Abstract
Nature-based, low technology wastewater treatment systems can benefit small and remote communities. Adding a constructed floating wetland (CFW) to waste stabilization ponds can enhance treatment efficacy at low cost, depending on appropriate macrophytes. In cold climates, harsh growing conditions may limit CFW success, requiring research under-ambient field conditions. Seven native macrophytes were assessed for the growth, biomass production, and root and shoot uptake of potential contaminants of concern from municipal wastewater in a facultative stabilization pond in Alberta, Canada. All macrophytes established. Scirpus microcarpus had high nitrogen and phosphorus in roots and shoots and phytoextraction potential. Metal and trace elements were highest in Glyceria grandis, Beckmannia syzigachne, and Scirpus microcarpus, mostly greater in roots than shoots, indicating phytostabilization. Tissue contaminant concentrations did not always indicate high contaminant accumulation in the CFW. Total uptake per unit area was greatest for Glyceria grandis, although chromium and molybdenum were greatest in Beckmannia syzigachne and Carex aquatilis, respectively. Beckmannia syzigachne and Scirpus microcarpus have potential for phytoremediation if biomass per unit area is increased. Species variability is high for contaminant accumulation and biomass; in unpredictable climates and wastewaters with suites of contaminants, different macrophytes for wetland water treatment systems are recommended.
1. Introduction
Wastewater stabilization ponds are frequently used to treat municipal and industrial wastewater in small and remote communities. Stabilization ponds can effectively reduce suspended particles, biochemical oxygen demand, coliform bacteria, nutrients, metals and micropollutants [1]. Common contaminants in municipal wastewater include nitrogen, phosphorus, lead, nickel, mercury, cadmium, chromium, copper, and zinc [2,3,4,5]. Adding a constructed floating wetland (CFW) to a stabilization pond can further assist in contaminant removal during wastewater treatment [6,7,8,9], providing the same benefits as natural wetlands for water management and purification. These floating platforms form terrestrial habitat in aquatic environments while helping improve quality of the water in which they float. In contrast to traditional constructed wetlands, floating ones are not impacted by fluctuations in water and plant roots are permanently in contact with the water. Planted with native macrophytes, phytoremediation is the main mechanism of water treatment and includes the physical filtering of suspended sediments, water aeration, direct uptake of contaminants, and a suite of rhizosphere facilitated processes including biofilm establishment and immobilization and the mineralization of contaminants.
All the mechanisms involved in water treatment with CFWs are not completely understood [10]. The indirect role plants play may far exceed their direct role in the removal of contaminants [11]. For example, filtering by plant roots reduces biological oxygen demand and total suspended solids [6], and anerobic waste stabilization ponds, common in the arctic [12], may benefit from aeration and oxidation by plants. While phytoremediation potential in highly contaminated sites may be reduced due to phytotoxicity and/or the length of time to meet regulatory criteria, in less toxic environments, such as municipal wastewater treatment, it can be part of a long-term solution [13]. CFWs are low technology, low cost, nature-based solutions for water treatment and could greatly benefit northern and remote communities.
In cold (northern temperate to subpolar) climates, establishment, growth, and survival of plant species are reduced relative to warmer (southern temperate to tropical) climates where the majority of CFWs are employed. In designing and implementing operational scale CFW projects in northern communities, a first step is selecting macrophytes appropriate to the environmental conditions. Macrophytes that readily establish, produce high biomass in a short period of time, adapt to site conditions, survive overwinter, and tolerate and accumulate a range of contaminants are desired [14]. Abundant (dense and fibrous) and deep penetrating root biomass provides an active and dynamic zone where root exudates, microorganisms and biofilm result in the sequestration and degradation of water contaminants. Brisson and Chazarenc [15] highlight in their review that environmental considerations are the greatest factor guiding plant species selection. The majority of studies to date on plant species selection for CFWs have been conducted in microcosms and mesocosms; however, the assessment of species performance in the field is needed [13,16,17]. Focusing on field studies can be a more cost-effective approach to developing operational systems.
A review of the literature on macrophytes with potential for phytoremediation show the genera Typha, Scirpus, Juncus, Eleocharis, and Carex are widely using in water treatment in North America [11,15,18,19]. However, genera alone cannot be relied on, as species differences within a specific genus are high [15,20,21,22]. Site conditions are consistently reported to affect a species’ phytoremediation efficacy [13,17,21]. Controlled environment experiments and surveys of natural vegetation to assess species tolerance or accumulation of nutrients, metals, and other elements cannot substitute for direct comparison under operational conditions. Non-native macrophytes such as Phragmites australis and Phalaris arundinacea remove contaminants, even in cold climates, but are not desirable as they can be invasive and add to environmental degradation [23,24]. Hyperaccumulators, those species capable of accumulating very high concentrations of contaminants, may be necessary for highly contaminated sites. In less toxic environments and where multiple contaminants are of concern, such as municipal wastewater, non-hyperaccumulators may be adequate for phytoremediation. Few macrophytes native to cold regions have been tested in treatment wetlands, and even fewer with data from multiple studies. The scientific literature shows half a dozen key macrophytes with potential for use in northern treatment wetlands, most notably Typha latifolia, Scirpus validus, Scirpus acutus, Juncus effusus, Typha angustifolia, and Carex aquatilis [18,25].
Very few cold climate and operational scale field studies have been conducted to determine the potential for phytoremediation of urban wastewater. Even if available, species comparisons among studies are difficult as differences in contaminants and their concentrations, water chemistry, climate, seasonal variability, plant material type and age, and overall plant health impact phytoremediation potential. In this field study, we compare performance of seven native macrophytes to assess their potential for phytoremediation in cold climate CFWs. The roles of shoots and roots were assessed as little research has been conducted on their relative contribution to remediation, an important factor for understanding phyto-uptake potential and optimizing plant growth conditions in CFWs.
2. Materials and Methods
2.1. Study Site
The study site was a facultative cell stabilization pond for the community of Violet Grove, near Drayton Valley (53.1634° N, 115.0378° W), in the natural foothill region of Alberta, Canada. The stabilization pond has a surface area of 2800 m2 and a maximum depth of 1.43 m. Treated water (hydraulic retention time of approximately 60 days) from the pond drains to a storage lagoon until discharged biannually to a natural drainage ditch, which enters the North Saskatchewan River. The region has long, cold winters and short, warm summers. Mean annual temperature is 3.4 °C and mean annual precipitation is 576 mm. There are approximately 125 growing days a year. The constructed floating wetland was retrofitted in the stabilization pond in May 2019 and comprised plastic modules, each approximately 2.0 m × 2.5 m. Each module contained 15 crates that were 58 cm × 36 cm × 16.5 cm deep. In each crate, 5 plants were present.
Seven emergent macrophytes were selected for this study, based on a literature review of phytoremediation potential, native to the region, ease of establishment, and availability from local suppliers. Plant seedlings, sourced from Alberta greenhouses as plugs, were planted in the crates, which were then filled with gravel to a depth of 10 cm. Initially there were 10 modules with half containing Carex aquatilis (water sedge) and half Scirpus microcarpus (panicled bulrush). In May 2021, 5 modules of each of these species were added to the main platform. A separate floating module was established to test 5 additional plant species, Beckmannia syzigachne (slough grass), Carex retrorsa (retrorse sedge), Glyceria grandis (tall manna grass), Juncus balticus (Baltic rush), and Schoenoplectus tabernaemontani (syn Scirpus validus) (soft stem bulrush). This module contained 3 crates of each species.
2.2. Field and Laboratory Measurements
Plant species were assessed over 20 weeks from 26 May to 13 October 2021. At the time of planting, seedling shoot heights were 10–15 cm and root lengths 5–10 cm. At 20 weeks, shoot height and root length were measured on 15 randomly selected plants of Carex aquatilis and Scirpus microcarpus, as well as all plants of the other 5 species since there were only 15 plants in total. Shoot height was measured as the distance from the gravel surface to the tip of the longest fully extended live leaf (cm). Root length was measured from the bottom of the crate to the tip of the longest root on crates placed in a monitoring stand. To account for the root portion in gravel, 10 cm was added to all final root lengths. For each crate containing the selected plants, total percent vegetation cover was ocularly estimated. Vegetation cover is used to estimate biomass when destructive sampling is not possible [26,27,28]. The presence of seed heads and evidence of herbivory were recorded.
At the beginning of the study and at week 20, ten plants each of Carex aquatilis, Scirpus microcarpus, Beckmannia syzigachne, and Glyceria grandis were harvested (total of forty plants) and shoot and root dry biomass were measured in the University of Alberta laboratory. Soil was gently removed by hand from the roots of each plant, which were then rinsed with distilled water to remove any remaining soil. Shoots and roots were washed following a standard protocol in preparation for the commercial laboratory. The vegetation washing solution was 0.05% Liquinox (Sigma-Aldrich, St. Louis, MO, USA) and 0.05% tetrasodium ethylenediaminetetraacetate (Na4EDTA) [29]. A squeeze bottle was filled with a 1% dilution of the wash solution with distilled water. In a wash basin, a shoot or root biomass sample from one plant was covered with a small amount of the diluted wash solution (~3 mL), using gentle agitation, washed by hand for 30 s in a sieve with a fine mesh (pore size: 1 mm) to prevent loss of biomass, and then triple rinsed with distilled water. Shoot and root biomasses were oven-dried at 80 °C to a constant weight. Shoots and roots of each plant were individually weighed to obtain dry weight biomass (g).
Of the 40 plants harvested, washed, and weighed, 20 plants (20 shoot samples and 20 root samples; 5 plants of each species) were randomly selected and submitted to a commercial laboratory for tissue analyses. Total nitrogen in plant tissue was determined by combustion in reduced nitrous oxide gas using a thermal conductivity detector (Canadian Society of Soil Science 22.4). Phosphorus, potassium, sulfur, calcium, magnesium, and 29 metals and trace elements (Table 1 plus antimony, beryllium, bismuth, cesium, selenium, tellurium, thallium, tin, and zirconium) were determined via hot block digestion with nitric and hydrochloric acid, followed by collision cell inductively coupled plasma mass spectrometry (ICP-MS) (Environmental Protection Agency 200.3/modified 6020A). Parameters were reported in mg kg−1 of plant tissue, except total nitrogen, which was reported as percent and converted to mg kg−1.

Table 1.
Mean (±SE) tissue concentration and translocation factor (TF) of nutrients, metals, and trace elements by species after 20 weeks. Different letters indicate significant differences at p = 0.05 with Bonferroni test. Highest tissue concentration across species and translocation factors (TF) > 1 in bold.
2.3. Data Analyses
All statistical analyses were conducted using the R statistics environment (R Core Team, 2020) and all references to significant results were at p ≤ 0.05. Before analyses, all data were tested for normality and homogeneity of variance using Shapiro–Wilk’s and Levene’s tests, respectively. Data failed to pass the normality test even after transformation, therefore Kruskal–Wallis one-way, non-parametric analysis of variance (ANOVA), was conducted to test for differences in root, shoot, cover percent, biomass, and element concentrations for species. If results from ANOVA indicated significant differences, multiple comparisons were conducted using Bonferroni’s method.
Biomass accumulation was calculated using the equation below. BM is total biomass accumulation m−2, DW is mean total dry weight biomass, and d is density of plants m−2. BM is not adjusted for the initial plant biomass.
Relative growth rate (RGR), which accounts for biomass accumulation relative to initial biomass, was calculated using the equation below. DW1 is initial mean dry weight biomass, DW2 is final mean dry weight biomass, T1, is initial day number, and T2 is final day number.
Translocation factor (TF) was calculated as the ratio of the concentration of contaminant in the plant’s above-ground parts to contamination in the plant’s below-ground parts and is used to evaluate a species’ ability to translocate nutrients, metals, and trace elements from their roots to shoots. This is important to evaluate a species potential for phytoextraction versus phytostabilization. A TF greater than one is considered effective for phytoextraction.
Potential total uptake (TU) of nutrients, metals, and trace elements by a plant species was calculated as mean biomass accumulation (BM) multiplied by mean nutrient or metal concentration (C) in mg kg−1 of plant tissue at end of the growing season. Total uptake was calculated for shoots and roots separately as follows.
3. Results
3.1. Plant Growth
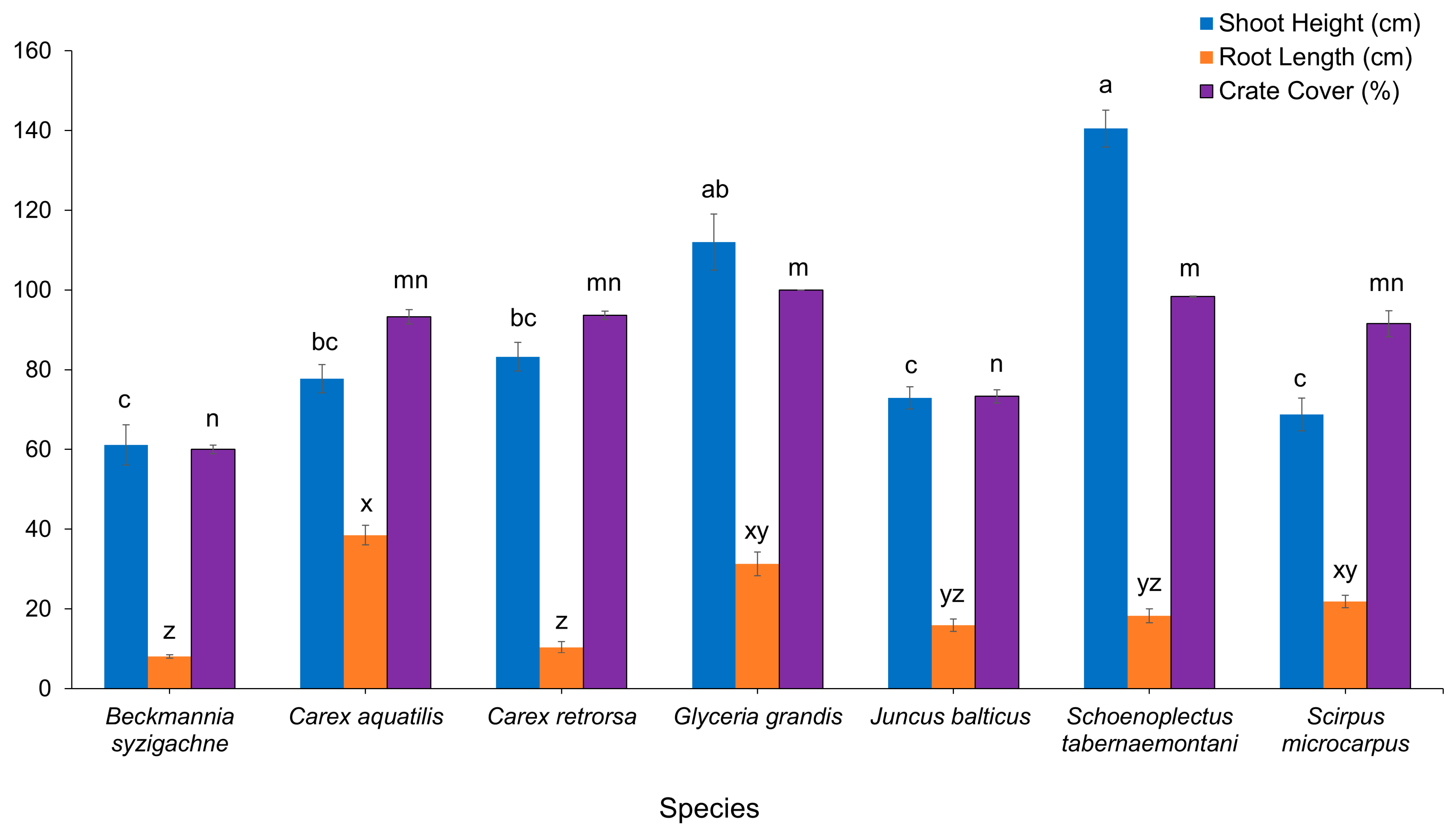
Schoenoplectus tabernaemontani had mean height of 142.2 cm, significantly greater height than all species except Glyceria grandis (Figure 1). Carex aquatilis had greater root length (49.5 cm) than most species except Glyceria grandis (42.3 cm) and Scirpus microcarpus (32.9 cm), which had significantly greater root length than Beckmannia syzigachne and Carex retrorsa. Beckmannia syzigachne and Carex retrorsa had mean heights of approximately 20 cm, indicating growth of only about 10 cm during the growing season. Beckmannia syzigachne and Juncus balticus had less vegetation cover than Glyceria grandis and Schoenoplectus tabernaemontani, and there were no differences among other species.
Figure 1.
Macrophyte growth at the end of growing season (20 weeks) in constructed floating wetland. Columns that do not share a common letter are significantly different at p ≤ 0.05.
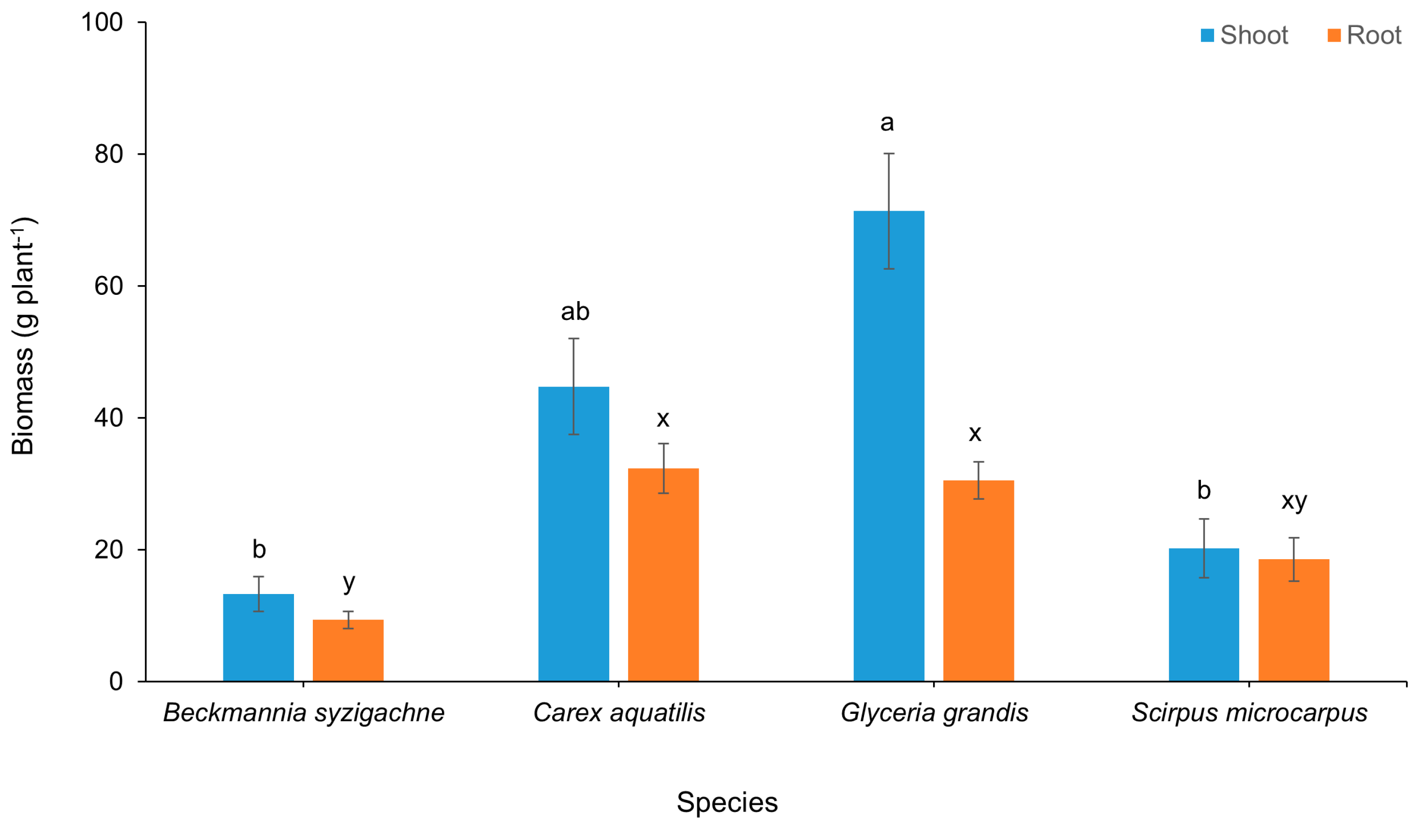
Glyceria grandis had significantly more shoot biomass than Beckmannia syzigachne or Scirpus microcarpus (Figure 2). Differences between Glyceria grandis and Carex aquatilus were not statistically significant; however, Glyceria grandis had almost double the shoot biomass (71.33 g plant−1 versus 44.73 g plant−1), which is of biological significance. Beckmannia syzigachne had significantly less root biomass than the other species (9.34 g plant−1 versus 18.52 to 32.30 g plant−1). There was significantly greater shoot than root biomass for Glyceria grandis and Carex aquatilis, with no differences for the other species. For the planted area of the CFW, total biomass accumulation was Glyceria grandis 2.4 kg m−2, Carex aquatilis 1.8 kg m−2, Scirpus microcarpus 0.9 kg m−2, and Beckmannia syzigachne 0.5 kg m−2.
Figure 2.
Macrophyte shoot and root biomass at the end of growing season (20 weeks) in constructed floating wetland. Columns that do not share a common letter are significantly different at p ≤ 0.05.
Relative growth rates for each species took initial biomass into account and were similar for Carex aquatilis and Scirpus microcarpus (0.020 to 0.025 g g−1 day−1). Beckamannia syzigachne had lower shoot and root growth rates (0.019 and 0.016 g g−1 day−1, respectively), and Glyceria grandis had a higher shoot growth rate (0.032 g g−1 day−1) than the other species. Differences between shoot and root growth rates for a species were low, except for Glyceria grandis.
More than 80% of the plants produced seed heads during the growing season, with the exception of Scirpus microcarpus where only 7% of plants produced them. Grazing was evident for all species, with Beckmannia syzigachne and Glyceria grandis being most affected (100% of plants) and Juncus balticus least affected (7% of plants).
3.2. Plant Uptake of Nutrients
In roots, Scirpus microcarpus had the greatest accumulation per unit mass of nitrogen, phosphorous, sulfur, and calcium—significantly more than all other species for nitrogen and sulphur and more than all species, except Beckmannia syzigachne, for phosphorus (Table 1). In shoots, nitrogen, phosphorus, sulfur, and calcium accumulation were greatest in Scirpus microcarpus—significantly greater than all other species for nitrogen and calcium, greater than Glyceria grandis for phosphorus, and greater than Beckmannia syzigachne and Carex aquatilis for sulfur. Glyceria grandis and Carex aquatilis had the greatest accumulation of potassium in shoots and roots—significantly more than Beckmannia syzigachne. Beckmannia syzigachne had the greatest magnesium in roots—significantly more than other species—but the lowest amount in shoots, with significantly less than Glyceria grandis and Scirpus microcarpus.
The translocation factor was greater than one for approximately half of the nutrients analyzed and greater than 0.8 for most others, providing evidence of translocation from roots to shoots and potential for phytoextraction (Table 1). Species had a translocation factor greater than one for calcium, magnesium, and potassium, except for Beckmannia syzigachne, which only did for potassium. Translocation factors for nitrogen and phosphorus were only greater than one for Beckmannia syzigachne and Carex aquatilis, respectively.
3.3. Plant Uptake of Metals and Trace Elements
Of the 29 metals and trace elements analyzed, 9 (antimony, beryllium, bismuth, cesium, selenium, tellurium, thallium, tin, and zirconium) were below detection limits in the majority of samples and not included in analyses. For the remaining 20, shoot accumulation per unit mass for the majority was greatest in Scirpus microcarpus (14 of 20) and root accumulation was greatest in Beckmannia syzigachne (10 of 20) or Glyceria grandis (9 of 20) (Table 1). Beckmannia syzigachne root concentrations of aluminium, barium, chromium, and nickel were more than double that of the other species, and for cobalt and nickel, these concentrations were at least double for two of the other three species. Glyceria grandis root concentrations of cadmium and sodium were 0.6–4× and 2.9 to 3.7× greater, respectively, relative to other species. While Scirpus microcarpus had high shoot concentrations, there were few significant differences, boron and strontium concentrations were significantly greater in shoots relative to the other species and molybdenum was significantly greater in roots.
The translocation factor was mostly less than one, indicating little movement of contaminants from roots to shoots for the four plant species (Table 1). No translocation factor was greater than one for Beckmannia syzigachne, indicating storage in roots. Translocation factors for the other three species were greater than one for barium, boron, manganese, and strontium; however, translocation factors were greater than one for sodium and molybdenum for Carex aquatilis only, and lithium for Glyceria grandis only.
3.4. Total Plant Uptake per Unit Area of CFW
Based on the biomass accumulation of each species and mean concentrations of nutrients, metals and trace elements, total uptake was estimated per m2 of the CFW (Table 2). Glyceria grandis had the greatest total uptake for nutrients and most metals and trace elements. The exceptions were chromium, which had the greatest uptake in Beckmannia syzigachne roots, and molybdenum, which had the greatest uptake in Carex aquatilus shoots, approximately double that of other species. The greatest accumulation of all nutrients was in the shoots. The greatest accumulation of metals and trace elements was divided between shoots and roots, depending upon the element.

Table 2.
Total uptake by native macrophytes of nutrients, metals, and trace elements (mg) per m2 of the planted constructed floating wetland area. Highest total uptake of contaminant per m2 across species in bold.
4. Discussion
The success of phytoremediation for municipal wastewater is dependent on the ability of the plants to establish and persist, gain below- and above-ground length and biomass, and uptake potential contaminants to roots and shoots. These three components are equally important for a self-sustaining treatment wetland system. Of the macrophytes selected for this study, Glyceria grandis showed the greatest potential for the remediation of wastewater in cold climates due to its combination of high biomass, growth rate, and tissue concentrations of potential contaminants. High contaminant concentrations in Beckmannia syzigachne and Scirpus microcarpus shoots and roots indicate potential for stabilization and extraction; however, biomass was low and would need to be mitigated with higher planting densities or the improvement of growing conditions. There was high growth potential for Schoenoplectus tabernaemontani in our study, and data on uptake in other studies [30,31,32] suggests that this species a good candidate for further field testing. With the exception of Carex aquatilis and Schoenoplectus tabernaemontani, little phytoremediation research has been conducted on the other species in our study. Very few temperate climate and operational scale field studies with any macrophytes have been conducted; thus, our study adds considerably to the scientific literature.
In municipal wastewater, nutrients such as nitrogen and phosphorus are common contaminants of concern. Scirpus microcarpus had the highest nitrogen and phosphorus accumulation in both shoots and roots. Nitrogen concentration was 25 to 65% greater in shoots and 60 to 100% greater in roots than for other plant species. Carex aquatilis, one of the most commonly used plants in northern treatment wetlands, is known to significantly reduce nitrogen [25,33]; while root nitrogen concentrations were second highest, shoot concentrations were less than in other species. Other studies found the Scirpus species to be efficient at nitrogen removal, e.g., [34], although studies specific to Scirpus microcarpus were not found. Schoenoplectus tabernaemontani (syn Scirpus validus) removed nitrogen from floating wetland mesocosms and above-ground biomass was positivity associated with that removal [35]. This species was the best accumulator of nitrogen and phosphorus in laboratory microcosms, outperforming Phalaris arundinacea and Typha latifolia [36,37]. It is more effective at accumulating nitrate than ammonia [37]. While biomass was not directly measured for Schoenoplectus tabernaemontani in our study, shoot height, root length, and cover were the greatest for this species and thus, alongside results from other studies, provides evidence of its potential for nutrient removal. Shoot height and cover have been positively associated with above-ground biomass, including in macrophytes [26,27,38,39].
Metals and trace elements are another group of contaminants frequently present in municipal wastewater. Large differences in concentrations in roots versus shoots and among species suggest varying abilities to uptake elements. Values for aluminum, arsenic, chromium, cobalt, iron, and vanadium in particular were orders of magnitude greater in roots than shoots for most species. Glyceria grandis and Scirpus validus were reported to have good removal rates for copper, lead, and zinc in laboratory experiments and average removal rates for cadmium relative to other studies [40]. Beckmannia syzigachne is efficient at lead and zinc removal [41]. Tissue values were much greater than in our study, with lead values up to 309 mg kg−1 and zinc up to 785 mg kg−1 of tissue. Carex aquatilis removed cadmium, copper, and nickel from industrial wastewater in a field study [42]. Copper tissue concentrations were greater than in our field study even though plants had much lower shoot and root biomass. Glyceria grandis and Beckmannia syzigachne had high and comparable copper, lead, and zinc root concentrations in our study. There are few other phytoremediation data published on elements such as barium, boron, manganese, and molybdenum, although these are of growing concern for water quality.
The movement of nutrients from roots to shoots was high based on translocation factor, indicating high potential for phytoextraction. Through the harvesting of biomass excess, nitrogen and phosphorous, in particular, can be removed from the wastewater. However, the movement of metals and trace elements from roots to shoots was low, indicating potential for phytostabilization but less so for phytoextraction. Even when phytoextraction in shoots is low, stabilization in roots is beneficial for water quality. Indirect plant effects, such as filtering, oxidation, microbial degradation, and phytostabilization on wastewater remediation may be considerable as part of the water treatment system [6,11]. Other studies report that the storage of various metals occurs mostly in macrophyte roots, including those of Carex aquatilis, Schoenoplectus tabernaemontani, Glyceria grandis, and Beckmannia syzigachne [40,41,43,44]. In two studies of 21 macrophytes with potential for water remediation in constructed wetlands, all had highest lead, cadmium, iron, chromium, copper, and/or zinc concentrations in roots [42,44]. Storage in roots may allow plants to tolerate higher concentrations of contaminants, as roots are not part of the photosynthetic process [30,44]. Our study found all metal and trace element concentrations were greater in roots than shoots for Beckmannia syzigachne and most elements for Glyceria grandis. The high root concentrations for Beckmannia syzigachne may also be due to the loss of above-ground biomass in late summer and regrowth at the end of the season. In cold climates, plants put their resources into below-ground growth as temperatures decrease in September and October to ensure they have sufficient below-ground biomass for overwintering [45]. Translocation from roots to shoots would therefore be reduced. This is further supported by the high metal and trace element concentrations in Scirpus microcarpus but low translocation factors. Scirpus microcarpus was observed to start senescing before the other species.
Most research on plant selection for wastewater treatment to date has been conducted in controlled environments such as mesocosms. In cold climates, the short growing season means selected plant species for CFWs must grow rapidly and be resilient to variability in environmental factors, such as water and air temperatures, precipitation, water chemistry, water levels, and herbivory. Temperature-related impacts on phytoremediation efficacy have been reported, with reduced phytoextraction with colder temperatures [33,46]. Beckmannia syzigachne had significant shoot dieback between 10 and 15 weeks, the cause unknown. New green regrowth was observed, although it was late in the season to regain significant biomass, thus affecting relative growth rate and phytoremediation potential. In a 2019 study at the Violet Grove CFW, Carex aquatilis and Scirpus microcarpus had double the shoot biomass at the end of the growing season relative to our study [39,47]. Carex aquatilis root biomass in our study was approximately 60% that of 2019, while Scirpus microcarpus root biomass was approximately 25% greater. Carex aquatilis growth rate was less, while that of Scirpus microcarpus was greater. Scirpus microcarpus seed heads were abundant in 2019, although infrequently observed in 2021. The lack of seed heads suggests poor plant vigour, which can affect species’ persistence year to year. Poorer quality planting stock and planting may have been factors. Herbivory occurred and was generally greatest when plant shoots were green and actively growing. Muskrats (Ondatra zibethicus) were thought to be the main grazers. Seasonal variability affects above and below-ground growth, and nature-based wastewater treatment systems, such as CFWs, need to consider this in their design. Planting a diversity of species would buffer the system against fluctuating environmental conditions.
The selection of the best macrophytes for phytoremediation in CFWs must consider more than tissue contaminant concentrations or biomass alone [48,49]. Plants adapted to cold climates are often smaller in stature and have reduced plant biomass, which in theory could reduce their ability to be good accumulators of contaminants. When species were compared per area, Glyceria grandis was the best accumulator of nutrients, metals, and trace elements. Glyceria grandis did not have the highest shoot or root concentrations; while it had the highest shoot biomass, root biomass was comparable to Carex aquatilis. For the most common metals of concern in wastewater, maximum accumulation for Glyceria grandis was in roots. Little research has been conducted on this species and our study is important for identifying it as a promising species for phytoremediation. Beckmannia syzigachne and Scirpus microcarpus had high tissue concentrations for metals and nutrients, respectively; they did not have high total update within the CFW with the exception of chromium for Beckmannia syzigachne. If they can produce a greater biomass, they would be more effective for phytoremediation. Carex aquatilis did not have the highest molybdenum shoot concentration; however, when accounting for biomass, it had double the total uptake of other species. The application of methods to increase biomass would be beneficial, such as reducing herbivory, the flooding of the CFW, and ensuring good quality plugs—higher biomass is feasible based on other studies [47]. Roots were not long for any species and perhaps with additional growing seasons this will improve. Species with denser and longer roots may have the advantage in facilitating other phytoremediation processes, including filtration, oxidation, and microbial facilitated processes. In constructed wetlands rhizofiltration has been proposed as the primary mechanism for metal remediation [6,21], and the relative contribution to wastewater remediation relative to phytoextraction should be further investigated. Planting a diversity of macrophyte species would be beneficial to increase the efficiency of water treatment, particularly when there is a complex matrix of contaminants, while providing higher quality habitats and buffering against fluctuating weather events and water chemistry in wastewater stabilization ponds.
5. Conclusions
As part of a constructed floating wetland (CFW), Glyceria grandis (tall manna grass) has high potential for the removal of potential contaminants, including nitrogen, phosphorus, aluminum, barium, boron, cobalt, chromium, copper, iron, manganese, nickel, sodium, and strontium from wastewater stabilization ponds. Plants grew vigorously, produced high shoot and root biomass, and had high phytoextraction in roots and shoots relative to the other species assessed. Little research has been conducted on this species and it merits further study. Carex aquatilis and Schoenoplectus tabernaemontani, two of the more well-studied species in our study, continued to demonstrate potential for phytoremediation under field and operational scale conditions; specifically, the potential of Carex aquatilis to remove molybdenum. Beckmannia syzigachne and Scirpus microcarpus showed promise for cold climate wastewater treatment systems, through the removal of metals (aluminum, barium, chromium, cobalt, nickel, and zinc) and nutrients (nitrogen, phosphorus, sulfur, and calcium), respectively, but only if their biomass per unit area is increased through enhanced planting strategies. Our research shows that the assessment of macrophytes for inclusion in wastewater treatment wetlands requires tolerance and the uptake of contaminants of concern, whether storage is in roots or shoots, and tolerance to site-specific growing conditions. Species selection, as well as a species’ potential for high contaminant accumulation in roots or shoots, must be based on the combination of plant tissue concentrations and plant biomass. Variability among species to accumulate contaminants and biomass is high, and in cold climates, where growing conditions are harsh, the use of different macrophytes for wetland water treatment systems is recommended.
Author Contributions
Conceptualization, M.A.N.; methodology, M.A.N. and S.R.W.; data curation and formal analysis, A.D. and S.R.W.; writing—original draft preparation, review, and editing, M.A.N. and S.R.W.; project administration, S.R.W.; funding acquisition, M.A.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Brazeau County and the Federation of Canadian Municipalities’—Green Municipal Fund. Research grant number RES0053206.
Data Availability Statement
The data presented in this study may be available on request from the corresponding author. The data are not publicly available due to privacy.
Acknowledgments
The authors acknowledge Terry Lucke and Christopher Walker of Covey Associates Pty Ltd., Zimran Khokhar of Brazeau County, and Mohamed Gamal El-Din and Pamela Chelme-Ayala of the Department of Civil and Environmental Engineering at the University of Alberta for their contributions to the conception and establishment of the CFW research program.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Mahapatra, S.; Samal, K.; Dash, R.R. Waste stabilization pond (WSP) for wastewater treatment: A review on factors, modelling and cost analysis. J. Environ. Manag. 2022, 308, 114668. [Google Scholar] [CrossRef]
- Al-Hashimi, M.A.I.; Hussain, H.T. Stabilization pond for wastewater treatment. Eur. Sci. J. 2013, 9, 279–294. [Google Scholar]
- Cantinho, P.; Matos, M.; Transcoso, M.A.; Correia dos Santos, M.M. Behaviour and fate of metals in urban wastewater treatment plants: A review. Int. J. Environ. Sci. Technol. 2015, 13, 359–386. [Google Scholar] [CrossRef]
- Hargraves, A.J.; Constantino, C.; Dotro, G.; Cartmell, E.; Campo, P. Fate and removal f metals in municipal wastewater treatment: A review. Environ. Technol. Rev. 2018, 7, 1–18. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, P. Wastewater stabilization ponds: Removal of emerging contaminants. J. Sustain. Dev. Energy Water Environ. Syst. 2020, 8, 344–359. [Google Scholar] [CrossRef]
- Borne, K.E.; Fassman, E.A.; Tanner, C.C. Floating treatment wetland retrofit to improve stormwater pond performance for suspended solids, copper and zinc. Ecol. Eng. 2013, 54, 173–182. [Google Scholar] [CrossRef]
- Pavlineri, N.; Skoulikidis, N.T.; Tsihrintzis, V.A. Constructed floating wetlands: A review of research, design, operation and management aspects, and data meta-analysis. Chem. Eng. J. 2017, 308, 1120–1132. [Google Scholar] [CrossRef]
- Lucke, T.; Walker, C.; Beecham, S. Experimental designs of field-based constructed floating wetland studies: A review. Sci. Total Environ. 2019, 660, 199–208. [Google Scholar] [CrossRef]
- Vymazal, J.; Zhao, Y.; Mander, Ü. Recent research challenges in constructed wetlands for wastewater treatment: A review. Ecol. Eng. 2021, 169, 106318. [Google Scholar] [CrossRef]
- Colares, G.S.; Dell-Osbel, N.; Wiesel, P.G.; Oliveria, G.A.; Lemos, P.H.Z.; da Silva, F.P.; Lutterbeck, C.A.; Kist, L.T.; Machado, E.L. Floating treatment wetlands: A review and bibliometric analysis. Sci. Total Environ. 2020, 714, 136776. [Google Scholar] [CrossRef]
- Kulshreshtha, N.M.; Verma, V.; Soti, A.; Brighu, U.; Gupta, A.B. Exploring the contribution of plant species in the performance of constructed wetlands for domestic wastewater treatment. Bioresour. Technol. Rep. 2022, 18, 101038. [Google Scholar] [CrossRef]
- Ragush, C.; Schmidt, J.J.; Krkosek, W.H.; Gagnon, G.A.; Truelstrup-Hansen, L.; Jamieson, R.C. Treatment performance of wastewater stabilization ponds in Canada’s far north. Ecol. Eng. 2015, 83, 413–421. [Google Scholar] [CrossRef]
- Susarla, S.; Medina, V.F.; McCutcheon, S.C. Phytoremediation: An ecological solution to organic chemical contamination. Ecol. Eng. 2002, 18, 647–658. [Google Scholar] [CrossRef]
- Solomou, A.D.; Germani, R.; Proutsos, N.; Petropoulou, M.; Koutroumpilas, P.; Galanis, C.; Maroulis, G.; Kolimenakis, A. Utilizing mediterranean plants to remove contaminants from the soil environment: A short review. Agriculture 2022, 12, 238. [Google Scholar] [CrossRef]
- Yeh, N.; Yeh, P.; Chang, Y.-H. Artificial floating islands for environmental improvement. Renew. Sustain. Energy Rev. 2015, 47, 616–622. [Google Scholar] [CrossRef]
- Berg, E.C.; Borges, A.C. Use of plants in the remediation of arsenic-contaminated waters. Water Environ. Res. 2020, 92, 1669–1676. [Google Scholar] [CrossRef]
- Brisson, J.; Chazarenc, F. Maximizing pollutant removal in constructed wetlands: Should we pay more attention to macrophyte species selection? Sci. Total Environ. 2009, 407, 3923–3930. [Google Scholar] [CrossRef]
- Vymazal, J. Emergent plants used in free water surface constructed wetlands: A review. Ecol. Eng. 2013, 61, 582–592. [Google Scholar] [CrossRef]
- Bhatia, M.; Goyal, D. Analyzing remediation potential of wastewater through wetland plants: A review. Environ. Prog. Sustain. Energy 2014, 33, 9–27. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, Y.; Dong, Y. Phytoremediation of polluted waters potentials and prospects of wetland plant. Engineering 2022, 22, 199–208. [Google Scholar] [CrossRef]
- Rai, P.K. Heavy metal phytoremediation from aquatic ecosystems with special reference to macrophytes. Crit. Rev. Environ. Sci. Technol. 2009, 39, 697–753. [Google Scholar] [CrossRef]
- Syranidou, E.; Christofilopoulos, S.; Kalogerakis, N. Juncus spp.—The helophyte for all (phyto)remediation purposes? New Biotechnol. 2017, 38, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Lavergne, S.; Molofsky, J. Reed canary grass (Phalaris arundinacea) as a biological model in the study of plant invasions. Crit. Rev. Plant Sci. 2004, 23, 415–429. [Google Scholar] [CrossRef]
- Rezania, S.; Park, J.; Rupani, P.F.; Darajeh, N.; Xu, X.; Shahrokhishahraki, R. Phytoremediation potential and control of Phragmites australis as a green phytomass: An overview. Environ. Sci. Pollut. Res. 2019, 26, 7428–7441. [Google Scholar] [CrossRef]
- Yates, C.N.; Wootton, B.C.; Murphy, S.D. Performance assessment of arctic tundra municipal wastewater treatment wetlands through an arctic summer. Ecol. Eng. 2012, 44, 160–173. [Google Scholar] [CrossRef]
- MacDonald, R.L.; Burke, J.M.; Chen, H.Y.H.; Prepas, E.E. Relationship between aboveground biomass and percent cover of ground vegetation in Canadian boreal plain riparian forests. For. Sci. 2012, 58, 47–53. [Google Scholar] [CrossRef]
- Porte, A.J.; Samalens, J.-C.; Dulhoste, R.; Teissier Du Cros, R.; Bosc, A.; Meredieu, C. Using cover measurements to estimate above ground understory biomass in maritime pine stands. Annu. For. Sci. 2009, 66, 307. [Google Scholar] [CrossRef]
- Röttgermann, M.; Steinlein, T.; Beyschlag, W.; Dietz, H. Linear relationships between aboveground biomass and plant cover in low open herbaceous vegetation. J. Veg. Sci. 2020, 11, 145–148. [Google Scholar] [CrossRef]
- Cooke, J.A.; Johnson, M.S.; Davison, A.W. Determination of fluoride in vegetation: A review of modern techniques. Environ. Pollut. 1976, 11, 257–268. [Google Scholar] [CrossRef]
- Skinner, K.; Wright, N.; Porter-Goff, E. Mercury uptake and accumulation by four species of aquatic plants. Environ. Pollut. 2007, 145, 234–237. [Google Scholar] [CrossRef]
- McQueen, A.D.; Maas, H.; Gasparib, D.P.; Kinleya, C.M.; Rodgers, J.H., Jr.; Castle, J.W. Performance of a hybrid pilot-scale constructed wetland system for treating oil sands process-affected water from the Athabasca oil sands. Ecol. Eng. 2017, 101, 152–165. [Google Scholar] [CrossRef]
- Jiang, B.; Xing, Y.; Zhang, B.; Cai, R.; Zhang, D.; Sun, G. Effective phytoremediation of low-level heavy metals by native macrophytes in a vanadium mining area, China. Environ. Sci. Pollut. Res. 2018, 25, 31272–31282. [Google Scholar] [CrossRef] [PubMed]
- Yates, C.N.; Varickanickal, J.; Cousins, S.; Wootton, B.C. Testing the ability to enhance nitrogen removal at cold temperatures with C. aquatilis in a horizontal subsurface flow wetland system. Ecol. Eng. 2016, 94, 344–351. [Google Scholar] [CrossRef]
- Picard, C.R.; Fraser, L.H.; Steer, D. The interacting effects of temperature and plant community type on nutrient removal in wetland microcosms. Bioresour. Technol. 2004, 96, 1039–1047. [Google Scholar] [CrossRef]
- Zhang, C.-B.; Liu, W.-L.; Pan, X.-C.; Guan, M.; Liu, S.-L.; Ge, Y.; Chang, J. Comparison of effects of plant and biofilm bacterial community parameters on removal performances of pollutants in floating island systems. Ecol. Eng. 2014, 73, 58–63. [Google Scholar] [CrossRef]
- Fraser, L.H.; Carty, S.M.; Steer, D. A test of four plant species to reduce total nitrogen and total phosphorus from soil leachate in subsurface wetland microcosms. Bioresour. Technol. 2004, 94, 185–192. [Google Scholar] [CrossRef]
- He, N.; Sun, Z.; Zhang, Y.; Liu, M. Nitrogen and phosphorus removal from simulated wastewater with aquatic macrophytes. Adv. Mater. Res. 2012, 518–523, 2597–2603. [Google Scholar] [CrossRef]
- Gourand, C.; Giroux, J.-F.; Mesleard, F.; Desnouhes, L. Non-destructive sampling of Schoenoplectus maritimus in southern France. Wetlands 2008, 28, 532–537. [Google Scholar] [CrossRef]
- Gamal El-Din, M.; Naeth, M.A. Evaluating the performance of constructed floating wetlands in treating wastewater in cold climates. In Final Report Prepared for Brazeau County, Alberta and Covey and Associates; 2020; pp. 1–40. [Google Scholar]
- Weiss, J.; Hondzo, M.; Biesboer, D.; Semmens, M. Laboratory study of heavy metal phytoremediation by three wetland macrophytes. Int. J. Phytoremed. 2006, 8, 245–259. [Google Scholar] [CrossRef]
- Deng, H.; Ye, Z.N.; Wong, M.H. Lead and zinc accumulation and tolerance in populations of six wetland plants. Environ. Pollut. 2006, 141, 69–80. [Google Scholar] [CrossRef]
- Khan, S.; Ahmad, I.; Shah, M.T.; Rehman, S.; Khaliq, A. Use of constructed wetland for the removal of heavy metals from industrial wastewater. J. Environ. Manag. 2009, 90, 3451–3457. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.S.; Weiss, P. Metal uptake, transport, and release by wetland plants: Implications for phytoremediation and restoration. Environ. Int. 2004, 30, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, Z.; Gao, P.; Liu, P. Selection of aquatic plants for phytoremediation of heavy metal in electroplate wastewater. Acta Physiol. Plant. 2013, 35, 355–364. [Google Scholar] [CrossRef]
- Gupta, V.; Courtemanche, J.; Gunn, J.; Mykytczuk, N. Shallow floating treatment wetland capable of sulfate reduction in acid mine drainage impacted waters in a northern climate. J. Environ. Manag. 2020, 262, 110351. [Google Scholar] [CrossRef]
- Taylor, C.R.; Hook, P.B.; Stein, O.R.; Zabinski, C.A. Seasonal effects of 19 plant species on COD removal in subsurface treatment wetland microcosms. Ecol. Eng. 2011, 37, 703–710. [Google Scholar] [CrossRef]
- Arslan, M.; Wilkinson, S.; Naeth, M.A.; Gamal El-Din, M.; Khokhar, K.; Walker, C.; Lucke, T. Performance of constructed floating wetlands in a cold climate waste stabilization pond. Sci. Total Environ. 2023, 880, 163115. [Google Scholar] [CrossRef]
- Vymazal, J. Concentration is not enough to evaluate accumulation of heavy metals and nutrients in plants. Sci. Total Environ. 2016, 544, 495–498. [Google Scholar] [CrossRef]
- Sharma, R.; Vymazal, J.; Malaviya, P. Application of floating treatment wetlands for stormwater runoff: A critical review of the recent developments with emphasis on heavy metals and nutrient removal. Sci. Total Environ. 2021, 777, 146044. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).