Phytoremediation Capacity of Water Hyacinth (Eichhornia crassipes) as a Nature-Based Solution for Contaminants and Physicochemical Characterization of Lake Water
Abstract
1. Introduction
2. Materials and Methods
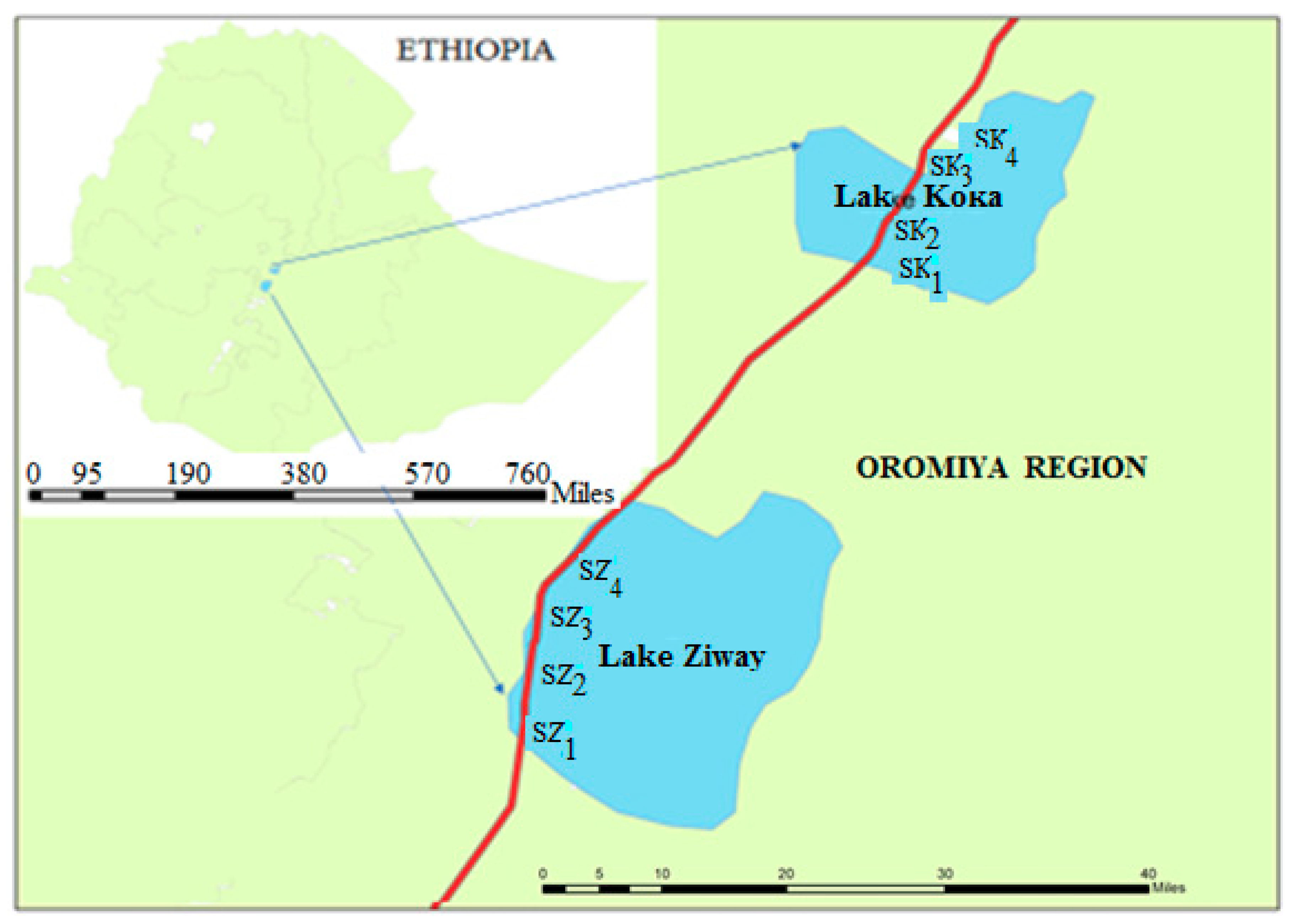
2.1. The Study Area
2.2. Sample Sites
2.3. Lakes’ Water Physico-Chemical Variables Test
2.4. Lakes’ Water Quality Measurement by Physico-Chemical Character
2.5. Water Sampling
2.6. Sample Analysis
3. Results and Discussion
3.1. Comparison of Wet and Dry Season Physico-Chemical Levels of Pollutants in the Lakes
3.2. The Effect of Water Hyacinth on the Physico-Chemical Levels of Pollutants in the Lakes
3.3. Comparison of the Physico-Chemical Levels of Pollutants in the Lakes at the Lake Covered by Water Hyacinth and Any Other Native Grasses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mulugeta, M.; Sahilu, R.; Kibret, Z.; Mersha, M. Level of Contamination in Lakes and Rivers of Ethiopia: An Overview. Arab. J. Chem. Environ. Res. 2020, 7, 158–174. [Google Scholar]
- Yan, S.H.; Song, W.; Guo, J.Y. Advances in management and utilization of invasive water hyacinth (Eichhornia crassipes) in aquatic ecosystems—A review. Crit. Rev. Biotechnol. 2017, 37, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Ting, W.H.T.; Tan, I.A.W.; Salleh, S.F.; Wahab, N.A. Journal of Water Process Engineering Application of water hyacinth (Eichhornia crassipes) for phytoremediation of ammoniacal nitrogen: A review. J. Water Process Eng. 2018, 22, 239–249. [Google Scholar] [CrossRef]
- Zaranyika, M.F.; Ndapwadza, T. Uptake of Ni, Zn, Fe, Co, Cr, Pb, Cu and Cd by water hyacinth (Eichhornia crassipes) in Mukuvisi and Manyame rivers, Zimbabwe. J. Environ. Sci. Health. 1995, 30, 157–169. [Google Scholar] [CrossRef]
- Navarro, L.; George, P. Water Hyacinth in Africa and the Middle East: A Survey of Problems and Solutions; International Development Research Centre: Ottawa, ON, Canada, 2000; 130p, Available online: http://books.google.com/books?hl=es&lr=&id=hhXqXNQ0WSQC&pgis=1 (accessed on 5 July 2023).
- Merga, L.B.; Mengistie, A.A.; Faber, J.H.; Van Den Brink, P.J.; Group, F. Trends in chemical pollution and ecological status of Lake Ziway, Ethiopia: A review focussing on nutrients, metals and pesticides. Afr. J. Aquat. Sci. 2020, 45, 386–400. [Google Scholar] [CrossRef]
- Lagoon, A.; Segbefia, A.Y.; Honlah, E.; Appiah, D.O. Effects of water hyacinth invasion on sustainability of fishing livelihoods along the River Tano and Effects of water hyacinth invasion on sustain- ability of fishing livelihoods along the River Tano and Abby-Tano Lagoon, Ghana. Cogent Food Agric. 2019, 5, 1654649. [Google Scholar] [CrossRef]
- Honlah, E.; Segbefia, A.Y.; Appiah, D.O.; Atakora, P.O. Effects of water hyacinth invasion on the health of the communities, and the education of children along River Tano and Abby-Tano Lagoon in Ghana. Cogent Soc. Sci. 2019, 5, 1619652. [Google Scholar] [CrossRef]
- Subash, T. Study on the benefits and impacts of Water Hyacinth at Pazhayar River Basin in Kanyakumari District, Tamilnadu, India—A Case Review. Int. J. Environ. Sci. Technol. 2016, 2, 69–74. [Google Scholar]
- Van Oijstaeijen, W.; Van Passel, S.; Cools, J.; de Bisthoven, L.J.; Hugé, J.; Berihun, D.; Ejigu, N.; Nyssen, J. Farmers’ preferences towards water hyacinth control: A contingent valuation study. J. Great Lakes Res. 2020, 46, 1459–1468. [Google Scholar] [CrossRef]
- Pagad, S.; Genovesi, P.; Carnevali, L.; Scalera, R.; Clout, M. IUCN SSC Invasive Species Specialist Group: Invasive alien species information management supporting practitioners, policy makers and decision takers. Manag. Biol. Invasions 2016, 6, 127–135. [Google Scholar] [CrossRef]
- Turbelin, A.J.; Malamud, B.D.; Francis, R.A. Mapping the global state of invasive alien species: Patterns of invasion and policy responses. Glob. Ecol. Biogeogr. 2017, 26, 78–92. [Google Scholar] [CrossRef]
- Shiferaw, W.; Demissew, S.; Bekele, T. Invasive alien plant species in Ethiopia: Ecological impacts on biodiversity a review paper. Int. J. Mol. Biol. 2018, 3, 169–176. [Google Scholar] [CrossRef]
- Getnet, H.; Kifle, D.; Fetahi, T. Water hyacinth (Eichhornia crassipes) affects the composition and abundance of zooplankton in the littoral region of Koka Reservoir, Ethiopia. Afr. J. Aquat. Sci. 2020, 45, 486–492. [Google Scholar] [CrossRef]
- Tewabe, D.; Asmare, E.; Zelalem, W.; Mohamed, B. Identification of impacts, some biology of water hyacinth (Eichhornia crassipes) and its management options in Lake Tana, Ethiopia. Net J. Agric. Sci. 2017, 5, 8–15. [Google Scholar] [CrossRef]
- Scholten, M.C.T.; Karman, C.C.; Huwer, S. Ecotoxicological risk assessment related to chemicals and pollutants in off-shore oil production. Toxicol. Lett. 2000, 113, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Lake, B.; Zerizghi, T.; Yang, Y.; Wang, W. Ecological risk assessment of heavy metal concentrations in sediment and fish of a shallow lake: A case study. Environ. Monit. Assess. 2020, 192, 154. [Google Scholar]
- Karakoc, G. Water quality and impacts of pollution sources for Eymir and Mogan Lakes (Turkey). Environ. Int. 2003, 29, 21–27. [Google Scholar] [CrossRef]
- Yabanl, M.; Yozukmaz, A.; Alparslan, Y. Evaluation of heavy metals and selenium contents in the muscle tissues of rainbow trout (Oncorhynchus mykiss Walbaum, 1792) in Western Anatolia. J. Food Agric. Environ. 2014, 3, 165–168. [Google Scholar]
- Yabanli, M.; Alparslan, Y.; Hasanhocaoglu Yapici, H.; Yapici, S.; Yozukmaz, A. Determination of heavy metal content in commercial marine fish hunted From Southeast Aegean Sea (Turkey) and their potential risk for public health. Casp. J. Environ. Sci. 2023, 14, 1–13. [Google Scholar]
- Keban, F.; Lake, D.A.M.; Aksu, Ö.; Yabanli, M.; Can, E.; Kutluyer, F. Comparison of Heavy Metals Bioaccumulation by Dreissena Polymorpha (Pallas, 1771) and Unio Elongatulus Eucirrus (Bourguignat, 1860). Fresenius Environ. Bull. 2012, 21, 1942–1947. [Google Scholar]
- Nash, D.A.H.; Abdullah, S.R.S.; Hasan, H.A.; Idris, M.; Muhammad, N.F.; Al-Baldawi, I.A.; Ismail, N.I. Phytoremediation of nutrients and organic carbon from sago mill effluent using water hyacinth (Eichhornia crassipes). J. Eng. Technol. Sci. 2019, 51, 573–584. [Google Scholar] [CrossRef]
- Sayago, U.F.C. Design and development of a biotreatment of E. crassipes for the decontamination of water with Chromium (VI). Sci. Rep. 2021, 11, 9326. [Google Scholar] [CrossRef]
- Coetzee, J.A.; Hill, M.P. The role of eutrophication in the biological control of water hyacinth, Eichhornia crassipes, in South Africa. BioControl 2012, 57, 247–261. [Google Scholar] [CrossRef]
- Honlah, E.; Segbefia, A.Y.; Appiah, D.O.; Honlah, E.; Segbefia, A.Y.; Appiah, D.O. Cogent Food & Agriculture the Effects of Water Hyacinth Invasion on Smallholder Farming along River Tano and Tano The Effects of Water Hyacinth Invasion on Smallholder Farming along River Tano and Tano. Cogent Food Agric. 2019, 5, 1567042. [Google Scholar] [CrossRef]
- World, S.; Assembly, H. Global vector control response: An integrated approach for the control of vector-borne diseases. In World Health Assembly Resolution WHA70; WHO: Geneva, Switzerland, 2017; pp. 15–17. [Google Scholar]
- Paulraj, M.; Prapakorn, G.; Santi, T.; Kraipat, C.; Sanket, C. Improvement of Water Hyacinth Bioconversion by Different Organic and Mineral Acid Pretreatment and the Effect of Post-Pretreatment Washing. BioEnergy Res. 2022. [Google Scholar] [CrossRef]
- Huynh, A.T.; Chen, Y.C.; Tran, B.N.T. A small-scale study on removal of heavy metals from contaminated water using water hyacinth. Processes 2021, 9, 1802. [Google Scholar] [CrossRef]
- Dersseh, M.G.; Kibret, A.A.; Tilahun, S.A.; Worqlul, A.W.; Moges, M.A.; Dagnew, D.C.; Abebe, W.B.; Melesse, A.M. Potential of water hyacinth infestation on Lake Tana, Ethiopia: A prediction using a GIS-based multi-criteria technique. Water 2019, 11, 1921. [Google Scholar] [CrossRef]
- Dersseh, M.G.; Tilahun, S.A.; Worqlul, A.W.; Moges, M.A.; Abebe, W.B.; Mhiret, D.A.; Melesse, A.M. Spatial and Temporal Dynamics of Water Hyacinth and Its Linkage with Lake-Level Fluctuation: Lake. Water 2020, 12, 1435. [Google Scholar] [CrossRef]
- Oliveira Junior, E.S.; van Bergen, T.J.H.M.; Nauta, J.; Budiša, A.; Aben, R.C.H.; Weideveld, S.T.J.; de Souza, C.A.; Muniz, C.C.; Roelofs, J.; Lamers, L.P.M.; et al. Water Hyacinth’s Effect on Greenhouse Gas Fluxes: A Field Study in a Wide Variety of Tropical Water Bodies. Ecosystems 2021, 24, 988–1004. [Google Scholar] [CrossRef]
- Ilo, O.P.; Simatele, M.D.; Nkomo, S.L.; Mkhize, N.M.; Prabhu, N.G. The benefits of water hyacinth (Eichhornia crassipes) for Southern Africa: A review. Sustainability 2020, 12, 9222. [Google Scholar] [CrossRef]
- Karouach, F.; Ben Bakrim, W.; Ezzariai, A.; Sobeh, M.; Kibret, M.; Yasri, A.; Hafidi, M.; Kouisni, L.; Ilo, O.P.; Simatele, M.D.; et al. A Comprehensive Evaluation of the Existing Approaches for Controlling and Managing the Proliferation of Water Hyacinth (Eichhornia crassipes): Review. Front. Environ. Sci. 2020, 12, 1–20. [Google Scholar] [CrossRef]
- Elbasiouny, H.; Darwesh, M.; Elbeltagy, H.; Abo-alhamd, F.G.; Amer, A.A.; Elsegaiy, M.A.; Khattab, I.A.; Elsharawy, E.A.; Ebehiry, F.; El-Ramady, H.; et al. Ecofriendly remediation technologies for wastewater contaminated with heavy metals with special focus on using water hyacinth and black tea wastes: A review. Environ. Monit. Assess. 2021, 193, 449. [Google Scholar] [CrossRef]
- Dsikowitzky, L.; Mengesha, M.; Dadebo, E.; Eduardo, C.; De Carvalho, V.; Sindern, S. Assessment of heavy metals in water samples and tissues of edible fish species from Awassa and Koka Rift Valley. Environ. Monit. Assess. 2013, 185, 3117–3131. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.S.; Ammar, N.S.; Soylak, M.; Ibrahim, M. Removal of Cd (II) and Pb (II) from aqueous solution using dried water hyacinth as a biosorbent. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 96, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Tabla-Hernandez, J.; Rodriguez-Espinosa, P.F.; Mendoza-Pérez, J.A.; Sánchez-Ortíz, E.; Martinez-Tavera, E.; Hernandez-Ramirez, A.G. Assessment of potential toxic metals in a ramsar wetland, Central Mexico and its self-depuration through Eichhornia crassipes. Water 2019, 11, 1248. [Google Scholar] [CrossRef]
- Liao, S.; Chang, W. Heavy Metal Phytoremediation by Water Hyacinth at Constructed Wetlands in Taiwan. J. Aquat. 2004, 110–118. [Google Scholar]
- Malik, A. Environmental challenge vis a vis opportunity: The case of water hyacinth. Environ. Int. 2007, 33, 122–138. [Google Scholar] [CrossRef]
- Eliku, T.; Leta, S. Spatial and seasonal variation in physicochemical parameters and heavy metals in Awash River, Ethiopia. Appl. Water Sci. 2018, 8, 177. [Google Scholar] [CrossRef]
- Shift, R.; Lake, T.; Ziway, L. Satellite Imageries and Field Data of Macrophytes Reveal a Regime Shift of a Tropical Lake (Lake Ziway, Ethiopia). Water 2021, 13, 396. [Google Scholar]
- Alamirew, T.; Zeleke, G. Spatiotemporal Dynamics of Water Quality Indicators in Koka. Remote Sens. 2023, 15, 1155. [Google Scholar]
- Gebregiorgis, F.Y. Management of Water Hyacinth (Eichhornia crassipes [Mart.] Solms) Using Bioagents in the Rift Valley of Ethiopia. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2017. [Google Scholar]
- Simpson, M.D.; Akbari, V.; Marino, A.; Prabhu, G.N.; Bhowmik, D.; Rupavatharam, S.; Datta, A.; Kleczkowski, A.; Sujeetha, J.A.R.P.; Anantrao, G.G.; et al. Detecting Water Hyacinth Infestation in Kuttanad, India, Using Dual-Pol Sentinel-1 SAR Imagery. Remote Sens. 2022, 14, 2845. [Google Scholar] [CrossRef]
- Gimeno-García, E.; Andreu, V.; Boluda, R. Heavy metals incidence in the application of inorganic fertilizers and pesticides to rice farming soils. Environ. Pollut. 1996, 92, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Teklay, A.; Amare, M. Water quality characteristics and pollution levels of heavy metals in Lake Haiq, Ethiopia. Ethiop. J. Sci. Technol. 2015, 8, 15. [Google Scholar] [CrossRef]
- Nizamutdinov, T.; Abakumov, E.; Morgun, E.; Loktev, R.; Kolesnikov, R. Agrochemical and pollution status of urbanized agricultural soils in the central part of yamal region. Energies 2021, 14, 4080. [Google Scholar] [CrossRef]
- Gizaw, A.; Zewge, F.; Kumar, A.; Mekonnen, A.; Tesfaye, M. A comprehensive review on nitrate and phosphate removal and recovery from aqueous solutions by adsorption. J. Water Supply Res. Technol. 2021, 70, 921–947. [Google Scholar] [CrossRef]
- Sasidharan, N.; Azim, T.; Devi, D.; Mathew, S. Water hyacinth for heavy metal scavenging and utilization as organic manure. Indian J. Weed Sci. 2013, 45, 204–209. [Google Scholar]
- Kumar, V.; Kumar, P.; Eid, E.M.; Singh, J.; Adelodun, B.; Kumar, P.; Kumari, S.; Choi, K.S. Modeling of water hyacinth growth and its role in heavy metals accumulation from unoperated old Ganga canal at Haridwar, India. Rend. Lincei 2021, 32, 805–816. [Google Scholar] [CrossRef]
- Sidek, N.M.; Abdullah, S.R.S.; Ahmad, N.U.; Draman, S.F.S.; Rosli, M.M.M.; Sanusi, M.F. Phytoremediation of abandoned mining lake by water hyacinth and water lettuces in constructed wetlands. J. Teknol. 2018, 80, 87–93. [Google Scholar] [CrossRef]
| Description | Lakes | |||||||
|---|---|---|---|---|---|---|---|---|
| Lake Koka | Lake Ziway | |||||||
| Study sites | Site 1 SK1 | Site 2 SK2 | Site 3 SK3 | Site 4 SK4 | Site 1 SZ1 | Site 2 SZ2 | Site 3 SZ3 | Site 4 SZ4 |
| Water hyacinth invasion or infestation level | Low (L) | Medium (M) | High (H) | Other grasses (G) | Low (L) | Medium (M) | High (H) | Other grasses (G) |
| Label | SK1L | SK2M | SK3H | SK4G | SZ1L | SZ2M | SZ3H | SZ4G |
| Lake Ziway | Season | WHO Stand | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Wet | Dry | ||||||||
| Parameters | SZ1L | SZ2M | SZ3H | SZ4G | SZ1L | SZ2M | SZ3H | SZ4G | |
| Cr | ND | ND | ND | ND | ND | ND | ND | ND | 0.05 |
| Pb | ND | ND | ND | ND | 0.69 | 0.68 | 0.69 | 0.71 | 0.05 |
| Cd | ND | ND | ND | ND | ND | ND | ND | ND | 0.01 |
| Zn | 0.05 | 0.10 | 0.08 | 0.04 | 0.59 | 0.38 | 0.57 | 0.53 | 0.01 |
| Cu | ND | ND | ND | 0.01 | ND | ND | ND | ND | 2 |
| EC | 347.3 | 315 | 289 | 284.4 | 337.3 | 306 | 283.6 | 280.4 | 300 |
| PO43-P | 24.7 | 17.1 | 13.1 | 28.8 | 0.6 | 0.60 | 0.8 | 0.8 | 5 |
| NO3-N | 15.3 | 27.0 | 36.6 | 18.2 | 8.4 | 9.5 | 9.6 | 7.3 | 50 |
| COD | 312 | 379 | 344 | 330.3 | 260 | 192 | 203 | 229 | 4.5 |
| BOD5 | 6.4 | 7.8 | 9.7 | 11.2 | 18.3 | 11.7 | 15.2 | 16.7 | 2 |
| pH | 6.5 | 6.0 | 6.0 | 7.8 | 6.0 | 5.9 | 5.5 | 7.9 | 6.5–8 |
| T | 25.5 | 25.5 | 26.5 | 23 | 26 | 28.3 | 29 | 23 | 30 |
| Lake Koka | Season | WHO Stand | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Wet | Dry | ||||||||
| Parameters | SK1L | SK2M | SK3H | SK4G | SK1L | SK2M | SK3H | SK4G | |
| Cr | ND | ND | ND | ND | ND | ND | ND | ND | 0.05 |
| Pb | ND | ND | 0.08 | ND | 0.49 | 0.66 | 0.56 | 0.56 | 0.05 |
| Cd | ND | ND | ND | ND | ND | ND | ND | ND | 0.01 |
| Zn | 0.18 | 0.03 | 0.02 | 0.19 | 0.34 | 0.45 | 0.37 | 0.72 | 0.01 |
| Cu | 0.07 | 0.04 | 0.03 | 0.07 | ND | ND | ND | ND | 2 |
| EC | 335.1 | 309 | 290.9 | 276.3 | 330.1 | 291 | 281.9 | 274.3 | 300 |
| PO43-P | 16.1 | 16.2 | 24.8 | 29.1 | 3.2 | 1.5 | 0.9 | 4.5 | 5 |
| NO3-N | 22.2 | 23.6 | 14.1 | 21.1 | 8.3 | 10.8 | 10.6 | 7.6 | 50 |
| COD | 291.0 | 263 | 333.7 | 323 | 246 | 536 | 264 | 305 | 4.5 |
| BOD5 | 8.4 | 7.5 | 9.6 | 8.1 | 26.25 | 37.5 | 22.5 | 31.5 | 2 |
| pH | 6.9 | 5.9 | 5.1 | 7.5 | 6.9 | 5.5 | 5.6 | 7.6 | 6.5–8 |
| T | 28.5 | 28.5 | 29.5 | 25 | 27 | 28 | 29 | 26 | 30 |
| Lakes | Parameters | Wet | Dry | ANOVA Seasonal |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Koka | Cr | NA | NA | NA |
| Pb | 0.02 ± 0.05 | 0.57 ± 0.07 | * 0.00 | |
| Cd | NA | NA | NA | |
| Zn | 0.11 ± 0.09 | 0.47 ± 0.17 | * 0.01 | |
| Cu | 0.07 ± 0.07 | 0.00 | 0.13 | |
| EC | 302.85 ± 25.32 | 294.35 ± 24.79 | 0.65 | |
| PO43-P | 21.55 ± 6.47 | 2.52 ± 1.64 | * 0.01 | |
| NO3-N | 20.25 ± 4.226 | 9.32 ± 1.62 | * 0.03 | |
| COD | 302.67 ± 32.07 | 337.75 ± 134.45 | 0.63 | |
| BOD5 | 8.4 ± 0.88 | 29.44 ± 6.52 | * 0.01 | |
| pH | 6.35 ± 1.07 | 6.4 ± 1.03 | 0.95 | |
| T | 27.87 ± 1.97 | 27.5 ± 1.29 | 0.76 | |
| Ziway | Cr | NA | NA | NA |
| Pb | 0.00 | 0.69 ± 0.01 | * 0.00 | |
| Cd | NA | NA | NA | |
| Zn | 0.067 ± 0.032 | 0.52 ± 0.095 | * 0.00 | |
| Cu | 0.01 ± 0.02 | 0.00 | 0.36 | |
| EC | 308.92 ± 28.91 | 301.85 ± 26.23 | 0.73 | |
| PO43-P | 20.925 ± 7.12 | 0.7 ± 0.12 | * 0.01 | |
| NO3-N | 24.275 ± 9.61 | 8.7 ± 1.08 | * 0.02 | |
| COD | 341.32 ± 28.33 | 221 ± 30.28 | * 0.01 | |
| BOD5 | 8.77 ± 2.11 | 15.47 ± 2.82 | * 0.01 | |
| pH | 6.57 ± 0.85 | 6.32 ± 1.07 | 0.73 | |
| T | 25.12 ± 1.49 | 26.32 ± 2.53 | 0.45 |
| Parameter | SK1L | SK2M | SK3H | ANOVA Spatial |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Cr W | NA | NA | NA | NA |
| Cr D | NA | NA | NA | NA |
| Pb W | 0.00 | 0.00 | 0.09 ± 0.111 | 0.21 |
| Pb D | 0.49 ± 0.12 | 0.66 ± 0.05 | 0.56 ± 0.04 | 0.08 |
| Cd W | NA | NA | NA | NA |
| Cd D | NA | NA | NA | NA |
| Zn W | 0.17 ± 0.01 | 0.02 ± 0.01 | 0.027 ± 0.06 | * 0.00 |
| Zn D | 0.34 ± 0.01 | 0.46 ± 0.01 | 0.37 ± 0.03 | * 0.01 |
| Cu W | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.79 |
| Cu D | NA | NA | NA | NA |
| EC W | 335 ± 4.58 | 309 ± 3.61 | 291 ± 2.00 | * 0.00 |
| EC D | 330 ± 3.46 | 291 ± 1.73 | 282 ± 1.00 | * 0.00 |
| PO43-P W | 3.17 ± 0.18 | 0.93 ± 0.01 | 1.47 ± 0.07 | * 0.00 |
| PO43-P D | 16.13 ± 0.06 | 16.20 ± 0.17 | 24.73 ± 0.06 | * 0.00 |
| NO3-N W | 22.17 ± 0.15 | 23.60 ± 0.2 | 14.13 ± 0.41 | * 0.00 |
| NO3-N D | 8.3 ± 0.20 | 10.80 ± 0.01 | 10.57 ± 0.06 | * 0.00 |
| COD W | 291 ± 2.00 | 263 ± 1.00 | 333.67 ± 3.23 | * 0.00 |
| COD D | 246 ± 0.01 | 535.67 ± 0.58 | 263.67 ± 0.58 | * 0.00 |
| BOD5 W | 8.33 ± 0.15 | 7.50 ± 0.10 | 9.6 ± 0.36 | * 0.01 |
| BOD5 D | 26.25 ± 0.25 | 37.50 ± 0.10 | 22.5 ± 0.10 | * 0.00 |
| pH W | 6.43 ± 0.40 | 5.87 ± 0.12 | 5.1 ± 0.10 | * 0.02 |
| pH D | 6.83 ± 0.06 | 5.37 ± 0.15 | 5.4 ± 0.35 | * 0.02 |
| T W | 28 ± 1.00 | 28.5 ± 0.50 | 29.33 ± 0.58 | 0.16 |
| TD | 27 ± 1.00 | 28 ± 0.00 | 28.67 ± 1.16 | 0.15 |
| Parameter | SZ1L | SZ2M | SZ3H | ANOVA Spatial |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Cr W | NA | NA | NA | NA |
| Cr D | NA | NA | NA | NA |
| Pb W | NA | NA | NA | NA |
| Pb D | 0.693 ± 0.07 | 0.69 ± 0.045 | 0.69 ± 0.03 | 0.97 |
| Cd W | NA | NA | NA | NA |
| Cd D | NA | NA | NA | NA |
| Zn W | 0.05 ± 0.01 | 0.17 ± 0.01 | 0.07 ± 0.01 | * 0.02 |
| Zn D | 0.59 ± 0.01 | 0.38 ± 0.01 | 0.57 ± 0.01 | * 0.00 |
| Cu W | NA | NA | NA | NA |
| Cu D | NA | NA | NA | NA |
| EC W | 347.33 ± 4.16 | 315.33 ± 4.04 | 289 ± 1 | * 0.000 |
| EC D | 337.33 ± 4.16 | 306.33 ± 5.51 | 283.67 ± 5.508 | * 0.000 |
| PO43-P W | 0.64 ± 0.02 | 0.6 ± 0.01 | 0.79 ± 0.02 | * 0.000 |
| PO43-P D | 24.73 ± 0.06 | 17.1 ± 0.01 | 13.13 ± 0.058 | * 0.000 |
| NO3-N W | 15.27 ± 0.38 | 26.97 ± 0.35 | 36.63 ± 1.159 | * 0.000 |
| NO3-N D | 8.40 ± 0.20 | 9.53 ± 0.06 | 9.6 ± 0.001 | * 0.000 |
| COD W | 312.33 ± 1.16 | 378.67 ± 2.52 | 344 ± 53.703 | 0.102 |
| COD D | 260.00 ± 2.65 | 192 ± 2 | 203 ± 0.001 | * 0.000 |
| BOD5 W | 6.40 ± 0.46 | 7.87 ± 0.15 | 9.7 ± 0.1 | * 0.000 |
| BOD5 D | 18.30 ± 0.20 | 11.7 ± 0.01 | 15.13 ± 0.058 | * 0.000 |
| pH W | 6.83 ± 0.29 | 6 ± 0.5 | 6 ± 0.866 | 0.226 |
| pH D | 6.07 ± 0.31 | 5.87 ± 0.12 | 5.33 ± 0.416 | 0.061 |
| T W | 25.33 ± 0.29 | 25.33 ± 1.16 | 26.67 ± 0.289 | 0.096 |
| T D | 25.50 ± 0.50 | 27.33 ± 0.58 | 28.83 ± 0.764 | * 0.002 |
| Lakes | Parameters | Hyacinth Infested | Other Native Grass Covered the Lake | ANOVA |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Koka | Cr W | NA | NA | NA |
| Cr D | NA | NA | NA | |
| Pb W | NA | NA | NA | |
| Pb D | 0.57 ± 0.10 | 0.54 | 0.59 | |
| Cd W | NA | NA | NA | |
| Cd D | NA | NA | NA | |
| Zn W | 0.08 ± 0.10 | 0.19 | 0.09 | |
| Zn D | 0.39 | 0.72 | * 0.01 | |
| Cu W | 0.05 | 0.07 | 0.09 | |
| Cu D | NA | NA | NA | |
| EC W | 311.67 ± 22.12 | 276.33 ± 32.12 | 0.06 | |
| EC D | 301 ± 25.51 | 274.33 ± 3.51 | 0.15 | |
| PO43-P W | 1.87 ± 1.19 | 4.51 ± 0.14 | * 0.03 | |
| PO43-P D | 19.03 ± 4.99 | 29.13 ± 0.01 | * 0.025 | |
| NO3-N W | 19.93 ± 5.19 | 21.07 ± 0.30 | 0.73 | |
| NO3-N D | 9.9 ± 1.39 | 7.6 ± 0.01 | * 0.05 | |
| COD W | 295.67 ± 35.23 | 323.33 ± 1.15 | 0.25 | |
| COD D | 348.67 ± 162.48 | 303.33 ± 3.05 | 0.65 | |
| BOD5 W | 8.5 ± 1.05 | 8.13 ± 0.01 | 0.58 | |
| BOD5 D | 28.73 ± 7.81 | 31.47 ± 0.01 | 0.58 | |
| pH W | 5.97 ± 0.91 | 7.43 ± 0.01 | * 0.05 | |
| pH D | 6 ± 0.78 | 7.4 ± 0.20 | * 0.04 | |
| T W | 28.83 ± 0.57 | 25 ± 1.00 | * 0.01 | |
| T D | 28 ± 1.00 | 25.67 ± 0.57 | * 0.025 | |
| Ziway | Cr W | NA | NA | NA |
| Cr D | NA | NA | NA | |
| Pb W | NA | NA | NA | |
| Pb D | 0.69 | 0.71 | 0.10 | |
| Cd W | NA | NA | NA | |
| Cd D | NA | NA | NA | |
| Zn W | 0.08 ± 0.03 | 0.04 | 0.09 | |
| Zn D | 0.513 ± 0.12 | 0.51 | 0.93 | |
| Cu W | NA | NA | NA | |
| Cu D | NA | NA | NA | |
| EC W | 317 ± 29.051 | 284.33 ± 0.57 | 0.13 | |
| EC D | 309 ± 26.63 | 280.33 ± 0.57 | 0.14 | |
| PO43-P W | 0.667 ± 0.11 | 0.77 ± 0.01 | 0.20 | |
| PO43-P D | 18.3 ± 5.89 | 28.77 ± 0.01 | * 0.04 | |
| NO3-N W | 26.3 ± 10.67 | 18.2 ± 0.30 | 0.26 | |
| NO3-N D | 9.17 ± 0.66 | 7.3 ± 0.10 | * 0.01 | |
| COD W | 345 ± 33.51 | 330.33 ± 1.53 | 0.49 | |
| COD D | 218.33 ± 36.50 | 229 ± 0.01 | 0.64 | |
| BOD5 W | 7.97 ± 1.66 | 11.2 ± 0.10 | * 0.028 | |
| BOD5 D | 15.07 ± 3.30 | 16.67 ± 0.01 | 0.45 | |
| pH W | 6.17 ± 0.28 | 7.73 ± 0.14 | * 0.01 | |
| pH D | 5.80 ± 0.26 | 7.9 ± 0.01 | * 0.00 | |
| T W | 25.83 ± 0.57 | 22.67 ± 0.57 | * 0.01 | |
| T D | 27.43 ± 1.50 | 23 ± 1.00 | * 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Churko, E.E.; Nhamo, L.; Chitakira, M. Phytoremediation Capacity of Water Hyacinth (Eichhornia crassipes) as a Nature-Based Solution for Contaminants and Physicochemical Characterization of Lake Water. Water 2023, 15, 2540. https://doi.org/10.3390/w15142540
Churko EE, Nhamo L, Chitakira M. Phytoremediation Capacity of Water Hyacinth (Eichhornia crassipes) as a Nature-Based Solution for Contaminants and Physicochemical Characterization of Lake Water. Water. 2023; 15(14):2540. https://doi.org/10.3390/w15142540
Chicago/Turabian StyleChurko, Esayas Elias, Luxon Nhamo, and Munyaradzi Chitakira. 2023. "Phytoremediation Capacity of Water Hyacinth (Eichhornia crassipes) as a Nature-Based Solution for Contaminants and Physicochemical Characterization of Lake Water" Water 15, no. 14: 2540. https://doi.org/10.3390/w15142540
APA StyleChurko, E. E., Nhamo, L., & Chitakira, M. (2023). Phytoremediation Capacity of Water Hyacinth (Eichhornia crassipes) as a Nature-Based Solution for Contaminants and Physicochemical Characterization of Lake Water. Water, 15(14), 2540. https://doi.org/10.3390/w15142540








