As one of the most sensitive aquatic invertebrates, mussels are affected by several parameters affecting water quality, which can be broadly divided into seven categories:
The existence of water quality standards for pollutants and their enforcement through NPDES permits based on the Clean Water Act is the main method followed to address and control water pollution, mainly from point sources. Therefore, it is helpful to compare Minnesota’s current water quality standards for major toxicants of freshwater mussels with corresponding federal criteria, and mussel tolerance values from the toxicological literature in order to provide a perspective on what is needed in terms of standard development and regulatory management of point sources to make the state’s water quality protective of mussels.
3.3.1. Nutrients
Freshwater mussels are more sensitive to nutrient pollution than most other macroinvertebrates [
105]. As mussels take up nutrients from the water column, excessive nutrients can affect mussels by impeding early life development. However, a complex indirect pathway exists through algae or phytoplankton. Mussels filter phytoplankton from the water column for food and excrete dissolved nitrogen and ammonia, which the phytoplankton can absorb [
37]. However, low filtration rates and/or increased phytoplankton blooms can lead to a decline in mussel numbers due to oxygen deficiency and/or the emergence of toxic cyanobacteria [
106]. Increased nutrient loadings from point and non-point sources can lead to such a decline in mussels, in turn leading to a loss in the ecosystem services they provide. Water pollution from excessive nitrogen loads takes the forms of nitrate (NO
3−), nitrite (NO
2−), and ammonia (NH
3), while phosphorus exists either in dissolved (soluble forms such as orthophosphorus) or particulate (attached to or contained in organic matter and sediment) form (
https://cms5.revize.com/revize/columbiaheights/document_center/Stormwater/Phosphorus_201412041423196128.pdf (accessed on 5 July 2023)).
Ammonia sensitivity. Freshwater mussels are among the most sensitive aquatic species to ammonia pollution. The USEPA considered freshwater mussels in its 1999 chronic and acute ammonia aquatic life criteria. These criteria are expressed in units of total ammonia nitrogen (TAN) comprising both ammonium ions: (NH
4+) and unionized ammonia: (NH
3-N), based on given temperature and pH values (chronic: 4.56 mg TAN/L; acute: 24 mg TAN/L, both at pH 7 and 20 °C) (
https://www.epa.gov/sites/production/files/2015-08/documents/aquatic-life-ambient-water-quality-criteria-for-ammonia-freshwater-2013.pdf (accessed on 5 July 2023)). Laboratory studies on ammonia tolerance levels for larval and juvenile freshwater mussels found tolerance values in the range 0.37–0.67 mg total ammonia N/L, (i.e., TAN/L) for growth, and 0.37–1.2 mg TAN/L for survival, which, is lower than the 1999 USEPA chronic and acute ammonia criteria [
107,
108]. A later study investigated the effect of pH on the toxicity of ammonia to juvenile fatmucket mussels (
Lampsilis siliquoidea), andcompared results with experiments on two other benthic invertebrates, amphipods (
Hyalella azteca) and oligochaetes (
Lumbriculus variegatus). The results showed that juvenile mussels were more sensitive to ammonia toxicity than other tested organisms and that mussel sensitivity to ammonia increased with increasing pH [
109]. Between the components of total ammonia, unionized ammonia is more toxic to freshwater mussels. It has been documented that unionized ammonia increases markedly with increases in pH, leading to an increase in the toxicity of total ammonia to freshwater mussels. As more nutrients are released into freshwater systems, these waters could become more eutrophic from excessive algal growth, which in turn would lead to an increase in water column pH from photosynthetic activity, resulting in waters less conducive for mussel survival [
110].
Based on studies such as these, the USEPA’s 2009 Ambient Water Quality Criteria (AWQC) for ammonia, included newly available data on the toxicity of ammonia to freshwater mussels, comprising 67 genera and 12 species compared to 34 genera considered in the 1999 AWQC [
111]. The USEPA revised the 2009 criteria in 2013 based on new toxicity data (
https://www.federalregister.gov/documents/2013/08/22/2013-20307/final-aquatic-life-ambient-water-quality-criteria-for-ammonia-freshwater-2013 (accessed on 5 July 2023)), and a reanalysis of data considered in the 1999 and 2009 ammonia criteria documents. The resulting ammonia criteria, which accounted for data on unionid mussels [
112], are: chronic: 1.9 mg TAN/L and acute: 17 mg TAN/L, both at pH 7 and 20 °C [
113]. Minnesota currently has a chronic ammonia standard expressed in µg/L of NH
3-N that is based on the USEPA 1980 criteria. The current criteria for ammonia at both the federal (USEPA-2013 guidance) and state level (MN-1981 water quality standards) are provided in
Table 1.
The USEPA chronic ammonia criteria at different pH and temperature values are provided in
Table 2. The Minnesota chronic ammonia criteria for Class 2A (cold water aquatic communities) and Class 2B (cool or warm water aquatic communities) waters in terms of TAN/L are provided in
Table 3 and
Table 4, respectively, for convenient comparison. Comparing the Minnesota chronic standard for ammonia in
Table 3 and
Table 4 to the USEPA ammonia criteria in
Table 2, we find that the Minnesota criteria for Class 2A waters (
Table 3) are protective of mussels at certain temperature–pH combinations, i.e., the MN cold water criteria are protective of mussels at temperatures higher than 100 °C and pH ≥ 7.5 (
Table 3), while the criteria for Class 2B waters (
Table 4) are not fully protective of mussels at any temperature–pH combination shown.
Minnesota currently does not have an acute standard for ammonia. Acute standards are based on pollutant concentrations that lead to mortality over short durations, while chronic standards are based on pollutant concentrations that would affect growth, survival, and reproduction over the longer term. Therefore, having both types of standards can help more comprehensively manage the multiple goals of clean water provisioning, including protection of beneficial uses, conservation of imperiled species such as freshwater mussels, and regulatory management of permitted discharges.
Consequently, the Minnesota Pollution Control Agency (MPCA) is revising its ammonia standard to adopt the USEPA 2013 ammonia criteria. The standard values would change according to temperature and pH conditions to reflect the underlying relationship between these variables and ammonia toxicity, as reflected in
Figure 3, which translates the USEPA 2013 criteria to Class 2 waters in Minnesota. The resulting standard accounts for ammonia sensitivity to a wider variety of aquatic life, including freshwater mussels in Minnesota lakes and streams [
114].
The USEPA 2013 ammonia criteria are expected to be protective of mussels in Minnesota based on a review of available ecotoxicological data. However, because many species of mussels are critically endangered, it helps to be mindful of the threats to their survival based on emerging drivers, such as climate change and increased human activity, that could affect the interaction between pollutants and water quality parameters.
For example, excessive unionized NH
3 can prevent juvenile mussel recruitment, with a significant effect on the total mussel population [
13]. This effect is worsened if increased nutrient loadings from human activity led to high rates of photosynthesis, which could increase pH, in turn leading to larger amounts of toxic unionized NH
3, which is particularly harmful for juvenile mussels. Apart from pH, temperature can also affect mussels’ sensitivity to ammonia. As noted in the USEPA’s 2013 ammonia criteria document, “In contrast to the pH–toxicity relationship, which applies to both vertebrates and invertebrates, the temperature–ammonia toxicity relationship only applies to invertebrates. Based on the results of the 1999 reanalysis of this relationship, it was determined that ammonia toxicity for invertebrates decreases with decreasing temperature to a temperature of approximately 7 °C, below which the relationship ends [
115]. As temperature increases in the aquatic environment, the toxicity of ammonia to mussels increases. The effect of temperature combined with pH on ammonia sensitivity was reported in a study of twenty river basins in China, which showed higher ammonia toxicity in mussels at sites with good water quality in summer and autumn, when water temperature and pH are higher [
116].
Recent research has noted higher ammonia sensitivity of freshwater mussels under certain conditions. For example, the LC
50 value or Lethal Concentration 50 (the concentration of the chemical at which 50% of test animals are reported killed during the observation period) for acute ammonia toxicity for the critically endangered freshwater mussel
Pseudunio auricularius in the disturbed and polluted Ebro basin of the Iberian Peninsula was found to be 7.53 mg of TAN/L based on 96 h acute toxicity tests [
117]. Chronic toxicity estimates for two species of freshwater mussels (the lotic
Velesunio sp. and the lentic
Velesunio angasi) in tropical northern Australia, characterized by soft waters (water hardness of <5 mg/L), were found to be 7–9.2 mg of TAN/L for V. angasi and 11.3 mg of TAN/L for Velesunio sp. at pH 6.0 and 27 ± 0.5 °C [
118]. When normalized to average temperate conditions of pH 7 and 20 °C, these freshwater mussel species were still found to be more sensitive than 8 of 16 other temperate, and 7 of 9 other tropical fish and invertebrate species. These differences in chronic ammonia toxicity were determined to be mainly due to the lower ionic strength of tropical fresh waters, based on the results of a study involving toxicity tests on six tropical species in Australia [
119]. These effects of ammonia toxicity can significantly affect freshwater mussels, altering the valuable services provided by mussels to freshwater ecosystems, and as such should be considered as part of an effective mussel conservation strategy.
Nitrate and Phosphorus sensitivity. A non-monotonic function was proposed to describe the multiple and complex pathways linking nutrients and mussels, by [
13] (
Figure 2 from [
13] reproduced here as
Figure 4). In thisrelationship, mussel abundance increases as total nitrogen and total phosphorus loads (TN and TP) increase from low levels and where fish hosts are more abundant, then reach a peak, after which proliferation of algal blooms cause eutrophication and may cause production of unionized ammonia (NH
3), which leads to a decline in juvenile mussels, and then in adult mussels. The parameters of this generic curve vary across mussel species.
Nitrate pollution is one of the main types of nutrient pollution nationally and globally and is recognized as being harmful to aquatic organisms. Recently, Minnesota revised a draft technical support document that developed endpoints for nitrate toxicity in a variety of aquatic organisms, including freshwater mussels [
120].
Nitrate, in addition to its indirect effect through algae, can have a toxic effect on freshwater mussels on its own. For example, experimental evidence of nitrate toxicity to mussels from the catchment of the Lužnice River, Czech Republic, was provided by [
121]. The results of a logistic regression based on mussel occurrence and nitrate-nitrogen concentration data showed that the spatial co-occurrence of five native mussel species declined with increasing nitrate concentrations, in agreement with earlier studies in Central European streams. Laboratory tests for acute (96 h) nitrate toxicity on adult freshwater mussels have found LC
50 values ranging between 357–937 mg of NO
3-N/L for the species
L. siliquoidea and
L. nervosa [
122]. Nitrate toxicity in the early life stages of freshwater mussels, particularly the glochidia (larval stage) and juvenile forms, was reported by [
92]. In this study, glochidia of two freshwater mussel species,
L siliquoidea and
Lampsilis fasciola, were exposed to nitrate solutions for 24 h (acute exposure) before being transported to a host fish. The results of this test showed a 28–35% reduction in juvenile production in
L. siliquoidea from exposure to 11 and 56 mg of NO
3-N/L, while no change was seen in
L. fasciola, showing differences between these closely related species. More recent acute toxicity tests with glochidia of four freshwater mussel species (
Hamiota altilis,
L. fasciola, L. siliquoidea,
Utterbackiana suborbiculata) have shown median effective concentrations (EC
50s) at 524–904 mg/L of NO
3-N/L in moderately hard water, i.e., 80–100 mg/L of CaCO
3 [
123] and 665 mg/L for
L. siliquoidea on its own [
124]. Chronic 28-day toxicity tests for nitrate for
L. siliquoidea found 20% effective concentrations (EC
20s) at 17 mg of NO
3-N/L [
124].
Phosphorus is typically the most limiting nutrient for algal growth in lakes and has a strong correlation with chlorophyll-
a [
125]. These indirect mechanisms affecting aquatic life can be significant in freshwater systems. Phosphorus negatively impacts freshwater mussels in three ways. Excess phosphorus loading leads to increased phytoplankton growth, which in turn leads to reduced DO levels [
126], which is detrimental to mussel survival. A study on the oxygen consumption (OC) rates of nine freshwater mussel species found that levels of dissolved oxygen (DO) concentrations in streams needed to be >2–4 mg/L for six of the species tested (
Pyganodon grandis,
Amblema plicata,
Quadrula pustulosa,
Elliptio complanata,
Elliptio fisheriana, and
Elliptio lanceolata), 3.5–4 mg/L and >4 mg/L, respectively, for two more sensitive species (
Pleurobema cordatum and
Villosa constricta), and >6 mg/L for the most sensitive species (
Villosa iris) in order to maintain normal OC under a temperature of 24 degrees Celsius [
127]. In addition, excess phosphorus can trigger toxic cyanobacterial blooms, specifically when N is low [
128], which reduces food quality for mussels. Finally, phosphorus leads to the production of toxins such as microcystins [
129] as well as unionized NH
3 from cyanobacteria, which is highly toxic to juvenile mussels [
113].
A water quality study of five rivers using principal component analysis of sampling events for 1998 and 2004 showed that the abundance of
L. fasciola was negatively correlated with the concentration of TP, nitrate, and nitrite (NO
2−), total Kjeldahl nitrogen (TKN), and turbidity [
93]. Mussels were found to be most abundant at TP < 0.05 mg/L and nitrate and nitrite < 3 mg/L, while no live mussels were found at TP > 0.10 mg/L and turbidity levels > 8 Jackson Turbidity Units (JTU) (Turbidity:
https://en.wikipedia.org/wiki/Turbidity (accessed on 5 July 2023)). The authors concluded that as higher N and P loadings lead to higher turbidity, it is plausible that the impacts of these contaminants on
L. fasciola are linked.
Minnesota’s Lake and River Eutrophication Standards, LES and RES, respectively, [
130,
131], are based on excess phosphorus as the causal agent of eutrophication associated with conditions of excess chlorophyll-
a, DO flux, and biochemical oxygen demand (BOD), all of which are harmful to aquatic life and reduce recreation quality. The RES are more explicitly based on aquatic life compared to the LES [See
https://www.pca.state.mn.us/sites/default/files/wq-s6-08.pdf (accessed on 5 July 2023)]. These criteria are not based directly on freshwater mussels, and incorporate data on many kinds of aquatic life, including fish and macroinvertebrates. The LES and RES include a cause criterion (i.e., TP), and response criteria comprising stressors (i.e., Chl-
a, BOD, and daily DO flux) [
130,
131]. The RES criteria, which are more explicitly based on aquatic life, are shown in
Table 6.
Turbidity is addressed by the criteria for total suspended sediments (TSS) in part 3 of the SONAR for the RES (MPCA document #: wq-rule4-06e, Eutrophication and TSS SONAR, Book 3:
https://www.pca.state.mn.us/sites/default/files/wq-rule4-06g.pdf; (accessed on 5 July 2023)). Streams that exceed both the cause and response criteria in
Table 6 are likely to be considered “impaired” for RES and are regulated through NPDES permits with total maximum daily loads (TMDLs) [
131]. They are additionally regulated through reasonable potential (RP) analyses to ensure that beneficial uses are met; such analyses include effluent limits in permits where there is RP to exceed a WQS [
131].
The Minnesota 2022 Impaired Waters list [
97] found that 40% of Minnesota’s lakes and streams are impaired by conventional pollutants [
132], including parameters for eutrophication. Conventional pollutants refer to a list of pollutants commonly used to assess the state’s waterbodies by watershed based on a 10-year assessment cycle, called Intensive Watershed Monitoring (
https://www.pca.state.mn.us/air-water-land-climate/watershed-approach-to-water-quality (accessed on 5 July 2023)). While controlling eutrophication is beneficial to mussels, which are particularly sensitive to ammonia from toxic algal blooms, the levels of stressors, including TP, that are deemed safe in RES were not developed incorporating data on freshwater mussels [
131].
The 2004 Canadian guidance framework document for phosphorus states: “The first response of an aquatic system to phosphorus additions is increased plant and algal productivity and biomass. Although this may be desirable in some cases, beyond a certain point, further phosphorus additions to phosphorus-limited systems can cause undesirable effects …When the excessive plant growth includes certain species of cyanobacteria, toxins may be produced, causing increased risk to aquatic life, livestock, and human health” ([
133], page 2). The framework document provides TP trigger ranges for Canadian lakes and rivers, which are shown in
Table 7.
In the absence of a stand-alone aquatic life standard for TP, and further considering that the RES was not explicitly developed to protect freshwater mussels, it is helpful to recall the results for freshwater mussel abundance found in the study of five rivers discussed before. As noted, mussels were found to be most abundant at TP < 0.05 mg/L and both nitrate and nitrite < 3 mg/L (in agreement with the Canadian chronic guideline for nitrate), while no mussels were found alive at TP > 0.10 mg/L and turbidity levels > 8 Jackson Turbidity Units (JTU) (Turbidity:
https://en.wikipedia.org/wiki/Turbidity (accessed on 5 July 2023)) [
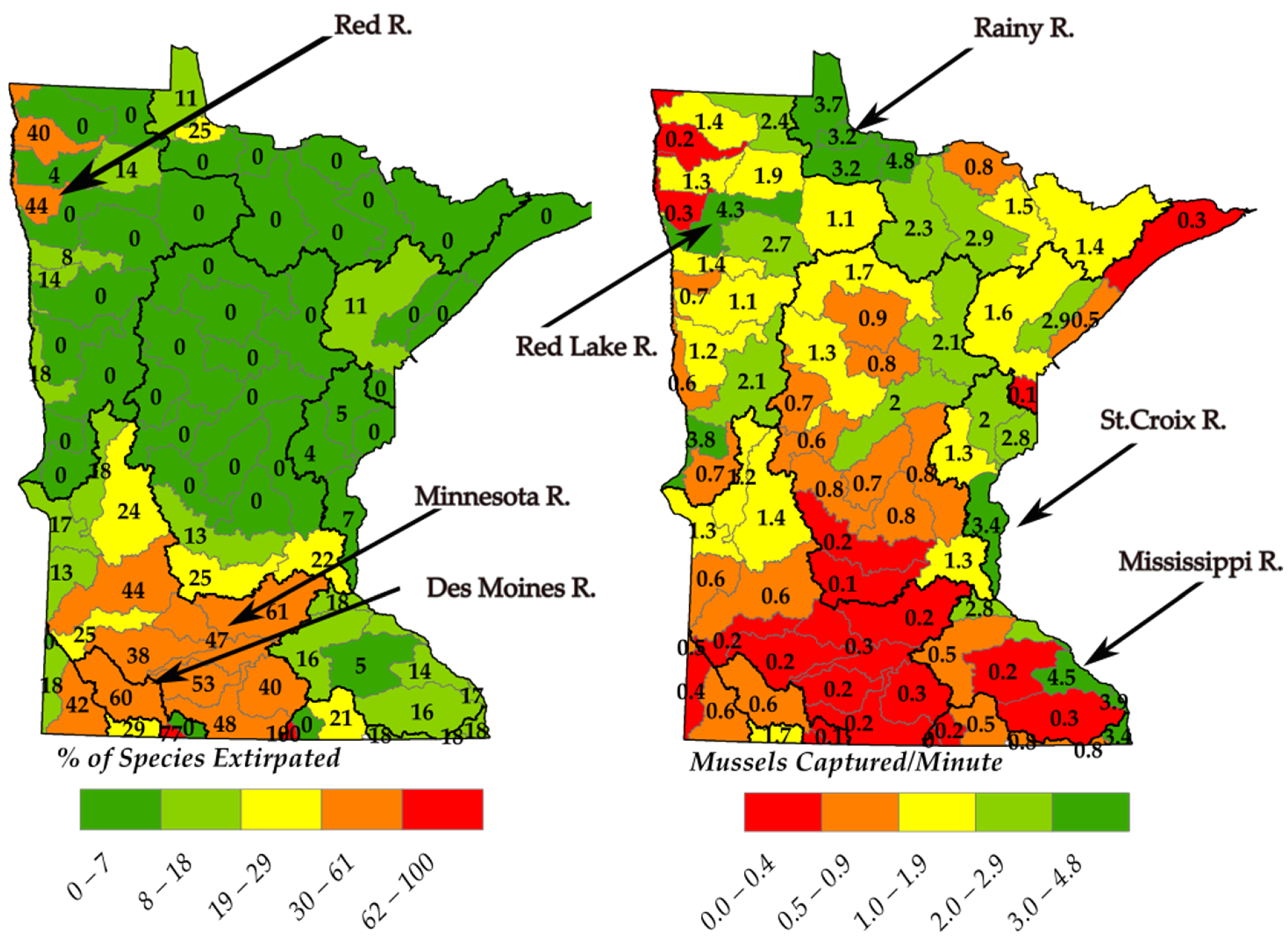
93]. Based on freshwater mussel survey data from the MDNR (
Figure 1), southern Minnesota has generally higher mussel extirpation rates as well as a larger contribution to pollution from nutrients; therefore, the corresponding TP standard of 0.15 mg/L for the South River nutrient region (
Table 6) may not be sufficiently protective.
It follows that for nutrients generally, current water quality standards in Minnesota may not be sufficiently protective of mussels, leaving scope for Minnesota’s proposed adoption of the 2013 USEPA ammonia criteria and revised nitrate TSD to be a step in the positive direction for freshwater mussel conservation [
114,
120].
3.3.2. Major Ions
Excessive loading of major ions or ‘salts’ from human activity in surface waters comprises cations such as sodium, potassium, calcium, and magnesium (Na, K, Ca, Mg) and anions such as chlorides, sulfates, and carbonates (Cl, SO
4, HCO
3). These salts contribute to salinization of surface water globally [
134]. Consequently, they are a serious concern for sensitive aquatic life such as freshwater mussels in the northeastern U.S. [
135,
136], and in Great Lakes states such as Minnesota [
137]. In addition, they can adversely affect drinking water supplies [
138]. The main anthropogenic sources for cations are mountaintop coal mining, valley filling, and mine drainage, while anions may result from road salt, alkali production, mine drainage, and water softeners, the latter further contributing to increased toxicity of salts to aquatic life by reducing water hardness [
139].
Major ions in various combinations of cations and anions contribute to total dissolved solids (TDS), which increases the specific conductivity of water, another parameter of concern for aquatic life [
140]. In chronic laboratory tests conducted with four invertebrate species (a cladoceran (
Ceriodaphnia dubia), a freshwater mussel (
L. siliquoidea), an amphipod (
H. azteca), and a mayfly (
Centroptilum triangulifer)) in water simulating Appalachian streams contaminated with either alkaline mine drainage from mountaintop mining with elevated Mg, Ca, K, SO
4, and HCO
3 ions or neutralized mine drainage with elevated Na, K, SO
4, and HCO
3 ions, both were toxic to freshwater mussels at the lowest tested concentration of 10%. The former was toxic to all except the cladoceran, and the latter was toxic to all except the mayfly, showing that elevated levels of total dissolved solids (TDS) based on the ionic composition, are particularly toxic to freshwater mussels, while being toxic to several other invertebrates [
141].
The acute toxicity of sodium chloride to glochidia of four species of freshwater mussels based on the 24-h EC
50 values, was found to be in the range 1265–1559 mg Cl/L for natural water (hardness 278–322 mg CaCO
3/L) and much lower for water reconstituted with salt (hardness: 100 mg CaCO
3/L): 113–1430 mg Cl/L (285 mg Cl/L for glochidia of
L. fasciola) [
89]. These findings show that glochidia of freshwater mussels are very sensitive to chloride toxicity, and additionally, are significantly more sensitive when hardness is lower [
89]. In addition, chloride levels in streams with endangered mussel habitats in the Great Lakes basin in Canada can exceed 1300 mg Cl/L, which is toxic to glochidia regardless of water hardness, raising a similar concern for freshwater mussel habitats in the Great Lakes region of the U.S. affected with salt pollution [
89,
137]. In another study testing the acute (96 h) toxicity of five species of juvenile mussels to ten chemicals, including chloride, the mussels had similar sensitivity based on EC
50s for most of the tested chemicals. In addition, the sensitivity of
L. siliquoidea, a commonly tested mussel, compared well with those of the other species, indicating
L. siliquoidea to be a representative species for toxicity testing [
90]. Chronic toxicity tests for sodium chloride or potassium chloride (NaCl or KCl) on juveniles of
L. siliquoidea found EC20s at 264 mg/L for NaCl and 8 mg/L for KCl, showing these mussels to be the most sensitive species to KCl toxicity and the second-most sensitive to NaCl toxicity among all freshwater organisms [
142].
Mussels have been documented to be even more sensitive to a combination of salts [
143]. As most chloride loading from WWTPs is due to home water softener use, the corresponding effluent can contain a high amount of chloride, which is toxic to aquatic life [
17]. Current research has documented that the type of salt matters for the extent of toxicity to freshwater aquatic life, with sodium chloride being more toxic compared to calcium or magnesium chloride [
144]. Chloride impairment of waterbodies is a growing problem in Minnesota, with 50 waters currently impaired and 75 at risk of impairment [
145].
However, current research has found that these North American chloride standards may not be protective of aquatic life due to the adverse effect of freshwater salinization from chloride pollution on zooplankton populations, which can in turn disrupt ecosystem functions related to lake food webs [
136,
148].
For sulfate, acute and chronic values for
Lampsilis abrupta have been recorded at 2362 and 1795 mg SO
4/L [
149]. Recent laboratory tests documented acute and chronic toxicity of sulfates in the pinkmucket mussel
L. abrupta and three other aquatic organisms: a cladoceran, a midge, and a fish (the fathead minnow) [
149]. The mussel (LC
50: 2362 mg SO
4/L) and the cladoceran (LC
50: 2441 mg SO
4/L) were found to be more acutely sensitive to sulfate, while the fish was the most chronically sensitive in the embryonic stage (305–477 mg SO
4/L for survival), followed by the mussel (LC
50: 1759 mg SO
4/L). The same study found that increased potassium (from 1 to 3 mg K/L) significantly reduced the toxicity of sulfate to fish embryos. Overall, these results highlight the toxicity of sulfate to aquatic life, including mussels, and the importance of considering complete toxicity data as well as the mitigating effect of potassium on sulfate toxicity when developing guidance values for sulfate.
3.3.3. Metals
Deposition of heavy metals such as cadmium, mercury, and lead, mainly from anthropogenic sources, has historically led to increases in metal concentration in waterbodies significantly in excess of natural background levels. This is particularly evident after the industrial revolution, as indicated by analysis of sediments in ice cores, lakes, and peat bogs in North America [
150]. While atmospheric deposition has declined in some areas of the northern hemisphere in the 21st century thanks to improvements in pollution control, metal concentrations can be elevated in many waterbodies [
151,
152] due to legacy concentrations in biota, potentially impacting biotic processes and interacting with exogenous drivers such as climate change [
153]. These elevated concentrations are toxic to mussels, which are exposed to metals dissolved in the water column or deposited in sediment and may bioaccumulate these toxins over their life cycle, resulting in a variety of effects including death and/or changes in growth, functionality, and behavior [
154]. In addition, metal deposition, particularly mercury deposition, can indirectly affect mussels by affecting their host fish species [
153,
155].
A review of metal toxicity to mussels has found that a possible cause of the widespread decline in freshwater mussels in North America could be chronic exposure to heavy metals such as cadmium, copper, lead, mercury, and zinc [
66]. Freshwater mussels have been foundto be particularly sensitive to mercury toxicity, with acute toxicity values for mercury ranked higher than those for cadmium and zinc [
156]. In laboratory tests with the rainbow mussel (
V. iris) mercury was confirmed to be toxic to both glochidia and juvenile mussels, with acute toxicity values in the range of 14–107 µg Hg/L for glochidia, and 99–162 µg Hg/L for juvenile mussels. In the chronic test, juvenile mussels stopped growing at 8 µg Hg/L. While these recorded toxicity values of mercury are much higher than Minnesota’s mercury WQS, there are multiple pathways by which mercury is capable of entering waterbodies, and it is currently the leading cause of surface water impairments in Minnesota [
97].
Acute and chronic toxicity of lead, cadmium, and zinc in the early life stages of freshwater mussels (glochidia and juveniles) in laboratory studies, was confirmed by [
157], while the same for acute and chronic toxicity of copper (2 and 12 µg of Cu/L, respectively), was confirmed by [
91,
108]. These results show that mussels are highly sensitive to all of the above metals compared to other freshwater species, except for chronic exposure to cadmium and zinc, for which the comparison is moderate. Current USEPA aquatic life criteria are protective of mussels against cadmium and lead toxicity, but not against zinc toxicity, for which chronic values were recorded in the range (63–68 µg Zn/L) [
157]. In addition, current USEPA criteria for copper are not protective of freshwater mussels [
91] (
https://www.epa.gov/wqc/national-recommended-water-quality-criteria-aquatic-life-criteria-table (accessed on 5 July 2023)). It is noteworthy that the toxicity of certain metals may depend on other factors, such as pH, ambient ions (CA, Mg/water hardness), and dissolved organic carbon (DOC) concentrations [
157,
158,
159,
160].
Minnesota has acute and chronic standards for cadmium (7.8 and 1.1 µg/L), copper (35 and 9.8 µg/L), lead (164 and 3.2 µg/L), and zinc (234 and 106 µg/L). It also has a human health-based chronic standard for mercury (6.9 ng/L) at a water hardness of 100 (
https://www.revisor.mn.gov/rules/7050.0222/ (accessed on 5 July 2023)). However, none of these standards are based on mussel toxicity information.
3.3.6. Contaminants of Emerging Concern (CECs)
Contaminants of emerging concern, or CECs, denote a wide variety of chemicals that are increasingly being detected in waterbodies as well as in soils and sediments [
171,
172,
173] and bound to plastics [
174,
175]. These pollutants, including “nanoparticles, pharmaceuticals and personal care products (PPCPs), estrogen-like compounds, flame retardants, detergents, and some industrial chemicals” (
https://www.epa.gov/columbiariver/chemicals-emerging-concern-columbia-river (accessed on 5 July 2023)), are mainly from anthropogenic sources, including agricultural runoff, air pollution, and wastewater effluent, with wastewater treatment plant discharges being the major sources for PPCPs [
176,
177]. Microplastics (MPs) are plastic particles up to five millimeters in diameter that include fragments from larger previously broken-down plastic items, such as clothing fibers (acrylic and polyester) and small particles referred to as microbeads (
https://en.wikipedia.org/wiki/Microbead (accessed on 5 July 2023))), while nanoplastics (NPs) consist of plastic particles less than 100 nm in diameter. Both can be viewed as CECs, as they are composed of organic polymers that may include chemicals classified as CECs or use CECs as additives to improve properties such as tensile strength, color, and use as flame-retardants [
178]. The prevalence of CECs in the U.S. [
179] and Minnesota waters (
https://www.pca.state.mn.us/water/pollutants-emerging-concern (accessed on 5 July 2023)) and in the Great Lakes system [
180,
181] represents a concern due to their potentially significant impacts on aquatic life and human health [
182]. These chemicals are often endocrine-active and can bioaccumulate in organisms even at very low concentrations, impacting growth, development, and reproductive functions over time [
183,
184,
185].
According to a recent report by the NOAA’s Mussel Watch Program (
https://www.fisheries.noaa.gov/inport/item/39400 (accessed on 5 July 2023)), which measured 237 CECs (PPCPs, pesticides and phenols) in the Great Lakes system, 99 (42%) CECs were detected in the tissues of freshwater mussels used as biomarkers for chemical contamination. While CECs were detected at all sites tested, they were more frequently found in sites located in river/harbor areas with known pollution sources including agrochemical runoff and wastewater treatment outfalls. In addition, CECs were found in several ‘reference’ sites located away from pollution sources, suggesting both the easy transport of these chemicals and their persistence over time [
186].
Freshwater mussels are among the most sensitive invertebrate species, are known to bioaccumulate, and feature differential exposure and impacts to pollutants across life stages [
187]. High CEC concentrations have been documented in mussels. For example, 145 PPCPs were found in mussels sampled from the Grand River in Ontario, Canada, which receives wastewater effluent from a major WWTP (
https://www.water-technology.net/projects/kitchener-wastewater-treatment-plant-upgrade-ontario/ (accessed on 5 July 2023)) [
188]. Moreover, freshwater mussels have been recognized to experience severe developmental impacts from CECs, including spawning in male mussels [
189]. Laboratory tests confirmed the acute and chronic toxicity of pesticide formulations including technical grade pesticides such as chlorpyrifos and atrazine (21-day exposure median 4.3 mg/l with visible effects at => 3.8 mg/l), as well as acute toxicity of the common pesticide glyphosate to both glochidia and juveniles of
L. siliquoidea at a concentration lower than any tested aquatic organism (48-h exposure at median 0.5 mg/L) [
86,
87,
88]. These results demonstrate that freshwater mussels are among the most sensitive aquatic organisms in this context. Chronic toxicity tests with juveniles of
L. siliquoidea exposed to high concentrations of PPCPs showed a decrease in functions such as feeding and filtering [
190]. Freshwater mussels have been documented to bioaccumulate and show toxicity to antimicrobial chemicals and microplastics, with significant declines in functions such as feeding, filtration rates, and enzyme activity [
191], ultimately leading to oxidative damage and neurotoxicity either alone or in combination with metals such as mercury [
192].
Considering the global spread of CECs in freshwater environments, including MPs and NPs [
172,
173], along with their documented toxicity on freshwater mussels, it is important to review the current regulatory guidelines defining the use of these chemicals. The U.S. passed the Microbead-Free Waters Act banning the manufacture of microbeads in 2017 (
https://en.wikipedia.org/wiki/Microbead-Free_Waters_Act_2015 (accessed on 5 July 2023)) and banned the sale of cosmetic products containing microbeads in 2018 in an effort to control the spread of microplastics in the environment [
172].
Currently, aquatic life criteria for CECs in general are not available at the national level. The USEPA issued a white paper to provide guidance to develop aquatic life criteria for CECs in 2008 [
193], as ”these chemicals have features that require additional consideration when applying existing ambient water quality criteria for the protection of aquatic life,” using the USEPA’s 1985 Guidelines for Deriving Numerical National Water Quality Criteria for the Protection of Aquatic Life and Their Uses’ (
https://www.epa.gov/wqc/contaminants-emerging-concern-including-pharmaceuticals-and-personal-care-products (accessed on 5 July 2023)). The white paper includes CEC toxicity information on many aquatic species, although it excludes freshwater mussels. Revised national aquatic life criteria on CECs are not available yet. Criteria exist for a few individual pollutants, such as the herbicide atrazine (draft criteria—Acute value: 3021 µg/L; Chronic value: 10 µg/L) and the petroleum derivative nonylphenol (Acute value: 55.5 µg/L; Chronic value 6.6 µg/L) [
194,
195]. However, these criteria do not incorporate toxicity information on freshwater mussels either. Canada has developed aquatic life criteria for more CECs; for example, Canada’s chronic criterion of 10 µg/L on carbamazepine (CBZ) (
https://ccme.ca/en/res/carbamazepine-en-canadian-water-quality-guidelines-for-the-protection-of-aquatic-life.pdf (accessed on 5 July 2023)) [
196], though these criteria also do not incorporate impacts on freshwater mussels. It is noteworthy that CBZ can transform into other chemical forms in the aquatic environment, which have different effects on aquatic life. For example, CBZ-DiOH, the main transformation product of CBZ in Canadian waters, is biologically inactive [
197] unlike, its counterpart in Minnesota waters, 10-OH-CBZ [
198].
Having water quality standards for CECs combined with the MDH framework for CECs, is helpful in monitoring risks to human health and the environment, and therefore, should pave the way for more standards based on aquatic life in the near future. However, in the context of protecting beneficial uses, including aquatic life and human health, it is important to note three points. First, concentrations of CECs in waterbodies much lower than HBVs may bioaccumulate in fish and in sensitive species such as freshwater mussels [
200,
201], leading to detrimental effects on growth and survival [
202,
203] as well as potential future effects on human health [
204]. Second, exposure to CECs is frequently accompanied by exposure to other environmental stressors, leading to multiple stressors that can vary with time, leading to adverse effects on the food web that could manifest across populations and through generations [
205]. Finally, in large freshwater systems such as the Great Lakes [
181] and their tributaries [
206], these chemicals have been detected at significantly higher concentrations (with several sites showing exceedances by a factor of four or more) compared even to existing water quality and human health benchmarks compiled from a variety of sources [
193,
207,
208,
209,
210]. This underscores the importance of developing aquatic life criteria for CECs and regulating point sources with corresponding WQBELs.