zhao, X.-C. Lipids production and nutrients recycling by microalgae mixotrophic culture in anaerobic digestate of sludge using wasted organics as carbon source. Bioresour. Technol. 2020, 297, 122379. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, M.; Kumar, P.; Bhatt, P.; Simsek, H.; Kumar, R.; Chaudhary, A.; Malik, A.; Prajapati, S.K. An integration of algae-mediated wastewater treatment and resource recovery through anaerobic digestion. J. Environ. Manag. 2023, 342, 118159. [Google Scholar] [CrossRef]
- Sheikh, M.; Harami, H.R.; Rezakazemi, M.; Valderrama, C.; Cortina, J.L.; Aminabhavi, T.M. Efficient NH3-N recovery from municipal wastewaters via membrane hybrid systems: Nutrient-Energy-Water (NEW) nexus in circular economy. Chem. Eng. J. 2023, 465, 142876. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y. Resource recovery from municipal wastewater: A critical paradigm shift in the post era of activated sludge. Bioresour. Technol. 2022, 363, 127932. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chang, J.-S.; Show, K.-Y.; Lee, D.-J. Anaerobic recalcitrance in wastewater treatment: A review. Bioresour. Technol. 2022, 363, 127920. [Google Scholar] [CrossRef] [PubMed]
- Vaz, S.A.; Badenes, S.M.; Pinheiro, H.M.; Martins, R.C. Recent reports on domestic wastewater treatment using microalgae cultivation: Towards a circular economy. Environ. Technol. Innov. 2023, 30, 103107. [Google Scholar] [CrossRef]
- Yang, J.; Xu, M.; Zhang, X.; Hu, Q.; Sommerfeld, M.; Chen, Y. Life-cycle analysis on biodiesel production from microalgae: Water footprint and nutrients balance. Bioresour. Technol. 2011, 102, 159–165. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Wang, G.; Zhou, Z.; Hao, S.; Wang, L. Microalgae cultivation using unsterilized cattle farm wastewater filtered through corn stover. Bioresour. Technol. 2022, 352, 127081. [Google Scholar] [CrossRef]
- Santhana Kumar, V.; Das Sarkar, S.; Das, B.K.; Sarkar, D.J.; Gogoi, P.; Maurye, P.; Mitra, T.; Talukder, A.K.; Ganguly, S.; Nag, S.K.; et al. Sustainable biodiesel production from microalgae Graesiella emersonii through valorization of garden wastes-based vermicompost. Sci. Total Environ. 2022, 807, 150995. [Google Scholar] [CrossRef] [PubMed]
- Josa, I.; Garfí, M. Social life cycle assessment of microalgae-based systems for wastewater treatment and resource recovery. J. Clean. Prod. 2023, 407, 137121. [Google Scholar] [CrossRef]
- Song, Y.; Wang, L.; Qiang, X.; Gu, W.; Ma, Z.; Wang, G. The promising way to treat wastewater by microalgae: Approaches, mechanisms, applications and challenges. J. Water Process Eng. 2022, 49, 103012. [Google Scholar] [CrossRef]
- Chen, J.; Dai, L.; Mataya, D.; Cobb, K.; Chen, P.; Ruan, R. Enhanced sustainable integration of CO2 utilization and wastewater treatment using microalgae in circular economy concept. Bioresour. Technol. 2022, 366, 128188. [Google Scholar] [CrossRef] [PubMed]
- Thanigaivel, S.; Vickram, S.; Manikandan, S.; Deena, S.R.; Subbaiya, R.; Karmegam, N.; Govarthanan, M.; Kim, W. Sustainability and carbon neutralization trends in microalgae bioenergy production from wastewater treatment: A review. Bioresour. Technol. 2022, 364, 128057. [Google Scholar] [CrossRef]
- Cheng, P.; Huang, J.; Song, X.; Yao, T.; Jiang, J.; Zhou, C.; Yan, X.; Ruan, R. Heterotrophic and mixotrophic cultivation of microalgae to simultaneously achieve furfural wastewater treatment and lipid production. Bioresour. Technol. 2022, 349, 126888. [Google Scholar] [CrossRef]
- Gao, F.; Yang, Z.-Y.; Zhao, Q.-L.; Chen, D.-Z.; Li, C.; Liu, M.; Yang, J.-S.; Liu, J.-Z.; Ge, Y.-M.; Chen, J.-M. Mixotrophic cultivation of microalgae coupled with anaerobic hydrolysis for sustainable treatment of municipal wastewater in a hybrid system of anaerobic membrane bioreactor and membrane photobioreactor. Bioresour. Technol. 2021, 337, 125457. [Google Scholar] [CrossRef]
- Kusmayadi, A.; Lu, P.-H.; Huang, C.-Y.; Leong, Y.K.; Yen, H.-W.; Chang, J.-S. Integrating anaerobic digestion and microalgae cultivation for dairy wastewater treatment and potential biochemicals production from the harvested microalgal biomass. Chemosphere 2022, 291, 133057. [Google Scholar] [CrossRef]
- López-Sánchez, A.; Silva-Gálvez, A.L.; Aguilar-Juárez, Ó.; Senés-Guerrero, C.; Orozco-Nunnelly, D.A.; Carrillo-Nieves, D.; Gradilla-Hernández, M.S. Microalgae-based livestock wastewater treatment (MbWT) as a circular bioeconomy approach: Enhancement of biomass productivity, pollutant removal and high-value compound production. J. Environ. Manag. 2022, 308, 114612. [Google Scholar] [CrossRef]
- Li, G.; Zhang, J.; Li, H.; Hu, R.; Yao, X.; Liu, Y.; Zhou, Y.; Lyu, T. Towards high-quality biodiesel production from microalgae using original and anaerobically-digested livestock wastewater. Chemosphere 2021, 273, 128578. [Google Scholar] [CrossRef]
- Rossi, S.; Díez-Montero, R.; Rueda, E.; Castillo Cascino, F.; Parati, K.; García, J.; Ficara, E. Free ammonia inhibition in microalgae and cyanobacteria grown in wastewaters: Photo-respirometric evaluation and modelling. Bioresour. Technol. 2020, 305, 123046. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Sewage, 20th ed.; Amer Public Health Assn: Washington, DC, USA, 1998. [Google Scholar]
- Almutairi, A.W.; Al-Hasawi, Z.M.; Abomohra, A.E.-F. Valorization of lipidic food waste for enhanced biodiesel recovery through two-step conversion: A novel microalgae-integrated approach. Bioresour. Technol. 2021, 342, 125966. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Rehman, F.; Khan, A.U.; Fazal, T.; Hafeez, A.; Rashid, N. Real textile industrial wastewater treatment and biodiesel production using microalgae. Biomass Bioenergy 2022, 165, 106559. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, S.; Mo, N.; Wang, Z.; Zeng, E.Y. Screening of freshwater oleaginous microalgae from South China and its cultivation characteristics in energy grass digestate. J. Clean. Prod. 2020, 276, 124193. [Google Scholar] [CrossRef]
- Dai, J.; Zheng, M.; He, Y.; Zhou, Y.; Wang, M.; Chen, B. Real-time response counterattack strategy of tolerant microalgae Chlorella vulgaris MBFJNU-1 in original swine wastewater and free ammonia. Bioresour. Technol. 2023, 377, 128945. [Google Scholar] [CrossRef]
- Paranjape, K.; Leite, G.B.; Hallenbeck, P.C. Effect of nitrogen regime on microalgal lipid production during mixotrophic growth with glycerol. Bioresour. Technol. 2016, 214, 778–786. [Google Scholar] [CrossRef]
- Al-Mallahi, J.; Ishii, K.; Sato, M.; Ochiai, S. Static supply of different simulated flue gases for native microalgae cultivation in diluted cow manure digestate. J. Environ. Manag. 2023, 335, 117557. [Google Scholar] [CrossRef]
- Tan, X.-B.; Zhang, Y.-L.; Zhao, X.-C.; Yang, L.-B.; Yangwang, S.-C.; Zou, Y.; Lu, J.-M. Anaerobic digestates grown oleaginous microalgae for pollutants removal and lipids production. Chemosphere 2022, 308, 136177. [Google Scholar] [CrossRef]
- Kwon, G.; Kim, H.; Song, C.; Jahng, D. Co-culture of microalgae and enriched nitrifying bacteria for energy-efficient nitrification. Biochem. Eng. J. 2019, 152, 107385. [Google Scholar] [CrossRef]
- Oviedo, J.A.; Muñoz, R.; Donoso-Bravo, A.; Bernard, O.; Casagli, F.; Jeison, D. A half-century of research on microalgae-bacteria for wastewater treatment. Algal Res. 2022, 67, 102828. [Google Scholar] [CrossRef]


| Unit | MW | AME | |
|---|---|---|---|
| Chemical oxygen demand (COD) | mg/L | 180–290 | 500–800 |
| Soluble COD (SCOD) | mg/L | 60–150 | 480–570 |
| Ammonia | mg/L | 20–30 | 150–200 |
| Total nitrogen (TN) | mg/L | 30–35 | 160–230 |
| Phosphate | mg/L | 0.8–2 | 10–20 |
| Total phosphate | mg/L | 1.5–5 | 12–27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Qu, X.; Chen, S.; Pu, Y.; He, X.; Zhou, Z.; Wang, H.; Jin, N.; Huang, J.; Shah, F.; et al. Microalgae Cultivation Using Municipal Wastewater and Anaerobic Membrane Effluent: Lipid Production and Nutrient Removal. Water 2023, 15, 2388. https://doi.org/10.3390/w15132388
Tang J, Qu X, Chen S, Pu Y, He X, Zhou Z, Wang H, Jin N, Huang J, Shah F, et al. Microalgae Cultivation Using Municipal Wastewater and Anaerobic Membrane Effluent: Lipid Production and Nutrient Removal. Water. 2023; 15(13):2388. https://doi.org/10.3390/w15132388
Chicago/Turabian StyleTang, Jialing, Xiangjiang Qu, Si Chen, Yunhui Pu, Xinrui He, Zhihui Zhou, Huijun Wang, Ni Jin, Jin Huang, Faisal Shah, and et al. 2023. "Microalgae Cultivation Using Municipal Wastewater and Anaerobic Membrane Effluent: Lipid Production and Nutrient Removal" Water 15, no. 13: 2388. https://doi.org/10.3390/w15132388
APA StyleTang, J., Qu, X., Chen, S., Pu, Y., He, X., Zhou, Z., Wang, H., Jin, N., Huang, J., Shah, F., Hu, Y., & Abomohra, A. (2023). Microalgae Cultivation Using Municipal Wastewater and Anaerobic Membrane Effluent: Lipid Production and Nutrient Removal. Water, 15(13), 2388. https://doi.org/10.3390/w15132388









