Assessment of Groundwater Quality in Relation to Organic versus Mineral Fertilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Area and Treatments Description
2.2. Methodology
2.3. Statistics
3. Results and Discussions
3.1. Physiochemical Characterization of the Groundwater Quality
3.2. Groundwater Pollution with Nitrates
3.3. The Study of the Simple Correlations
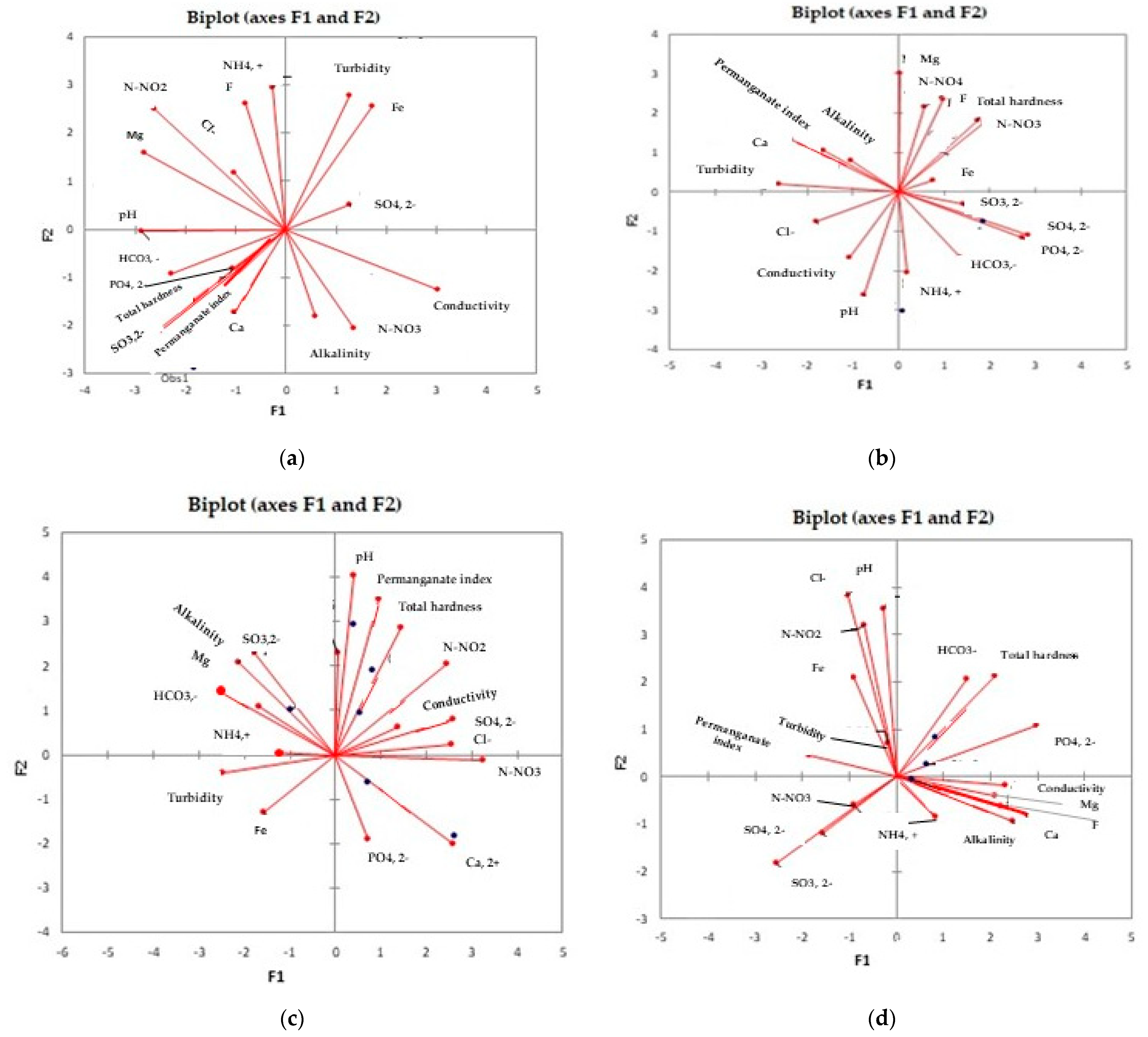
3.4. Principal Components Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roș, V.; Nașcu, H.; Chira, R.; Ghereș, M.I.; Fechete, L.V. Control of Water Pollution in Agriculture; Todesco Publishing House: Cluj-Napoca, Romania, 2003. (In Romanian) [Google Scholar]
- Canhong, G.; El-Sawah, A.M.; Dina, F.I.A.; Yousef, A.H.; Hiba, S.; Mohamed, S.S. The Integration of Bio and Organic Fertilizers Improve Plant Growth, Grain Yield, Quality and Metabolism of Hybrid Maize (Zea mays L.). Agronomy 2020, 10, 319. [Google Scholar]
- Rizzo, A.; Sarti, C.; Nardini, A.; Conte, G.; Masi, F.; Pistocchi, A. Nature-based solutions for nutrient pollution control in European agricultural regions: A literature review. Ecol. Eng. 2023, 186, 106772. [Google Scholar] [CrossRef]
- Liu, L.; Wu, J.; He, S.; Wang, L. Occurrence and distribution of groundwater fluoride and manganese in the Weining Plain (China) and their probabilistic health risk quantifcation. Expo Health 2022, 14, 263–279. [Google Scholar] [CrossRef]
- Rakib, M.A.; Sasaki, J.; Matsuda, H.; Quraishi, S.B.; Mahmud, M.J.; Bodrud Doza, M.; Ullah, A.; Fatema, K.J.; Newaz, M.A.; Bhuiyan, M. Groundwater salinization and associated co-contamination risk increase severe drinking water vulnerabilities in the southwestern coast of Bangladesh. Chemosphere 2020, 246, 125646. [Google Scholar] [CrossRef]
- Alramthi, S.M.; Ali, G.H.; Shaban, A.M.; Abdou, T.A.; Elthagafi, A.M.; Eldosari, S.H.; Zhu, B.-K.; Safaa, H.M. Quality Characterization of Groundwater for Drinking Purposes and Its Network Distribution to Assure Sustainability in Southern Region of Saudi Arabia. Water 2022, 14, 3565. [Google Scholar] [CrossRef]
- Munthali, C.; Kinoshita, R.; Onishi, K.; Rakotondrafara, A.; Mikami, K.; Koike, M.; Tani, M.; Palta, J.; Aiuchi, D. A Model Nutrition Control System in Potato Tissue Culture and Its Influence on Plant Elemental Composition. Plants 2022, 11, 2718. [Google Scholar] [CrossRef]
- Panneerselvam, B.; Muniraj, K.; Duraisamy, K.; Pande, C.; Karuppannan, S.; Thomas, M. An integrated approach to explore the suitability of nitrate-contaminated groundwater for drinking purposes in a semiarid region of India. Environ. Geochem. Health 2023, 45, 647–663. [Google Scholar] [CrossRef]
- Gaikwad, R.W.; Warade, A.R. Removal of Nitrate from Groundwater by Using Natural Zeolite of Nizarneshwar Hills of Western India. J. Water Resour. Hydraul. Eng. 2014, 3, 74–80. [Google Scholar]
- Valin, H.; Sands, R.D.; van der Mensbrugghe, D.; Nelson, G.C.; Ahammad, H.; Blanc, E.; Bodirsky, B.; Fujimori, S.; Hasegawa, T.; Havlik, P.; et al. The future of food demand: Understanding differences in global economic models. Agric. Econ. 2014, 45, 51–67. [Google Scholar] [CrossRef]
- Shukla, S.; Saxena, A. Global Status of Nitrate Contamination in Groundwater: Its Occurrence, Health Impacts, and Mitigation Measures. In Handbook of Environmental Materials Management; Hussain, C.M., Ed.; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Malyan, S.K.; Singh, R.; Rawat, M.; Kumar, M.; Pugazhendhi, A.; Kumar, A.; Kumar, V.; Kumar, S.S. An overview of carcinogenic pollutants in groundwater of India. Biocatal. Agric. Biotechnol. 2019, 21, 101288. [Google Scholar] [CrossRef]
- Mohammed, A.M.; Refaee, A.; El-Din, G.K.; Harb, S. Hydrochemical characteristics and quality assessment of shallow groundwater under intensive agriculture practices in arid region, Qena, Egypt. Appl. Water Sci. 2022, 12, 92. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Yang, S.; Zhao, X.; Ouyang, L.; Lai, C. Evaluating Surface Water Nitrogen Pollution via Visual Clustering in Megacity Chengdu. Water 2023, 15, 2113. [Google Scholar] [CrossRef]
- Vasilache, N.; Diacu, E.; Modrogan, C.; Chiriac, F.L.; Paun, I.C.; Tenea, A.G.; Pirvu, F.; Vasile, G.G. Groundwater Quality Affected by the Pyrite Ash Waste and Fertilizers in Valea Calugareasca, Romania. Water 2022, 14, 2022. [Google Scholar] [CrossRef]
- Ramalingam, S.; Panneerselvam, B.; Kaliappan, S.P. Effect of high nitrate contamination of groundwater on human health and water quality index in semi-arid region, South India. Arab. J. Geosci. 2022, 15, 242. [Google Scholar] [CrossRef]
- Mester, T.; Szabó, G.; Sajtos, Z.; Baranyai, E.; Szabó, G.; Balla, D. Environmental Hazards of an Uncultivated Liquid Waste Disposal Site on Soil and Groundwater. Water 2022, 14, 226. [Google Scholar] [CrossRef]
- Alshehri, F.; Abdelrahman, K. Integrated approach for the investigation of groundwater quality using hydrochemical and geostatistical analyses in Wadi Fatimah, western Saudi Arabia. Front. Earth Sci. 2023, 11, 1166153. [Google Scholar] [CrossRef]
- Mester, T.; Szabó, G.; Balla, D. Assessment of Shallow Groundwater Purification Processes after the Construction of a Municipal sewerage Network. Water 2021, 13, 1946. [Google Scholar] [CrossRef]
- Tanwer, N.; Deswal, M.; Khyalia, P.; Laura, J.S.; Khosla, B. Fluoride and nitrate in groundwater: A comprehensive analysis of health risk and potability of groundwater of Jhunjhunu district of Rajasthan, India. Environ. Monit. Assess. 2023, 195, 267. [Google Scholar] [CrossRef]
- Wang, Y.; Li, R.; Wu, X.; Yan, Y.; Wei, C.; Luo, M.; Xiao, Y.; Zhang, Y. Evaluation of Groundwater Quality for Drinking and Irrigation Purposes Using GIS-Based IWQI, EWQI and HHR Model. Water 2023, 15, 2233. [Google Scholar] [CrossRef]
- Wszelaczynska, E.; Poberezny, J.; Keutgen, A.J.; Keutgen, N.; Goscinna, K.; Milczarek, D.; Tatarowska, B.; Flis, B. Antinutritional Nitrogen Compounds Content in Potato (Solanum tuberosum L.) Tubers Depending on the Genotype and Production System. Agronomy 2022, 12, 2415. [Google Scholar] [CrossRef]
- Wadas, W. Effect of Foliar Silicon Application on Nutrient Content in Early Crop Potato Tubers. Agronomy 2022, 12, 2706. [Google Scholar] [CrossRef]
- Hansen, B.; Thorling, L.; Schullehner, J.; Termansen, M.; Dalgaard, T. Groundwater nitrate response to sustainable nitrogen management. Sci. Rep. 2017, 7, 8566. [Google Scholar] [CrossRef] [PubMed]
- Nyiraneza, J.; Chen, D.; Fraser, T.; Comeau, L.-P. Improving Soil Quality and Potato Productivity with Manure and High-Residue Cover Crops in Eastern Canada. Plants 2021, 10, 1436. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Su, C.; Wang, M.; Abbas, H.; Baloch, M.Y.J.; Ghani, J.; Ullah, Z.; Huq, M.E. Groundwater fluoride and nitrate contamination and associated human health risk assessment in South Punjab, Pakistan. Environ. Sci. Pollut. Res. 2023, 30, 61606–61625. [Google Scholar] [CrossRef]
- Eslamian, S.; Harooni, Y.; Sabzevari, Y. Simulation of nitrate pollution and vulnerability of groundwater resources using MODFLOW and DRASTIC models. Sci. Rep. 2023, 13, 8211. [Google Scholar] [CrossRef] [PubMed]
- Șara, A.; Odagiu, A. Determination of Fodder Quality; AcademicPres Publishing House: Cluj-Napoca, Romania, 2002. (In Romanian) [Google Scholar]
- ISO 5667-3; Water Quality. Sampling-Part 3: Preservation and Handling of Water Samples. International Standardization Organization: Geneve, Switzerland, 2018.
- EN ISO 7027; Water Quality. Determination of Turbidity. European Committee for Standardization: Brussels, Belgium, 2012.
- Internal Laboratory Procedures for Water Quality Assessment; Somes Water Company, Water Analysis Laboratory of the Water Treatment Plant: Gilău, Romania, 2019. (In Romanian)
- ISO 10523; Water Quality. Determination of pH. International Standardization Organization: Geneve, Switzerland, 2012.
- EN 27888; Water quality. Determination of electrical conductivity. International Standardization Organization: Geneve, Switzerland, 1997.
- ISO 9297; Water Quality. Determination of Cloride. Silver Nitrate Titration with Chromate Indicator (Mohr’s Method). International Standardization Organization: Geneve, Switzerland, 2001.
- EN ISO 8467; Water quality — Determination of permanganate index. European Committee for Standardization: Brussels, Belgium, 2019.
- ISO 7150-1; Water Quality. Determination of Ammonium. Part 1: Manual Spectrometric Method. International Standardization Organization: Geneve, Switzerland, 2001.
- EN ISO 26777; Water Quality. Determination of Nitrite. Molecular Absorption Spectrometric Method. European Committee for Standardization: Brussels, Belgium, 2006.
- ISO 7890; Water Quality. Determination of Nitrate. Part 3: Spectrometric Method Using Sulfosalicylic Acid. International Standardization Organization: Geneve, Switzerland, 2000.
- ISO 6058; Water Quality. Determination of Calcium. EDTA Titrimetric Method. International Standardization Organization: Geneve, Switzerland, 2008.
- ISO 6059; Water Quality. Determining the Sum of Calcium and Magnesium. EDTA Titrimetric Method. International Standardization Organization: Geneve, Switzerland, 2008.
- U.S. EPA. 1997; Method 300.1: Determination of Inorganic Anions in Drinking Water by Ion Chromatography, Revision 1.0. United States Environmental Protection Agency: Cincinnati, OH, USA, 1997.
- U.S. EPA 2018; Methylene Blue Method. Measures total sulfides, H2S, HS–, and some metal sulfides in groundwater, wastewater, brines and seawater. United States Environmental Protection Agency: Colorado, CO, USA, 2018.
- EN ISO 9963-1; Water quality—Determination of alkalinity—Part 1: Determination of total and composite alkalinity, 2002. International Standardization Organization: Geneve, Switzerland, 2002.
- U.S. EPA 1989; EZ1301 Iron, FerroVer® Method, for water, wastewater and seawater; digestion is required for determining total iron. United States Environmental Protection Agency: Colorado, CO, USA, 1989.
- EN ISO 6878; Water quality—Determination of phosphorus—Ammonium molybdate spectrometric method, 2004. International Standardization Organization: Geneve, Switzerland, 2004.
- Merce, E.; Merce, C. Statistică—Paradigme Consacrate şi Paradigme Întregitoare; AcademicPres Publishing House: Cluj-Napoca, Romania, 2009. (In Romanian) [Google Scholar]
- Bahrami, M.; Zarei, A.R.; Rostami, F. Temporal and spatial assessment of groundwater contamination with nitrate by nitrate pollution index (NPI) and GIS (case study: Fasarud plain, southern Iran). Environ. Geochem. Health 2020, 43, 3119–3130. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Q.; Xie, C.; Lu, X. Source identification and health risks of nitrate contamination in shallow groundwater: A case study in Subei Lake basin. Environ. Sci. Pollut. Res. 2023, 30, 13660–13670. [Google Scholar] [CrossRef]
- Suthar, S.; Bishnoi, P.; Singh, S.; Mutiyar, P.K.; Nema, A.K.; Patil, N.S. Nitrate contamination in groundwater of some rural areas of Rajasthan, India. J. Hazard. Mater. 2009, 171, 189–199. [Google Scholar] [CrossRef]
- Cardona, A.; Carrillo-Rivera, J.J.; Huizar-Aolvarez, R.; Graniel-Castro, E. Salinization in coastal aquifers of arid zones: An example from santo domingo, baja California sur, Mexico. Environ. Geol. 2004, 45, 350–366. [Google Scholar] [CrossRef]
- Chae, G.T.; Kim, K.; Yun, S.T.; Kim, K.H.; Kim, S.O.; Choi, B.Y.; Kim, H.S.; Rhee, C.W. Hydro geochemistry of alluvial groundwaters in an agricultural area: An implication for groundwater contamination susceptibility. Chemosphere 2004, 55, 369–378. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, H.; Guo, S.; Fu, K.; Liao, L.; Xu, Y.; Cheng, S. Groundwater pollution source apportionment using principal component analysis in a multiple land-use area in southwestern China. Environ. Sci. Pollut. Res. 2020, 27, 9000–9011. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamdany, N.; Al-Shaker, Y.M.S.; Al-Saffawi, A.Y.T. Application of nitrate pollution index (Npi) to evaluate the health safety of wells water for some quarters of the leftside of Mosul City, Iraq. Biochem. Cell. Arch. 2020, 20, 6063–6068. [Google Scholar]
- Al-Sinjari, W.E.A.; Al-Shanoona, R.A.A.; Al-Saffawi, A.Y.T. Valuation of Drinking Water Human Health Risk Using Nitrate Pollution Index (NPI): A Case Study for The Ground Water of Al-Hamdaniya District. Iraq. HIV Nurs. 2023, 23, 486–490. [Google Scholar]
- El Mountassir, O.; Bahir, M.; Ouazar, D.; Chehbbouni, A.; Careirra, P.M. Temporal and spatial assessment of groundwater contamination with nitrate using nitrate pollution index (NPI), groundwater pollution index (GPI), and GIS (case study: Essaouira basin, Morocco). Environ. Sci. Pollut. Res. 2022, 29, 17132–17149. [Google Scholar] [CrossRef]
- Obeidat, M.M.; Awawdeh, M.; Al-Rub, F.A.; Al-Ajlouni, A. An innovative nitrate pollution index and multivariate statistical investigations of groundwater chemical quality of Umm Rijam Aquifer (B4), North Yarmouk River Basin, Jordan. In Water Quality Monitoring and Assessment; Vouddouris, K., Voutsa, D., Eds.; InTech: Rijeka, Croatia, 2012; pp. 169–188. [Google Scholar]
- Pang, T.; Zhang, H.; Wen, L.; Tang, J.; Zhou, B.; Yang, Q.; Li, Y.; Wang, J.; Chen, A.; Zeng, Z. Quantitative Analysis of a Weak Correlation between Complicated Data on the Basis of Principal Component Analysis. J. Anal. Methods Chem. 2021, 2021, 8874827. [Google Scholar] [CrossRef]
- Rovetta, A. Raiders of the Lost Correlation: A Guide on Using Pearson and Spearman Coefficients to Detect Hidden Correlations in Medical Sciences. Cureus 2020, 12, e11794. [Google Scholar] [CrossRef]
- Brindha, K.; Vaman, K.N.; Srinivasan, K.; Babu, M.S.; Elango, L. Identifcation of surface water-groundwater interaction by hydrogeochemical indicators and assessing its suitability for drinking and irrigational purposes in Chennai. South. India Appl. Water Sci. 2014, 4, 159–174. [Google Scholar] [CrossRef]
- Dartan, G.; Taspinar, F.; Toroz, I. Assessment of heavy metals in agricultural soils and their source apportionment: A Turkish district survey. Environ. Monit. Assess. 2015, 187, 99. [Google Scholar] [CrossRef]
- Bhuiyan, M.A.H.; Bodrud-Doza, M.; Islam, A.R.M.T.; Rakib, M.A.; Rahman, M.S.; Ramanathan, A.L. Assessment of groundwater quality of Lakshimpur district of Bangladesh using water quality indices, geostatistical methods, and multivariate analysis. Environ. Earth Sci. 2016, 75, 1020. [Google Scholar] [CrossRef]
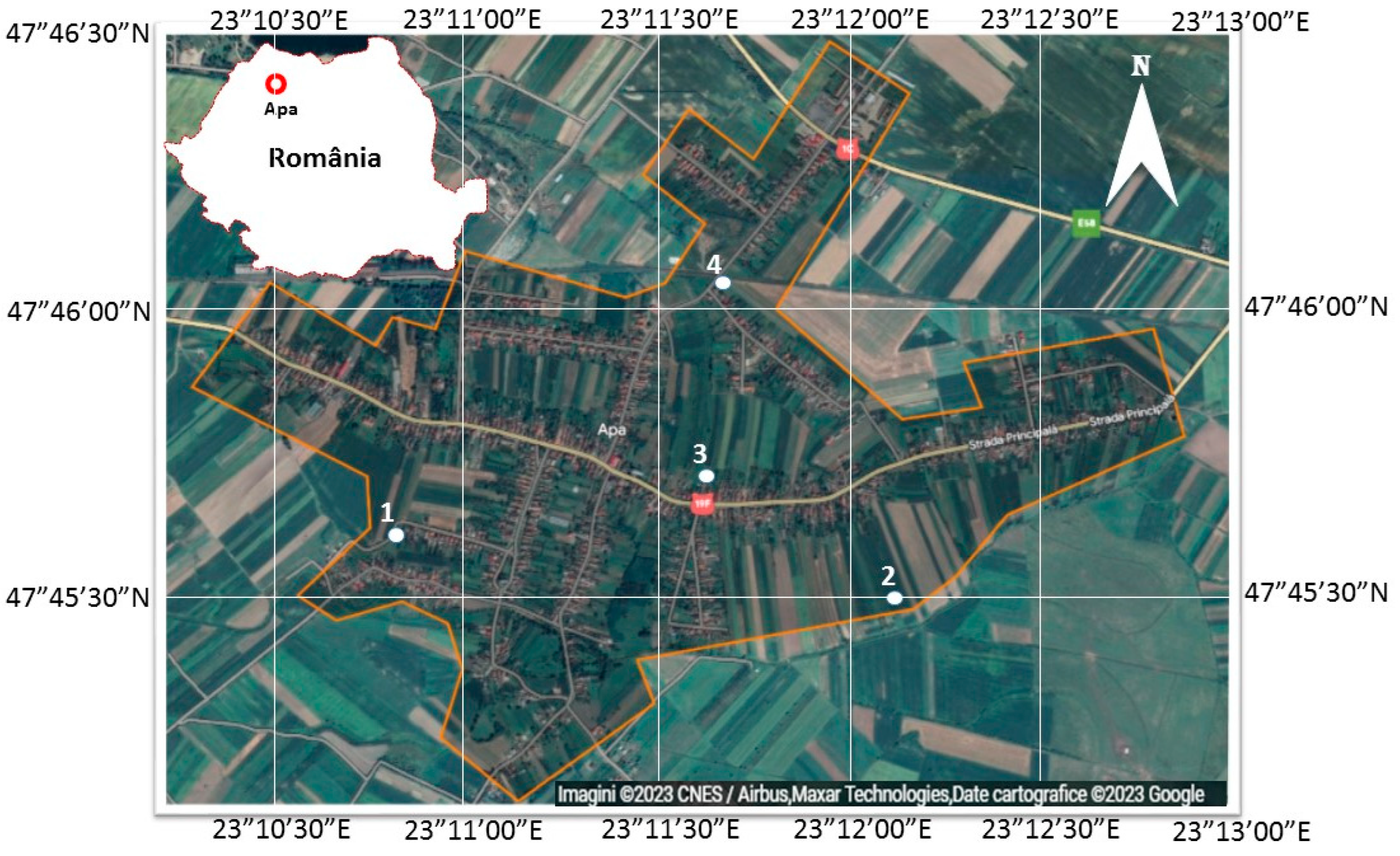
| Plot Number as Shown in Figure 1 | Type of Fertilization | Administered Doses | Date of Administration |
|---|---|---|---|
| 1 | Organic | 39.52 t/Ha | 10 February 2023 |
| 2 | Organic | 79.04 t/Ha | 10 February 2023 |
| 3 | Organic | 118.56 t/Ha | 10 February 2023 |
| 4 | Mineral | NPK: 14:7:21 | 18 April 2022 |
| Parameter | Measure Unit | Limit According to the Law 458/2002 Modified by the Law 311/2004 | Method | |
|---|---|---|---|---|
| Standard/Method | Internal Laboratory Procedure (PLST) | |||
| Turbidity | NTU | ≤5 | SR EN ISO 7027-1:2016 [30] | PSLT—02 [31] |
| pH | unit. pH | ≥6.5, ≤9.5 | SR EN ISO 10523:2012 [32] | PSLT—07 [31] |
| Electric conductivity at 20 °C | µS/cm | <2500 | SR EN 27888:1997 [33] | PSLT—13 [31] |
| Chlorides | mg/L | 250 | SR ISO 9297:2001 [34] | PSLT—06 [31] |
| Permanganate index | mg O2/L | 5 | SR EN ISO 8467:2001 [35] | PSLT—11 [31] |
| Ammonium | mg/L | 0.5 | SR ISO 7150-1:2001 [36] | PSLT—10 [31] |
| Nitrites | mg/L | 0.5 | SR EN 26777:2002/C91:2006 [37] | PSLT—09 [31] |
| Nitrates | mg/L | 50 | SR ISO 7890-3:2000 [38] | PSLT—08 [31] |
| Total hardness | °G | ≥5 | SR ISO 6059:2008 [39] | PSLT—05 [31] |
| Calcium | mg/L | - | SR ISO 6058:2008 [40] | PSLT—03 [31] |
| Magnesium | mg/L | - | SR ISO 6059:2008 [39] | PSLT—05 [31] |
| Sulfates | mg/L | 250 | SR ISO 6059:2008 [39] | PSLT—05 [31] |
| Fluorides | mg/L | 1.2 | Method 8029 HACH [41] | PSLT—27 [31] |
| Sulfides | µg/L | 100 | Method 8131 HACH [42] | PSLT—26 [31] |
| Alkalinity | mL HCl 0.1 N | - | SR ISO 9963-1:2002 [43] | PSLT—17 [31] |
| Bicarbonates | mg/L | - | SR ISO 9963-1:2002 [43] | PSLT—17 [31] |
| Iron | µg/L | 200 | Method LCK521 HACH [44] | PSLT—22 [31] |
| Phosphates | mg/L | - | SR EN ISO 6878:2008 [45] | PSLT—16 [31] |
| Issue | N * | Site 1-Sheep Manure, 39.52 t/ha | Site 2-Sheep Manure, 79.04 t/Ha | Site 3-Sheep Manure, 118.56 t/Ha | Site 4-Mineral Fertilization N14:P7:K21 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | s | CV, % | Mean | s | CV, % | Mean | s | CV, % | Mean | s | CV, % | ||
| Turbidity, NTU | 10 | 0.22a | 0.01 | 4.54 | 0.20a | 0.01 | 5.00 | 0.23a | 0.06 | 26.08 | 0.24a | 0.01 | 4.16 |
| pH | 10 | 7.68a | 0.77 | 10.02 | 7.08a | 0.69 | 9.74 | 6.37b | 0.72 | 11.30 | 7.57a | 0.49 | 6.47 |
| Conductivity, µS/cm | 10 | 415.49a | 7.13 | 1.71 | 421.24a | 6.98 | 1.65 | 438.00b | 4.59 | 1.47 | 429.44a | 7.56 | 1.76 |
| Chlorides, mg/L | 10 | 30.44a | 1.61 | 5.29 | 31.03a | 1.35 | 4.35 | 31.88a | 0.85 | 2.66 | 31.49a | 1.49 | 4.73 |
| Permanganate index, mg O2/L | 10 | 0.25a | 0.02 | 8.00 | 0.26a | 0.02 | 7.69 | 0.32b | 0.06 | 19.76 | 0.24a | 0.01 | 4.16 |
| Ammonium, mg/L | 10 | 0.009a | 0.002 | 22.23 | 0.010a | 0.002 | 20.00 | 0.012a | 0.001 | 10.39 | 0.013a | 0.002 | 15.38 |
| Nitrites, mg/L | 10 | 0.004a | 0.001 | 25.00 | 0.005a | 0.001 | 20.00 | 0.008a | 0.002 | 22.82 | 0.007a | 0.002 | 28.57 |
| Nitrates, mg/L | 10 | 44.69a | 2.66 | 5.96 | 43.58b | 1.58 | 3.62 | 64.29b | 1.63 | 2.54 | 44.68a | 2.12 | 4.74 |
| Total hardness, °G | 10 | 10.53a | 0.96 | 9.11 | 10.45a | 1.10 | 10.52 | 10.60a | 1.23 | 11.63 | 10.66a | 1.35 | 12.67 |
| Calcium, mg/L | 10 | 51.28a | 1.28 | 2.49 | 52.08a | 1.14 | 2.20 | 52.70a | 1.48 | 2.81 | 52.56a | 0.98 | 1.87 |
| Magnesium, mg/L | 10 | 13.48a | 1.26 | 9.36 | 13.76a | 1.08 | 7.85 | 13.97a | 1.39 | 9.96 | 13.99a | 0.96 | 6.87 |
| Sulfates, mg/L | 10 | 61.60a | 2.23 | 3.62 | 62.60a | 1.96 | 3.13 | 68.19a | 1.43 | 2.10 | 63.20a | 1.88 | 2.97 |
| Fluoride, mg/L | 10 | 0.15a | 0.01 | 8.89 | 0.16a | 0.01 | 7.99 | 0.18a | 0.01 | 4.54 | 0.17a | 0.01 | 6.55 |
| Sulfides, µg/L | 10 | 3.27a | 0.35 | 10.70 | 3.35a | 0.42 | 12.53 | 3.40a | 0.52 | 15.19 | 3.29a | 0.44 | 13.37 |
| Alkalinity, mL HCl 0.1 N | 10 | 1.89a | 0.31 | 16.40 | 1.72a | 0.34 | 19.76 | 1.40b | 0.27 | 19.28 | 1.68a | 0.30 | 17.85 |
| Bicarbonates, mg/L | 10 | 92.36a | 1.25 | 1.35 | 89.94a | 1.15 | 1.27 | 85.40b | 1.01 | 1.19 | 89.44a | 1.28 | 1.43 |
| Iron, µg/L | 10 | 34.54a | 0.50 | 1.44 | 34.74a | 0.64 | 1.84 | 35.00a | 1.39 | 3.98 | 34.92a | 0.69 | 1.96 |
| Phosphates, mg/L | 10 | 0.041a | 0.001 | 3.19 | 0.042a | 0.001 | 3.12 | 0.043a | 0.001 | 0.29 | 0.052a | 0.003 | 5.76 |
| Issue | Mean | S 1 | CV 2, % |
|---|---|---|---|
| Site 1-Sheep manure, 39.52 t/ha | 1.23 | 0.04 | 3.25 |
| Site 2-Sheep manure, 79.04 t/Ha | 1.17 | 0.03 | 2.56 |
| Site 3-Sheep manure, 118.56 t/Ha | 2.21 | 0.07 | 3.16 |
| Site 4-Mineral fertilization N14:P7:K21 | 1.24 | 0.04 | 3.23 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.35 | −0.57 | 0.20 | 0.52 | 0.22 | −0.09 | 0.13 | −0.29 | 0.02 | −0.12 | −0.08 | −0.10 | −0.13 | 0.16 | −0.15 | −0.17 | −0.32 | |
| 2 | 0.35 | 0.65 | −0.04 | −0.09 | −0.31 | −0.64 | −0.68 | 0.25 | 0.19 | 0.07 | 0.15 | 0.62 | −0.14 | −0.18 | 0.01 | 0.10 | 0.77 | |
| 3 | −0.57 | 0.65 | 0.20 | −0.19 | −0.58 | −0.36 | −0.25 | −0.21 | 0.08 | 0.14 | 0.17 | 0.15 | 0.23 | −0.16 | −0.04 | 0.24 | 0.17 | |
| 4 | 0.20 | −0.04 | 0.20 | −0.22 | −0.11 | 0.68 | 0.02 | −0.07 | 0.18 | 0.17 | 0.19 | 0.23 | −0.14 | −0.04 | 0.12 | 0.15 | 0.16 | |
| 5 | 0.52 | −0.09 | −0.19 | −0.22 | 0.56 | −0.18 | −0.13 | −0.15 | 0.16 | −0.17 | −0.06 | −0.11 | 0.13 | −0.23 | 0.02 | −0.24 | 0.25 | |
| 6 | 0.22 | −0.31 | −0.58 | −0.11 | 0.56 | 0.15 | −0.13 | −0.13 | −0.03 | 0.16 | 0.14 | 0.18 | −0.14 | −0.17 | 0.16 | 0.20 | −0.53 | |
| 7 | −0.09 | −0.64 | −0.36 | 0.68 | −0.18 | 0.15 | −0.61 | 0.14 | −0.05 | 0.60 | −0.05 | 0.17 | 0.11 | −0.19 | 0.14 | 0.13 | 0.14 | |
| 8 | 0.13 | −0.68 | −0.25 | 0.02 | −0.13 | −0.33 | −0.61 | −0.02 | 0.10 | −0.25 | −0.15 | −0.13 | 0.01 | −0.17 | −0.03 | 0.05 | −0.01 | |
| 9 | −0.29 | 0.25 | −0.21 | −0.07 | −0.15 | −0.13 | 0.14 | −0.02 | 0.05 | 0.11 | −0.03 | −0.02 | 0.21 | 0.21 | 0.61 | 0.16 | −0.13 | |
| 10 | 0.02 | 0.19 | 0.08 | 0.18 | 0.16 | −0.03 | −0.05 | 0.10 | 0.05 | 0.26 | 0.22 | −0.14 | 0.12 | 0.25 | 0.15 | −0.58 | 0.15 | |
| 11 | −0.12 | 0.07 | 0.14 | 0.17 | −017 | 0.16 | 0.60 | −0.25 | 0.11 | 0.26 | −0.18 | 0.26 | 0.01 | −0.24 | 0.55 | −0.18 | −0.28 | |
| 12 | −0.08 | 0.15 | 0.17 | 0.19 | −0.06 | 0.14 | −0.05 | −0.15 | −0.03 | 0.22 | −0.18 | 0.21 | 0.22 | 0.14 | −0.17 | −0.06 | −0.24 | |
| 13 | −0.10 | 0.62 | 0.15 | 0.23 | −0.11 | 0.18 | 0.17 | −0.13 | 0.02 | −0.14 | 0.26 | 0.21 | 0.19 | −0.16 | 0.09 | −0.19 | 0.18 | |
| 14 | −0.13 | −0.14 | 0.23 | −0.14 | 0.13 | −0.14 | 0.11 | 0.01 | 0.21 | 0.12 | 0.01 | 0.22 | 0.19 | 0.02 | 0.19 | −0.11 | −0.30 | |
| 15 | 0.16 | −0.18 | −0.16 | −0.04 | −0.23 | −0.17 | −0.19 | −0.17 | 0.21 | 0.25 | −0.24 | 0.14 | −0.16 | 0.02 | 0.17 | 0.53 | −0.28 | |
| 16 | −0.15 | 0.01 | −0.04 | 0.12 | 0.02 | 0.16 | 0.14 | −0.03 | 0.61 | 0.15 | 0.55 | −0.17 | 0.09 | 0.19 | 0.17 | −0.54 | −0.14 | |
| 17 | −0.17 | 0.10 | 0.24 | 0.15 | −0.24 | 0.20 | 0.13 | 0.05 | 0.16 | −0.58 | −0.18 | −0.06 | −0.19 | −0.11 | 0.53 | −0.54 | −0.19 | |
| 18 | −0.32 | 0.77 | 0.17 | 0.16 | −0.53 | 0.25 | 0.14 | −0.01 | −0.13 | 0.15 | −0.28 | −0.24 | 0.18 | −0.30 | −0.28 | −0.14 | −0.19 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.35 | −0.57 | 0.20 | 0.52 | 0.22 | −0.09 | 0.13 | −0.29 | 0.02 | −0.12 | −0.08 | −0.10 | −0.13 | 0.16 | −0.15 | −0.17 | −0.32 | |
| 2 | 0.35 | 0.65 | −0.04 | −0.09 | −0.31 | −0.64 | −0.68 | 0.25 | 0.19 | 0.07 | 0.15 | 0.62 | −0.14 | −0.18 | 0.01 | 0.10 | 0.77 | |
| 3 | −0.57 | 0.65 | 0.20 | −0.19 | −0.58 | −0.36 | −0.25 | −0.21 | 0.08 | 0.14 | 0.17 | 0.15 | 0.23 | −0.16 | −0.04 | 0.24 | 0.17 | |
| 4 | 0.20 | −0.04 | 0.20 | −0.22 | −0.11 | 0.68 | 0.02 | −0.07 | 0.18 | 0.17 | 0.19 | 0.23 | −0.14 | −0.04 | 0.12 | 0.15 | 0.16 | |
| 5 | 0.52 | −0.09 | −0.19 | −0.22 | 0.56 | −0.18 | −0.13 | −0.15 | 0.16 | −0.17 | −0.06 | −0.11 | 0.13 | −0.23 | 0.02 | −0.24 | 0.25 | |
| 6 | 0.22 | −0.31 | −0.58 | −0.11 | 0.56 | 0.15 | −0.13 | −0.13 | −0.03 | 0.16 | 0.14 | 0.18 | −0.14 | −0.17 | 0.16 | 0.20 | −0.53 | |
| 7 | −0.09 | −0.64 | −0.36 | 0.68 | −0.18 | 0.15 | −0.61 | 0.14 | −0.05 | 0.60 | −0.05 | 0.17 | 0.11 | −0.19 | 0.14 | 0.13 | 0.14 | |
| 8 | 0.13 | −0.68 | −0.25 | 0.02 | −0.13 | −0.33 | −0.61 | −0.02 | 0.10 | −0.25 | −0.15 | −0.13 | 0.01 | −0.17 | −0.03 | 0.05 | −0.01 | |
| 9 | −0.29 | 0.25 | −0.21 | −0.07 | −0.15 | −0.13 | 0.14 | −0.02 | 0.05 | 0.11 | −0.03 | −0.02 | 0.21 | 0.21 | 0.61 | 0.16 | −0.13 | |
| 10 | 0.02 | 0.19 | 0.08 | 0.18 | 0.16 | −0.03 | −0.05 | 0.10 | 0.05 | 0.26 | 0.22 | −0.14 | 0.12 | 0.25 | 0.15 | −0.58 | 0.15 | |
| 11 | −0.12 | 0.07 | 0.14 | 0.17 | −017 | 0.16 | 0.60 | −0.25 | 0.11 | 0.26 | −0.18 | 0.26 | 0.01 | −0.24 | 0.55 | −0.18 | −0.28 | |
| 12 | −0.08 | 0.15 | 0.17 | 0.19 | −0.06 | 0.14 | −0.05 | −0.15 | −0.03 | 0.22 | −0.18 | 0.21 | 0.22 | 0.14 | −0.17 | −0.06 | −0.24 | |
| 13 | −0.10 | 0.62 | 0.15 | 0.23 | −0.11 | 0.18 | 0.17 | −0.13 | 0.02 | −0.14 | 0.26 | 0.21 | 0.19 | −0.16 | 0.09 | −0.19 | 0.18 | |
| 14 | −0.13 | −0.14 | 0.23 | −0.14 | 0.13 | −0.14 | 0.11 | 0.01 | 0.21 | 0.12 | 0.01 | 0.22 | 0.19 | 0.02 | 0.19 | −0.11 | −0.30 | |
| 15 | 0.16 | −0.18 | −0.16 | −0.04 | −0.23 | −0.17 | −0.19 | −0.17 | 0.21 | 0.25 | −0.24 | 0.14 | −0.16 | 0.02 | 0.17 | 0.53 | −0.28 | |
| 16 | −0.15 | 0.01 | −0.04 | 0.12 | 0.02 | 0.16 | 0.14 | −0.03 | 0.61 | 0.15 | 0.55 | −0.17 | 0.09 | 0.19 | 0.17 | −0.54 | −0.14 | |
| 17 | −0.17 | 0.10 | 0.24 | 0.15 | −0.24 | 0.20 | 0.13 | 0.05 | 0.16 | −0.58 | −0.18 | −0.06 | −0.19 | −0.11 | 0.53 | −0.54 | −0.19 | |
| 18 | −0.32 | 0.77 | 0.17 | 0.16 | −0.53 | 0.25 | 0.14 | −0.01 | −0.13 | 0.15 | −0.28 | −0.24 | 0.18 | −0.30 | −0.28 | −0.14 | −0.19 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.35 | −0.57 | 0.20 | 0.52 | 0.22 | −0.09 | 0.13 | −0.29 | 0.02 | −0.12 | −0.08 | −0.10 | −0.13 | 0.16 | −0.15 | −0.17 | −0.32 | |
| 2 | 0.35 | 0.65 | −0.04 | −0.09 | −0.31 | −0.64 | −0.68 | 0.25 | 0.19 | 0.07 | 0.15 | 0.62 | −0.14 | −0.18 | 0.01 | 0.10 | 0.77 | |
| 3 | −0.57 | 0.65 | 0.20 | −0.19 | −0.58 | −0.36 | −0.25 | −0.21 | 0.08 | 0.14 | 0.17 | 0.15 | 0.23 | −0.16 | −0.04 | 0.24 | 0.17 | |
| 4 | 0.20 | −0.04 | 0.20 | −0.22 | −0.11 | 0.68 | 0.02 | −0.07 | 0.18 | 0.17 | 0.19 | 0.23 | −0.14 | −0.04 | 0.12 | 0.15 | 0.16 | |
| 5 | 0.52 | −0.09 | −0.19 | −0.22 | 0.56 | −0.18 | −0.13 | −0.15 | 0.16 | −0.17 | −0.06 | −0.11 | 0.13 | −0.23 | 0.02 | −0.24 | 0.25 | |
| 6 | 0.22 | −0.31 | −0.58 | −0.11 | 0.56 | 0.15 | −0.13 | −0.13 | −0.03 | 0.16 | 0.14 | 0.18 | −0.14 | −0.17 | 0.16 | 0.20 | −0.53 | |
| 7 | −0.09 | −0.64 | −0.36 | 0.68 | −0.18 | 0.15 | −0.61 | 0.14 | −0.05 | 0.60 | −0.05 | 0.17 | 0.11 | −0.19 | 0.14 | 0.13 | 0.14 | |
| 8 | 0.13 | −0.68 | −0.25 | 0.02 | −0.13 | −0.33 | −0.61 | −0.02 | 0.10 | −0.25 | −0.15 | −0.13 | 0.01 | −0.17 | −0.03 | 0.05 | −0.01 | |
| 9 | −0.29 | 0.25 | −0.21 | −0.07 | −0.15 | −0.13 | 0.14 | −0.02 | 0.05 | 0.11 | −0.03 | −0.02 | 0.21 | 0.21 | 0.61 | 0.16 | −0.13 | |
| 10 | 0.02 | 0.19 | 0.08 | 0.18 | 0.16 | −0.03 | −0.05 | 0.10 | 0.05 | 0.26 | 0.22 | −0.14 | 0.12 | 0.25 | 0.15 | −0.58 | 0.15 | |
| 11 | −0.12 | 0.07 | 0.14 | 0.17 | −017 | 0.16 | 0.60 | −0.25 | 0.11 | 0.26 | −0.18 | 0.26 | 0.01 | −0.24 | 0.55 | −0.18 | −0.28 | |
| 12 | −0.08 | 0.15 | 0.17 | 0.19 | −0.06 | 0.14 | −0.05 | −0.15 | −0.03 | 0.22 | −0.18 | 0.21 | 0.22 | 0.14 | −0.17 | −0.06 | −0.24 | |
| 13 | −0.10 | 0.62 | 0.15 | 0.23 | −0.11 | 0.18 | 0.17 | −0.13 | 0.02 | −0.14 | 0.26 | 0.21 | 0.19 | −0.16 | 0.09 | −0.19 | 0.18 | |
| 14 | −0.13 | −0.14 | 0.23 | −0.14 | 0.13 | −0.14 | 0.11 | 0.01 | 0.21 | 0.12 | 0.01 | 0.22 | 0.19 | 0.02 | 0.19 | −0.11 | −0.30 | |
| 15 | 0.16 | −0.18 | −0.16 | −0.04 | −0.23 | −0.17 | −0.19 | −0.17 | 0.21 | 0.25 | −0.24 | 0.14 | −0.16 | 0.02 | 0.17 | 0.53 | −0.28 | |
| 16 | −0.15 | 0.01 | −0.04 | 0.12 | 0.02 | 0.16 | 0.14 | −0.03 | 0.61 | 0.15 | 0.55 | −0.17 | 0.09 | 0.19 | 0.17 | −0.54 | −0.14 | |
| 17 | −0.17 | 0.10 | 0.24 | 0.15 | −0.24 | 0.20 | 0.13 | 0.05 | 0.16 | −0.58 | −0.18 | −0.06 | −0.19 | −0.11 | 0.53 | −0.54 | −0.19 | |
| 18 | −0.32 | 0.77 | 0.17 | 0.16 | −0.53 | 0.25 | 0.14 | −0.01 | −0.13 | 0.15 | −0.28 | −0.24 | 0.18 | −0.30 | −0.28 | −0.14 | −0.19 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.35 | −0.57 | 0.20 | 0.52 | 0.22 | −0.09 | 0.13 | −0.29 | 0.02 | −0.12 | −0.08 | −0.10 | −0.13 | 0.16 | −0.15 | −0.17 | −0.32 | |
| 2 | 0.35 | 0.65 | −0.04 | −0.09 | −0.31 | −0.64 | −0.68 | 0.25 | 0.19 | 0.07 | 0.15 | 0.62 | −0.14 | −0.18 | 0.01 | 0.10 | 0.77 | |
| 3 | −0.57 | 0.65 | 0.20 | −0.19 | −0.58 | −0.36 | −0.25 | −0.19 | 0.08 | 0.14 | 0.17 | 0.15 | 0.23 | −0.16 | −0.04 | 0.24 | 0.17 | |
| 4 | 0.20 | −0.04 | 0.20 | −0.22 | −0.11 | 0.68 | 0.02 | −0.07 | 0.18 | 0.17 | 0.19 | 0.23 | −0.14 | −0.04 | 0.12 | 0.15 | 0.16 | |
| 5 | 0.52 | −0.09 | −0.19 | −0.22 | 0.56 | −0.18 | −0.13 | −0.15 | 0.16 | −0.17 | −0.06 | −0.11 | 0.13 | −0.23 | 0.02 | −0.24 | 0.25 | |
| 6 | 0.22 | −0.31 | −0.58 | −0.11 | 0.56 | 0.15 | −0.13 | −0.13 | −0.03 | 0.16 | 0.14 | 0.18 | −0.14 | −0.17 | 0.16 | 0.20 | −0.53 | |
| 7 | −0.09 | −0.64 | −0.36 | 0.68 | −0.18 | 0.15 | −0.61 | 0.14 | −0.05 | 0.60 | −0.05 | 0.17 | 0.11 | −0.19 | 0.14 | 0.13 | 0.14 | |
| 8 | 0.13 | −0.68 | −0.25 | 0.02 | −0.13 | −0.33 | −0.61 | −0.02 | 0.10 | −0.25 | −0.15 | −0.13 | 0.01 | −0.17 | −0.03 | 0.05 | −0.01 | |
| 9 | −0.29 | 0.25 | −0.19 | −0.07 | −0.15 | −0.13 | 0.14 | −0.02 | 0.05 | 0.11 | −0.03 | −0.02 | 0.21 | 0.21 | 0.61 | 0.16 | −0.13 | |
| 10 | 0.02 | 0.19 | 0.08 | 0.18 | 0.16 | −0.03 | −0.05 | 0.10 | 0.05 | 0.26 | 0.22 | −0.14 | 0.12 | 0.25 | 0.15 | −0.58 | 0.15 | |
| 11 | −0.12 | 0.07 | 0.14 | 0.17 | −017 | 0.16 | 0.60 | −0.25 | 0.11 | 0.26 | −0.18 | 0.26 | 0.01 | −0.24 | 0.55 | −0.18 | −0.23 | |
| 12 | −0.08 | 0.15 | 0.17 | 0.19 | −0.06 | 0.14 | −0.05 | −0.15 | −0.03 | 0.22 | −0.18 | 0.21 | 0.22 | 0.14 | −0.17 | −0.06 | −0.24 | |
| 13 | −0.10 | 0.62 | 0.15 | 0.23 | −0.11 | 0.18 | 0.17 | −0.13 | 0.02 | −0.14 | 0.26 | 0.21 | 0.19 | −0.16 | 0.09 | −0.19 | 0.18 | |
| 14 | −0.13 | −0.14 | 0.23 | −0.14 | 0.13 | −0.14 | 0.11 | 0.01 | 0.21 | 0.12 | 0.01 | 0.22 | 0.19 | 0.02 | 0.19 | −0.11 | −0.30 | |
| 15 | 0.16 | −0.18 | −0.16 | −0.04 | −0.23 | −0.17 | −0.19 | −0.17 | 0.21 | 0.25 | −0.24 | 0.14 | −0.16 | 0.02 | 0.17 | 0.53 | −0.28 | |
| 16 | −0.15 | 0.01 | −0.04 | 0.12 | 0.02 | 0.16 | 0.14 | −0.03 | 0.61 | 0.15 | 0.55 | −0.17 | 0.09 | 0.19 | 0.17 | −0.54 | −0.14 | |
| 17 | −0.17 | 0.10 | 0.24 | 0.15 | −0.24 | 0.20 | 0.13 | 0.05 | 0.16 | −0.58 | −0.18 | −0.06 | −0.19 | −0.11 | 0.53 | −0.54 | −0.19 | |
| 18 | −0.32 | 0.77 | 0.17 | 0.16 | −0.53 | 0.25 | 0.14 | −0.01 | −0.13 | 0.15 | −0.23 | −0.24 | 0.18 | −0.30 | −0.28 | −0.14 | −0.19 |
| Issues | Site 1-Sheep Manure, 39.52 t/ha | Site 2-Sheep Manure, 79.04 t/Ha | Site 3-Sheep Manure, 118.56 t/Ha | Site 4-Mineral Fertilization N14:P7:K21 | ||||
|---|---|---|---|---|---|---|---|---|
| Component | Component | Component | Component | |||||
| PC1 | PC2 | PC1 | PC2 | PC1 | PC2 | PC1 | PC2 | |
| Turbidity, NTU | 0.750 | −0.042 | −0.773 | −0.386 | −0.733 | 0.043 | −0.108 | 0.087 |
| pH | −0.263 | 0.850 | 0.389 | −0.749 | 0.413 | 0.688 | 0.424 | 0.124 |
| Conductivity, µS/cm | −0.527 | 0.022 | −0.433 | −0.370 | 0.016 | −0.785 | −0.034 | −0.633 |
| Chlorides, mg/L | −0.702 | −0.032 | 0.162 | −0.553 | −0.242 | 0.289 | 0.531 | 0.187 |
| Permanganate index, mg O2/L | −0.150 | 0.733 | −0.501 | −0.194 | 0.423 | 0.162 | 0.289 | 0.561 |
| Ammonium, mg/L | 0.159 | 0.152 | 0.444 | −0.314 | −0.626 | 0.403 | 0.390 | −0.246 |
| Nitrites, mg/L | −0.701 | 0.482 | 0.114 | 0.795 | −0.259 | 0.938 | −0.792 | 0.316 |
| Nitrates, mg/L | −0.873 | 0.017 | −0.054 | 0.589 | 0.368 | −0.532 | 0.897 | 0.076 |
| Total hardness, °G | −0.381 | 0.586 | −0.074 | 0.776 | 0.598 | 0.301 | −0.261 | −0.535 |
| Calcium, mg/L | −0.666 | −0.424 | 0.071 | −0.247 | 0.525 | 0.022 | 0.143 | −0.862 |
| Magnesium, mg/L | 0.550 | 0.004 | −0.700 | 0.430 | 0.088 | 0.679 | −0.808 | −0.256 |
| Sulfates, mg/L | −0.766 | 0.101 | 0.854 | 0.296 | −0.292 | −0.239 | −0.591 | 0.420 |
| Fluoride, mg/L | 0.093 | 0.543 | −0.402 | 0.727 | −0.509 | 0.531 | 0.221 | −0.731 |
| Sulfides, µg/L | 0.212 | 0.391 | 0.747 | 0.053 | 0.649 | 0.307 | 0.593 | 0.507 |
| Alkalinity, mL HCl 0.1 N | 0.466 | 0.649 | −0.303 | −0.033 | 0.322 | −0.376 | 0.397 | −0.718 |
| Bicarbonates, mg/L | 0.485 | 0.407 | 0.714 | −0.022 | 0.531 | 0.420 | 0.627 | −0.319 |
| Iron, µg/L | 0.134 | −0.253 | 0.453 | 0.150 | −0.762 | −0.073 | −0.039 | 0.422 |
| Phosphates, mg/L | −0.258 | −0.391 | 0.709 | 0.308 | 0.323 | 0.137 | −0.639 | −0.531 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Covaciu, D.C.; Balint, A.C.; Neamțu, C.V.; Moșneag, S.C.; Bordea, D.; Dîrjan, S.; Odagiu, A.C.M. Assessment of Groundwater Quality in Relation to Organic versus Mineral Fertilization. Water 2023, 15, 2895. https://doi.org/10.3390/w15162895
Covaciu DC, Balint AC, Neamțu CV, Moșneag SC, Bordea D, Dîrjan S, Odagiu ACM. Assessment of Groundwater Quality in Relation to Organic versus Mineral Fertilization. Water. 2023; 15(16):2895. https://doi.org/10.3390/w15162895
Chicago/Turabian StyleCovaciu (Neamțu), Diana Cătălina, Ana Claudia Balint, Călin Vasile Neamțu, Silvia Claudia Moșneag, Daniela Bordea, Sorina Dîrjan, and Antonia Cristina Maria Odagiu. 2023. "Assessment of Groundwater Quality in Relation to Organic versus Mineral Fertilization" Water 15, no. 16: 2895. https://doi.org/10.3390/w15162895
APA StyleCovaciu, D. C., Balint, A. C., Neamțu, C. V., Moșneag, S. C., Bordea, D., Dîrjan, S., & Odagiu, A. C. M. (2023). Assessment of Groundwater Quality in Relation to Organic versus Mineral Fertilization. Water, 15(16), 2895. https://doi.org/10.3390/w15162895








