Response of Heavy Metals to Microseism in Coal Mining Subsidence Water of Huainan, China
Abstract
:1. Introduction
2. Materials and Methods
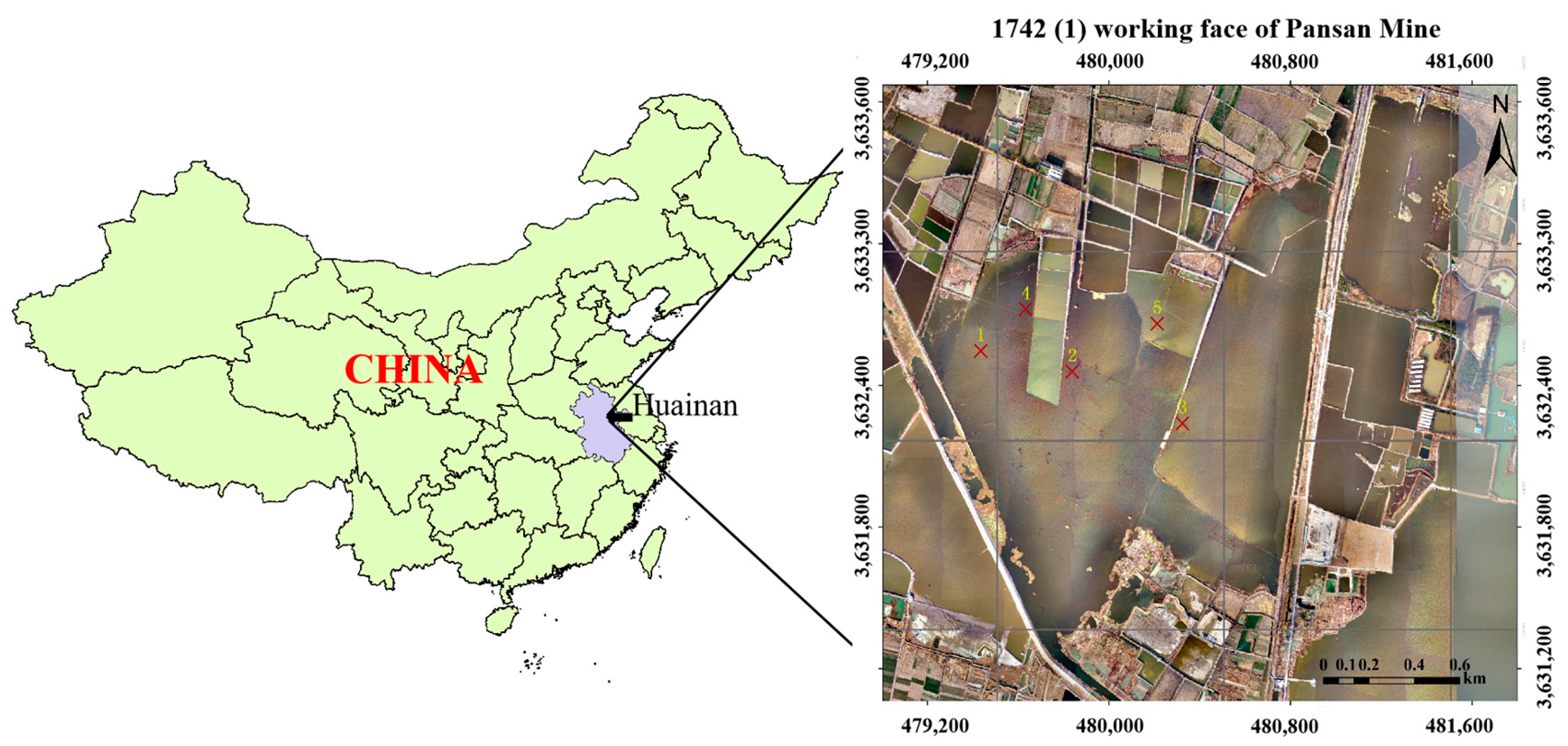
2.1. Description of the Study Area
2.2. Collection and Treatment of Samples
2.3. Statistical Analysis and Health Risk Appraisal
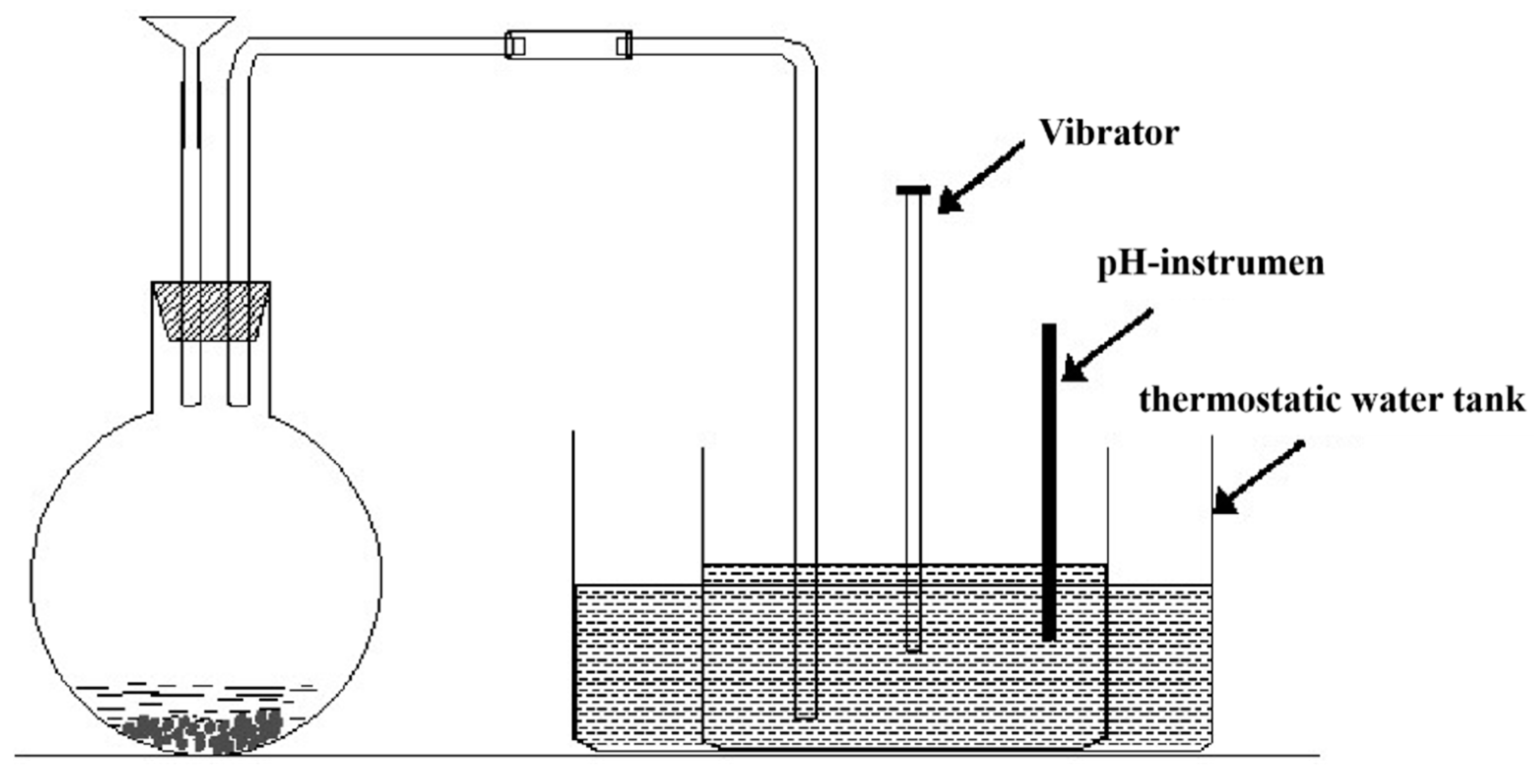
2.4. Design and Fabrication of the Microseism Similarity Model Experiment
2.5. Similarity Model Experimental Process
3. Results and Discussion
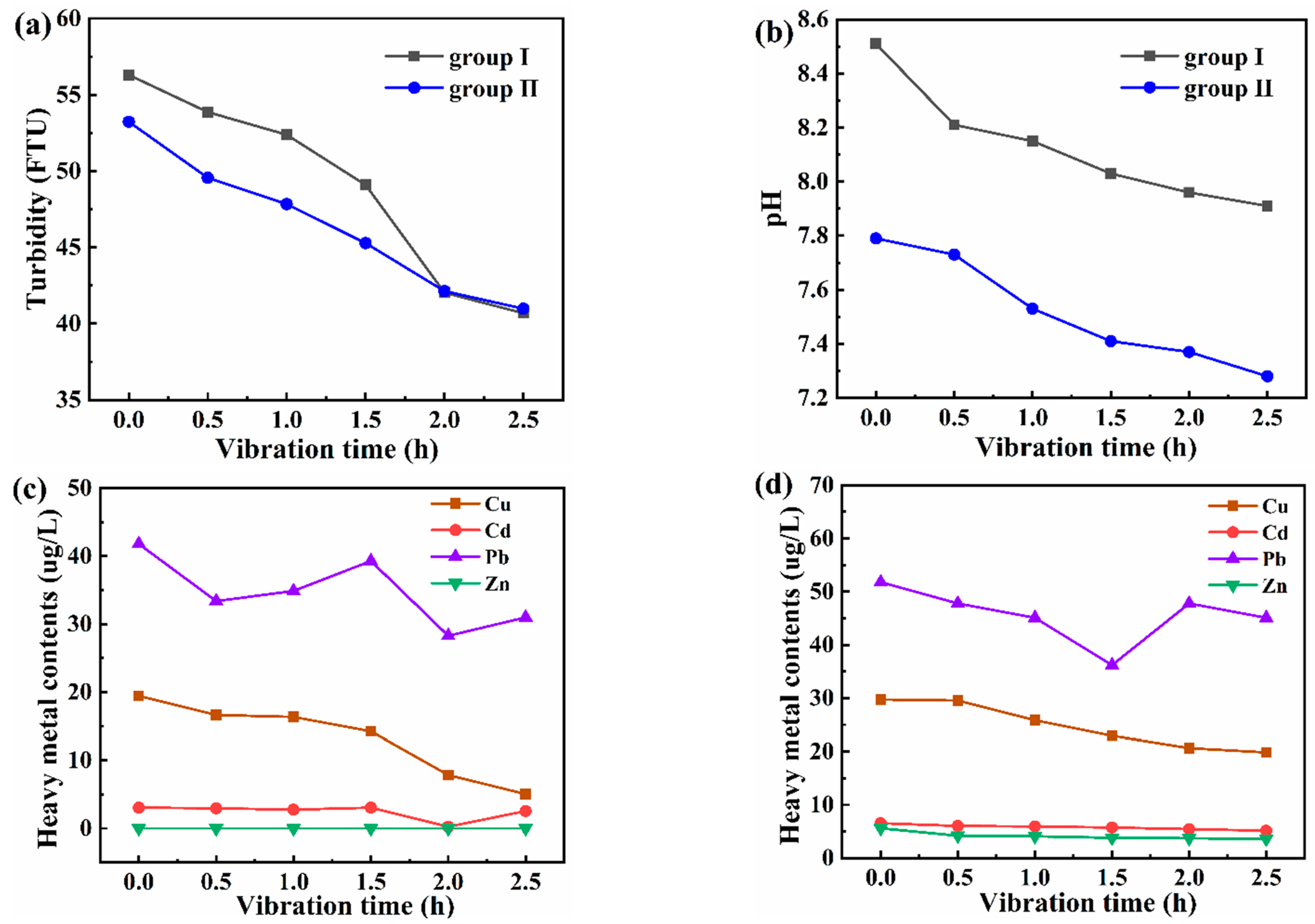
3.1. Distribution Characteristics of Turbidity in the Subsided Water Area
3.2. Influence of Microseism on Heavy Metals in the Subsided Water
3.3. Heavy Metal Variation in Microseismic Simulation Experiment
3.3.1. Evolution of Heavy Metals in Water
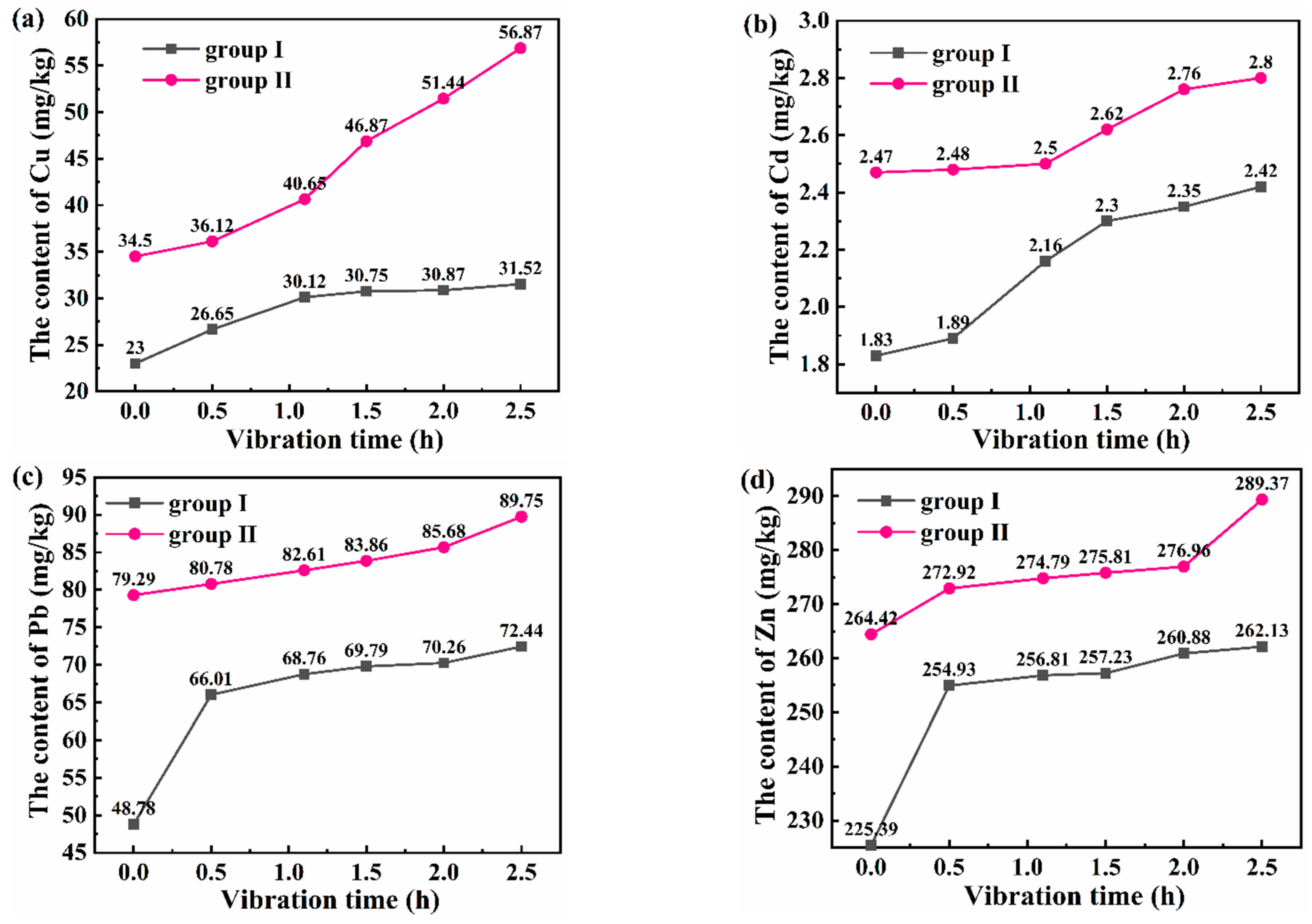
3.3.2. Evolution of Heavy Metals in Sediment
3.4. Mechanism of Microseismic Action on Heavy Metals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, X.; Jiang, X.-W.; Chen, Y.-F.; Tian, H.; Xu, N.-X. The influences of mining subsidence on the ecological environment and public infrastructure: A case study at the Haolaigou Iron Ore Mine in Baotou, China. Environ. Earth Sci. 2009, 59, 803–810. [Google Scholar] [CrossRef]
- Dong, S.; Samsonov, S.; Yin, H.; Yao, S.; Xu, C. Spatio-temporal analysis of ground subsidence due to underground coal mining in Huainan coalfield, China. Environ. Earth Sci. 2014, 73, 5523–5534. [Google Scholar] [CrossRef]
- Zhang, B.; Lu, C.; Wang, J.; Sun, Q.; He, X.; Cao, G.; Zhao, Y.; Yan, L.; Gong, B. Using storage of coal-mining subsidence area for minimizing flood. J. Hydrol. 2019, 572, 571–581. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, C.; Yang, Y.; Ansari, U.; Han, Y.; Li, X.; Cheng, Y. Preliminary experimental investigation on long-term fracture conductivity for evaluating the feasibility and efficiency of fracturing operation in offshore hydrate-bearing sediments. Ocean. Eng. 2023, 281, 114949. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, D.; Yin, J.; Zhou, X.; Li, Y.; Chi, P.; Han, Y.; Ansari, U.; Cheng, Y. Sediment Instability Caused by Gas Production from Hydrate-bearing Sediment in Northern South China Sea by Horizontal Wellbore: Evolution and Mechanism. Nat. Resour. Res. 2023, 32, 1595–1620. [Google Scholar] [CrossRef]
- Gilsbach, L.; Schütte, P.; Franken, G. Applying water risk assessment methods in mining: Current challenges and opportunities. Water Resour. Ind. 2019, 22, 100118. [Google Scholar] [CrossRef]
- Hu, Y.; Dong, Z.; Liu, G. Distribution and potential ecological risk of heavy metals accumulated in subsidence lakes formed in the Huainan Coalfield, China. Environ. Forensics 2017, 18, 251–257. [Google Scholar] [CrossRef]
- Vishwakarma, A.K.; Mishra, V.N.; Rai, R.; Shrivastva, B.K. Quantitative assessment of the effect of mining subsidence on the health of native floras using remote sensing techniques. Results Geophys. Sci. 2021, 8, 100031. [Google Scholar] [CrossRef]
- Zheng, L.; Chen, X.; Dong, X.; Wei, X.; Jiang, C.; Tang, Q. Using delta(34)S-SO4 and delta(18)O-SO4 to trace the sources of sulfate in different types of surface water from the Linhuan coal-mining subsidence area of Huaibei, China. Ecotoxicol. Environ. Saf. 2019, 181, 231–240. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Y.; Huang, H.; Yang, F. Deciphering soil bacterial community structure in subsidence area caused by underground coal mining in arid and semiarid area. Appl. Soil Ecol. 2021, 163, 103916. [Google Scholar] [CrossRef]
- Chen, G.; Wang, X.; Wang, R.; Liu, G. Health risk assessment of potentially harmful elements in subsidence water bodies using a Monte Carlo approach: An example from the Huainan coal mining area, China. Ecotoxicol. Environ. Saf. 2019, 171, 737–745. [Google Scholar] [CrossRef]
- Loupasakis, C.; Angelitsa, V.; Rozos, D.; Spanou, N. Mining geohazards—Land subsidence caused by the dewatering of opencast coal mines: The case study of the Amyntaio coal mine, Florina, Greece. Nat. Hazards 2013, 70, 675–691. [Google Scholar] [CrossRef]
- Song, Z.; Song, G.; Tang, W.; Zhao, Y.; Yan, D.; Zhang, W. Spatial and temporal distribution of Mo in the overlying water of a reservoir downstream from mining area. J. Env. Sci. (China) 2021, 102, 256–262. [Google Scholar] [CrossRef]
- Wang, H.; Ge, M. Acoustic emission/microseismic source location analysis for a limestone mine exhibiting high horizontal stresses. Int. J. Rock Mech. Min. Sci. 2008, 45, 720–728. [Google Scholar] [CrossRef]
- Barthwal, H.; van der Baan, M. Microseismicity observed in an underground mine: Source mechanisms and possible causes. Geomech. Energy Environ. 2020, 22, 100167. [Google Scholar] [CrossRef]
- Cheng, J.; Song, G.; Sun, X.; Wen, L.; Li, F. Research Developments and Prospects on Microseismic Source Location in Mines. Engineering 2018, 4, 653–660. [Google Scholar] [CrossRef]
- Chen, D.; Martin Mai, P. Automatic identification model of micro-earthquakes and blasting events in Laohutai coal mine based on the measurement of source parameter difference. Measurement 2021, 184, 109883. [Google Scholar] [CrossRef]
- Chen, D.; Wang, E.-Y.; Li, N. Study on the rupture properties and automatic identification model of micro-earthquakes and blasting events in a coal mine. Soil Dyn. Earthq. Eng. 2021, 146, 106759. [Google Scholar] [CrossRef]
- Ghosh, G.K.; Sivakumar, C. Application of underground microseismic monitoring for ground failure and secure longwall coal mining operation: A case study in an Indian mine. J. Appl. Geophys. 2018, 150, 21–39. [Google Scholar] [CrossRef]
- Leake, M.R.; Conrad, W.J.; Westman, E.C.; Ghaychi Afrouz, S.; Molka, R.J. Microseismic monitoring and analysis of induced seismicity source mechanisms in a retreating room and pillar coal mine in the Eastern United States. Undergr. Space 2017, 2, 115–124. [Google Scholar] [CrossRef]
- Wang, N.; Han, J.; Wei, Y.; Li, G.; Sun, Y. Potential Ecological Risk and Health Risk Assessment of Heavy Metals and Metalloid in Soil around Xunyang Mining Areas. Sustainability 2019, 11, 4828. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Li, C.; Jia, C.; Zhang, H.; Guan, Q.; Wu, X.; Wang, J.; Lv, M. Health risk assessment of groundwater nitrate contamination: A case study of a typical karst hydrogeological unit in East China. Environ. Sci. Pollut. Res. Int. 2020, 27, 9274–9287. [Google Scholar] [CrossRef]
- Liu, M.; Han, Z.; Yang, Y. Accumulation, temporal variation, source apportionment and risk assessment of heavy metals in agricultural soils from the middle reaches of Fenhe River basin, North China. RSC Adv. 2019, 9, 21893–21902. [Google Scholar] [CrossRef]
- Saha, N.; Rahman, M.S.; Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Industrial metal pollution in water and probabilistic assessment of human health risk. J. Environ. Manag. 2017, 185, 70–78. [Google Scholar] [CrossRef]
- Yan, J.; Qu, Z.; Li, F.; Li, H. Heavy metals in the water environment of Yangtze River Economic Belt: Status, fuzzy environmental risk assessment and management. Urban Clim. 2021, 40, 100981. [Google Scholar] [CrossRef]
- Jehan, S.; Ullah, I.; Khan, S.; Muhammad, S.; Khattak, S.A.; Khan, T. Evaluation of the Swat River, Northern Pakistan, water quality using multivariate statistical techniques and water quality index (WQI) model. Environ. Sci. Pollut. Res. Int. 2020, 27, 38545–38558. [Google Scholar] [CrossRef]
- Mondal, S.; Singh, G. Pollution evaluation, human health effect and tracing source of trace elements on road dust of Dhanbad, a highly polluted industrial coal belt of India. Environ. Geochem. Health 2021, 43, 2081–2103. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Huang, Y.; Zhang, W.; Shi, Z.; Yu, D.; Chen, Y.; Liu, C.; Wang, R. Effect of different industrial activities on soil heavy metal pollution, ecological risk, and health risk. Environ. Monit. Assess. 2021, 193, 20. [Google Scholar] [CrossRef]
- Pejman, A.; Nabi Bidhendi, G.; Ardestani, M.; Saeedi, M.; Baghvand, A. Fractionation of heavy metals in sediments and assessment of their availability risk: A case study in the northwestern of Persian Gulf. Mar. Pollut. Bull. 2017, 114, 881–887. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Q.; Zhang, G.; Zhao, Y.; Zhu, P.; Ma, X.; Li, X. Study on the coupling evolution of air and temperature field in coal mine goafs based on the similarity simulation experiments. Fuel 2021, 283, 118905. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, C. Similarity simulation of bolt support in a coal roadway in a tectonic stress field. Min. Sci. Technol. (China) 2010, 20, 718–722. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Shang, Z.; Ren, T.; Chen, P.; Wang, Z.; Shi, Z.; Lv, P. Experimental study on the preparation method of coal-like materials based on similarity of material properties and drilling parameters. Powder Technol. 2022, 395, 26–42. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, G.; Jia, Z.; Zheng, C.; Wang, W. Similarity simulation of mining-crack-evolution characteristics of overburden strata in deep coal mining with large dip. J. Pet. Sci. Eng. 2018, 165, 477–487. [Google Scholar] [CrossRef] [Green Version]
- Guang-li, G.; Jian-feng, Z.; Xie-xing, M.; Qiang, W.; Xian-ni, Z. Similar material and numerical simulation of strata movement laws with long wall fully mechanized gangue backfilling. Procedia Earth Planet. Sci. 2009, 1, 1089–1094. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chen, Y.; Bai, Y.; Li, S. Similarity theory for the physical simulation of natural gas hydrate reservoir development. Min. Sci. Technol. (China) 2010, 20, 782–788. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, S.; Wen, G.; Dai, L.; Wang, B. Coal-like material for coal and gas outburst simulation tests. Int. J. Rock Mech. Min. Sci. 2015, 74, 151–156. [Google Scholar] [CrossRef]
- Protsenko, V.S.; Danilov, F.I. Application of dimensional analysis and similarity theory for simulation of electrode kinetics described by the Marcus–Hush–Chidsey formalism. J. Electroanal. Chem. 2012, 669, 50–54. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Liang, R.; Liang, B.; Zhang, T. Virtual-joint based motion similarity criteria for human–robot kinematics mapping. Robot. Auton. Syst. 2020, 125, 103412. [Google Scholar] [CrossRef]
- Liu, J.-p.; Feng, X.-t.; Li, Y.-h.; Xu, S.-d.; Sheng, Y. Studies on temporal and spatial variation of microseismic activities in a deep metal mine. Int. J. Rock Mech. Min. Sci. 2013, 60, 171–179. [Google Scholar] [CrossRef]
- Tang, C.; Li, Y.; He, C.; Acharya, K. Dynamic behavior of sediment resuspension and nutrients release in the shallow and wind-exposed Meiliang Bay of Lake Taihu. Sci. Total Environ. 2020, 708, 135131. [Google Scholar] [CrossRef]
- Jalil, A.; Li, Y.; Zhang, K.; Gao, X.; Wang, W.; Khan, H.O.S.; Pan, B.; Ali, S.; Acharya, K. Wind-induced hydrodynamic changes impact on sediment resuspension for large, shallow Lake Taihu, China. Int. J. Sediment Res. 2019, 34, 205–215. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Z.; van der Kuijp, T.J.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468–469, 843–853. [Google Scholar] [CrossRef]
- Miljojkovic, D.; Trepsic, I.; Milovancevic, M. Assessment of physical and chemical indicators on water turbidity. Phys. A Stat. Mech. Appl. 2019, 527, 121171. [Google Scholar] [CrossRef]
- Ji, H.; Ding, H.; Tang, L.; Li, C.; Gao, Y.; Briki, M. Chemical composition and transportation characteristic of trace metals in suspended particulate matter collected upstream of a metropolitan drinking water source, Beijing. J. Geochem. Explor. 2016, 169, 123–136. [Google Scholar] [CrossRef]
- Helali, M.A.; Zaaboub, N.; Oueslati, W.; Added, A.; Aleya, L. Suspended particulate matter fluxes along with their associated metals, organic matter and carbonates in a coastal Mediterranean area affected by mining activities. Mar. Pollut. Bull. 2016, 104, 171–181. [Google Scholar] [CrossRef]
- Zeng, J.; Han, G.; Yang, K. Assessment and sources of heavy metals in suspended particulate matter in a tropical catchment, northeast Thailand. J. Clean. Prod. 2020, 265, 121898. [Google Scholar] [CrossRef]
- Lu, Y.-F.; Allen, H.E. Partitioning of copper onto suspended particulate matter in river waters. Sci. Total Environ. 2001, 277, 119–132. [Google Scholar] [CrossRef]
- Fan, J.-Y.; He, X.-Y.; Wang, D.-Z. Experimental study on the effects of sediment size and porosity on contaminant adsorption/desorption and interfacial diffusion characteristics. J. Hydrodyn. 2013, 25, 20–26. [Google Scholar] [CrossRef]
- Miranda, L.S.; Ayoko, G.A.; Egodawatta, P.; Goonetilleke, A. Adsorption-desorption behavior of heavy metals in aquatic environments: Influence of sediment, water and metal ionic properties. J. Hazard. Mater. 2022, 421, 126743. [Google Scholar] [CrossRef]
- Ji, Z.; Zhang, Y.; Zhang, H.; Huang, C.; Pei, Y. Fraction spatial distributions and ecological risk assessment of heavy metals in the sediments of Baiyangdian Lake. Ecotoxicol. Environ. Saf. 2019, 174, 417–428. [Google Scholar] [CrossRef]
- Kongsune, P.; Rattanapan, S.; Chanajaree, R. The removal of Pb2+ from aqueous solution using mangosteen peel activated carbon: Isotherm, kinetic, thermodynamic and binding energy calculation. Groundw. Sustain. Dev. 2021, 12, 100524. [Google Scholar] [CrossRef]
- Yang, P.; Liu, C.; Guo, Q.; Liu., Y. Variation of activation energy determined by a modified Arrhenius approach: Roles of dynamic recrystallization on the hot deformation of Ni-based superalloy. J. Mater. Sci. Technol. 2021, 72, 162–171. [Google Scholar] [CrossRef]

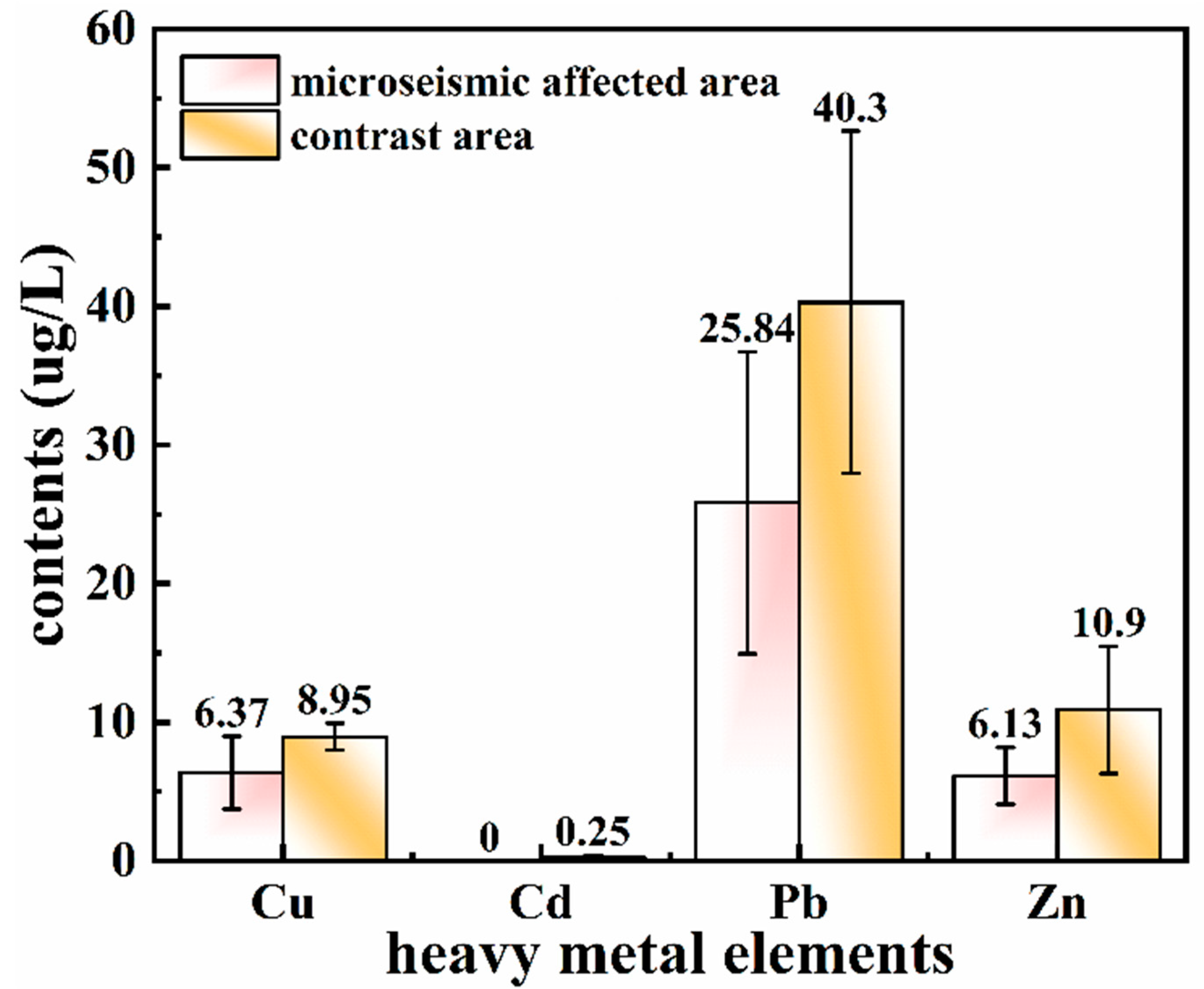

| Sampling Point | Carcinogenic Risk | Non-Carcinogenic Risk | Hazard Index | Impact Conditions | ||
|---|---|---|---|---|---|---|
| Cd | Cu | Pb | Zn | |||
| 1 | ― | 1.25 × 10−4 | 4.32 × 10−4 | 4.17 × 10−6 | 5.61 × 10−4 | Microseism-affected area |
| 2 | ― | 7.76 × 10−5 | 4.74 × 10−4 | 4.37 × 10−6 | 5.56 × 10−4 | |
| 3 | ― | 1.12 × 10−4 | 5.43 × 10−4 | 3.97 × 10−6 | 6.59 × 10−4 | |
| 4 | 1.19 × 10−7 | 1.46 × 10−4 | 8.48 × 10−4 | 7.95 × 10−6 | 1.00 × 10−3 | Contrast area |
| 5 | 1.39 × 10−7 | 1.49 × 10−4 | 6.70 × 10−4 | 2.62 × 10−5 | 8.45 × 10−4 | |
| Predictive Variable (Y) | Linear Regression Equation | R2 | F Examine Value | Sig. |
|---|---|---|---|---|
| Turbidity | Y = −0.2X + 10.562 | 0.985 | 264.253 | 0.001 |
| pH | Y = −4.480X + 34.929 | 0.960 | 95.772 | 0.004 |
| Cu | Y = −0.212X + 6.497 | 0.958 | 91.083 | 0.000 |
| Cd | Y = −1.885X + 12.217 | 0.970 | 127.895 | 0.002 |
| Pb | Y = −0.069X + 4.397 | 0.172 | 0.829 | 0.414 |
| Zn | Y = −1.079X + 5.747 | 0.728 | 10.692 | 0.031 |
| Dependent variable (X): vibration time | ||||
| Correlation is significant at the 0.05 level. | ||||
| 25 °C | 30 °C | |||||
|---|---|---|---|---|---|---|
| Time (s) | pH | [S2−] (mol/L) | v | pH | [S2−] (mol/L) | v |
| 16 | 6.06 | 1.22 × 10−10 | ― | 6.52 | 1.01 × 10−9 | ― |
| 24 | 5.41 | 6.08 × 10−12 | 1.45 × 10−11 | 6.10 | 1.48 × 10−10 | 6.75 × 10−10 |
| 32 | 5.37 | 5.07 × 10−12 | 1.29 × 10−13 | 5.98 | 8.36 × 10−11 | 3.22 × 10−11 |
| 40 | 5.34 | 4.42 × 10−12 | 8.0 × 10−14 | 5.76 | 3.03 × 10−11 | 1.35 × 10−11 |
| 48 | 5.30 | 3.67 × 10−12 | 9.35 × 10−14 | 5.57 | 1.29 × 10−11 | 2.75 × 10−12 |
| 56 | 5.28 | 3.35 × 10−12 | 4.13 × 10−14 | 5.40 | 5.91 × 10−12 | 7.06 × 10−13 |
| K25 °C = 4.57 × 104 | K30 °C = 7.96 × 104 | |||||
| 25 °C | 30 °C | |||||
|---|---|---|---|---|---|---|
| Time (s) | pH | [S2−] (mol/L) | v | pH | [S2−] (mol/L) | v |
| 16 | 6.68 | 2.12 × 10−9 | ― | 7.12 | 1.63 × 10−8 | ― |
| 24 | 6.21 | 2.43 × 10−10 | 2.03 × 10−9 | 6.44 | 6.97 × 10−10 | 8.50 × 10−8 |
| 32 | 6.08 | 1.33 × 10−10 | 7.32 × 10−11 | 6.23 | 2.67 × 10−10 | 6.18 × 10−10 |
| 40 | 5.86 | 4.84 × 10−11 | 2.85 × 10−11 | 6.10 | 1.46 × 10−10 | 1.38 × 10−10 |
| 48 | 5.67 | 2.01 × 10−11 | 5.86 × 10−12 | 5.99 | 6.85 × 10−11 | 5.35 × 10−11 |
| 56 | 5.51 | 9.63 × 10−12 | 1.48 × 10−12 | 5.66 | 1.92 × 10−11 | 1.64 × 10−11 |
| K25 °C = 8.10 × 104 | K30 °C = 1.30 × 104 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Wang, J.; Zhang, K. Response of Heavy Metals to Microseism in Coal Mining Subsidence Water of Huainan, China. Water 2023, 15, 2624. https://doi.org/10.3390/w15142624
Xu L, Wang J, Zhang K. Response of Heavy Metals to Microseism in Coal Mining Subsidence Water of Huainan, China. Water. 2023; 15(14):2624. https://doi.org/10.3390/w15142624
Chicago/Turabian StyleXu, Liangji, Jiayi Wang, and Kun Zhang. 2023. "Response of Heavy Metals to Microseism in Coal Mining Subsidence Water of Huainan, China" Water 15, no. 14: 2624. https://doi.org/10.3390/w15142624
APA StyleXu, L., Wang, J., & Zhang, K. (2023). Response of Heavy Metals to Microseism in Coal Mining Subsidence Water of Huainan, China. Water, 15(14), 2624. https://doi.org/10.3390/w15142624





