Tradescantia-Based Test Systems Can Be Used for the Evaluation of the Toxic Potential of Harmful Algal Blooms
Abstract
1. Introduction
2. Materials and Methods
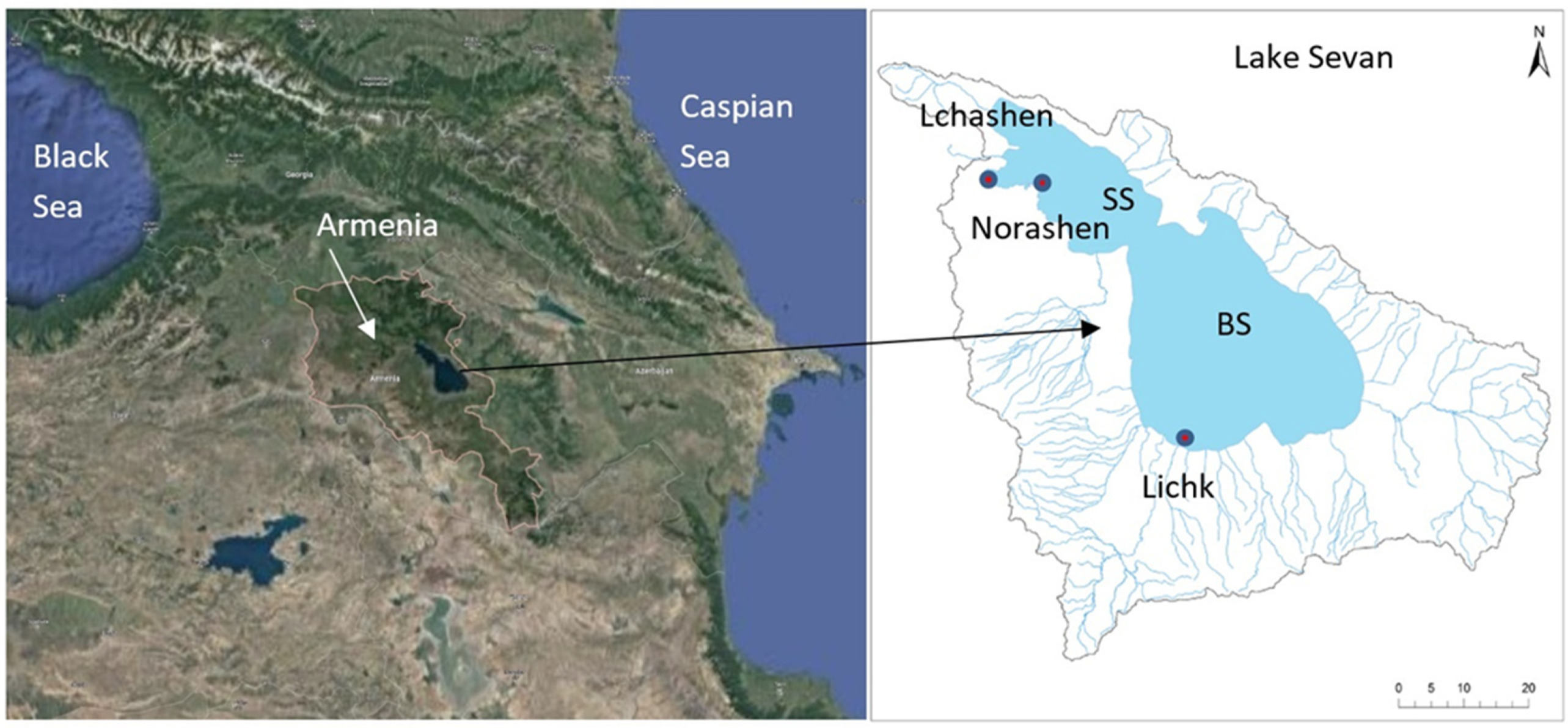
- Study Sites and Sampling
- Chemical analysis
- Hydrometeorological data
- Algae and cyanobacteria
- Bioassays
- Statistical analysis
3. Results
3.1. Chemical Analysis
3.2. Biological and Ecotoxicological Analyses
3.3. Integrated Analysis of Biological and Ecotoxicological Data
4. Discussion
- Algal nutrients availability and algal blooming
- HAB and genotoxic responses of Tradescantia
- Suitability of Tradescantia-based test systems for the evaluation of toxic potential of HABs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gusik, K.D.; Sayler, G.S. An overview on the marine neurotoxin, saxitoxin: Genetics, molecular targets, methods of detection and ecological functions. Mar. Drugs 2013, 11, 991–1018. [Google Scholar] [CrossRef]
- Drobac, D.; Tokodi, N.; Simeunovic, J.; Baltic, V.; Stanic, D.; Svircev, Z. Human exposure to cyanotoxins and their effects on health. Arch. Ind. Hyg. Tocixol. 2013, 64, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gautam, S.; Kumar, S. Phycotoxins. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 25–29. [Google Scholar] [CrossRef]
- Zhang, Y.; Whalen, J.K.; Cai, C.; Shan, K.; Zhou, H. Harmful cyanobacteria-diatom/dinoflagellate blooms and their cyanotoxins in freshwaters: A nonnegligible chronic health and ecological hazard. Water Res. 2023, 233, 119807. [Google Scholar] [CrossRef]
- Nyakairu, G.W.; Nagawa, C.B.; Mbabazi, J. Assessment of cyanobacteria toxins in freshwater fish: A case study of Murchison Bay (Lake Victoria) and Lake Mburo, Uganda. Toxicon 2010, 55, 939–946. [Google Scholar] [CrossRef]
- Dionysiou, D. Overview: Harmful algal blooms and natural toxins in fresh and marine waters—Exposure, occurrence, detection, toxicity, control, management and policy. Toxicon 2010, 55, 907–908. [Google Scholar] [CrossRef]
- Anton, A.; Teoh, P.L.; Mohd-Shaleh, S.R.; Mohammad-Noor, N. First occurrence of Cochlodinium blooms in Sabah, Malaysia. Harmful Algae 2008, 7, 331–336. [Google Scholar] [CrossRef]
- Richlen, M.L.; Morton, S.L.; Jamali, E.A.; Rajan, A.; Anderson, D.M. The catastrophic 2008–2009 red tide in the Arabian gulf region, with observations on the identification and phylogeny of the fish-killing dinoflagellate Cochlodinium polykrikoides. Harmful Algae 2010, 9, 163–172. [Google Scholar] [CrossRef]
- Whyte, J.N.S.; Haigh, N.; Ginther, N.G.; Keddy, L.J. First record of blooms of Cochlodinium sp. (Gymnodiniales, Dinophyceae) causing mortality to aquacultured salmon on the west coast of Canada. Phycologia 2001, 40, 298–304. [Google Scholar] [CrossRef]
- Papantoniou, G.; Cladas, Y.; Ketsilis-Rinis, V.; Vaitsi, Z.; Fragopoulu, N. Effects of HABs and a dystrophic event on zooplankton community structure in a Mediterranean lagoon (W Greece). Estuar. Coast. Shelf Sci. 2020, 245, 106985. [Google Scholar] [CrossRef]
- Turner, J.T. Planktonic marine copepods and harmful algae. Harmful Algae 2014, 32, 81–93. [Google Scholar] [CrossRef]
- Wells, M.L.; Karlson, B.; Wulff, A.; Kudela, R.; Trick, C.; Asnaghi, V.; Berdalet, E.; Cochlan, W.; Davidson, K.; De Rijcke, M.; et al. Future HAB science: Directions and challenges in a changing climate. Harmful Algae 2020, 91, 101632. [Google Scholar] [CrossRef]
- Gabrielyan, B.; Khosrovyan, A.; Schultze, M. A review of anthropogenic stressors on Lake Sevan, Armenia. J. Limnol. 2022, 81, 2061. [Google Scholar] [CrossRef]
- Hovhanissian, R.O. Lake Sevan Yesterday, Today, Tomorrow; Gitutyn: Yerevan, Armenia, 1994. [Google Scholar]
- Legovich, N. Qualitative changes in the phytoplankton community of Lake Sevan under the water level lowering. Biol. J. Armen. 1968, 21, 31–42. (In Russian) [Google Scholar]
- Hovsepyan, A.A.; Khachikyan, T.G. Phytoplankton of littoral zone and submerged areas of Lake Sevan coast. In Lake Sevan. Ecological State during the Period of Water Level Change; Krylov, A.V., Ed.; Filigran: Yaroslavel, Russia, 2016; pp. 109–113. [Google Scholar]
- Nietch, C.T.; Gains-Germain, L.; Lazorchak, J.; Keely, S.P.; Youngstrom, G.; Urichich, E.M.; Astifan, B.; DaSilva, A.; Mayfield, H. Development of a Risk Characterization Tool for Harmful Cyanobacteria Blooms on the Ohio River. Water 2022, 14, 644. [Google Scholar] [CrossRef]
- Son, G.; Kim, D.; Kim, Y.D.; Lyu, S.; Kim, S. A Forecasting Method for Harmful Algal Bloom (HAB)-Prone Regions Allowing Preemptive Countermeasures Based only on Acoustic Doppler Current Profiler Measurements in a Large River. Water 2020, 12, 3488. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, R.; Li, F.; Chen, L. Effect of flow rate on environmental variables and phytoplankton dynamics: Results from field enclosures. Chin. J. Oceanol. Limnol. 2015, 33, 430–438. [Google Scholar] [CrossRef]
- Graham, J.L.; Dubrovsky, N.M.; Foster, G.M.; King, L.R.; Loftin, K.A.; Rosen, B.H.; Stelzer, E.A. Cyanotoxin occurrence in large rivers of the United States. Inland Waters 2020, 10, 109–117. [Google Scholar] [CrossRef]
- Loftin, K.A.; Clark, J.M.; Journey, C.A.; Kolpin, D.W.; Van Metre, P.C.; Bradley, P.M. Spatial and temporal variation in microcystins occurrence in wadeable streams in the southeastern USA. Environ. Toxicol. Chem. 2016, 35, 2281–2287. [Google Scholar] [CrossRef] [PubMed]
- Ujvárosi, A.Z.; Riba, M.; Garda, T.; Gyémánt, G.; Vereb, G.; Hamvas, M.M.; Vasas, G.; Máthé, C. Attack of Microcystis aeruginosa bloom on a Ceratophyllum submersum field: Ecotoxicological measurements in real environment with real microcystin exposure. Sci. Total Environ. 2019, 662, 735–745. [Google Scholar] [CrossRef]
- Banno, K.; Oda, T.; Nagai, K.; Nagai, S.; Tanaka, Y.; Basti, L. Deleterious Effects of Harmful Dinoflagellates and Raphidophytes on Egg Viability and Spermatozoa Swimming Velocity in the Japanese Pearl Oyster Pinctada fucata martensii. J. Shellfish Res. 2018, 37, 41–48. [Google Scholar] [CrossRef]
- Lopez-Flores, R.; Boix, D.; Badosa, A.; Brucet, S.; Quintana, X.D. Is Microtox (R) toxicity related to potentially harmful algae proliferation in Mediterranean salt marshes? Limnetica 2010, 29, 257–267. [Google Scholar] [CrossRef]
- Aghajanyan, E.A.; Avalyan, R.E.; Simonyan, A.E.; Atoyants, A.L.; Gabrielyan, B.; Aroutiounian, R.M.; Khosrovyan, A. Clastogenecity evaluation of water of Lake Sevan (Armenia) using Tradescantia micronucleus assay. Chemosphere 2018, 209, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Khosrovyan, A.; Aghajanyan, E.; Avalyan, R.; Atoyants, A.; Sahakyan, L.; Gabrielyan, B.; Aroutiounian, R. Assessment of the mutagenic potential of the water of an urban river by means of two Tradescantia-based test systems. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2022, 876, 503449. [Google Scholar] [CrossRef] [PubMed]
- Misik, M.; Pichler, C.; Rainer, B.; Nersesyan, A.; Misikova, K.; Knasmueller, S. Micronucleus assay with tetrad cells of Tradescantia. Methods Mol. Biol. 2019, 2031, 325–335. [Google Scholar] [CrossRef]
- Placencia, F.; Fadic, X.; Janes, K.; Cereceda-Balic, F. Tradescantia as a biomonitor for genotoxicity evaluation of diesel and biodiesel exhaust emissions. Sci. Total Environ. 2019, 173, 2297–2306. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for Drinking Water Quality. 2017. Available online: https://apps.who.int/iris/bitstream/handle/10665/254637/9789241549950-eng.pdf (accessed on 4 June 2023).
- Abakumov, V.A. Guide on Methods for the Hydrobiological Analysis of Surface Water and Bottom Sediments (Guide); Gidrometeoizdat: Leningrad, Russia, 1983. (In Russian) [Google Scholar]
- Abakumov, V.A.; Talskikh, V.N.; Popchenko, V.I.; Bulgakov, G.P.; Svirskaya, N.L.; Krineva, S.V.; Popcheko, I.I.; Semin, V.A.; Khromov, V.M.; Raspopov, I.M.; et al. Guide on the Hydrobiological Monitoring of Freshwater Ecosystems (Guide); Gidrometeoizdat: Saint-Petesburg, Russia, 1992. (In Russian) [Google Scholar]
- Tsarenko, P.M. Short Determinant Chlorococcus Algae of the Ukraine; Naukova Dumka: Kyiv, Ukraine, 1990. (In Russian) [Google Scholar]
- Streble, H.; Krauter, D. Das Leben im Wassertropfen; Kosmos: Stuttgart, Germany, 2001. [Google Scholar]
- Berg, L.K.; Hoef-Emden, K.; Melkonian, M. Der Kosmos-Algenfuhrer: Die Wichtigsten Subwasseralgen im Mikroskop; Kosmos: Stuttgart, Germany, 2012. [Google Scholar]
- Hambaryan, L.; Shahazizyan, I. Determinant and Educational Manual for Genera of Freshwater Algae; YSU Press: Yerevan, Armenia, 2014. (In Armenian) [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase; World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 2023; Available online: https://www.algaebase.org (accessed on 4 June 2023).
- Devlin, M.; Best, M.; Bresnan, E.; Scanlan, C.; Baptie, M. Water Framework Directive: The development and status of phytoplankton tools for ecological assessment of coastal and transitional waters. In Technical Report WFD-UKTAG; Water Framework Directive—United Kingdom Technical Advisory Group (WFD-UKTAG): UK, 2014. [Google Scholar]
- Kitaev, S.P. Fundamentals of Limnology for Hydrobiologists and Ichthyologists; Karel. Nauchn. Center, Russ. Akad. Sciences: Petrozavodsk, Russia, 2007. [Google Scholar]
- Ma, T.H.; Cabrera, G.L.; Cebulska-Wasilevska, A.; Chen, R.; Loarca, A.I.; Vandenberg, A.L.; Salamone, M.F. Tradescantia stamen hair mutation bioassay. Mutat. Res. 1994, 310, 211–220. [Google Scholar] [CrossRef]
- Ma, T.H.; Cabrera, G.L.; Chen, R.; Gill, B.S.; Sandhu, S.S.; Vandenberg, A.L.; Salamone, M.F. Tradescantia micronucleus bioassay. Mutat. Res. 1994, 310, 220–230. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality, Incorporating The 1st Addendum (Chapters). Chemical Aspects. Technical Document, 4th ed.; World Health Organization (WHO): Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/m/item/guidelines-for-drinking-water-quality-4th-ed.-incorporating-the-1st-addendum-(chapters) (accessed on 4 June 2023).
- Li, M.; Li, Y.; Zhang, Y.; Xu, Q.; Iqbal, M.S.; Xi, Y.; Xiang, X. The significance of phosphorus in algae growth and the subsequent ecological response of consumers. J. Freshwater Ecol. 2022, 37, 57–69. [Google Scholar] [CrossRef]
- Britto, D.T.; Siddiqi, M.Y.; Glass, A.D.M.; Kronzucker, H.J. Futile transmembrane NH4+ cycling: A cellular hypothesis to explain ammonium toxicity in plants. Proc. Natl. Acad. Sci. USA 2001, 98, 4255–4258. [Google Scholar] [CrossRef]
- Muro-Pastor, M.I.; Florencio, F.J. Regulation of ammonium assimilation in cyanobacteria. Plant Physiol. Biochem. 2003, 41, 595–603. [Google Scholar] [CrossRef]
- Sheath, R.G.; Wehr, J.D. Introduction to freshwater algae. In Aquatic Ecology, Freshwater Algae of North America; John, D., Wehr, R.S., Eds.; Academic Press: Cambridge, MA, USA, 2003; pp. 1–9. [Google Scholar] [CrossRef]
- Sommer, U.; Adrian, R.; Bauer, B.; Winder, M. The response of temperate aquatic ecosystems to global warming: Novel insights from a multidisciplinary project. Mar. Biol. 2012, 159, 2367–2377. [Google Scholar] [CrossRef]
- Sukenik, A.; Quesada, A.; Salmaso, N. Global expansion of toxic and non-toxic cyanobacteria: Effect on ecosystem functioning. Biodivers. Conserv. 2015, 24, 889–908. [Google Scholar] [CrossRef]
- Malviya, S.; Scalco, E.; Audic, S.; Vincent, F.; Veluchamy, A.; Poulain, J.; Wincker, P.; Iudicone, D.; Vargas, C.; Bittner, L.; et al. Insights into global diatom distribution and diversity in the world’s ocean. Proc. Natl. Acad. Sci. USA 2016, 113, E1516–E1525. [Google Scholar] [CrossRef]
- Song, X.L.; Liu, Z.W.; Pan, H.K.; Yang, G.J.; Chen, Y.W. Phytoplankton community structure in Meiliang Bay and Lake Wuli of Lake Taihu. J. Lake Sci. 2007, 19, 643–651. [Google Scholar]
- Wang, P.; Shen, H.; Xie, P. Can Hydrodynamics Change Phosphorus Strategies of Diatoms?—Nutrient Levels and Diatom Blooms in Lotic and Lentic Ecosystems. Microb. Ecol. 2012, 63, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Tang, T.; Tan, L.; Gu, Y.; Jiang, W.; Cai, Q. Identifying community thresholds for lotic benthic diatoms in response to human disturbance. Sci. Rep. 2017, 7, 4134. [Google Scholar] [CrossRef]
- Altamirano, R.C.; Sierra-Beltrán, A.P. Biotoxins from freshwater and marine harmful algal blooms occurring in Mexico. Toxin Rev. 2008, 27, 27–77. [Google Scholar] [CrossRef]
- Metcaf, J.S.; Codd, G.A. Co-occurrence of Cyanobacteria and Cyanotoxins with other Environmental Health Hazards: Impacts and Implications. Toxins 2020, 12, 629. [Google Scholar] [CrossRef]
- Watson, S.B.; Ridal, J.; Boyer, G.L. Taste and odour and cyanobacterial toxins: Impairment, prediction, and management in the Great Lakes. Can. J. Fish. Aquat. Sci. 2008, 65, 1779–1796. [Google Scholar] [CrossRef]
- Gevorgyan, G.; Rinke, K.; Schultze, M.; Mamyan, A.; Kuzmin, A.; Belykh, O.; Sorokovikova, E.; Hayrapetyan, A.; Hovsepyan, A.; Khachikyan, T.; et al. First report about toxic cyanobacterial bloom occurrence in Lake Sevan, Armenia. Int. Rev. Hydrobiol. 2020, 105, 131–142. [Google Scholar] [CrossRef]
- Hambaryan, L.; Khachikyan, T.; Ghukasyan, E. Changes in the horizontal development of phytoplankton of the littoral of Lake Sevan (Armenia) in conditions of water level fluctuations. Limnol. Freshwater Biol. 2020, 4, 662–664. [Google Scholar] [CrossRef]
- Oh, H.-M.; Lee, S.J.; Jang, M.-H.; Yoon, B.-D. Microcystin Production by Microcystis aeruginosa in a Phosphorus-Limited Chemostat. Appl. Environ. Microbiol. 2000, 66, 176–179. [Google Scholar] [CrossRef]
- Sivonen, K. Cyanobacterial toxins. In Encyclopedia of Microbiology, 3rd ed.; Schaechter, M., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 290–307. [Google Scholar] [CrossRef]
- Otero, P.; Silva, M. Chapter 7—The role of toxins: Impact on human health and aquatic environments. In The Pharmacological Potential of Cyanobacteria; Lopes, G., Silva, M., Vasconcelos, V., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 173–199. [Google Scholar] [CrossRef]
- Pflugmacher, S. Possible allelopathic effects of cyanotoxins, with reference to microcystin-LR, in aquatic ecosystems. Environ. Toxicol. 2002, 17, 407–413. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency Office of Water (USEPA). Health Effects Support Document for the Cyanobacterial Toxin Anatoxin-A. 2015. Available online: https://www.epa.gov/sites/default/files/2017-06/documents/anatoxin-a-report-2015.pdf (accessed on 4 June 2023).
- Stevens, D.K.; Krieger, R.I. Stability studies on the cyanobacterial nicotinic alkaloid snatoxin-A. Toxicon 1991, 29, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, G.; Yin, R.; Zhang, T.; Zheng, Y.; Wu, C.; Liu, C.; Huang, K.; Wang, F. Effects of molecular-level component variation of fulvic acid on photodegradation of Microcystin-LR under solar irradiation. Chem. Eng. J. 2022, 449, 137553. [Google Scholar] [CrossRef]
- Jones, G.J.; Negri, A.P. Persistence and degradation of cyanobacterial paralytic shellfish poisons (PSPs) in freshwaters. Water Res. 1997, 31, 525–533. [Google Scholar] [CrossRef]
- Lahti, K.; Rapala, J.; Färdig, M.; Niemelä, M.; Sivonen, K. Persistence of cyanobacterial hepatotoxin, microcystin-LR in particulate material and dissolved in lake water. Water Res. 1997, 31, 1005–1012. [Google Scholar] [CrossRef]
- Zastepa, A.; Pick, F.R.; Blais, J.M. Fate and Persistence of Particulate and Dissolved Microcystin-LA from Microcystis Blooms. Hum. Ecol. Risk Assess. Int. J. 2014, 20, 1670–1686. [Google Scholar] [CrossRef]
- Preece, E.P.; Hardy, F.J.; Moore, B.C.; Bryan, M. A review of microcystin detections in estuarine and marine waters: Environmental implications and human health risk. Harmful Algae 2017, 61, 31–45. [Google Scholar] [CrossRef]
- Gaysina, L.A.; Saraf, A.; Singh, P. Chapter 1—Cyanobacteria in diverse habitats. In Cyanobacteria; Mishra, A.K., Tiwari, D.N., Rai, A.N., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 1–28. [Google Scholar] [CrossRef]
- Gerashkin, S.; Evseeva, T.; Oudalova, A. Plants as a tool for the environmental health assessment. In Encyclopedia of Environmental Health, 2nd ed.; Nriagu, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 239–248. [Google Scholar] [CrossRef]
- Chiang, I.-Z.; Huang, W.-Y.; Wu, J.-T. Allelochemicals of Botryococcus brauna (Chlorophyceae). J. Phycol. 2004, 40, 474–480. [Google Scholar] [CrossRef]
- Suriyanti, S.N.P.; Usup, G. First report of the toxigenic Nitzschia navis-varingica (Bacillariophyceae) isolated from Tebrau Straits, Johor, Malaysia. Toxicon 2015, 108, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Thoha, H.; Kotaki, Y.; Panggabean, L.; Lundholm, N.; Ogawa, H.; Lim, P.T.; Takata, Y.; Kodama, M.; Fukuyo, Y. Screening of diatoms that produce ASP toxins in Southermost Asian waters. Coast. Mar. Sci. 2012, 35, 34–38. [Google Scholar]
- Dhar, B.C.; Cimarelli, L.; Singh, K.S.; Brandi, L.; Brandi, A.; Puccinelli, C.; Marcheggiani, S.; Spurio, R. Molecular Detection of a Potentially Toxic Diatom Species. Int. J. Environ. Res. Public Health 2015, 12, 4921–4941. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.L.J.; Lirdwitayaprasit, T.; Kotaki, Y.; Relox, J.J.; Furio, E.F.; Terada, R.; Yokoyama, T.; Kodama, M.; Fukuuo, Y. Isolation of ASP toxin-producing Nitzschia navis-varingica from Thailand. Mar. Res. Indones. 2008, 33, 225–228. [Google Scholar] [CrossRef]








| K− | Ca2+ | Na+ | Mg2+ | F− | Cl− | SO42− | HCO3− | Fe Total | Cu2+ | Zn2+ | Pb2+ | Cd2+ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg L−1 | |||||||||||||
| Spring | |||||||||||||
| Lichk | 7.8 | 31 | 43 | 21 | 0.4 | 44 | 19 | 220 | 0.26 | <dL | <dL | <dL | <dL |
| Norashen | 15 | 27 | 55 | 46 | 0.65 | 53 | 39 | 366 | 0.04 | <dL | <dL | <dL | <dL |
| Lchashen | 15 | 27 | 65 | 45 | 0.68 | 52 | 38 | 366 | 0.04 | <dL | <dL | <dL | <dL |
| Summer | |||||||||||||
| Lichk | 20.2 | 27 | 60 | 40 | 0.65 | 56 | 34 | 337 | 0.07 | <dL | <dL | <dL | <dL |
| Norashen | 28.27 | 28 | 65 | 45 | 0.65 | 67 | 35 | 366 | 0 | <dL | <dL | <dL | <dL |
| Lchashen | 34.09 | 27 | 63 | 45 | 0.68 | 69 | 33 | 366 | 0.04 | <dL | <dL | <dL | <dL |
| Autumn | |||||||||||||
| Lichk | 12.35 | 27 | 54 | 42 | <dL | <dL | <dL | <dL | 0.02 | <dL | <dL | <dL | <dL |
| Norashen | 13.5 | 27 | 61 | 45 | <dL | <dL | <dL | <dL | 0.04 | <dL | <dL | <dL | <dL |
| Lchashen | 13.5 | 27 | 60 | 46 | <dL | <dL | <dL | <dL | 0.02 | <dL | <dL | <dL | <dL |
| “average” water quality * | 4 × B | 200 | 4 × B | 100 | - | 150 | 150 | - | 0.5 | 0.05 | 0.2 | 0.025 | B + 0.002 |
| “good” quality * | 2 × B | 100 | 2 × B | 50 | - | 2 × B | 2 × B | - | 2 × B | B + 0.02 | |||
| “excellent” quality * | B | B | B | B | - | B | B | - | B | B | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khosrovyan, A.; Avalyan, R.; Atoyants, A.; Aghajanyan, E.; Hambaryan, L.; Aroutiounian, R.; Gabrielyan, B. Tradescantia-Based Test Systems Can Be Used for the Evaluation of the Toxic Potential of Harmful Algal Blooms. Water 2023, 15, 2500. https://doi.org/10.3390/w15132500
Khosrovyan A, Avalyan R, Atoyants A, Aghajanyan E, Hambaryan L, Aroutiounian R, Gabrielyan B. Tradescantia-Based Test Systems Can Be Used for the Evaluation of the Toxic Potential of Harmful Algal Blooms. Water. 2023; 15(13):2500. https://doi.org/10.3390/w15132500
Chicago/Turabian StyleKhosrovyan, Alla, Rima Avalyan, Anahit Atoyants, Evelina Aghajanyan, Lusine Hambaryan, Rouben Aroutiounian, and Bardukh Gabrielyan. 2023. "Tradescantia-Based Test Systems Can Be Used for the Evaluation of the Toxic Potential of Harmful Algal Blooms" Water 15, no. 13: 2500. https://doi.org/10.3390/w15132500
APA StyleKhosrovyan, A., Avalyan, R., Atoyants, A., Aghajanyan, E., Hambaryan, L., Aroutiounian, R., & Gabrielyan, B. (2023). Tradescantia-Based Test Systems Can Be Used for the Evaluation of the Toxic Potential of Harmful Algal Blooms. Water, 15(13), 2500. https://doi.org/10.3390/w15132500








